Introduction
Hepatocellular carcinoma (HCC) is one of the most
lethal malignancies endangering global health, while the diagnostic
assessment and routine follow-up measures are far from satisfactory
(1). In general, the foremost
significant risk factor for developing HCC is underlying liver
cirrhosis (LC). Hence, biannual HCC surveillance strategies in
daily medical practice are broadly recommended, including imaging
with abdominal ultrasonography (US) with or without monitoring
serum alpha-fetoprotein (AFP) (2).
This screening tool, nevertheless, has inadequate performance
characteristics for early HCC detection and evaluation due to
modest early-stage sensitivity, interobserver variation and limited
patient adherence (3).
While most cancers have decreasing mortality, HCC
continues to be one of the leading causes of cancer-related death,
with an overall five-year survival of 15% (1). Inadequate early detection, the lack of
curative options for individuals found at advanced stages and
conflicting risks of death from concomitant LC all contribute to
this high mortality rate (1,3,4).
Curative treatment modalities, such as radiofrequency ablation
(RFA), liver surgery and transplantation, are only available for
early-stage disease (5), with
five-year survival rates above 60%. Relapse within those five years
is prevalent, even after receiving optimal treatment. Accordingly,
vigilant monitoring with attention to HCC development and
prediction of recurrence is required to decrease disease-related
mortality.
Serum is a useful tool for detecting HCC because
many serum proteins are produced and secreted by the liver, and
aberrant serum proteins may act as molecular indicators of liver
disease progression and carcinogenesis. In the last decades,
several serum proteins have been described as possible biomarkers
for HCC diagnosis and prognosis, but most require further
validation before being applicable in routine clinical practice.
Lens culinary agglutinin-reactive fraction of AFP (AFP-L3),
des-gamma-carboxyprothrombin (DCP) and cell-free DNA, for example,
have all been studied (3). In
cancer, protein glycosylation has emerged as a major field of
interest since it plays various roles in cellular activities
(6). Humans experience two main
forms of glycosylation: N- and O-linked glycosylation. The most
common N-linked type involves sugar molecules attached to a
nitrogen atom in an asparagine residue as part of a specific
protein sequon. Different monosaccharides can be consecutively
attached to each other and processed by several glycan-modifying
enzymes without the use of a template, resulting in the dynamic
manufacture of substantial glycopeptide heterogeneity. When cancer
cells begin to develop abnormally, cell-cell interactions change
and altered N-glycan structures become apparent during cancer
progression, such as core fucosylation, β1,6-GlcNAc branching,
bisecting GlcNAc and sialylation (6–8).
Consequently, detecting and quantifying specific
glycans associated with tumour progression in patients with liver
disease provides insight into cancer growth and could be a
promising approach for personalised HCC management. In this study,
we performed a systematic review of the potential value of
N-glycomics as prognostic biomarkers in HCC.
Materials and methods
Protocol
This systematic review was performed concordant with
the PRISMA 2020 statement (https://www.prisma-statement.org/prisma-2020), and was
registered on PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=283324).
Literature search. A scoping review was performed to
exclude existing systematic reviews on the same topic using the
search terms ‘glycosylation’ and ‘hepatocellular carcinoma’. A
comprehensive literature search was conducted through the databases
Medline (PubMed interface), EMBASE (embase.com interface), Web of
Science Core Collection and Scopus through December 20, 2022, with
no start date restriction. An update was executed through August 17
and December 29, 2023. With the assistance of an experienced
librarian (N.P.), optimised search terms for the concepts
‘glycosylation’, ‘hepatocellular carcinoma’ and ‘biomarker’ were
identified. Searches were limited to studies in English.
Complementary, cited references (e.g., reference list of recent
review articles) were explored, and grey literature (e.g., Google
Scholar, Biblio UGent) was screened for eligibility. The search
strategy for all databases is detailed in Table SI. The search databases used are
available at the following URLs: https://pubmed.ncbi.nlm.nih.gov/; https://www.embase.com/#advancedSearch/;
https://www.webofscience.com; https://www.scopus.com/search/form.uri?display=basic#basic.
