Introduction
Multiple myeloma (MM) is the second most common
hematological malignancy and is characterized by the abnormal
proliferation of plasma cells in the bone marrow (1–3). The
heterogeneous nature of MM results in varied response rates and
survival outcomes among patients receiving identical treatments
(4,5). Given that MM remains incurable,
accurate risk stratification is essential for evaluating patient
prognosis and determining optimal treatment strategies.
Cytogenetic abnormalities are critical prognostic
factors in patients with MM. Among these, 1q21+ is one of the most
frequently observed chromosomal aberrations, occurring in ~40% of
newly diagnosed MM cases and 70% of relapsed/refractory cases
(6). Previous studies have
consistently identified 1q21+ as a poor prognostic marker and a
high-risk factor in patients with MM (7–9). The
introduction of novel drugs, including the proteasome inhibitor
bortezomib, has significantly improved the prognosis of patients
with MM (10). While some studies
suggest that bortezomib may alleviate the negative impact of 1q21
aberration (11,12), others report conflicting outcomes
(13,14). Therefore, a systematic review is
necessary to clarify the prognostic significance of 1q21+ in
patients with MM undergoing bortezomib-based treatment.
Relying solely on individual studies often fails to
yield definitive conclusions. By contrast, meta-analyses have the
potential to overcome the limitations of single studies by
consolidating findings from multiple sources and resolving
discrepancies. Therefore, a meta-analysis was conducted in the
present study to evaluate the prognostic significance of 1q21+ in
patients with MM undergoing bortezomib-based treatment.
Materials and methods
Literature screening to identify
related studies
The present meta-analysis was conducted following
the guidelines of the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses guidelines (15). In the present study, two authors
independently screened relevant studies from the Embase (http://www.embase.com), PubMed (http://pubmed.ncbi.nlm.nij.gov/) and Cochrane Library
databases (http://www.cochranelibrary.com). The search was
restricted to publications in English and included studies
published from inception until April 1, '23. The search focus was
on studies that compared survival rates or response rates between
patients with and without the 1q21+ aberration who underwent
bortezomib-based treatment. The search strategy used various
combinations of the following keywords: (((+1q21) OR (Gain(1q))) OR
(Amp(1q))) OR (Chromosome 1 abnormality)) AND (bortezomib).
Inclusion and exclusion criteria
The inclusion criteria for the present study were as
follows: i) Studies investigating the prognostic significance of
1q21+ in patients with MM treated with bortezomib; ii)
administration of bortezomib-based treatment to patients; iii)
division of patients into two groups based on the presence or
absence of 1q21+; iv) inclusion of at least one of the following
three indicators for analysis: Overall survival (OS),
progression-free survival (PFS) or complete response (CR) rate; and
v) publication in a peer-reviewed journal. The exclusion criteria
were as follows: i) Reviews, meeting abstracts and letters that did
not include a full text in English; ii) non-human studies; and iii)
studies lacking usable data.
Data extraction from the related
studies
The first two authors independently extracted
detailed data, including the first author, year of publication,
country, sample size, OS, PFS and CR rate. In cases where
discrepancies arose during the data extraction process, consensus
was reached through discussion with another author.
Quality assessment
The quality of the included studies was
independently evaluated by two investigators using the
Newcastle-Ottawa Scale (NOS). The NOS assesses research quality
across three domains: Selection (up to 4 points), comparability (up
to 2 points) and outcome assessment (up to 3 points). Each study
received a total score ranging from 0 to 9, with a score of ≥7
indicating a high-quality study (16).
Statistical analysis
The hazard ratios (HRs) for PFS and OS, along with
their corresponding 95% confidence intervals (CIs), as well as the
CR rates, were extracted directly from the included studies.
Heterogeneity among the studies was assessed using the Cochran Q
test and the I2 statistic. Since heterogeneity is always
expected for the intervention effects among multiple studies from
different groups and geographical locations, a random effects model
was used to account for this. Publication bias was evaluated using
a funnel plot. All statistical analyses were performed using Review
Manager 5.4 (The Cochrane Collaboration), and P<0.05 was
considered to indicate a statistically significant difference
(17).
