Introduction
Gastric cancer (GC) presents a major risk to human
health, which is a highly ranked tumor in terms of occurrence and
death rates. Notably, the incidence and mortality rates of gastric
cancer rank fifth among all tumors worldwide (1). Globally, >50% of new cases and
fatalities as a result of GC are documented in East Asia. Compared
with other types of cancer, the outlook for GC is typically
unfavorable, exhibiting notable differences across regions.
Globally, GC mortality is highest in East Asia, followed by central
and South Asia and Eastern Europe, and lowest in South Africa
(2–4). The rising trend of aging populations
is expected to contribute to elevated incidences and deaths due to
GC in the coming years. The unfavorable prognosis associated with
GC primarily stems from its propensity for invasion and metastasis
upon diagnosis (5). Identifying
potential molecular targets implicated in the onset and advancement
of GC is crucial for informing treatment strategies and evaluating
prognosis.
Glycosyltransferases are ubiquitously present in
nature and are vital for preserving the structural variability of
natural compounds. These enzymes facilitate the transfer of
glycosyl groups to proteins or lipids, thereby altering their
properties, modulating protein function and participating in
various biological processes (6).
The enzyme glucoside xylosyltransferase 2 (GXYLT2), which belongs
to the human glycosyltransferase 8 group, is responsible for
producing a protein comprising 443 amino acids that operates as a
xylosyltransferase. This enzyme facilitates the incorporation of
xylose into the O-glucose (O-Glc) segment of epidermal growth
factor (EGF) and the repeat sequences of various proteins, leading
to the extension of the structure through xylose addition and
resulting in a xylose-xylose-glucose trisaccharide (7,8).
The Notch gene encodes a group of evolutionarily
conserved cell surface receptors. The pathway mediated by Notch is
crucial in controlling cell differentiation, cell proliferation and
programmed cell death, as well as the creation of cell boundaries.
Intercellular communication is facilitated by adjacent cells
transmitting signals through the interaction between Notch
receptors and ligands, which enhances and stabilizes molecular
distinctions between cells, ultimately influencing cell fate
(9). Notably, the extracellular
region of the Notch receptor consists of repeats similar to EGF
(10,11). Post-translation,
glycosyltransferases within the endoplasmic reticulum and Golgi
apparatus alter the EGF repeat sequence in the Notch protein along
with glycans. The initial addition of xylose is facilitated by
GXYLT enzymes GXYLT1 and GXYLT2.
Epithelial-mesenchymal transition (EMT) involves the
dedifferentiation of epithelial cells, resulting in polarity loss,
decreased cell-cell and cell-matrix interactions, and increased
motility and migration. The process involves a steady decrease or
disappearance of epithelial indicators, such as E-cadherin, coupled
with an increase in mesenchymal markers, such as vimentin,
N-cadherin, α-SMA, Snail and Slug (12). Numerous studies have demonstrated
the presence of EMT in the physiological context of embryonic
development, and the pathological context of tumor invasion and
metastasis (13–15). EMT entails the differentiation of
epithelial-derived tumor cells, originating from the endoderm and
ectoderm, into mesenchymal-like cells from the mesoderm, causing
enhanced metastatic and invasive capabilities.
It was hypothesized that GXYLT2 may act as a
controlling factor in the spread and metastasis of GC. As a
glycosyltransferase, GXYLT2 has been shown to exhibit an aberrant
expression in GC, leading to atypical glycosylation of the Notch1
protein (16). This phenomenon, in
turn, can modulate the Notch signaling pathway, facilitate the EMT
process, diminish cell-stroma adhesion, and enhance the metastatic
and invasive capabilities of cancer cells. Consequently, the
infiltration and metastasis of GC ensue, ultimately contributing to
a poor prognosis for affected individuals.
The present study aimed to explore the role of
GXYLT2 in the development of GC by examining the levels of GXYLT2,
Notch1 and EMT-associated indicators, E-cadherin and vimentin, in
GC tissues. The study aimed to offer new perspectives in
identifying possible molecular targets for diagnosing and treating
GC.
Materials and methods
Specimens
Specimens, including 338 GC tissues and 30
paracancerous tissues, were randomly chosen from paraffin-embedded
samples from Department of Pathology, The First Affiliated Hospital
of Bengbu Medical University (Bengbu, China) obtained between
January 2018 and December 2019. Samples for participation in this
study were obtained with patient consent. The patients whose
samples were used in the present study had not received any other
treatment before surgery, and excluded minors, pregnant women and
patients with serious underlying diseases, such as severe
hypertension and diabetes. Paracancerous tissues were collected
from the surgical margins of 30 of the 338 patients with GC, at a
distance of ≥3 cm from the lesion. The pathological assessments for
all cases were collaboratively conducted by two senior
pathologists. Comprehensive clinical information was accessible for
all patients, with 230 individuals being monitored until September
2023 or their death. Before undergoing surgery, no patient received
either radiotherapy or chemotherapy. The present study was approved
by the Ethics Committee of Bengbu Medical University [approval no.
(2023)245].
Immunohistochemistry
GC and paracancerous samples were fixed in 10%
neutral formalin at room temperature for 24 h and embedded in
paraffin, and the paraffin-embedded specimens were sliced into 4-µm
sections. Antigen retrieval was performed by heating the
paraffin-embedded sections after deparaffinization and hydration;
the heating temperature of antigen retrieval was 60–70°C and the
reagents used were citric acid antigen repair solution and EDTA
antigen repair solution. To block endogenous peroxidase, the
sections were placed in 3% hydrogen peroxide solution and incubated
at room temperature in the dark for 20 min. Subsequently, 5% BSA
(Wuhan Servicebio Technology Co., Ltd.) blocking solution was
applied to the tissue sections dropwise and was incubated at room
temperature for 30 min. The sections were then incubated with a
primary antibody solution consisting of 50 µl anti-GXYLT2 (rabbit
polyclonal; 1:250; cat. no. bs-16377R; BIOSS), anti-Notch1 (rabbit
polyclonal; 1:200; cat. no. bs-11976R; BIOSS), anti-E-cadherin
(mouse monoclonal; ready-to-use; cat. no. MAB-0738; Fuzhou Maixin
Biotechnology Development Co., Ltd.) or anti-vimentin (mouse
monoclonal; ready-to-use; cat. no. RMA-0547; Fuzhou Maixin
Biotechnology Development Co., Ltd.) overnight at 4°C.
