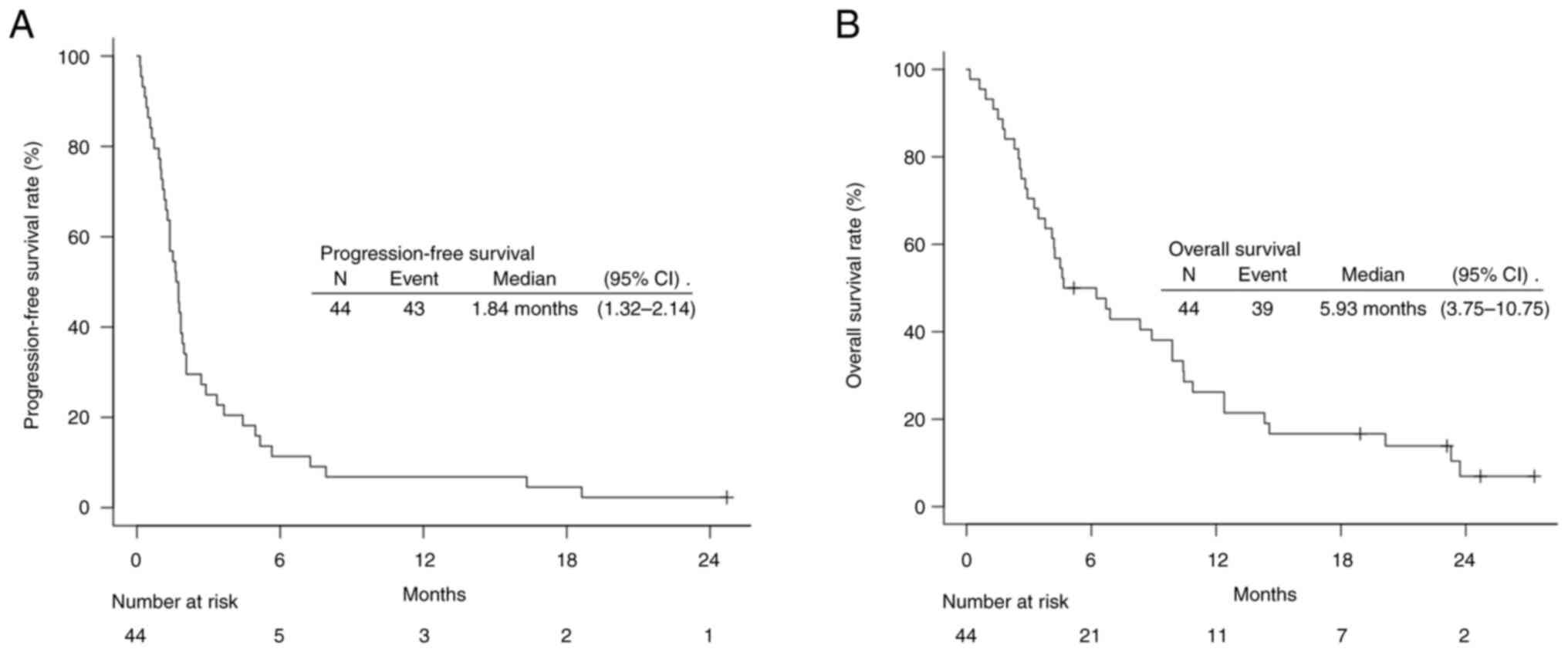
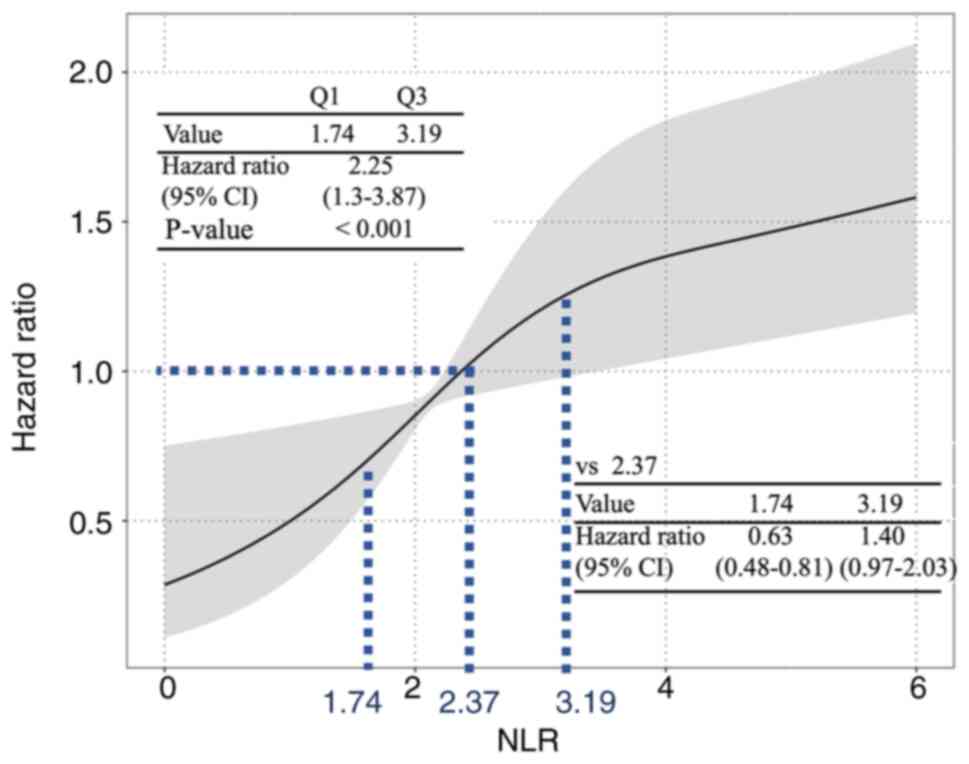
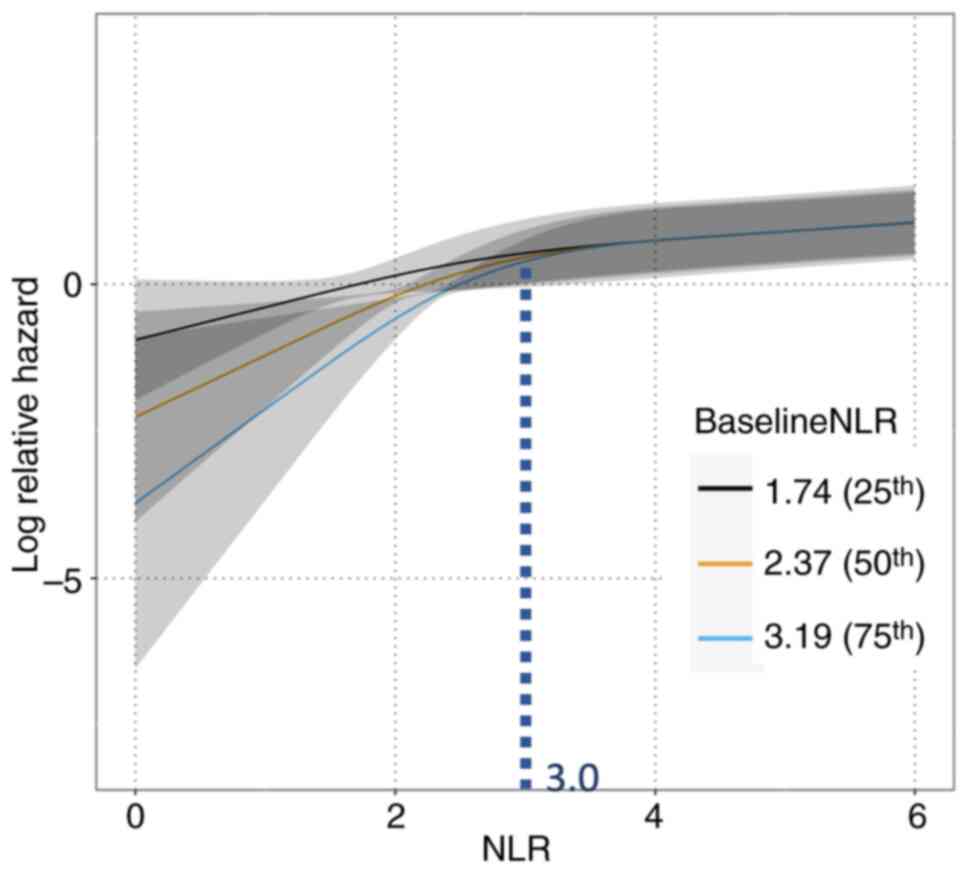
|
1
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar
|
|
2
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous Non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar
|
|
3
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar
|
|
4
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar
|
|
5
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomized,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar
|
|
6
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomized, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar
|
|
7
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomized, multicentre, double-blind,
Placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247. 2022.
View Article : Google Scholar
|
|
8
|
Japanese Gastric Cancer Association, .
Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition).
Gastric Cancer. 26:1–25. 2023. View Article : Google Scholar
|
|
9
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN Clinical Practice Guidelines
in Oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar
|
|
10
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar
|
|
11
|
Iwasa S, Kudo T, Takahari D, Hara H, Kato
K and Satoh T: Practical guidance for the evaluation of disease
progression and the decision to change treatment in patients with
advanced gastric cancer receiving chemotherapy. Int J Clin Oncol.
