Introduction
Epithelial-myoepithelial carcinoma (EMC), previously
known as malignant myoepithelial tumor and myoepithelial carcinoma,
was first reported in 1972 by Donath et al (1). In 1992, the World Health Organization
classified it as a separate salivary gland tumor (2). EMC is a rare, low-grade malignant
salivary gland-type tumor arising in the head and neck region,
occurring in ~1% of salivary gland adenomas and ~2% of all
malignant tumors of the salivary gland (3). The parotid gland is the most common
site of EMC, followed by the submandibular gland and minor salivary
glands, and occasionally the lacrimal gland (4), maxillary sinus (5), nasopharynx (6), pituitary gland (7), tongue (8), trachea (9), esophagus (10), lungs (11) and mammary glands (12). Despite its propensity for local
recurrence and low metastatic potential, it rarely exhibits
invasive behavior in remote tissues and organs (13). A positive surgical margin is
associated with an increased risk of recurrence, whereas adjuvant
radiotherapy was associated with a reduced risk of local disease
recurrence. The optimal management strategy for EMC remains
undetermined. In addition to complete surgical treatment,
postoperative radiation or chemotherapy is administered depending
on the patient condition (5). Most
of the relevant literature records to date, especially for distant
organ metastasis of EMC, are case reports, which generally lack
more detailed imaging data and morphological feature descriptions.
In the present report, the relevant data of a case of lung
metastasis from parotid epithelial-muscle epithelial carcinoma were
collected, its imaging manifestations and pathological features
were analyzed in detail, and the relevant literature was reviewed,
to deepen the understanding of the disease, highlight the malignant
potential of the disease and provide a reference for its clinical
diagnosis.
Case report
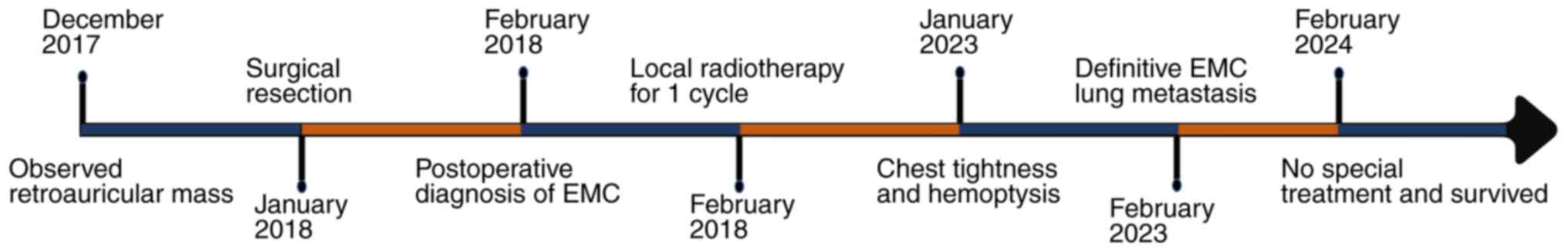
A 60-year-old man presented to Binzhou Medical
University Hospital (Binzhou, China) in December 2017 with a left
retroauricular swelling for >1 month, accompanied by tinnitus
and numbness on the left side of the face after cold stimulation
for the previous 10 days. On physical examination, a painless,
poorly mobile mass measuring 3×3×2 cm was detected deep to the
lower border of the mandible. Initial ultrasound imaging revealed