Subsequently, the samples were washed three times with PBS,
incubated with 50 µl polymer reinforcement (Reagent A,
ready-to-use; cat. no. KIT-9902; Fuzhou Maixin Biotechnology
Development Co., Ltd.) at room temperature for 20 min, and washed
again three times with PBS. After removal of the PBS solution, each
section was incubated with 50 µl enzyme-labeled anti-mouse/rabbit
polymer (Reagent B, ready-to-use; cat. no. KIT-9902; Fuzhou Maixin
Biotechnology Development Co., Ltd.) for 30 min at room
temperature. Subsequently, the samples were washed three times with
PBS and were stained at room temperature for 3–5 min with
3,3′-diaminobenzidine for microscopic examination. Hematoxylin was
used for counterstaining at room temperature for 3 min. PBS
functioned as the negative control, while recognized positive
sections treated with primary antibodies (from the companies of the
reagents) were employed as the positive control. The procedures
were executed according to the guidelines outlined in the
Elivision™ Plus detection kit (Cat. no. KIT-9902; Fuzhou Maixin
Biotechnology Development Co., Ltd.).
Criteria for determining positive
results
Light yellow to brownish-yellow particles within the
cytoplasm or cell membrane suggested the existence of positive
staining, under a light microscope. The immunohistochemical results
were assessed using a semi-quantitative scoring method that
integrated the percentage of positive cells and the intensity of
staining. Five high-power fields (magnification, ×400) were
randomly selected from each section, and 200 tumor cells were
counted. The scoring system for positive cell proportion was as
follows: 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; 4, >75%. The
staining intensity of positively stained cells was semi-quantified
using a scoring system ranging from 0 to 3: 0, no staining; 1,
light yellow; 2, brown yellow 2; 3, tan. Multiplying the scores for
staining intensity and the number of positive cells in each case
allowed for the classification of negative expression (<3
points) and positive expression (≥3 points). Every outcome was
evaluated using a double-blind approach, conducted three times.
Statistical analysis
The data were analyzed using SPSS 26.0 statistical
software (IBM Corp.). The χ2 test was used to analyze
count data among the various groups, and this test was also used to
investigate the association between two factors. The coefficient of
contingency (C) was used to show the association between
categorical variables in a contingency table for χ2
tests. When the contingency table is a 2×2 table, C can be
represented by the symbol φ, which represents the degree of
association between two variables, φ=x2n+x2 The Kaplan-Meier technique was
used for univariate survival analysis and the log-rank test was
used to assess the effect of different factors on survival.
Univariate cox regression was used to analyze the effect of each
factor on survival. Furthermore, the Cox regression analysis was
employed for multivariate survival analysis. P<0.05, falling
within a 95% confidence interval, was considered to indicate a