Results
Characteristics of the included
studies
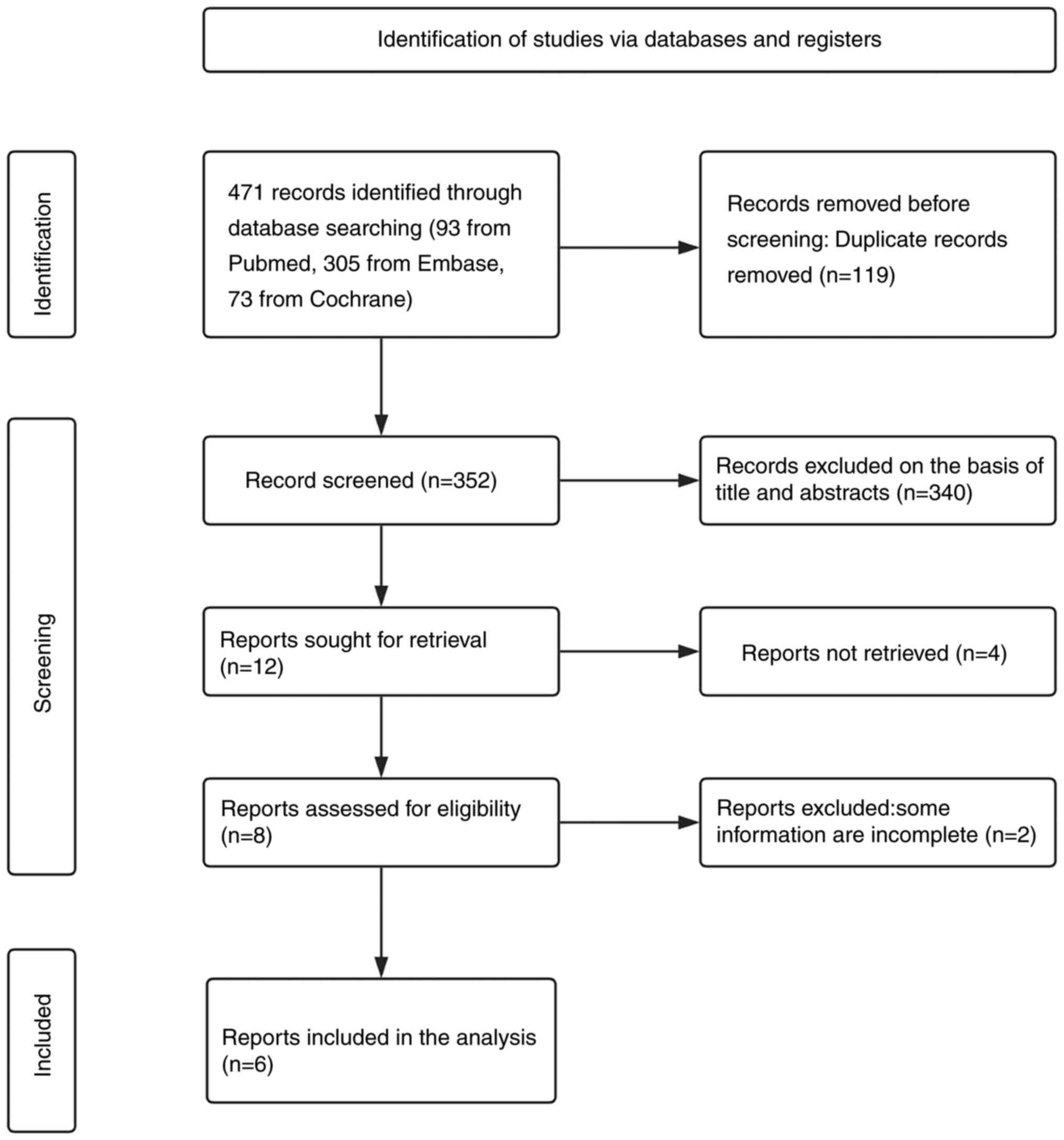
Following a comprehensive full-text screening, 8
articles were identified that initially met the inclusion criteria
for analysis. However, 2 of these articles, authored by Sonneveld
et al (12) and Smetana
et al (11), were excluded
as they did not provide HRs for PFS or OS. Consequently, the final
analysis included 6 studies involving a total of 1,575 patients, as
illustrated in Fig. 1. These
studies were published between 2010 and 2022, with 1 study
published in 2011 (14), 2 in 2019
(18,19), 1 in 2020 (20) and 2 in 2022 (21,22).
The research was conducted across various countries, including
China, Canada and the United States. The objective of these studies
was to investigate the prognostic value of 1q21+ in patients with
MM treated with bortezomib-based regimens. This was assessed by
comparing PFS, OS and the response rates between patients with
1q21+ and those without. Among the 6 studies, 5 identified the
presence of 1q21 when patients were newly diagnosed with MM, except
the study by Chang et al (14). The study conducted by Chang et
al (14) identified the
chromosome aberrations at the relapsed stage of the disease prior
to bortezomib therapy. The examination of the presence of 1q21+ was
performed before the patients received bortezomib-based treatment
in all studies. The observation periods of all studies were
sufficient to calculate the PFS rate both in patients with 1q21+
and those without 1q21+ and were sufficient to calculate the OS
rate in patients with 1q21+. The OS of patients without 1q21+ was
not reached in the studies by Li et al (18) and Du et al (20).
The quality of the included studies was assessed
using the NOS, with scores ranging from 7 to 8, indicating high
quality. Among the included studies, 5 studies, comprising a total
of 1,240 patients (with individual study sample sizes ranging from
85 to 414), reported PFS rates, along with the HR for PFS and the
corresponding 95% CI (14,18,19,20,21).
Additionally, 4 studies, involving 1,031 patients (with sample
sizes ranging from 85 to 414), examined OS, HR for OS and 95% CI
for HR (14,18,20,22).