that the mass was a solid, space-occupying lesion occurring in the
parotid gland (data not shown). In January 2018, computed
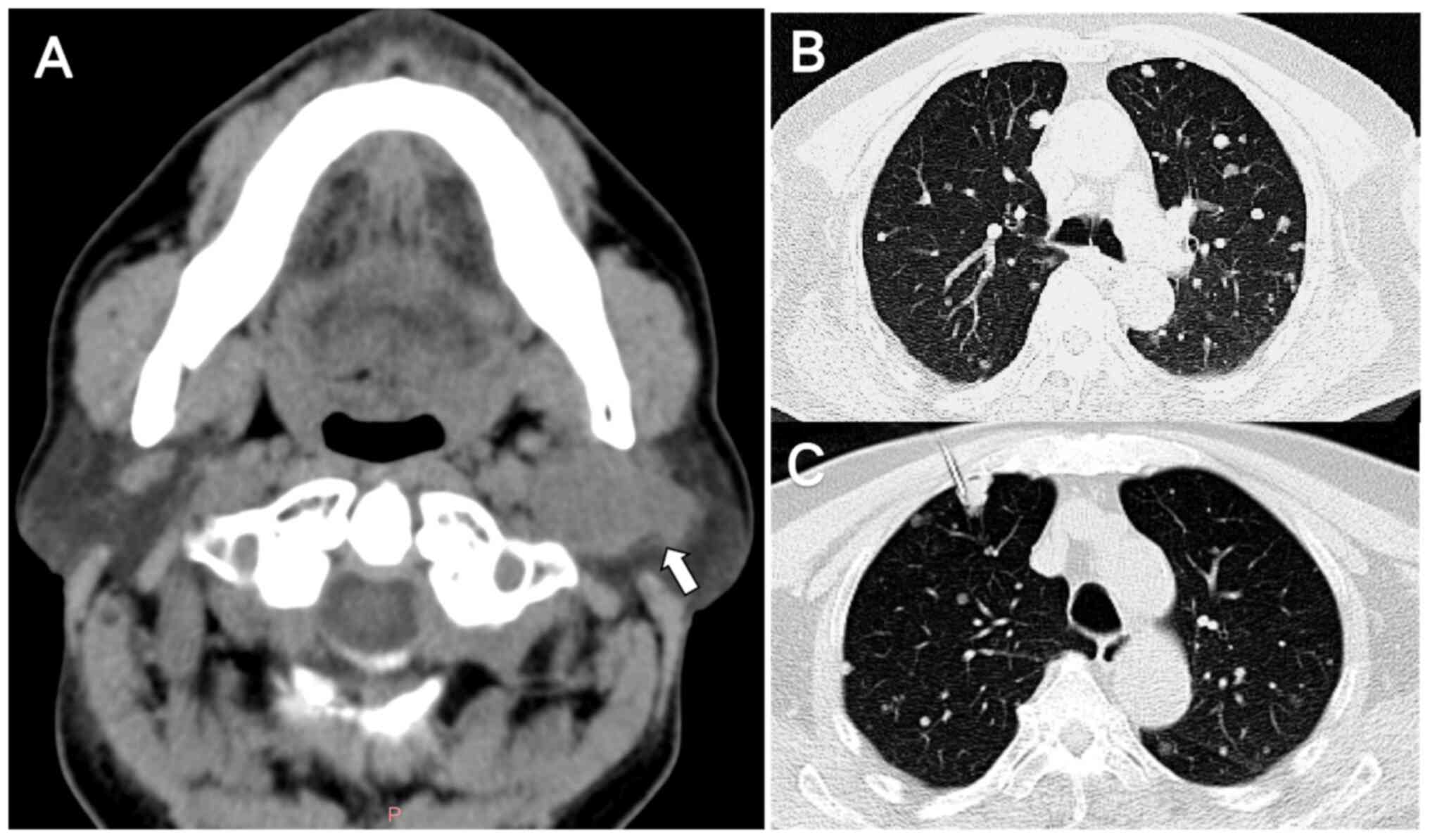
tomography (CT) of the parotid gland further confirmed that there
was a space-involving lesion in the parotid region (Fig. 1A). Additionally, enhanced
dual-source spiral CT of the oropharynx revealed the presence of
foci of abnormal enhancement within the parotid gland, thereby
suggesting a heightened probability of the lesion being malignant.
The patient denied any previous history of hypertension, infectious
diseases, hereditary familial disorders or smoking. Consequently,
in January 2018, the patient underwent a left parotidectomy,
lumpectomy and cervical lymphatic dissection. Intraoperatively, it
was observed that certain branches of the facial nerve were
adherent to the tumor in the deep lobe, necessitating the careful
performance of facial nerve anastomosis. Postoperative pathological
observation revealed a greyish-yellow solid texture of the tumor
section and no metastasis in the cervical lymph nodes.
Immunohistochemistry demonstrated positivity for P63,
pan-cytokeratin (CK), CK7, CD117, S-100 and P63. The final
diagnosis was left parotid EMC and the patient was discharged after
receiving one course of local radiotherapy.
In January 2023, the patient returned to the
hospital reporting chest tightness and hemoptysis. Initial
abdominal ultrasound revealed no significant abnormal lesions;
however, axial chest CT indicated the presence of multiple randomly
distributed nodules in both lungs, suggesting the possibility of
lung metastasis (Fig. 1B). Routine
blood, liver biochemistry, stool and urine testing, as well as
examination of tumor markers such as carcinoembryonic antigen,
non-small cell lung cancer antigen, neurogen-specific enolase and
squamous cell carcinoma-associated antigen showed no significant
abnormalities. The patient underwent
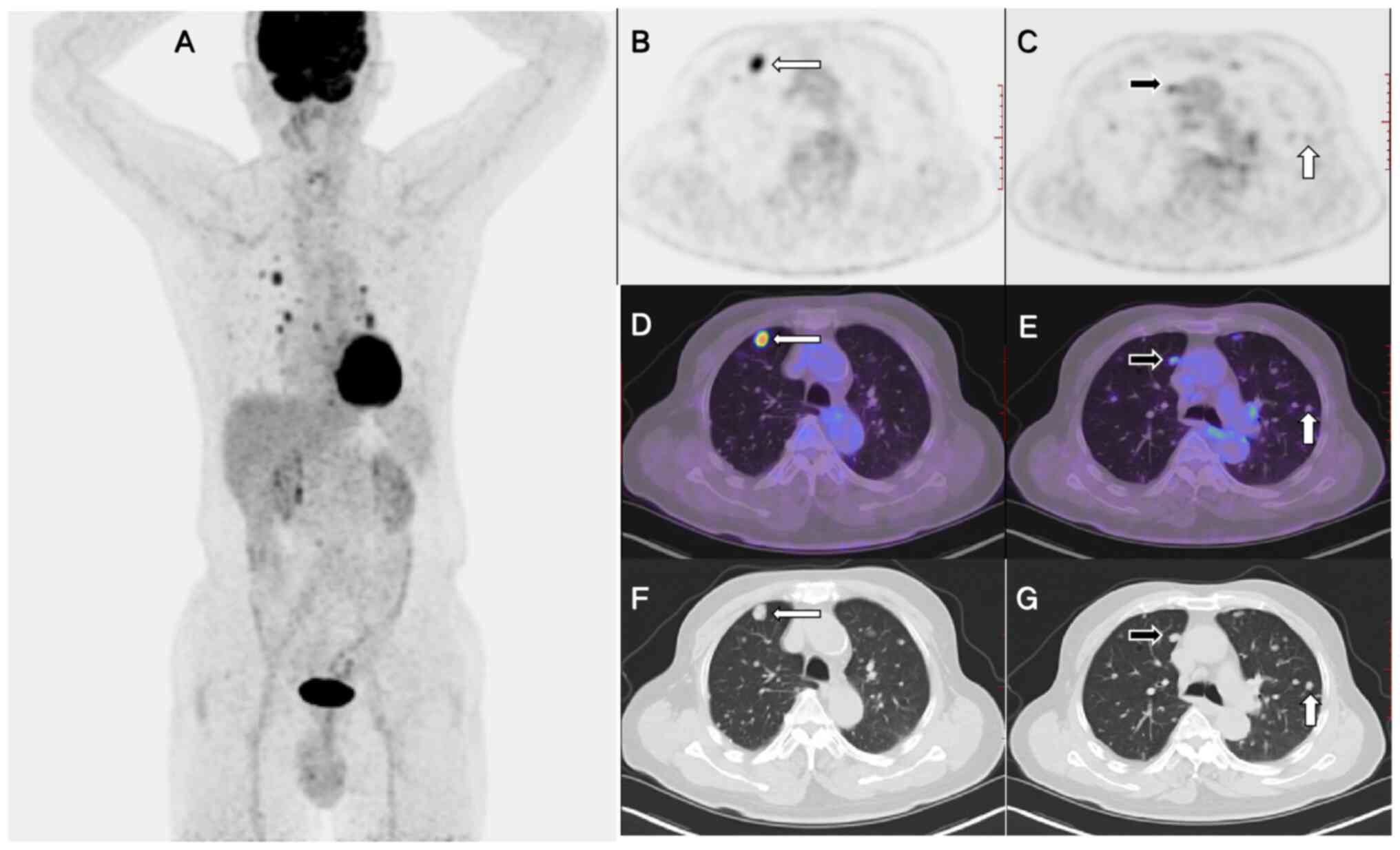
18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET)/CT and fine-needle aspiration biopsy (Fig. 1C) for further diagnosis. The maximal
intensity projection revealed numerous FDG-avid lesions in the
chest (Fig. 2A). The PET and PET/CT
fusion images demonstrated varying degrees of FDG uptake among
these nodules (Fig. 2C and F).