statistically significant difference.
Results
Fundamental details of the
patients
The patients with GC included in the present study
were aged between 26 and 89 years (median age, 65 years; mean ± SD,
63±9.98 years). Among the cases, 109 individuals were <60 years
old and 229 individuals were ≥60 years old. Of the cases, 206 had
tumor diameters <5 cm, whereas 132 cases had tumor diameters ≥5
cm. Regarding tumor differentiation, 7 cases exhibited high
differentiation, 194 cases showed medium differentiation and 137
cases had low differentiation. In terms of infiltration depth,
cancer cells were limited to the mucosa or submucosa in 21 cases,
extended into the lamina propria in 72 cases, reached the serous
membrane in 219 cases, and breached the serous membrane or
disseminated to adjacent tissues in 26 cases. A total of 210 cases
exhibited lymph node metastasis, whereas 128 were devoid of such
metastases. Concerning vascular and nerve infiltration, 42 cases
demonstrated such infiltration, while 296 cases did not. Following
the 8th American Joint Committee on Cancer/Union for International
Cancer Control (14) pathological
tumor-node-metastasis (pTNM) classification for gastric carcinoma,
63 cases were identified as stage I, 134 as stage II, 139 as stage
III and 2 as stage IV.
Levels of GXYLT2, Notch1, E-cadherin
and vimentin expression in GC and paracancerous tissues
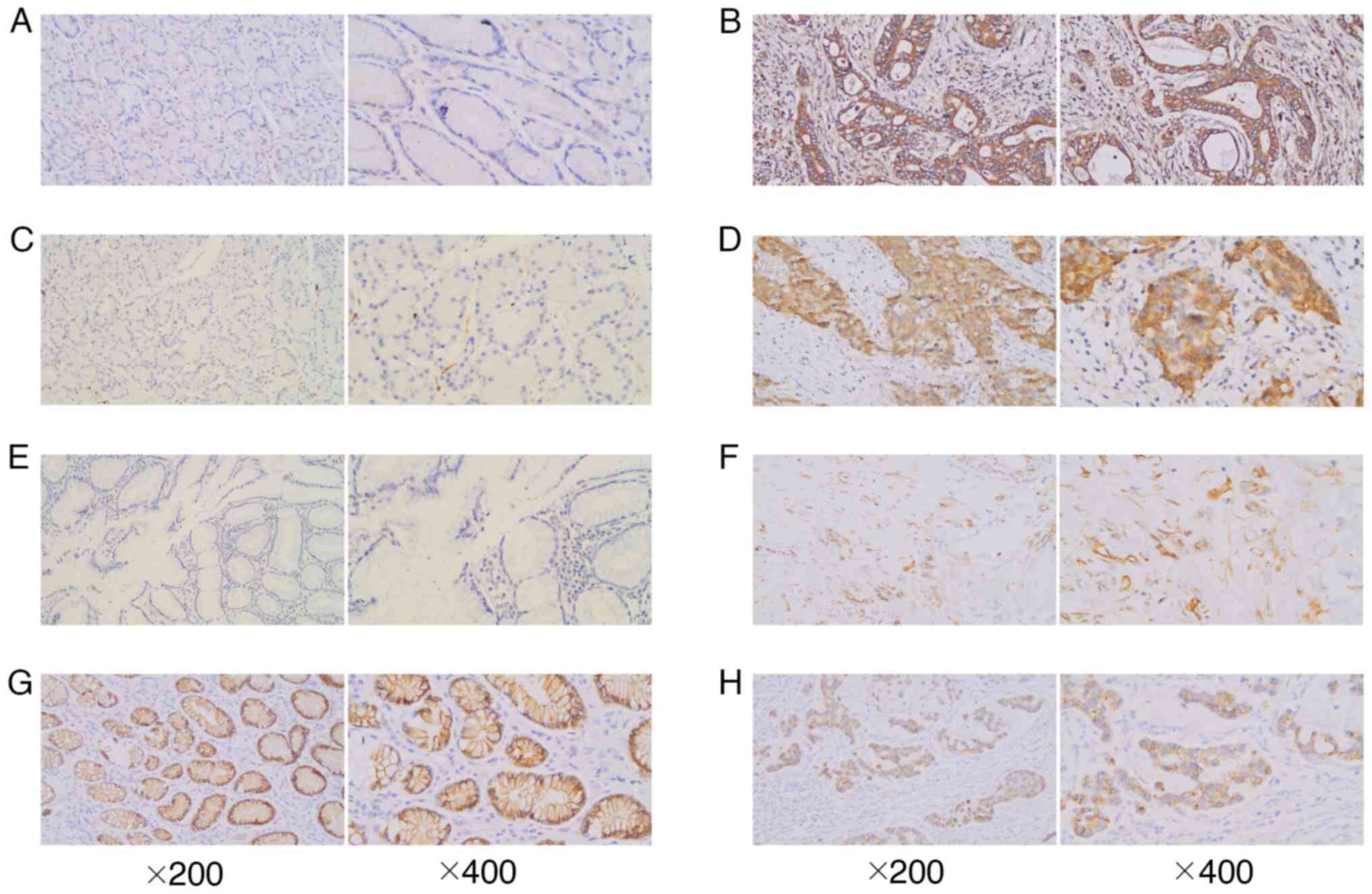
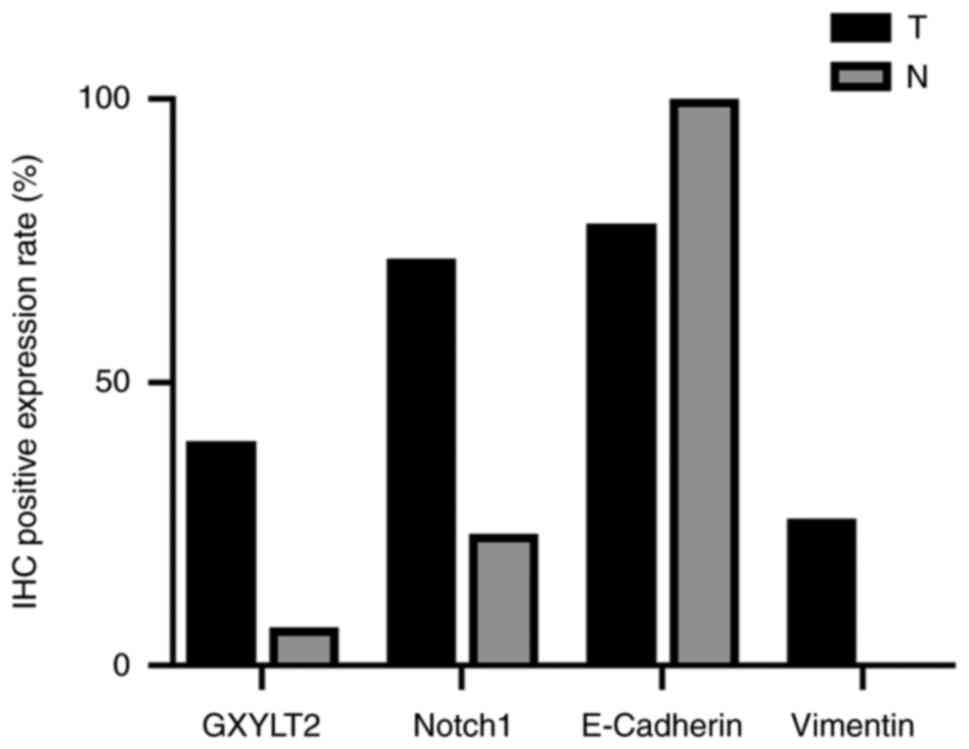
GXYLT2 staining exhibited localization to the cell
membrane, characterized by a brown-yellow hue, with a positive
expression rate of 39.64% (134/338) in GC samples. These outcomes
are presented in Figs. 1 and
2. By contrast, only 2 cases in the
paracancerous group showed this staining trend (2/30), leading to a
statistically notable disparity between the groups
(χ2=12.862; P<0.05). Notch1 expression was observed
in the cytoplasm, presenting as brown-yellow particles. The
positive rate of Notch1 was significantly higher in GC tissues
(71.89%; 243/338) than in adjacent tissues (23.33%; 7/30)
(χ2=29.828; P<0.05). In GC tissues, 26.04% (88/338)
showed positive vimentin staining, whereas no vimentin staining was
observed in the paracancerous tissues (0/30), highlighting a
notable disparity between the two groups (χ2=10.265;
P<0.05). E-cadherin staining was predominantly localized toward
the cell membrane but could also be expressed in the cytoplasm. In
the control group, E-cadherin exhibited a strong positive
expression (30/30), whereas in GC cells, its expression was notably
diminished, displaying a light yellow hue. The positive expression
rate of E-cadherin in GC tissues was 78.11% (264/338), and a
notable difference was noted between the control and GC tissues
(χ2=8.221; P<0.05).
Association between the expression levels of GXYLT2,
Notch1, E-cadherin and vimentin in GC tissues, and
clinicopathological factors. The findings indicated a significant
association between the upregulation of GXYLT2 in GC tissues, and
tumor differentiation degree and pTNM stage (P<0.05). The
results are shown in Table I.