Furthermore, 3 studies, including 1,031 patients (with sample sizes
ranging from 250 to 414), reported on the CR rate (18,20,22). A
summary of the main characteristics of the included studies is
detailed in Table I.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Author, year | Country | Design | Sample size, n | Sex, % of males | Median β2M (range),
mg/l | Median LDH (range),
units/l | 1q21+positive, n | 1q21+negative, n | Outcomes | Median follow-up
(range), months | Treatment | NOS | (Refs.) |
|---|
| Chang et al,
2011 | Canada | Retro | 85 | 61 | 2.4 0.4–19.9) | / | 31 | 54 | OS and PFS | 37 | VTD, VCD, VRD, VMP,
PAD and VD | 8 | (14) |
| Li et al,
2019 | China | Retro | 414 | 58 | / | 172.0
(70.0–1356.0) | 156 | 258 | CR rate | 23.8 (0.5–129.5) | Bortezomib-based | 7 | (18) |
| Schmidt et al,
2019 | America | Retro | 201 | 57 | 2.95 | 150 | 94 | 107 | PFS | 48 | VRD | 8 | (19) |
| Du et al,
2020 | China | Retro | 367 | / | / | / | 203 | 164 | OS, PFS and CR
rate | 43 (3–113) | VAD and VCD | 7 | (20) |
| Chen et al,
2022 | China | Retro | 258 | 57 | 3.90
(1.03–40.1) | 185 (79–5758) | 127 | 131 | PFS | 24.1
(1.1–71.0) | VD | 7 | (21) |
| Tang et al,
2022 | China | Retro | 250 | 55 | / | / | 167 | 83 | OS and CR rate | / | VTD | 7 | (22) |
Meta-analysis results
Association between 1q21+ and the PFS rate of
patients with MM
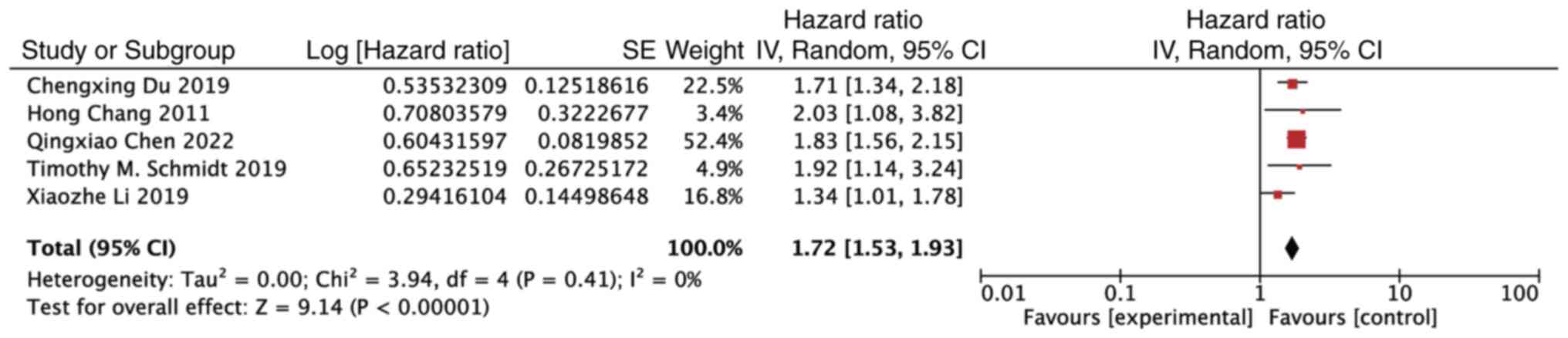
The findings of the meta-analysis revealed that the
presence of 1q21+ was a detrimental prognostic factor for PFS in
patients with MM undergoing bortezomib treatment (HR, 1.72; 95% CI,
1.53–1.93; P<0.00001). Notably, there was no observed
heterogeneity among the included studies (P=0.41, I2=0%)
(Fig. 2).