While certain nodules exhibited markedly elevated FDG uptake
[maximum standardized uptake value (SUVmax), 8.8;
Fig. 2B and D], others displayed
lower (SUVmax, 3.3) or negligible (SUVmax,
1.0; Fig. 2E and G) FDG uptake.
Additionally, lymph nodes with increased FDG uptake were observed
in the hila and mediastinum. Pathological analysis of the puncture
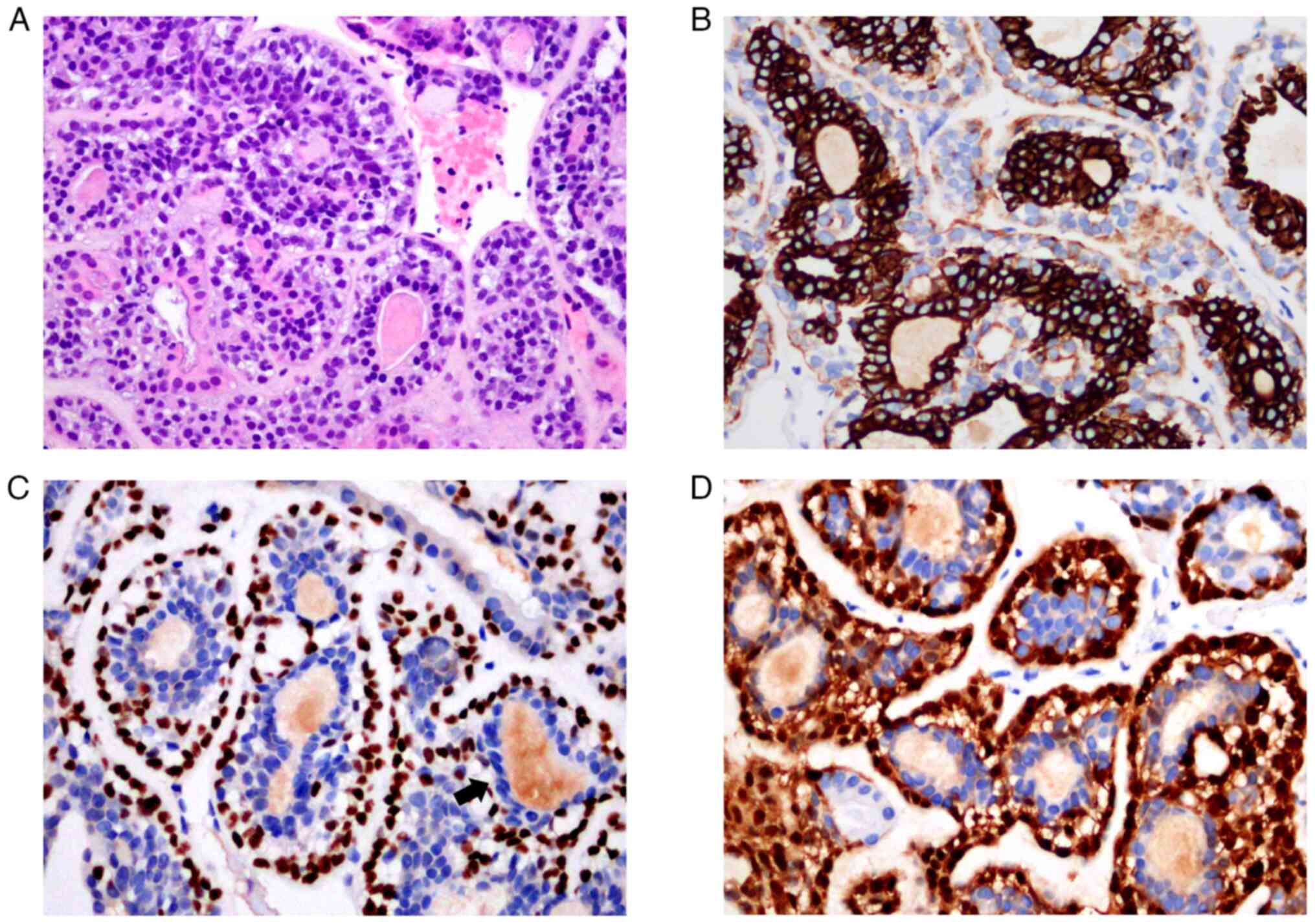
tissues from the pulmonary nodules confirmed the presence of
epithelial-myoepithelial cells (Fig.