Eligibility criteria and study
selection
Published studies of any design, except review
articles and guidelines, were included. Animal studies, studies on
children (<18 years) and abstracts without the availability of a
full-text paper were excluded. Eligible studies assessed the
potential of serum N-glycomics as prognostic biomarkers for HCC
regardless of aetiology, disease stage, comorbidities or treatment.
After deduplication, using the Endnote software application, all
records were screened independently by at least two authors (N.S.,
E.B. or X.V.) in Rayyan (doi: 10.1186/s13643-016-0384-4), with
discrepancies resolved by consensus.
Outcome measures
The primary goal was to evaluate the capability of
N-glycomics to predict the risk of developing HCC in adults with
chronic liver disease and, if HCC was present, to predict the
overall survival or survival rate. As a secondary goal, the
potential to predict HCC recurrence was assessed. The
predictability of the glycomics-based biomarker was expressed as
the area under the receiver operating characteristic curve (AUC)
with its optimal cut-off index (COI) value obtained by the
maximised Youden index and corresponding sensitivity and
specificity. P-values of the log-rank test, comparing high and low
levels of the biomarker in the Kaplan-Meier analysis, were reported
when available. Statistical significance was set at a two-tailed
Pvalue of <0.05.
Data extraction
Relevant data were extracted from the full-text
articles by two independent authors (N.S. and E.B.) using a
standardised form designed a priori. The following information was
retrieved from each study: author, year, study design, sample size
of different study cohorts (training, control, validation), patient
and disease characteristics, HCC aetiology, glycoprotein or
N-glycan with its analytical technique and statistical methods.
Quality assessment
The QUALSYST quality assessment tool (doi:
10.7939/R37M04F16) was applied to assess the overall risk of bias.
As such, the methodological quality of the included studies was
determined based on 14 criteria. A score was given depending on the
extent to which the specific criterion was disclosed (yes=2;
partial=1; no=0; N/A=not applicable). Table SII outlines the summary score
created for every article by adding the scores obtained across the