Association between 1q21+ and the OS
rate of patients with MM
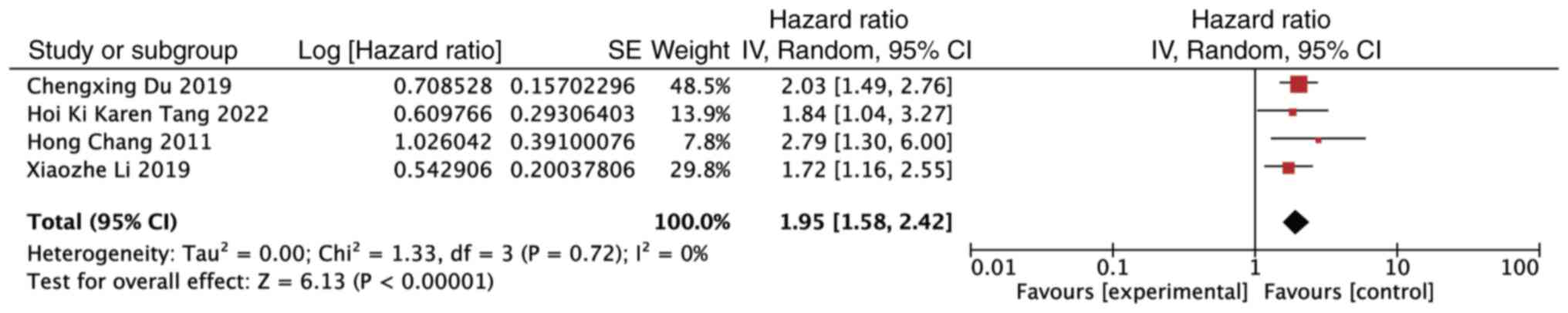
Similarly, the analysis of the OS demonstrated that
1q21+ was an unfavorable prognostic factor for patients with MM
receiving bortezomib treatment (HR, 1.95; 95% CI, 1.58–2.42;
P<0.00001). Notably, no significant heterogeneity was detected
among the studies (P=0.72, I2=0%) (Fig. 3).
Association between 1q21+ and the CR
rate of patients with MM
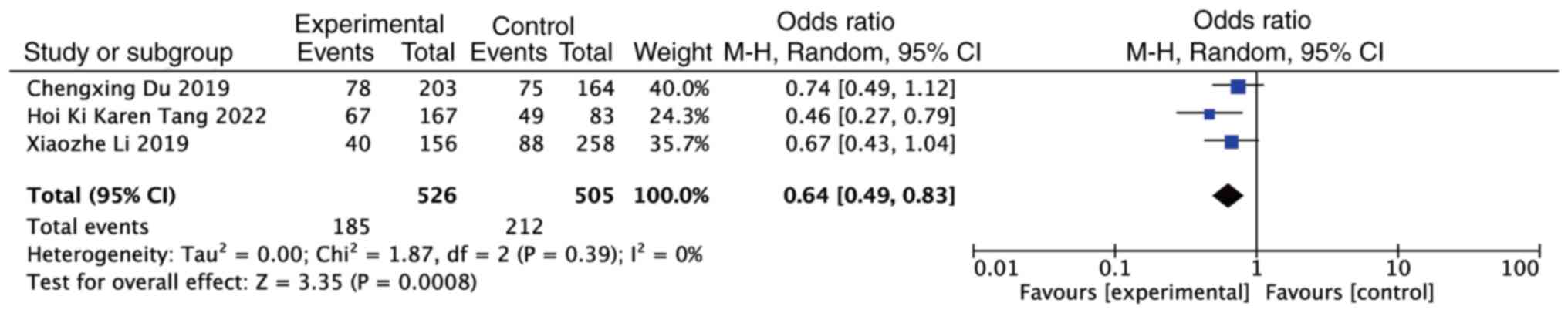
By contrast, the meta-analysis revealed that the
presence of 1q21+ acted as a protective factor for the CR rate in
patients with MM treated with bortezomib (OR, 0.64; 95% CI,
0.49–0.83; P=0.0008). However, no heterogeneity was observed among
the studies (P=0.39, I2=0%) (Fig. 4).
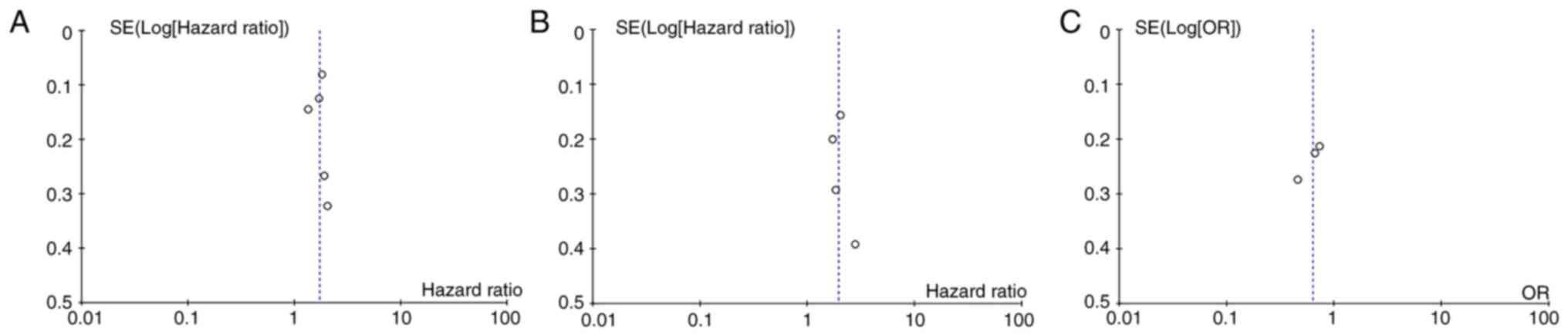
Publication bias
No evidence of publication bias was detected in the
analyses of OS, PFS and CR rate (Fig.
5).