3A). Immunohistochemical staining showed columnar cells inside
the glandular ducts were CK+, EMA+, CD117+, CK7+ (Fig. 3B), and the clear cells outside the
glandular ducts were P63+ (Fig.
3C), S-100+ (Fig. 3D), and the
Ki-67 positive rate was about 10%. The pathological diagnosis was
EMC. Based on an extensive evaluation considering the medical
history of the patient, a conclusive diagnosis of pulmonary
metastatic EMC was made. The case timeline is presented in Fig. 4. After the diagnosis of lung
metastases from EMC, an expert opinion from a radiation oncologist
was obtained, and the patient was advised to undergo postoperative
radiation therapy. Treatment was refused by the patient and family
and therefore, a regular strict follow-up (including regular
physical and CT examinations) was planned. A total of 6 years after
left parotidectomy and 20 months after confirmation of lung
metastases, without the use of any drugs or treatment, the patient
had not experienced any local recurrence or abnormally enlarged
lung nodules.
Staining methods
For staining, the tissue was fixed with 4% neutral
formalin for 24 h, embedded in paraffin, and made into 3 µm
continuous sections, which were placed in an oven at 60°C for 60
min. HE staining was performed using a standardized program stainer
(Tissue-TekFilm-JC2) for 70 min and observed using an OLYMPUS
optical microscope (OLYMPUS BX53-DP22). For immunohistochemical
staining, 4 µm sections were used for staining. The procedures were
all performed using the BOND-MAX issued by Leica and the Benchmark
Ultra fully automatic immunohistochemical stainer issued by Roche.
The immunohistochemical experimental procedures were standardized
for staining. The primary and secondary antibodies were antibodies
produced by Fuzhou Maixin Biotechnology Co., Ltd. and Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd. The sections were
observed using an OLYMPUS optical microscope (OLYMPUS
BX53-DP22).
Literature review
The PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science
databases (https://www.webofscience.com/wos/woscc/basic-search;
as of August 1, 2024) were searched for case reports and case
series of EMC with lung metastasis. Adults with relevant imaging
information were considered first. Inclusion criteria were case
report studies on EMC with lung metastasis (e.g.,
epithelial-myoepithelial carcinoma and lung metastasis) written in
English and published after 1990, with a description of pulmonary
metastatic nodules in the literature. Exclusion criteria comprised
studies published before 1990 and those penned in languages other
than English. For each relevant case report, the first author,
publication year and country, as well as the sex, age, primary
tumor location, time of lung metastases detection, CT and PET-CT
imaging findings, methods of diagnosis, treatment and follow-up
results of the patient were recorded (Table I).
 | Table I.Clinical and imaging features of the
cases of epithelial-myoepithelial carcinoma with lung metastases
based on the literature review. |
Table I.
Clinical and imaging features of the
cases of epithelial-myoepithelial carcinoma with lung metastases
based on the literature review.
| First author/s,
year | Country | Sex | Age, years | Primary EMC
location | Time of
metastases | Chest X-ray/CT | PET-CT | Methods of
diagnosis | Treatment | Follow up | (Refs.) |
|---|
| Civan et al,
2024 | Turkey | F | 51 | Parotid gland | 5 years after
surgery | Multiple nodular
lesions in both lungs | Mild to moderate FDG
uptake | Biopsy | Chemotherapy | NA | (14) |
| Mäkelä et
al, 2020 | Finland | F | 36 | Salivary gland | 4 years after
surgery | Multiple small
nodules in right lung | NA | Biopsy | Chemotherapy and
targeted medical therapy | Died ~11 months
later | (15) |
| Chen et al,
2017 | Australia | M | 52 | Base of tongue | Found along with
the primary lesion | NA | Bilateral multiple
pulmonary nodules. No significant FDG uptake | NA | Palliative
care | Died after 18
months | (16) |
| Hsieh et al,
2016 | Taiwan | M | 43 | Right parotid
gland | 3 years prior to
the primary lesion | A total of three
nodular lesions in left lung | NA | Postoperative
pathology | Thoracoscopic
surgery | NA | (17) |
| Yamazaki et
al, 2013 | Japan | M | 35 | Right parotid
gland | 10 months after
surgery | Small nodular
lesions in both lungs | NA | Progression
lesions | Adjuvant
radiotherapy | No recurrence or
metastasis in 2 years | (18) |
| Yang et al,
2012 | China | F | 60 | Left submandibular
gland | 15 months after
surgery | Multiple nodular
lesions in both lungs | NA | NA | NA | Lost to
follow-up | (19) |
| Pierard et
al, 2006 | Belgium | M | 51 | Submandibular
gland | 3 years after
surgery | Multiple
parenchymal nodules in both lungs | Bilateral multiple
pulmonary nodules | Biopsy |
Chemoradiotherapy | Died after 30
months | (20) |
| Kasper et
al, 1999 | Germany | F | 58 | Left parotid
gland | 12 years after
surgery | Multiple nodular
lesions in right lung | NA | Biopsy of the mass
lesion | Palliative
chemotherapy | Dies after 3
years | (21) |
| Noel et al,
1992 | USA | M | 63 | Left parotid
gland | 14 years after
surgery | Multiple pulmonary
nodules in both lungs | NA | Biopsy | NA | Minimal tumor
growth 5 months later | (22) |
A systematic database search revealed that nine
studies (14–22) reported EMC with lung metastases
prior to the case reported in the present study. Of these, 5
patients were male and 4 were female. There was no regional bias in
the occurrence of EMC diseases. Most of the lesions occurred in the
parotid gland. Most lung metastases from EMC appeared as multiple
nodules in both lungs on chest X-ray or CT. However, PET-CT was
performed in only three studies, and two of them characterized the
FDG uptake of the nodules. As in the present case, one study
demonstrated mild to moderate FDG uptake. A total of 6 patients in
these studies received radiation or chemotherapy, and only 1 had a
good prognosis so far.