Specifically, a positive expression of GXYLT2 was observed in
34.52% (68/197) of patients with pTNM stages I and II, which was
lower than the 46.81% (66/141) positive expression detected in
patients with pTNM stages III and IV; this disparity between the
two groups was statistically significant. Furthermore, the positive
expression rate of GXYLT2 was 44.78% (90/201) in well/moderately
differentiated cases, compared with 32.12% (44/137) poorly
differentiated cases. No notable link was determined between GXYLT2
expression and factors such as sex, age, tumor dimension, depth of
infiltration, lymph node metastasis and vascular and nerve
infiltration (P>0.05). Nonetheless, the expression of Notch1 was
positively associated with the vascular and nerve infiltration
(P<0.05). In instances of vascular and nerve invasion, the rate
of positive expression stood at 85.71% (36/42), markedly surpassing
the 69.93% (207/296) rate in situations lacking vascular and nerve
invasion. Notch1 expression did not exhibit any association with
the other clinicopathological parameters. Notably, positive
E-cadherin expression was associated with factors such as age,
tumor dimension, differentiation, depth of infiltration, lymph node
metastasis and pTNM stage (P<0.05), but not vascular and nerve
infiltration. A notable link was also identified between the
positive expression of vimentin in GC tissues and several
clinicopathological factors, such as age, tumor dimension,
differentiation level, depth of infiltration, lymph node metastasis
and pTNM stage (P<0.05), although vimentin was not associated
with tumor vascular and nerve infiltration.
 | Table I.Association between the expression of
GXYLT2, Notch1, E-cadherin and vimentin in gastric cancer tissues
and various clinicopathological factors. |
Table I.
Association between the expression of
GXYLT2, Notch1, E-cadherin and vimentin in gastric cancer tissues
and various clinicopathological factors.
|
| GXYLT2 |
| Notch1 |
| E-cadherin |
| Vimentin |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | + | - | P-value | + | - | P-value | + | - | P-value | + | - | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
| Male | 102 | 148 | 0.464 | 185 | 65 | 0.146 | 198 | 52 | 0.431 | 64 | 186 | 0.758 |
|
Female | 32 | 56 |
| 58 | 30 |
| 66 | 22 |
| 24 | 64 |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
≥60 | 91 | 138 | 0.960 | 169 | 60 | 0.259 | 189 | 40 | 0.004 | 50 | 179 | 0.011 |
|
<60 | 43 | 66 |
| 74 | 35 |
| 75 | 34 |
| 38 | 71 |
|
| Diameter, cm |
|
|
|
|
|
|
|
|
|
|
|
|
| ≥5 | 58 | 74 | 0.196 | 92 | 40 | 0.472 | 95 | 37 | 0.029 | 46 | 86 | 0.003 |
|
<5 | 76 | 130 |
| 151 | 55 |
| 169 | 37 |
| 42 | 164 |
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Well/Moderate | 90 | 111 | 0.019 | 147 | 54 | 0.539 | 178 | 23 | <0.001 | 27 | 174 | <0.001 |
|
Poor | 44 | 93 |
| 96 | 41 |
| 86 | 51 |
| 61 | 76 |
|
| Depth of
infiltration |
|
|
|
|
|
|
|
|
|
|
|
|
| T1 | 5 | 16 | 0.463 | 14 | 7 | 0.598 | 20 | 1 | 0.001 | 3 | 18 | 0.004 |
| T2 | 28 | 44 |
| 49 | 23 |
| 65 | 7 |
| 10 | 62 |
|
| T3 | 91 | 128 |
| 159 | 60 |
| 160 | 59 |
| 63 | 156 |
|
| T4 | 10 | 16 |
| 21 | 5 |
| 19 | 7 |
| 12 | 14 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 87 | 123 | 0.391 | 147 | 63 | 0.321 | 151 | 59 | <0.001 | 69 | 141 | <0.001 |
| No | 47 | 81 |
| 96 | 32 |
| 113 | 15 |
| 19 | 109 |
|
| pTNM |
|
|
|
|
|
|
|
|
|
|
|
|
|
I+II | 68 | 129 | 0.023 | 137 | 60 | 0.256 | 171 | 26 | <0.001 | 31 | 166 | <0.001 |
|
III+IV | 66 | 75 |
| 106 | 35 |
| 93 | 48 |
| 57 | 84 |
|
| Vascular and nerve
infiltration |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes | 18 | 24 | 0.649 | 36 | 6 | 0.033 | 30 | 12 | 0.263 | 11 | 31 | 0.980 |
| No | 116 | 180 |
| 207 | 89 |
| 234 | 62 |
| 77 | 219 |
|
Relationship between the expression
levels of GXYLT2, Notch1, E-cadherin and vimentin in GC
tissues
In the group exhibiting a positive GXYLT2
expression, the incidence of Notch1-positive expression stood at
80.60% (108/134), compared with 66.18% (135/204) in the group with
negative GXYLT2 expression, as shown in Table II. A statistically significant
difference was observed between the two groups, indicating an
association between GXYLT2 and Notch1 expression in GC (P=0.004,
φ=0.155). In addition, a notable positive association was detected
between GXYLT2 and vimentin expression in GC tissues. In the group
with positive GXYLT2 expression, the rate of positive vimentin
expression was 34.33% (46/134), markedly surpassing the 20.59% rate
(42/204) observed in the group with negative GXYLT2 expression.
Notably, GXYLT2 expression was positively associated with vimentin
expression in GC tissues (P=0.005, φ=0.151). A positive association
was also observed between Notch1 and vimentin expression in GC
tissues (P=0.033, φ=0.115), as shown in Table III. The prevalence of vimentin
positivity in the Notch1-positive group was 29.22% (71/243),
implying an increase compared with that in the Notch1-negative
group (17.89%; 17/95). Conversely, the incidence of vimentin
positivity in the E-cadherin-positive expression group was 12.50%
(33/264), which was markedly lower than that in the
E-cadherin-negative expression group (74.32%; 55/74). Notably, a
significant negative association existed between E-cadherin
expression and vimentin expression (P<0.001, φ=−0.503).
 | Table II.Relationship between the expression
of GXYLT2, Notch1 and vimentin in gastric cancer tissues. |
Table II.
Relationship between the expression
of GXYLT2, Notch1 and vimentin in gastric cancer tissues.
|
| GXYLT2 |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | + | - | χ2 | P-value | φ |
|---|
| Notch1 |
|
| 8.323 | 0.004 | 0.155 |
| + | 108 | 135 |
|
|
|
| - | 26 | 69 |
|
|
|
| Vimentin |
|
| 7.929 | 0.005 | 0.151 |
| + | 46 | 42 |
|
|
|
| - | 88 | 162 |
|
|
|
 | Table III.Relationship between the expression
levels of Notch1, E-cadherin and vimentin in gastric cancer
tissues. |
Table III.