rated criteria. The quality of the included articles was assessed
by two independent reviewers (E.B. and N.S.), with discrepancies
resolved by consensus.
Results
General results
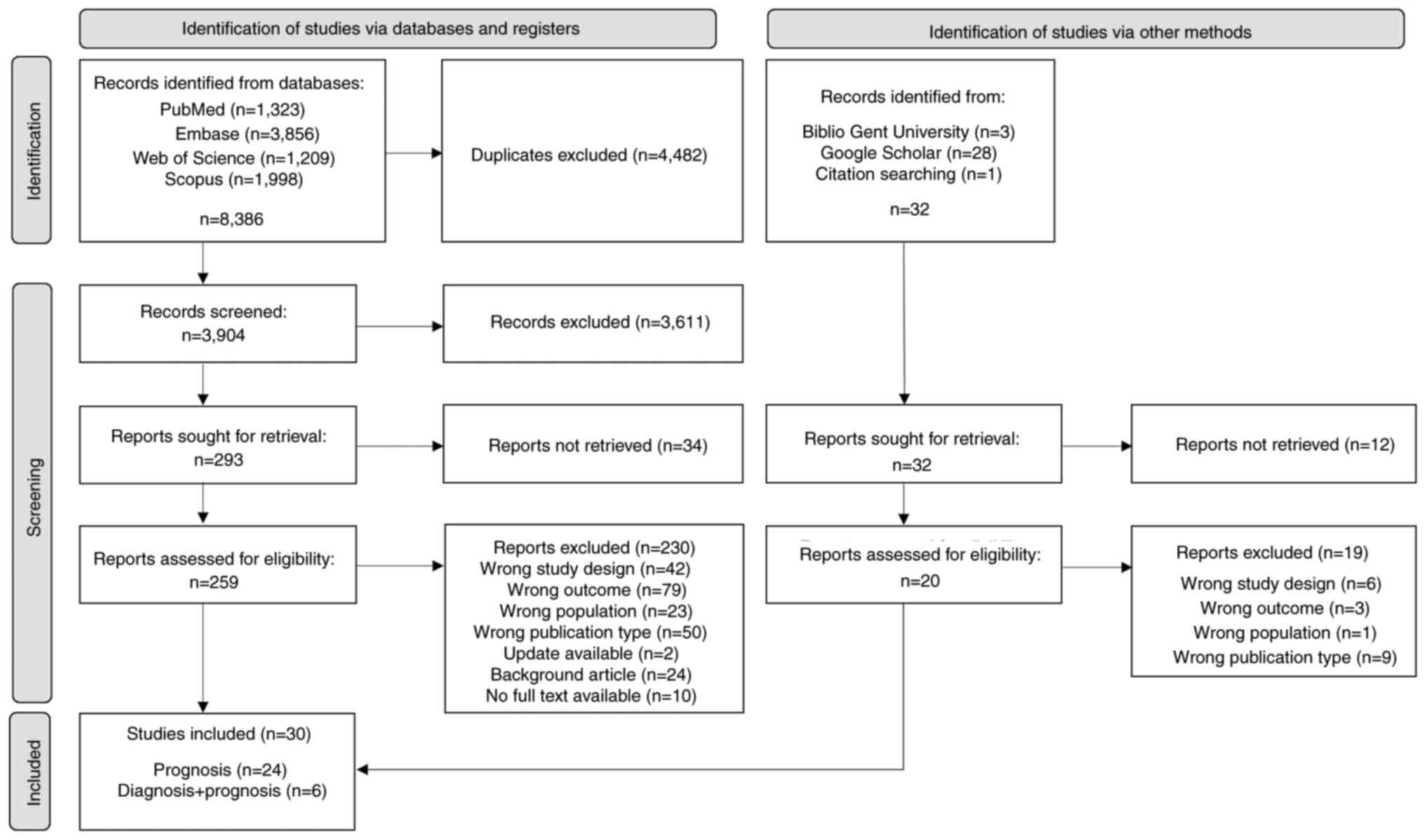
A database search generated 8,386 studies, while 32
studies emerged through other methods. In total, 3,936 paper
abstracts were reviewed, and 279 were considered eligible.
Following a full-text evaluation, 249 publications were omitted,
providing 30 studies examining serum N-glycomics as a predictive
biomarker in HCC (Fig. 1). The
features of the included reports are tabulated by the main examined
outcome: prediction of HCC development in chronic liver disease
(Table I), survival or mortality
(Table SIII) and recurrence
(Table SIV) in HCC. The most
relevant descriptives are highlighted for all articles and the
subset of articles on M2BPGi and for each outcome by itself
(Table SV). Fig. 2 illustrates an overview of the
investigated glycans and glycoproteins per outcome.
 | Table I.Prediction of HCC development in
chronic liver disease. |
Table I.
Prediction of HCC development in
chronic liver disease.
| First author, year
of publication | Patients with
HCC | Controls | External
validation | Analytical
technique | Biomarker
characteristics |
|
|---|
|
|
|---|
| Glycan | Prognostic
value | AUC | (Refs.) |
|---|
| Verhelst et
al, | n=125 LC | N/A | N/A | DSA-FACE | Whole serum | High baseline |
GlycoCirrhoTest | (9) |
| 2017 | n=34 HCC |
|
|
| GlycoCirrhoTest=Log
[NA2FB]/ | GlycoCirrhoTest
(≥0.2) and | AUC=0.71 |
|
|
| Median FU |
|
|
| [NA3]
GlycoHCCRiskScore | GlycoHCCRiskScore
are | GlycoHCCRisk
Score |
|
|
| time: 66.7 m |
|
|
| =[(NGA2FB ×
0.137) | predictive for | AUC=0.73 |
|
|
|
|
|
|
| + (NA2FB ×
−0.044) | developing HCC in
LC | AFP AUC=0.56 |
|
|
|
|
|
|
| + (NA3 ×
−0.216) |
|
|
|
|
|
|
|
|
| + NA3Fb ×
0.158) |
|
|
|
|
|
|
|
|
| + (NA3Fbc ×
0.796) |
|
|
|
|
|
|
|
|
| + (NA4 ×
−0.764)] |
|
|
|
| Liu et al,
2017 | n=357 | n=713 | N/A | Chemilumine- | M2BPGi | High baseline
M2BPGi | 1-2 years
model: | (15) |
|
| HBV-related | CHB |
| scent enzyme- |
| (≥2) and the
prediction | AUC=0.84
M2BPGi: |
|
|
| HCC |
|
| linked |
| model (M2BPGi + AFP
+ | AUC=0.79 AFP: |
|
|
| FU-time: 7 y |
|
| immunoassay |
| HBsAg) are
predictive | AUC=0.69 |