Discussion
EMC is a rare low-grade malignancy in which >70%
of patients are >60 years of age, evenly distributed in sex, or
have a slight female predominance (23). Different from the clinical
presentation of common salivary gland malignancies (faster growth,
more painful symptoms and invasion of nerves), this tumor usually
presents as a slow-growing asymptomatic mass (23).
Despite being a low-grade malignancy, EMC has a
tendency to recur locally and can invade adjacent nerves, blood
vessels and bones. However, regional lymph node metastasis and
distant metastasis are uncommon, occurring in <5% of cases
(13). Luna et al (24) reviewed the data of 9 cases of EMC,
of which 5 had tumor recurrence and 1 had metastases to the
cervical lymph nodes and lungs. A case of a patient with
epithelial-muscle epithelial cell carcinoma of the parotid gland
who developed lung metastases and two local recurrences 14 years
after the initial resection has also been reported in the
literature (22). In a review of 58
patients with EMCs, only 3 patients (5.2%) showed signs of
metastatic disease, with just 1 case exhibiting distant metastasis
to the iliac bone (25). In the
largest review of 246 cases of EMCs, 11 patients (4.47%) had
distant metastases; however, the specific locations were not
mentioned (26). In the present
case, lung metastasis occurred 5 years after parotid EMC,
indicating that the aggressive nature of EMC should not be
underestimated and warrants careful examination in clinical
practice. Furthermore, it has been reported that tumor necrosis is
associated with distant metastasis and poor clinical prognosis
(27,28). Seethala et al (25) reported that positive margins,
vascular lymphatic infiltration, tumor necrosis and myoepithelial
abnormalities, including severe nuclear atypia or pleomorphism,
were notably associated with tumor recurrence. As a result, close
monitoring is crucial, particularly if the tumor shows signs of
necrosis and exhibits aggressive characteristics upon pathological
examination.
The diagnosis of EMC relies on pathologic patterns,
as well as immunohistochemical staining. Gross pathology reveals a
multinodular white mass, the majority of which are well-defined and
often lack or only partially have an enclosing membrane (29). Infiltrative growth patterns are
observed in only ~12% of cases, and identifying tumor infiltration
visually is challenging. Most sections exhibit a solid, gray or
grayish-yellow appearance along with hemorrhage and necrosis,
whereas a few have cystic areas with internal papillary
projections. Most of these cystic or necrotic areas may arise from
high-grade cancerous areas or from poorly differentiated or
undifferentiated areas, and as a result, exhibit the presence of
inactive nodules on PET-CT radiographic images (SUV for tumor
activity) (3). In the present case,
the tumor microscopically showed a mixture of lobulated, tubular
and solid nests or sheets. The typical histopathology of EMC
involves a biphasic tubular structure, consisting of inner layer
lined with eosinophilic ductal epithelial cells and an outer layer
of hyaline myoepithelial cells. The cytoplasm of clear muscle
epithelial cells contains glycogen (30). Cellular uptake of 18F-FDG
is proportional to the rate of glycogen metabolism. In most cases,
tumor cells do not exhibit malignant characteristics. However,
recurrences, particularly those with a predominance of hyaline
cells, display distinct anisotropy, karyorrhexis and necrosis,
occurring in ~18% of cases. This structure demonstrates
infiltrative growth, and the ratio between the two cell types can
vary (3). Additionally, a network
of different histological subtypes may be present, contributing to
the difficulty in diagnosing EMC due to its histological diversity,
complexity and heterogeneity (3).
These microscopic manifestations can be reflected by the differing
metabolic rates of each nodule on PET-CT (31), as observed in the present case, but
are not feasible with conventional examinations. PET-CT is useful
in determining the activity of tissue metabolism (32). Analyzing the characteristics of
tumor biological behavior, we hypothesize that the unusual
presentation of lung metastatic nodules may be related to the
presence of varying degrees of necrosis or histological complexity
within the tumor. A previous study also reported that the
histologic grading of EMC appeared to be associated with the level
of FDG uptake (33).
Immunohistochemistry reveals varying degrees of positivity for
specific markers of epithelial cells such as CK, CK7 and epithelial
cell membrane antigen in most EMCs. Specific markers such as S-100,
smooth muscle actin, P63 and Calponin are also positively expressed
to varying degrees in tumor cells (34). If the characteristic double-layered
tubular structure is seen in the biopsy specimen, combined with
immunohistochemical dual expression of the epithelial myoepithelial
component, this can lead to a definitive diagnosis. In the present
case, a double-layered tubular structure was observed in the lung
biopsy tissue, and immunohistochemistry also confirmed the presence
of CK7, p63 and S-100 with varying degrees of positive expression.
The morphological spectrum of EMCs is broad, with multiple
histologic subtypes and variants, and metastatic sites may show
multiple growth patterns (25).
Therefore, in addition to incorporating a significant medical
history at the time of diagnosis, it is crucial to be able to
incorporate PET-CT manifestations that reflect the atypical spread
patterns of these nodules.
Increased availability of imaging modalities such as
ultrasound, CT and MRI has improved the diagnostic sensitivity for
epithelial-muscle-epithelial (EME) cancers presenting as neck
masses. It can also increase the detection of EME cancers that are
incidentally found using diagnostic imaging for unrelated reasons
(35). In a case of left parotid
EMC (36), MRI revealed a
well-defined lobulated mass with low signal intensity on
T1-weighted images and high signal intensity on T2-weighted images.