Relationship between the expression
levels of Notch1, E-cadherin and vimentin in gastric cancer
tissues.
|
| Vimentin |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | + | - | χ2 | P-value | φ |
|---|
| Notch1 |
|
| 4.548 | 0.033 | 0.115 |
| + | 71 | 172 |
|
|
|
| - | 17 | 78 |
|
|
|
| E-cadherin |
|
| 114.722 | <0.001 | −0.503 |
| + | 33 | 231 |
|
|
|
| - | 55 | 19 |
|
|
|
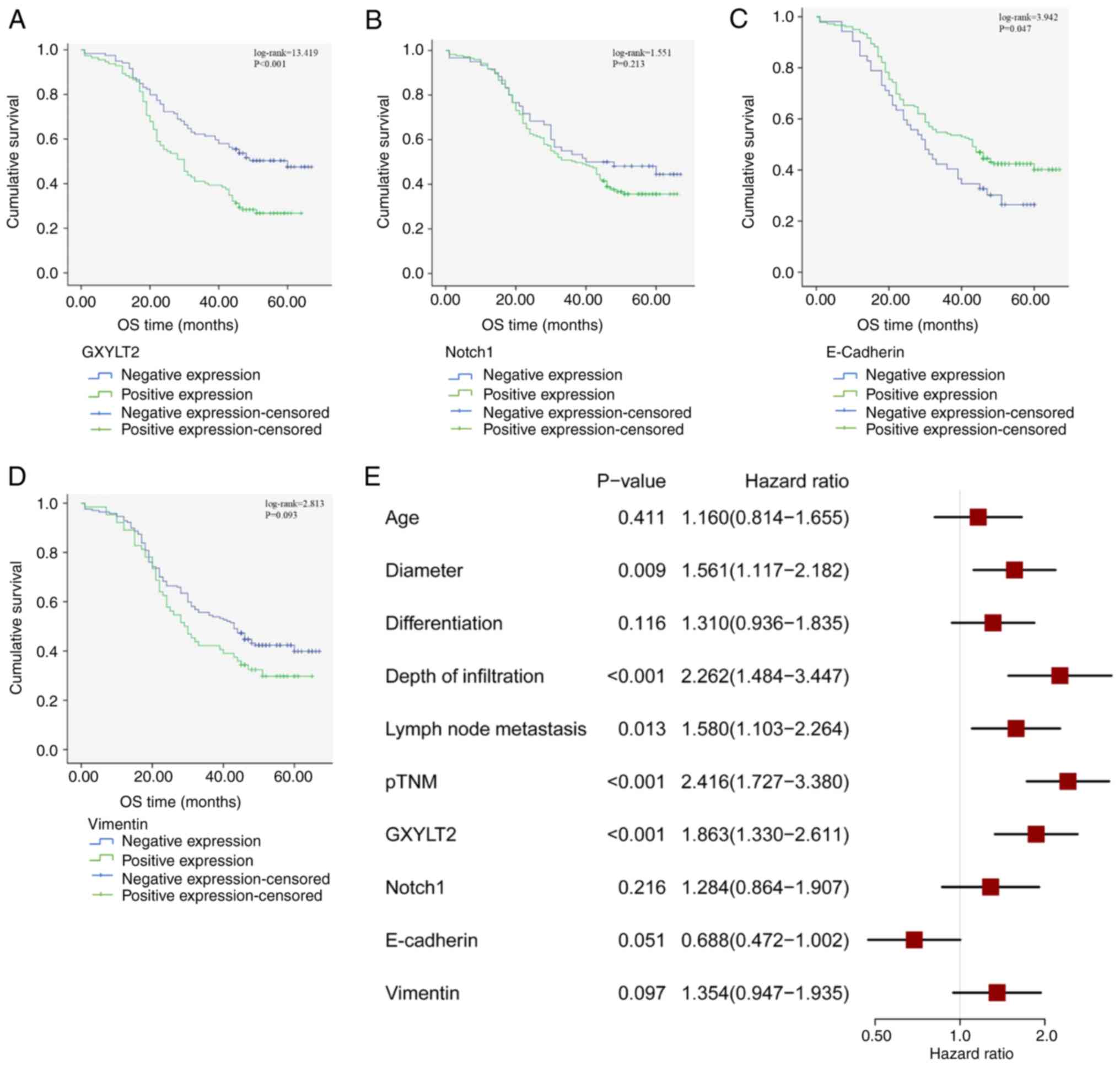
GXYLT2 as an important prognostic
factor
A follow-up study was carried out on 231 patients to
explore the predictive effects of GXYLT2, Notch1, E-cadherin and
vimentin on patients with GC. Kaplan-Meier survival and
multivariate Cox regression survival analyses were conducted. The
findings demonstrated a mean survival time of 36.04±17.43 months,
with a postoperative survival rate of 39.40%. Among the 231
patients with follow-up data, 112 patients exhibited positive
GXYLT2 expression. The overall survival rate was 27.68% for
GXYLT2-positive patients and 50.42% for GXYLT2-negative patients.
The Kaplan-Meier analysis revealed a statistically significant
disparity in survival rates between the two groups
(log-rank=13.419; P<0.001) (Fig.