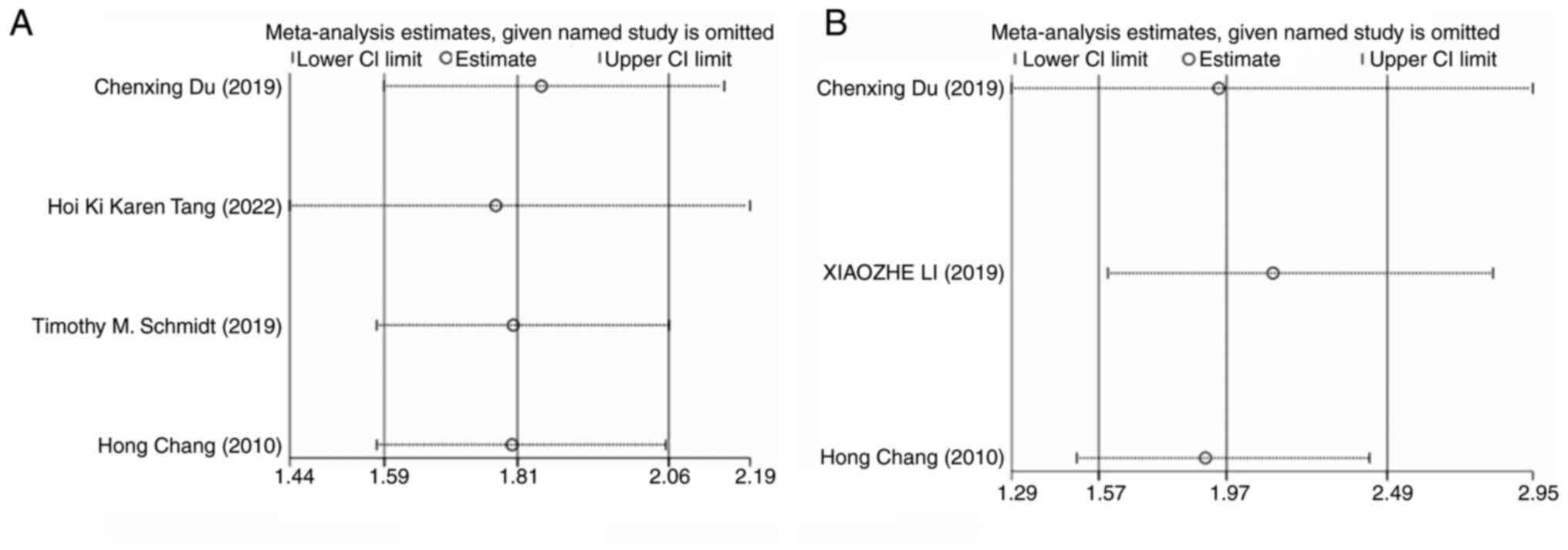
Sensitivity analysis of PFS and
OS
The sensitivity analysis for PFS included 4 studies.
When any single study was excluded from the analysis, the combined
results of the remaining studies consistently aligned with the
original pooled estimates, indicating the robustness of the
findings. Similarly, the sensitivity analysis for OS, which
included 3 studies, showed that excluding any individual study did
not alter the overall results, further confirming the stability of
the study findings (Fig. 6).
Discussion
In total, ~40% of patients with MM exhibit 1q21+, a
genetic aberration associated with poor prognosis (23). Over the past decades, the
introduction of novel therapeutics has notably improved outcomes
for patients with MM (24). Despite
these advancements, the prognosis for patients with 1q21+ remains a
significant challenge (25). Some
studies suggest that, with appropriate treatment strategies,
patients harboring certain high-risk factors can achieve survival
outcomes comparable to those with standard risk. For instance, a
large trial demonstrated that a treatment regimen comprising
bortezomib-based induction, early autologous stem cell
transplantation and bortezomib maintenance resulted in a median OS
time of ~8 years (with an 8-year survival rate of 52%) for patients
with del(17p), matching the survival rates of patients with
standard-risk MM (26). In the
HOVON-65/GMMG-HD4 trial, patients were randomized to receive either
three cycles of VAD (arm A: vincristine, adriamycin and
dexamethasone) or PAD (arm B: bortezomib, adriamycin and
dexamethasone). With a median follow-up time of 40.3 months, the
trial revealed that patients with 1q21+ experienced significantly
improved OS rates when treated with bortezomib (3-year OS rates:
Arm A, 59%, arm B, 83%; P=0.016) (10). Given these findings, it is
imperative to explore whether bortezomib-based treatments can
mitigate the adverse prognostic impact of 1q21+, thereby enabling
patients with this genetic aberration to achieve survival outcomes
comparable to those without the gain.