Suto et al (29) summarized
the cases of 7 patients with EMC of the parotid gland and found
that although the MRI features of EMC were similar to those of
benign salivary gland tumors or low-grade malignant salivary
adenocarcinomas, a multinodular structure and internal septa were
characteristic of EMC after cross-referencing with histological
findings. There have also been case reports of PET/CT scans
revealed high 18F-FDG affinity at the EMC primary site (37) or peripheral regions exhibiting an
irregular ring of strong FDG uptake with a lack of central photons
(38). Due to the low rate of
distant organ metastasis in EMC, imaging manifestations associated
with metastasis are less commonly reported. In patients with lung
metastasis, chest CT often shows a well-defined rounded mass with
multiple satellite nodules extending from the lung lobes to the
left lower lobe (22) or scattered
multiple nodules (39).
Histologically, the lung tumor is similar to a previously detected
parotid tumor that could serve as an important diagnostic basis.
However, there are few reports on the PET/CT imaging manifestations
of consistent with the observations in the present case. Only one
recent case report demonstrated multiple lung lesions with mild to
moderate FDG uptake similar to the present case (14). In comparison with the present case,
the number of lesions reported in the literature is relatively
smaller, with larger nodule sizes and similar degrees of FDG
uptake. Notably, the present case presents with small and numerous
EMC lung metastasis nodules with varying FDG uptake. However,
nodules with high SUV values should be monitored in subsequent
follow-up. PET/CT is not only quantitative but can also provide
valuable information about the pathophysiology of tumors, receptor
expression, metabolism or morphological and functional
characteristics, such as oxygenation or tissue density, and
pharmacodynamic properties of drugs (40). In recent years, the use of more
specific imaging agents for PET/CT has not only opened up novel
avenues for tumor imaging, but has also brought the integration of
diagnosis and therapy to a novel stage of development. Due to the
recent advancements in imaging techniques, this presentation of
atypical findings on PET-CT has a significant value in differential
diagnosis and follow-up of disease recurrence.
Due to the low prevalence of EMC, there are fewer
treatment experiences to draw on and the relevant studies lack big
data support (3). Most reports
emphasize its diagnosis and pathology, with fewer research advances
involving its treatment. Surgery is the preferred treatment option
and thoroughness is essential. As the rate of hematogenous
metastasis of EMC is not low, it is crucial to perform liver CT,
lung CT, bone scans or whole-body PET-CT examinations to detect any
potential distant metastases. Postoperative chemotherapy may be
beneficial in combating hematogenous metastasis. Additionally, head
and neck EMC is often associated with HRAS mutations (30.0–82.7%)
and the mutation sites are mostly concentrated in codon 61 of exon
3 (HRAS Q61R) (41). Consequently,
HRAS codon 61 mutations could serve as important molecular markers
for EMC. Therefore, intensive clinical, imaging, pathologic and
molecular examinations are necessary to assess EMC risk
stratification and management.
In conclusion, the present report describes a rare
case of EMC with pulmonary metastases that exhibited distinctive
imaging manifestations. Diffusely distributed lung nodules that
exhibit varying degrees of FDG uptake can be considered as unique
characteristics resembling metastasis on EMC imaging. Furthermore,
the present report emphasizes that the malignant potential of EMC
cannot be underestimated. Careful physical examination, meticulous
pathologic and molecular observation, comprehensive treatment and
frequent imaging follow-ups are important factors in tightly
monitoring disease recurrence and metastasis.
Acknowledgements
Not applicable.
Funding
The present study received funding from the Natural Science
Foundation of Shandong Province (grant no. ZR2024QH216), the
Medical Health Science and Technology Program of Shandong Province
(grant no. 202309020311) and the Postdoctoral Research Fund from
Affiliated Hospital of Jining Medical University (grant no.
JYFY362641).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YCW, NJG, YNZ, WBX and HSS contributed to the study
conception and design. NJG, YNZ and WBX analyzed data. YCW and HSS
wrote the manuscript. YCW, NJG, YNZ, WBX and HSS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures in the present study were performed
in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
Declaration.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMC
|
epithelial-myoepithelial carcinoma
|
|
FDG
|
fluorodeoxyglucose
|
|
CT
|
computed tomography
|
|
PET/CT
|
positron emission tomography-CT
|
|
MRI
|
magnetic resonance imaging
|
|
CK
|
creatine kinase
|
References
|
1
|
Donath K, Seifert G and Schmitz R:
Diagnosis and ultrastructure of the tubular carcinoma of salivary
gland ducts. Epithelial-myoepithelial carcinoma of the intercalated
ducts. Virchows Arch A Pathol Pathol Anat. 356:16–31. 1972.(In
German). View Article : Google Scholar
|
|
2
|
Seifert G and Sobin LH: The World Health
Organization's histological classification of salivary gland
tumors. A commentary on the second edition. Cancer. 70:379–385.
1992. View Article : Google Scholar
|
|
3
|
Nakaguro M and Nagao T:
Epithelial-myoepithelial carcinoma. Surg Pathol Clin. 14:97–109.