3A). There was no significant difference between the expression
of Notch1 protein and survival of patients with GC (log-rank=1.551;
P>0.05) (Fig. 3B). By contrast,
a significant association was identified between E-cadherin
expression and survival (log-rank=3.94; P<0.05) (Fig. 3C). However, the expression of
vimentin protein was not associated with the survival of patients
with GC (log-rank=2.813; P>0.05) (Fig. 3D). Furthermore, the univariate Cox
regression analysis indicated that tumor diameter, depth of
infiltration, lymph node metastasis, pTNM stage and GXYLT2
expression were significant factors influencing the survival
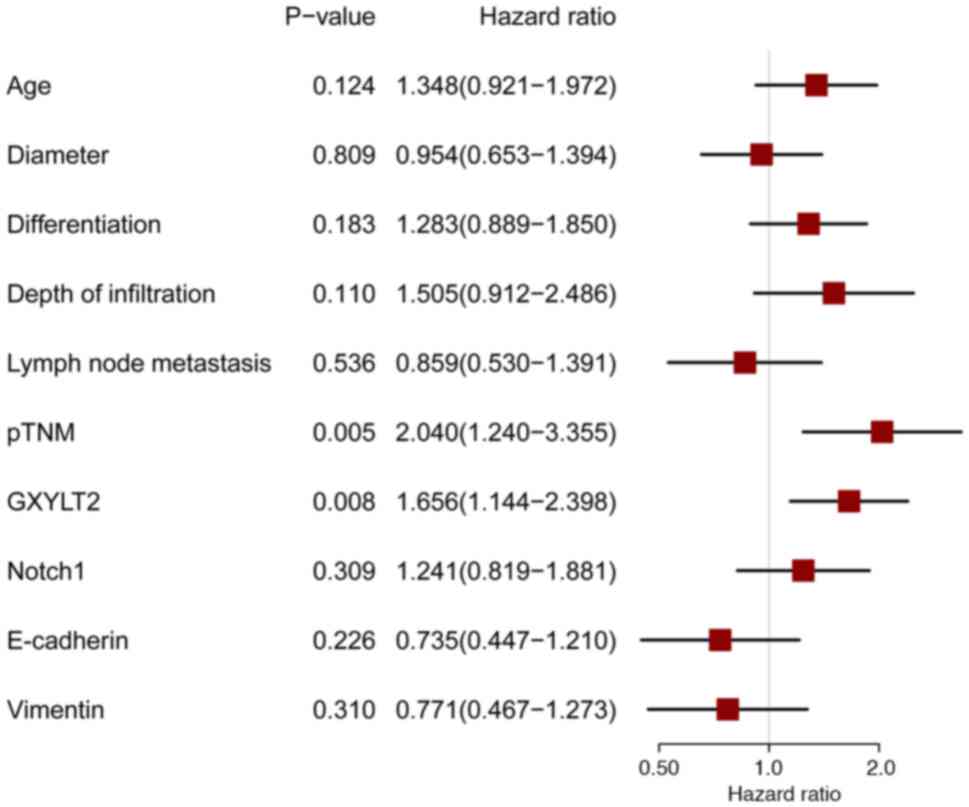
duration of patients with GC (P<0.05) (Fig. 3E). Findings from the multivariate
Cox regression analysis indicated that pTNM (P<0.001; Table IV; Fig.
4) and GXYLT2 (P<0.001) independently and significantly
predicted the prognosis of patients with GC, whereas the other
factors analyzed showed no notable association with prognosis.
 | Table IV.Comprehensive Cox regression analysis
of overall survival. |
Table IV.
Comprehensive Cox regression analysis
of overall survival.
|
|
|
|
|
|
|
| 95.0% CI for Exp
(B) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | df | P-value | Exp (B) | Lower | Upper |
|---|
| Age | −0.296 | 0.194 | 2.327 | 1 | 0127 | 0.744 | 0.509 | 1.088 |
| Diameter | 0.042 | 0.193 | 0.048 | 1 | 0.827 | 1.043 | 0.714 | 1.524 |
|
Differentiation | −0.246 | 0.187 | 1.733 | 1 | 0.188 | 0.782 | 0.542 | 1.128 |
| Depth of
infiltration | −0.408 | 0.256 | 2.541 | 1 | 0.111 | 0.665 | 0.403 | 1.098 |
| Lymph node
metastasis | 0.149 | 0.246 | 0.367 | 1 | 0.544 | 1.161 | 0.717 | 1.878 |
| pTNM | −0.702 | 0.253 | 7.676 | 1 | 0.006 | 0.496 | 0.302 | 0.814 |
| GXYLT2 | −0.500 | 0.189 | 7.015 | 1 | 0.008 | 0.607 | 0.419 | 0.878 |
| Notch1 | −0.213 | 0.212 | 1.008 | 1 | 0.315 | 0.808 | 0.534 | 1.225 |
| E-cadherin | 0.310 | 0.254 | 1.490 | 1 | 0.222 | 1.363 | 0.829 | 2.241 |
| Vimentin | 0.257 | 0.255 | 1.019 | 1 | 0.313 | 1.293 | 0.785 | 2.141 |
Discussion
GC is a frequent type of malignant tumor. Recently,
advancements in all-encompassing surgical treatment techniques have
led to a reduction in mortality rates (17); however, s GC mortality rates are
still high. A primary cause of the unfavorable outlook for GC lies
in its discovery at a late stage. Furthermore, GC has significant
heterogeneity and a complex pathogenesis, characterized by a
multifaceted process of multiple gene alterations and aberrant
expression (18). The investigation
of molecular mechanisms associated with GC is crucial for enhancing
the specificity of diagnosis and treatment targets, with the aim of
facilitating the advancement of personalized and comprehensive
clinical interventions for patients with this disease.
The process of glycosylation, a vital
post-translational protein alteration, is crucial for preserving
the functionality of proteins and aiding their participation in
numerous physiological activities, such as growth, development and
immune protection (19–22). N-glycosylation and O-glycosylation
exist in human cells, and O-glycosylation is completed by various
glycosyltransferases, including GXYLT2. Research has demonstrated
that abnormal glycosylation can significantly influence the onset
and progression of cancer by modulating tumor proliferation,
invasion, metastasis and angiogenesis (23–26).
However, limited research exists on the role of GXYLT2 as a
regulator in GC. Our previous bioinformatics analysis confirmed
that GXYLT2 may be an important target for the diagnosis and
treatment of GC (27).
The present study detected a notable upregulation in
the expression of GXYLT2 within GC tissues, indicating a
potentially pivotal role of GXYLT2 in the pathogenesis and
progression of GC. In vitro experimental studies have
documented a reduced expression of GXYLT2 in colorectal cancer and
an increased expression in renal cell carcinoma (28,29).
Abnormal expression of GXYLT has been shown to trigger anomalous
glycosylation of intracellular proteins in triple-negative breast
cancer, particularly the O-Glc trisaccharide modification of EGF
repeats, and the xylose extension of O-Glc glycans on Notch1 is
crucial for their transport (16,30).
Therefore, the present study further detected the expression of
Notch1 in GC tissues; the results revealed that it was
significantly increased and that it was positively associated with
the expression of GXYLT2. This finding suggested that GXYLT2 may
lead to abnormal glycosylation of Notch1, and affect the occurrence
and development of tumors. Research has indicated that the Notch
signaling pathway exhibits abnormal activation in numerous
malignant tumors and is of paramount importance in regulating the
EMT process (31), suggesting its
potential as a novel target for tumor therapy.