According to a study by Smetana et al
(11), there was no significant
difference in the overall response rate between patients with or
without 1q21+ when treated with a bortezomib-based regimen (44.8
vs. 44.4%; P=0.996). Additionally, the differences in time to
progression (TTP) and OS between the two groups were not
statistically significant (TTP, 12.9 vs. 13.9 months; P=0.983; and
OS, 29 vs. 37.1 months; P=0.146). Based on these findings, the
study suggested that 1q21+ might not serve as an unfavorable
prognostic factor in patients undergoing bortezomib treatment
(11). However, a conflicting
report by Du et al (20)
indicated that patients with 1q21+ had a significantly shorter
median PFS and OS time compared with those without the gain in a
bortezomib-based cohort. Given these contradictory findings, a
meta-analysis was conducted in the present study, including 6
studies and a total of 1,575 patients, to clarify the prognostic
significance of 1q21+ in this context.
The results of the present study indicated that
patients with 1q21+ were more likely to achieve a CR than those
without it when undergoing bortezomib-based treatment (OR, 0.64;
95% CI, 0.49–0.83; P=0.0008). However, it is important to note that
1q21+ still conferred a poor prognostic outcome following
bortezomib treatment, as evidenced by the PFS (HR, 1.81; 95% CI,
1.59–2.06; P<0.00001) and OS (HR, 2.06; 95% CI, 1.60–2.66;
P<0.00001) results. Notably, no publication bias was identified,
underscoring the reliability of these findings and providing
valuable guidance for clinicians when selecting treatment
strategies for patients with high-risk MM. Given the limited
efficacy of bortezomib in improving outcomes for patients with
1q21+, we recommend that clinicians consider alternative treatment
regimens for these individuals.
In the present study, the paradoxical results
indicated that a higher percentage of patients with 1q21+ achieved
CR but with a poor prognosis. The poor prognosis of patients with
1q21+ can be attributed to findings from the Total Therapy 3 trial.
Specifically, it has been proposed that this poor efficacy is
linked to the upregulation of proteasome 26S subunit ubiquitin
receptor, non-ATPase 4 (PSMD4), a proteasome subunit encoded at
1q21. Notably, PSMD4 showed a strong correlation with the copy
number of 1q and emerged as a significant poor prognostic factor in
both the GEP-70 and GEP-80 models developed by the UAMS-MIRT group.
The upregulation of PSMD4 appears to contribute to resistance
against bortezomib, and the presence of additional copies of 1q21
further intensifies this resistance (27). There have been no relevant
fundamental studies to determine why patients with 1q21+ are more
likely to achieve CR than patients without 1q21+. We speculate that
myeloma cells with 1q21+ may have a higher proteasome activity,
with which bortezomib-based treatment can exert greater efficacy at
first; therefore, a higher percentage of patients with MM harboring
1q21+ achieve CR with bortezomib-based regimens. However, as the
disease develops, patients are highly susceptible to drug
resistance, so even after achieving a CR after initial treatment,
these patients will relapse early and eventually have a poor
prognosis. Additionally, Cao et al (28) hypothesized that ageing of bone
marrow mesenchymal stem cells is associated with the progression of
MM. In the study, a prognostic risk model was established based on
this assumption, in which a copy number of 1q21 >2 had a higher
risk score and was associated with early progression (28). These findings provide support for
considering 1q21+ as a high-risk factor, even for patients
receiving bortezomib. However, more studies are needed to explain
why 1q21+ is associated with achieving a CR.
Generally, 1q21 refers to the presence of additional
copies of the 21 portions of chromosome 1q (29). The number of these copies can
significantly impact the prognostic significance of 1q21+, with an
increased copy number often associated with worse outcomes
(13,25,30).
According to a study by Gao et al (31), 1q21+ becomes an unfavorable
prognostic factor when ≥4 copies are present. Additionally, while
bortezomib-based treatment may improve PFS in patients with 3
copies, it appears less effective in those with ≥4 copies (31). Unfortunately, due to the limited
number of studies in this area, it was not possible to conduct a
subgroup analysis based on varying copy numbers of 1q21. Future
research should focus on examining the prognostic value of
different 1q21 copy numbers under treatment with novel therapeutic
agents.
The present study specifically investigated the
impact of bortezomib on patients with 1q21+. However, further
research is needed to explore the effectiveness of other novel
therapies, such as immunomodulatory drugs and anti-CD38 monoclonal
antibodies, in patients with MM harboring 1q21+. Such studies will
be crucial in identifying the most appropriate treatment strategies
for these patients. Several limitations of the present study must
be acknowledged. First, most of the included studies were
retrospective in nature, which is a significant limitation. Second,
the geographic scope of the included studies was predominantly
confined to three countries, which may introduce some bias. Lastly,
there is variability in the treatment strategies employed across
the different studies, which could potentially influence the
results.
Additionally, several strengths of the present study
should be highlighted. First, a large sample size of 1,575 patients
was included in the analysis, allowing for a robust quantitative
assessment of the prognostic significance of 1q21+. This
comprehensive analysis offered a powerful evaluation of this
important issue. Second, the present study incorporated 5 studies
published between 2019 and 2022, ensuring the timeliness and
relevance of the findings. Lastly, the analysis was not impacted by
publication bias, further strengthening the reliability of the
conclusions.