2021. View Article : Google Scholar
|
|
4
|
Sharma D, Neiweem A, Davis K, Prendes M,
Chundury R and Illing E: Epithelial-myoepithelial carcinoma of the
lacrimal sac and literature review of the lacrimal system. Allergy
Rhinol (Providence). 11:21526567209206002020. View Article : Google Scholar
|
|
5
|
Wockner RS, Seethala RR, Emeto TI, McCaul
JA and Subramaniam SS: Epithelial-myoepithelial carcinoma of the
maxillofacial and sinonasal region: A systematic review of
presenting characteristics, treatment modalities, and associated
outcomes. Int J Oral Maxillofac Surg. 52:1–12. 2023. View Article : Google Scholar
|
|
6
|
Zhang W, Wang XX, Wang XL, Zhang Y, Li XF,
Li Y, Cai YY, Ren HQ, Zhang YX and Hao FR: Epithelial-myoepithelial
carcinoma of the nasopharynx: A case report and review of the
literature. Front Oncol. 12:9235792022. View Article : Google Scholar
|
|
7
|
Lavin V, Callipo F, Donofrio CA,
Ellwood-Thompson R, Metcalf R, Djoukhadar I, Higham CE, Kearney T,
Colaco R, Gnanalingham K and Roncaroli F: Primary
epithelial-myoepithelial carcinoma of the pituitary gland.
Neuropathology. 40:261–267. 2020. View Article : Google Scholar
|
|
8
|
Sanz Sánchez CI, Pérez Villa L and Cazorla
Ramos OE: Epithelial-myoepithelial carcinoma of the base of tongue.
Acta Otorrinolaringol Esp (Engl Ed). 72:198–200. 2021.(In English,
Spanish). View Article : Google Scholar
|
|
9
|
Huang HC, Zhao L, Cao XH, Meng G, Wang YJ
and Wu M: Primary salivary gland tumors of the lung: Two cases date
report and literature review. Respir Med Case Rep.
32:1013332020.
|
|
10
|
Wu H, Zhang F, Peng J, Wu Z, Zhang X and
Wu X: Epithelial-myoepithelial carcinoma of the esophagus: A case
report. Front Surg. 9:9420192022. View Article : Google Scholar
|
|
11
|
Sharma S, Tayal A, Khatri S, Mohapatra SG
and Mohanty SK: Primary pulmonary epithelial-myoepithelial
carcinoma: Report of a rare and under-diagnosed low-grade
malignancy. J Cancer Res Ther. 18:795–800. 2022. View Article : Google Scholar
|
|
12
|
Grenier K, Altinel G, Dastani Z and
Omeroglu A: Epithelial-myoepithelial carcinoma of the breast with
rhabdoid features. Case Rep Pathol. 2020:88790352020.
|
|
13
|
Gore MR: Epithelial-myoepithelial
carcinoma: A population-based survival analysis. BMC Ear Nose
Throat Disord. 18:152018. View Article : Google Scholar
|
|
14
|
Civan C, Has Şimşek D, Vurallı Bakkaloğlu
D and Kuyumcu S: 18F-FDG and 68Ga-FAPI-04
PET/CT findings of a rare epithelial-myoepithelial carcinoma
arising from ex pleomorphic adenoma of parotid. Mol Imaging
Radionucl Ther. 33:125–128. 2024.
|
|
15
|
Mäkelä R, Arjonen A, Suryo Rahmanto A,
Härmä V, Lehtiö J, Kuopio T, Helleday T, Sangfelt O, Kononen J and
Rantala JK: Ex vivo assessment of targeted therapies in a rare
metastatic epithelial-myoepithelial carcinoma. Neoplasia.
22:390–398. 2020. View Article : Google Scholar
|
|
16
|
Chen MY, Vyas V and Sommerville R:
Epithelial-myoepithelial carcinoma of the base of tongue with
possible lung metastases. Case Rep Otolaryngol.
2017:49735732017.
|
|
17
|
Hsieh MS, Chen JS, Lee YH and Chou YH:
Epithelial-myoepithelial carcinoma of the salivary gland harboring
HRAS codon 61 mutations with lung metastasis. Int J Surg Pathol.
24:227–231. 2016. View Article : Google Scholar
|
|
18
|
Yamazaki H, Ota Y, Aoki T and Kaneko A:
Lung metastases of epithelial-myoepithelial carcinoma of the
parotid gland successfully treated with chemotherapy: A case
report. J Oral Maxillofac Surg. 71:220–226. 2013. View Article : Google Scholar
|
|
19
|
Yang S and Chen X:
Epithelial-myoepithelial carcinoma with high grade transformation.
Int J Oral Maxillofac Surg. 41:810–813. 2012. View Article : Google Scholar
|
|
20
|
Pierard S, Gregoire V, Weynand B and
Machiels JP: Epithelial-myoepithelial carcinoma of the
submandibular gland with symptomatic lung metastases treated with
chemotherapy. Eur Arch Otorhinolaryngol. 263:1158–1160. 2006.
View Article : Google Scholar
|
|
21
|
Kasper HU, Mellin W, Kriegsmann J,
Cheremet E, Lippert H and Roessner A: Epithelial-myoepithelial
carcinoma of the salivary gland-a low grade malignant neoplasm?
Report of two cases and review of the literature. Pathol Res Pract.
195:189–192. 1999. View Article : Google Scholar
|
|
22
|
Noel S and Brozna JP:
Epithelial-myoepithelial carcinoma of salivary gland with
metastasis to lung: Report of a case and review of the literature.
Head Neck. 14:401–406. 1992. View Article : Google Scholar
|
|
23
|
Skálová A, Hyrcza MD and Leivo I: Update
from the 5th edition of the World Health Organization
classification of head and neck tumors: Salivary glands. Head Neck
Pathol. 16:40–53. 2022. View Article : Google Scholar
|
|
24
|
Luna MA, Ordonez NG, Mackay B, Batsakis JG
and Guillamondegui O: Salivary epithelial-myoepithelial carcinomas
of intercalated ducts: A clinical, electron microscopic, and
immunocytochemical study. Oral Surg Oral Med Oral Pathol.