25:1223–1232. 2020. View Article : Google Scholar
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
13
|
Hasegawa H, Fujitani K, Nakazuru S, Hirao
M, Yamamoto K, Mita E and Tsujinaka T: Optimal treatment change
criteria for advanced gastric cancer with Non-measurable peritoneal
metastasis: Symptom/tumor Marker-based versus CT-based. Anticancer
Res. 34:5169–5174. 2014.
|
|
14
|
Ota Y, Takahari D, Suzuki T, Osumi H,
Nakayama I, Oki A, Wakatsuki T, Ichimura T, Ogura M, Shinozaki E,
et al: Changes in the Neutrophil-to-lymphocyte ratio during
nivolumab monotherapy are associated with gastric cancer survival.
Cancer Chemother Pharmacol. 85:265–272. 2020. View Article : Google Scholar
|
|
15
|
Namikawa T, Yokota K, Tanioka N, Fukudome
I, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M,
et al: Systemic inflammatory response and nutritional biomarkers as
predictors of nivolumab efficacy for gastric cancer. Surg Today.
50:1486–1495. 2020. View Article : Google Scholar
|
|
16
|
Fujita K, Haruki N, Kurehara H, Ochi N,
Yamakawa Y, Harata S, Tsumoto C, Tsuji T, Ito T, Izumi A, et al:
Neutrophil-lymphocyte ratio as a prognostic indicator in patients
treated with nivolumab for gastric cancer. Gan To Kagaku Ryoho.
47:923–926. 2020.(In Japanese).
|
|
17
|
Ogata T, Satake H, Ogata M, Hatachi Y,
Inoue K, Hamada M and Yasui H: Neutrophil-to-lymphocyte ratio as a
predictive or prognostic factor for gastric cancer treated with
nivolumab: A multicenter retrospective study. Oncotarget.
9:34520–34527. 2018. View Article : Google Scholar
|
|
18
|
Tsujimoto H, Ono S, Ichikura T, Matsumoto
Y, Yamamoto J and Hase K: Roles of inflammatory cytokines in the
progression of gastric cancer: Friends or foes? Gastric Cancer.
13:212–221. 2010. View Article : Google Scholar
|
|
19
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
20
|
Granja S, Tavares-Valente D, Queirós O and
Baltazar F: Value of pH regulators in the diagnosis, prognosis and
treatment of cancer. Semin Cancer Biol. 43:17–34. 2017. View Article : Google Scholar
|
|
21
|
Nakaya A, Kurata T, Yoshioka H, Takeyasu
Y, Niki M, Kibata K, Satsutani N, Ogata M, Miyara T and Nomura S:
Neutrophil-to-lymphocyte ratio as an early marker of outcomes in
patients with advanced non-small-cell lung cancer treated with
nivolumab. Int J Clin Oncol. 23:634–640. 2018. View Article : Google Scholar
|
|
22
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar
|
|
23
|
Ohashi H, Takeuchi S, Miyagaki T and
Kadono T: Increase of lymphocytes and eosinophils, and decrease of
neutrophils at an early stage of anti-PD-1 antibody treatment is a
favorable sign for advanced malignant melanoma. Drug Discov Ther.
14:117–121. 2020. View Article : Google Scholar
|
|
24
|
Nakao M, Muramatsu H, Kagawa Y, Suzuki Y,
Sakai Y, Kurokawa R, Fujita K and Sato H: Immunological status may
predict response to nivolumab in non-small cell lung cancer without
driver mutations. Anticancer Res. 37:3781–3786. 2017.
|
|
25
|
Suzuki K, Terakawa T, Furukawa J, Harada
K, Hinata N, Nakano Y and Fujisawa M: C-reactive protein and the
neutrophil-to-lymphocyte ratio are prognostic biomarkers in
metastatic renal cell carcinoma patients treated with nivolumab.
Int J Clin Oncol. 25:135–144. 2020. View Article : Google Scholar
|
|
26
|
Maehara Y, Sugimachi K, Akagi M, Kakegawa
T, Shimazu H and Tomita M: Serum carcinoembryonic antigen level
increases correlate with tumor progression in patients with
differentiated gastric carcinoma following noncurative resection.
Cancer Res. 50:3952–3955. 1990.
|
|
27
|
Kodera Y, Yamamura Y, Torii A, Uesaka K,
Hirai T, Yasui K, Morimoto T, Kato T and Kito T: The prognostic
value of preoperative serum levels of CEA and CA19-9 in patients
with gastric cancer. Am J Gastroenterol. 91:49–53. 1996.
|