In conclusion, the results of the present study,
based on 6 studies involving 1,575 patients diagnosed with MM,
consistently demonstrated that 1q21+ remains a significant
high-risk factor among patients with MM treated with bortezomib.
Although patients with MM harboring 1q21+ may exhibit a higher
likelihood of achieving CR under bortezomib treatment compared with
those without this aberration, it is clear that bortezomib cannot
fully mitigate the adverse prognostic impact associated with 1q21+.
Therefore, clinicians are encouraged to consider alternative
treatment strategies for patients with MM harboring 1q21+
aberration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ and SL confirm the authenticity of all the raw
data. XZ contributed to the conception and design of the study,
data analysis and interpretation, as well as manuscript writing. SL
contributed to manuscript writing and data analysis and
interpretation. KL contributed to data analysis and interpretation.
JH and TL contributed to the design of the study, submission and
communication. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar
|
|
2
|
Pulte D, Jansen L, Castro FA, Emrich K,
Katalinic A, Holleczek B and Brenner H; GEKID Cancer Survival
Working Group, : Trends in survival of multiple myeloma patients in
Germany and the United States in the first decade of the 21st
century. Br J Haematol. 171:189–196. 2015. View Article : Google Scholar
|
|
3
|
Cullis J: Haematology: Multiple myeloma.
Clin Med (Lond). 19:1882019. View Article : Google Scholar
|
|
4
|
Sonneveld P, Avet-Loiseau H, Lonial S,
Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle
RA, et al: Treatment of multiple myeloma with high-risk
cytogenetics: A consensus of the International Myeloma Working
Group. Blood. 127:2955–2962. 2016. View Article : Google Scholar
|
|
5
|
Soekojo CY and Chng WJ: Treatment horizon
in multiple myeloma. Eur J Haematol. 109:425–440. 2022. View Article : Google Scholar
|
|
6
|
Burroughs Garcìa J, Eufemiese RA, Storti
P, Sammarelli G, Craviotto L, Todaro G, Toscani D, Marchica V and
Giuliani N: Role of 1q21 in multiple myeloma: From pathogenesis to
possible therapeutic targets. Cells. 10:13602021. View Article : Google Scholar
|
|
7
|
Hanamura I: Multiple myeloma with
High-risk cytogenetics and its treatment approach. Int J Hematol.
115:762–777. 2022. View Article : Google Scholar
|
|
8
|
D'Agostino M, Cairns DA, Lahuerta JJ,
Wester R, Bertsch U, Waage A, Zamagni E, Mateos MV, Dall'Olio D,
van de Donk NWCJ, et al: Second revision of the international
staging system (R2-ISS) for overall survival in multiple myeloma: A
european myeloma network (EMN) report within the HARMONY project. J
Clin Oncol. 40:3406–3418. 2022. View Article : Google Scholar
|
|
9
|
Hassan H and Szalat R: Genetic predictors
of mortality in patients with multiple myeloma. Appl Clin Genet.
14:241–254. 2021. View Article : Google Scholar
|
|
10
|
McBride A and Ryan PY: Proteasome
inhibitors in the treatment of multiple myeloma. Expert Rev
Anticancer Ther. 13:339–538. 2013. View
Article : Google Scholar
|
|
11
|
Smetana J, Berankova K, Zaoralova R, Nemec
P, Greslikova H, Kupska R, Mikulasova A, Frohlich J, Sevcikova S,
Zahradova L, et al: Gain(1)(q21) is an unfavorable genetic
prognostic factor for patients with relapsed multiple myeloma
treated with thalidomide but not for those treated with bortezomib.
Clin Lymphoma Myeloma Leuk. 13:123–130. 2013. View Article : Google Scholar
|
|
12
|
Sonneveld P, Schmidt-Wolf IG, van der Holt
B, El Jarari L, Bertsch U, Salwender H, Zweegman S, Vellenga E,
Broyl A, Blau IW, et al: Bortezomib induction and maintenance
treatment in patients with newly diagnosed multiple myeloma:
Results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin
Oncol. 30:2946–2955. 2012. View Article : Google Scholar
|
|
13
|
An G, Xu Y, Shi L, Shizhen Z, Deng S, Xie
Z, Sui W, Zhan F and Qiu L: Chromosome 1q21 gains confer inferior
outcomes in multiple myeloma treated with bortezomib but copy
number variation and percentage of plasma cells involved have no
additional prognostic value. Haematologica. 99:353–359. 2014.
View Article : Google Scholar
|
|
14
|
Chang H, Trieu Y, Qi X, Jiang NN, Xu W and
Reece D: Impact of cytogenetics in patients with relapsed or
refractory multiple myeloma treated with bortezomib: Adverse effect
of 1q21 gains. Leuk Res. 35:95–98. 2011. View Article : Google Scholar
|
|
15
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar
|
|
16
|
Wells GA, Shea BJ, O'Connell D, Peterson J
and Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the
quality of non-randomized studies in meta-analysis. Sci Educ;
2000
|
|
17
|
Lee YH: An overview of meta-analysis for
clinicians. Korean J Intern Med. 33:277–283. 2018. View Article : Google Scholar
|
|
18
|
Li X, Chen W, Wu Y, Li J, Chen L, Fang B,
Feng Y, Liu J, Chen M, Gu J, et al: 1q21 Gain Combined with
High-risk factors is a heterogeneous prognostic factor in newly
diagnosed multiple myeloma: A multicenter study in China.