To clarify the possible involvement of GXYLT2 and
Notch1 in the EMT process of GC, the expression of E-cadherin and
vimentin was measured, both of which are crucial factors linked to
EMT, in GC tissues. The results of the present study showed a
notable reduction in E-cadherin levels and a substantial increase
in vimentin levels in GC tissues. A negative association existed
between the levels of E-cadherin and vimentin, whereas a positive
association existed between GXYLT2, Notch1 and vimentin, indicating
the significant role of GXYLT2 and Notch1 in the EMT mechanism of
GC. Research has shown that reducing GXYLT2 levels hinders the
proliferation, spread and metastasis of cancer cells, whereas
increasing GXYLT2 expression intensifies these effects (32). Suppressing GXYLT2 signaling has been
reported to result in a significant decrease in innate Notch
intracellular domain levels and a substantial increase in Notch1
protein levels, suggesting that GXYLT2 serves a role in activating
the Notch1 signaling pathway in human cells (32). Subsequent administration of the
Notch1 inhibitor DAPT may result in the inhibition of cell
proliferation and migration, as well as the complete reversal of
the EMT process (32). This
previous study supported the present hypothesis that GXYLT2 may
facilitate tumor cell proliferation, migration and EMT through the
Notch signaling pathway. Moreover, the present study, to the best
of our knowledge, is the first to examine the effect of GXYLT2 on
prognosis and survival from a histological standpoint. A link was
identified between the survival rate of patients and GXYLT2 protein
expression, in which elevated GXYLT2 protein levels were associated
with a poor prognosis for patients with GC. Therefore, the presence
of GXYLT2 could act as an independent predictive indicator for
patients with GC. The present study further validated the
association between GXYLT2 expression and pTNM, as well as the
association between Notch1 protein expression and nerve vessel
invasion in tumors. The heightened expression of GXYLT2 and Notch1
could potentially increase the invasive and metastatic capabilities
of GC cells.
A previous study suggested that GXYLT2 expression
was mostly negatively correlated with tumor mutational burden (TMB)
and microsatellite instability (MSI) in 33 tumor tissues from a
public database (33), and TMB and
MSI are considered genomic biomarkers for identifying patients with
cancer who may benefit from treatment with immune checkpoint
inhibitors (34–36). Therefore, it could be hypothesized
that GXYLT2 may serve as a prognostic marker and potential
immunotherapeutic target for GC, providing new directions for
future studies.
Despite the valuable findings of the present study,
its limitations include the restricted number of clinical specimens
and the inadequate sample size. Literature examining the direct
effect of GXYLT2 on GC is also lacking; we aim to explore this
topic in our forthcoming studies. In addition, the present study
only demonstrated an association between the protein levels, but
did not assess how GXYLT2 regulates Notch1 signaling and EMT at the
molecular level. In the future, we aim to explore how GXYLT2
regulates Notch1 signaling and EMT at the molecular level by
knocking down or overexpressing GXYLT2.
In conclusion, the present results indicated that
GXYLT2 may affect the EMT of GC through abnormal alteration of
Notch1 EGF sequences, suggesting that GXYLT2 may be an effective
prognostic marker for GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Education Fund Item of
Anhui Province (grant no. 2022AH051525) and the Natural Science
Foundation of Bengbu Medical University (grant no.
2020byzd035).
Availability of data and materials
The data generated in this study can be requested
from the corresponding author.
Authors' contributions
YZ was involved in conceptualization, methodology
and first draft writing, and acquired funding. LL wrote, reviewed
and edited the manuscript, analyzed and interpretated the data, and
processed the images. ZC was involved in conceptualization,
writing, reviewing and editing, and data collection and processing.
YZ and LL confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Bengbu Medical College (approval no. [2023]245). All
research participants or their legal representatives provided
written informed consent. All methods were conducted in accordance
with The Declaration of Helsinki (37).
Patient consent for publication
All research participants or their legal
representatives provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar
|
|
2
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000–14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar
|
|
3
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021. View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar
|
|
5
|
Miao ZF, Chen H, WangZ N, Jia-Fu JI, Liang
H, Xu HM and Wang J: Progress and remaining challenges in
comprehensive gastric cancer treatment. Holistic Integrative
Oncology. 1:2731–4529. 2022. View Article : Google Scholar
|
|
6
|
Lairson LL, Henrissat B, Davies GJ and
Withers SG: Glycosyltransferases: Structures, functions, and
mechanisms. Annu Rev Biochem. 77:521–555. 2008. View Article : Google Scholar
|
|
7
|
Lee TV, Sethi MK, Leonardi J, Rana NA,
Buettner FF, Haltiwanger RS, Bakker H and Jafar-Nejad H: Negative
regulation of notch signaling by xylose. PLoS Genet.
9:e10035472013. View Article : Google Scholar
|
|
8
|
Sethi MK, Buettner FFR, Krylov VB,
Takeuchi H, Nifantiev NE, Haltiwanger RS, Gerardy-Schahn R and
Bakker H: Identification of glycosyltransferase 8 family members as
xylosyltransferases acting on O-glucosylated notch epidermal growth
factor repeats. J Biol Chem. 285:1582–1586. 2010. View Article : Google Scholar
|
|
9
|
Shi Q, Xue C, Zeng Y, Yuan X, Chu Q, Jiang
S, Wang J, Zhang Y, Zhu D and Li L: Notch signaling pathway in
cancer: From mechanistic insights to targeted therapies. Signal
Transduct Target Ther. 27:1282024. View Article : Google Scholar
|
|
10
|
Bakker H and Gerardy-Schahn R: A sweet
development in Notch regulation. J Biol Chem. 292:15974–15975.