59:482–490. 1985. View Article : Google Scholar
|
|
25
|
Seethala RR, Barnes EL and Hunt JL:
Epithelial-myoepithelial carcinoma: A review of the
clinicopathologic spectrum and immunophenotypic characteristics in
61 tumors of the salivary glands and upper aerodigestive tract. Am
J Surg Pathol. 31:44–57. 2007. View Article : Google Scholar
|
|
26
|
Vázquez A, Patel TD, D'Aguillo CM, Abdou
RY, Farver W, Baredes S, Eloy JA and Park RCW:
Epithelial-myoepithelial carcinoma of the salivary glands: An
analysis of 246 cases. Otolaryngol Head Neck Surg. 153:569–574.
2015. View Article : Google Scholar
|
|
27
|
Kong M, Drill EN, Morris L, West L,
Klimstra D, Gonen M, Ghossein R and Katabi N: Prognostic factors in
myoepithelial carcinoma of salivary glands: A clinicopathologic
study of 48 cases. Am J Surg Pathol. 39:931–938. 2015. View Article : Google Scholar
|
|
28
|
Skálová A, Weinreb I, Hyrcza M, Simpson
RHW, Laco J, Agaimy A, Vazmitel M, Majewska H, Vanecek T, Talarčik
P, et al: Clear cell myoepithelial carcinoma of salivary glands
showing EWSR1 rearrangement: Molecular analysis of 94 salivary
gland carcinomas with prominent clear cell component. Am J Surg
Pathol. 39:338–348. 2015. View Article : Google Scholar
|
|
29
|
Suto T, Kato H, Kawaguchi M, Kobayashi K,
Miyazaki T, Ando T, Noda Y, Hyodo F, Matsuo M, Ishihara H and Ogawa
T: MRI findings of epithelial-myoepithelial carcinoma of the
parotid gland with radiologic-pathologic correlation. Jpn J Radiol.
40:578–585. 2022. View Article : Google Scholar
|
|
30
|
Urano M, Nakaguro Y, Yamamoto Y, Hirai H,
Tanigawa M, Saigusa N, Shimizu A, Tsukahara K, Tada Y, Sakurai K,
et al: Diagnostic significance of HRAS mutations in
epithelial-myoepithelial carcinomas exhibiting a broad
histopathologic spectrum. Am J Surg Pathol. 43:984–994. 2019.
View Article : Google Scholar
|
|
31
|
Kim JW, Oh JS, Roh JL, Kim JS, Choi SH,
Nam SY, et al: Prognostic significance of standardized uptake value
and metabolic tumour volume on 18F-FDG PET/CT in
oropharyngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging.
42:1353–1361. 2015. View Article : Google Scholar
|
|
32
|
Çimen F, Aloglu M, Düzgün S, Şentürk A,
Atikcan Ş and Özmen Ö: What is the effect of tumor diameter, lymph
node metastases, and SUVmax value on prognosis in limited-stage
small cell lung cancer? Rev Assoc Med Bras (1992). 68:1252–1258.
2022. View Article : Google Scholar
|
|
33
|
Kim CH, Jeong JS, Kim SR and Lee YC:
Endobronchial epithelial-myoepithelial carcinoma of the lung.
Thorax. 73:593–594. 2018. View Article : Google Scholar
|
|
34
|
El Hallani S, Udager AM, Bell D, Fonseca
I, Thompson LDR, Assaad A, Agaimy A, Luvison AM, Miller C, Seethala
RR and Chiosea S: Epithelial-myoepithelial carcinoma: Frequent
Morphologic and molecular evidence of preexisting pleomorphic
adenoma, common HRAS mutations in PLAG1-intact and HMGA2-intact
cases, and occasional TP53, FBXW7, and SMARCB1 alterations in
high-grade cases. Am J Surg Pathol. 42:18–27. 2018. View Article : Google Scholar
|
|
35
|
Rumboldt Z, Gordon L, Gordon L, Bonsall R
and Ackermann S: Imaging in head and neck cancer. Curr Treat
Options Oncol. 7:23–34. 2006. View Article : Google Scholar
|
|
36
|
Inan HC and Issin G: Epithelial
myoepithelial carcinoma of the parotid gland: A rare tumor with
oncocytic changes. Niger J Clin Pract. 23:266–269. 2020. View Article : Google Scholar
|
|
37
|
Dzuko Kamga J, Leclere JC, Uguen A, Amrane
K and Abgral R: Case report: Nasal cavity epithelial-myoepithelial
carcinoma with high fluoro-D-glucose uptake on positron emission
tomography/computed tomography. Front Med (Lausanne). 8:6645202021.
View Article : Google Scholar
|
|
38
|
Takumi K, Fukukura Y, Kamiyama T, Nakajo
M, Ohori J, Kurono Y and Higashi M: Epithelial-myoepithelial
carcinoma of the parotid gland: Correlation of dynamic magnetic
resonance imaging, (18)F-fluorodeoxyglucose-positron emission
tomography, and pathological findings. Jpn J Radiol. 28:618–622.
2010. View Article : Google Scholar
|
|
39
|
Saleh D and Al Ghamdi D: Rare development
of primary parotid gland epithelial-myoepithelial carcinoma in a
child. Case Rep Pathol. 2020:58376592020.
|
|
40
|
Schwenck J, Sonanini D, Cotton JM,
Rammensee HG, la Fougère C, Zender L and Pichler BJ: Advances in
PET imaging of cancer. Nat Rev Cancer. 23:474–490. 2023. View Article : Google Scholar
|
|
41
|
Yanagawa N, Sato A, Nishiya M, Suzuki M,
Sugimoto R, Osakabe M, Uesugi N, Saito H and Sugai T: Pulmonary
epithelial-myoepithelial carcinoma without AKT1, HRAS or PIK3CA
mutations: A case report. Diagn Pathol. 15:1052020. View Article : Google Scholar
|