Oncologist. 24:e1132–e1140. 2019. View Article : Google Scholar
|
|
19
|
Schmidt TM, Barwick BG, Joseph N, Heffner
LT, Hofmeister CC, Bernal L, Dhodapkar MV, Gupta VA, Jaye DL, Wu J,
et al: Gain of Chromosome 1q is associated with early progression
in multiple myeloma patients treated with lenalidomide, bortezomib,
and dexamethasone. Blood Cancer J. 9:942019. View Article : Google Scholar
|
|
20
|
Du C, Mao X, Xu Y, Yan Y, Yuan C, Du X,
Liu J, Fan H, Wang Q, Sui W, et al: 1q21 gain but not t(4;14)
indicates inferior outcomes in multiple myeloma treated with
bortezomib. Leuk Lymphoma. 61:1201–1210. 2020. View Article : Google Scholar
|
|
21
|
Chen Q, Han X, Zheng G, Yang Y, Li Y,
Zhang E, Yang L, Dong M, He D, He J and Cai Z: The adverse impact
of a gain in chromosome 1q on the prognosis of multiple myeloma
treated with bortezomib-based regimens: A retrospective
single-center study in China. Front Oncol. 12:10846832022.
View Article : Google Scholar
|
|
22
|
Tang HKK, Fung CY, Morgan GJ, Kumar S, Siu
L, Ip HWA, Yip SF, Lau KNH, Lau CK, Lee H, et al: The impact of
bortezomib-based induction in newly diagnosed multiple myeloma with
chromosome 1q21 gain. Ther Adv Hematol. 13:204062072210820432022.
View Article : Google Scholar
|
|
23
|
Bisht K, Walker B, Kumar SK, Spicka I,
Moreau P, Martin T, Costa LJ, Richter J, Fukao T, Macé S, et al:
Chromosomal 1q21 abnormalities in multiple myeloma: A review of
translational, clinical research, and therapeutic strategies.
Expert Rev Hematol. 14:1099–1114. 2021. View Article : Google Scholar
|
|
24
|
Kumar SK, Dispenzieri A, Lacy MQ, Gertz
MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, et
al: Continued improvement in survival in multiple myeloma: Changes
in early mortality and outcomes in older patients. Leukemia.
28:1122–1128. 2014. View Article : Google Scholar
|
|
25
|
Schmidt TM, Fonseca R and Usmani SZ:
Chromosome 1q21 abnormalities in multiple myeloma. Blood Cancer J.
11:832021. View Article : Google Scholar
|
|
26
|
Rajkumar SV: Multiple myeloma: 2022 update
on diagnosis, risk stratification, and management. Am J Hematol.
97:1086–1107. 2022. View Article : Google Scholar
|
|
27
|
Shaughnessy JD Jr, Qu P, Usmani S, Heuck
CJ, Zhang Q, Zhou Y, Tian E, Hanamura I, van Rhee F, Anaissie E, et
al: Pharmacogenomics of bortezomib test-dosing identifies
hyperexpression of proteasome genes, especially PSMD4, as novel
high-risk feature in myeloma treated with Total Therapy 3. Blood.
118:3512–3524. 2011. View Article : Google Scholar
|
|
28
|
Cao YJ, Zheng YH, Li Q, Zheng J, Ma LT,
Zhao CJ and Li T: MSC Senescence-related genes are associated with
myeloma prognosis and lipid metabolism-mediated resistance to
proteasome inhibitors. J Oncol. 2022:47056542022. View Article : Google Scholar
|
|
29
|
Walker BA, Mavrommatis K, Wardell CP,
Ashby TC, Bauer M, Davies F, Rosenthal A, Wang H, Qu P, Hoering A,
et al: A high-risk, Double-Hit, group of newly diagnosed myeloma
identified by genomic analysis. Leukemia. 33:159–170. 2019.
View Article : Google Scholar
|
|
30
|
Neben K, Lokhorst HM, Jauch A, Bertsch U,
Hielscher T, van der Holt B, Salwender H, Blau IW, Weisel K,
Pfreundschuh M, et al: Administration of bortezomib before and
after autologous stem cell transplantation improves outcome in
multiple myeloma patients with deletion 17p. Blood. 119:940–948.
2012. View Article : Google Scholar
|
|
31
|
Gao L, Liu Y, Li Y, Feng L, Wang Z, Wen L,
Wang F, Huang X, Lu J and Lai Y: The Importance of FISH Signal
Cut-off value and copy number variation for 1q21 in newly diagnosed
multiple myeloma: Is it underestimated? Clin Lymphoma Myeloma Leuk.
22:535–544. 2022. View Article : Google Scholar
|