2017. View Article : Google Scholar
|
|
11
|
Wharton KA, Johansen KM, Xu T and
Artavanis-Tsakonas S: Nucleotide sequence from the neurogenic locus
notch implies a gene product that shares homology with proteins
containing EGF-like repeats. Cell. 43:567–581. 1985. View Article : Google Scholar
|
|
12
|
Blavier L, Lazaryev A, Shi X-H, Dorey FJ,
Shackleford GM and DeClerck YA: Stromelysin-1 (MMP-3) is a target
and a regulator of Wnt1-induced epithelial-mesenchymal transition
(EMT). Cancer Biol Ther. 10:198–208. 2010. View Article : Google Scholar
|
|
13
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar
|
|
14
|
Kim DH, Xing T, Yang Z, Dudek R, Lu Q and
Chen YH: Epithelial mesenchymal transition in embryonic
development, tissue repair and cancer: A comprehensive overview. J
Clin Med. 7:12017. View Article : Google Scholar
|
|
15
|
Amack JD: Cellular dynamics of EMT:
Lessons from live in vivo imaging of embryonic development. Cell
Commun Signal. 19:792021. View Article : Google Scholar
|
|
16
|
Urata Y, Saiki W, Tsukamoto Y, Sago H,
Hibi H, Okajima T and Takeuchi H: Xylosyl Extension of O-Glucose
glycans on the extracellular domain of NOTCH1 and NOTCH2 regulates
notch cell surface trafficking. Cells. 9:12202020. View Article : Google Scholar
|
|
17
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a Population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar
|
|
18
|
Weiser TG, Haynes AB, Molina G, Lipsitz
SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR and
Gawande AA: Size and distribution of the global volume of surgery
in 2012. Bull World Health Organ. 94:201–209F. 2016. View Article : Google Scholar
|
|
19
|
Cisło M, Filip AA, Arnold Offerhaus GJ,
Ciseł B, Rawicz-Pruszyński K, Skierucha M and Polkowski WP:
Distinct molecular subtypes of gastric cancer: From Laurén to
molecular pathology. Oncotarget. 9:19427–19442. 2018. View Article : Google Scholar
|
|
20
|
Hancock CN, Kent L and McClure BA: The
stylar 120 kDa glycoprotein is required for S-specific pollen
rejection in Nicotiana. Plant J. 43:716–723. 2005. View Article : Google Scholar
|
|
21
|
Josè-Estanyol M and Puigdomènech P: Plant
cell wall glycoproteins and their genes. Plant Physiol Biochem.
38:97–108. 2000. View Article : Google Scholar
|
|
22
|
Pearce G, Siems WF, Bhattacharya R, Chen
YC and Ryan CA: Three hydroxyproline-rich glycopeptides derived
from a single petunia polyprotein precursor activate defensin I, a
pathogen defense response gene. J Biol Chem. 282:17777–17784. 2007.
View Article : Google Scholar
|
|
23
|
Tan Z, Lu W, Li X, Yang G, Guo J, Yu H, Li
Z and Guan F: Altered N-Glycan expression profile in
epithelial-to-mesenchymal transition of NMuMG cells revealed by an
integrated strategy using mass spectrometry and glycogene and
lectin microarray analysis. J Proteome Res. 13:2783–2795. 2014.
View Article : Google Scholar
|
|
24
|
Fuster MM and Esko JD: The sweet and sour
of cancer: Glycans as novel therapeutic targets. Nat Rev Cancer.
5:526–542. 2005. View
Article : Google Scholar
|
|
25
|
Munkley J and Elliott DJ: Hallmarks of
glycosylation in cancer. Oncotarget. 7:35478–35489. 2016.
View Article : Google Scholar
|
|
26
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View
Article : Google Scholar
|
|
27
|
Zhao Y, Hu S, Zhang J, Cai Z, Wang S, Liu
M, Dai J and Gao Y: Glucoside xylosyltransferase 2 as a diagnostic
and prognostic marker in gastric cancer via comprehensive analysis.
Bioengineered. 12:5641–5654. 2021. View Article : Google Scholar
|
|
28
|
Wu Y and Liao Q: MicroRNA-204-5p hampers
the malignant progression of clear cell renal cell carcinoma
through GXYLT2 downregulation. Kidney Blood Press Res. 47:654–663.
2022. View Article : Google Scholar
|
|
29
|
Zhou ZH, Wang QL, Mao LH, Li XQ, Liu P,
Song JW, Liu X, Xu F, Lei J and He S: Chromatin accessibility
changes are associated with enhanced growth and liver metastasis
capacity of Acid-adapted colorectal cancer cells. Cell Cycle.
18:511–522. 2019. View Article : Google Scholar
|
|
30
|
Rana NA, Nita-Lazar A, Takeuchi H, Kakuda
S, Luther KB and Haltiwanger RS: O-glucose trisaccharide is present
at high but variable stoichiometry at multiple sites on mouse
Notch1. J Biol Chem. 286:31623–31637. 2011. View Article : Google Scholar
|
|
31
|
Dongre A and Weinberg RA: New insights
into the mechanisms of Epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar
|
|
32
|
Cui Q, Xing J, Gu Y, Nan X, Ma W, Chen Y
and Zhao H: GXYLT2 accelerates cell growth and migration by
regulating the Notch pathway in human cancer cells. Exp Cell Res.
376:1–10. 2019. View Article : Google Scholar
|
|
33
|
Song YB, Bao WG, Liu DH, Wei LQ, Yang ST,
Miao XJ, Lin CY, Li HJ, Lan D and He HM: Pan-cancer analysis of the
prognostic significance and oncogenic role of GXYLT2. Medicine
(Baltimore). 102:e356642023. View Article : Google Scholar
|
|
34
|
McNamara MG, Jacobs T, Lamarca A, Hubner
RA, Valle JW and Amir E: Impact of high tumor mutational burden in
solid tumors and challenges for biomarker application. Cancer Treat
Rev. 89:1020842020. View Article : Google Scholar
|
|
35
|
Palmeri M, Mehnert J, Silk AW, Jabbour SK,
Ganesan S, Popli P, Riedlinger G, Stephenson R, de Meritens AB,
Leiser A, et al: Real-world application of tumor mutational
burden-high (TMB-high) and microsatellite instability (MSI)
confirms their utility as immunotherapy biomarkers. ESMO Open.
7:1003362022. View Article : Google Scholar
|
|
36
|
Picard E, Verschoor CP, Ma GW and Pawelec
G: Relationships between immune landscapes, genetic subtypes and
responses to immunotherapy in colorectal cancer. Front Immunol.
11:3692020. View Article : Google Scholar
|
|
37
|
World Medical Association: World Medical
Association Declaration of Helsinki: ethical principles for medical
research involving human subjects. JAMA. Nov. 310:2191–2194.
2013.
|