|
|
|
|
|
| (HISCL-5000) |
| for developing HCC
in | 2-5 years
model: |
|
|
|
|
|
|
|
| treatment-naive
CHB | AUC=0.81
M2BPGi: |
|
|
|
|
|
|
|
|
| AUC=0.74 AFP: |
|
|
|
|
|
|
|
|
| AUC=0.63 |
|
| Jun et
al, | n=692 CHB | N/A | N/A | Chemilumine- | M2BPGi | High baseline
M2BPGi | 10 years CHB
M2BPGi: | (16) |
| 2019 | n=47 HCC |
|
| scent enzyme- |
| (>1.09) is
predictive for | AUC=0.84 AFP: |
|
|
| Median FU |
|
| linked |
| developing HCC
intreatment- | AUC=0.75 |
|
|
| time: 6.8 y |
|
| immunoassay |
| naive CHB |
|
|
|
|
|
|
| (HISCL-5000) |
|
|
|
|
| Mak et
al, | n=207 CHB | N/A | N/A | Chemilumine- | M2BPGi | High M2BPGi (≥0.68)
within 3 | AUC=0.88 | (10) |
| 2019 | n=14 HCC |
|
| scent enzyme- |
| years after
HBeAg |
|
|
|
| Median FU |
|
| linked |
| seroconversion is
predictive for |
|
|
|
| time: 13.1 y |
|
| immunoassay |
| developing HCC
in |
|
|
|
|
|
|
| (HISCL-5000) |
| treatment-naive
CHB |
|
|
| Heo et
al, | n=95 CHB | N/A | N/A | Chemilumine- |
WFA+-M2BP | High baseline
WFA+- | P=0.016 | (13) |
| 2016 | n=7 HCC |
|
| scent enzyme- |
| M2BP (≥1.8) is
predictive for |
|
|
|
| Median FU- |
|
| linked |
| developing HCC in
CHB |
|
|
|
| time: 45 m |
|
| immunoassay |
| (82% antiviral
therapy) |
|
|
|
|
|
|
| (HISCL-5000) |
|
|
|
|
| Murata et
al, | n=147 CHB | N/A | N/A | Chemilumine- | M2BPGi | High M2BPGi
(>1.5) | AUC=0.83 | (18) |
| 2020 | n=14 HCC |
|
| scent enzyme- |
| at 48 weeks NA
therapy | P<0.001 |
|
|
| Median FU |
|
| linked |
| is predictive for
developing |
|
|
|
| time: 6.6 y |
|
| immunoassay |
| HCC in CHB |
|
|
|
|
|
|
| (HISCL-5000) |
|
|
|
|
| Su et
al, | n=126 LC | N/A | n=145 LC | Chemilumine- | M2BPGi | High M2BPGi (≥3)
at | AUC=0.79 | (17) |
| 2020 | n=20 HBV- |
| n=30 | scent enzyme- |
| antiviral
therapy-induced | P<0.0001 |
|
|
| related HCC |
| HBV- | linked |
| VR is predictive
for |
|
|
|
| Mean FU |
| related | immunoassay |
| developing HCC in
CHB |
|
|
|
| time: 50.3 m |
|
| (HISCL-5000) |
|
|
|
|
| Shinkai et
al, | n=234 CHB | N/A | N/A | Chemilumine- | M2BPGi | High M2BPGi
(≥1.21) | P<0.001 | (19) |
| 2018 | n=24 HCC |
|
| scent enzyme- |
| at 48 weeks of NA
therapy (VR) | Low AFP: |
|
|
| Median FU |
|
| linked |
| is predictive for
developing | P=0.011 |
|
|
| time: 51 m |
|
| immunoassay |
| HCC in CHB |
|
|
|
|
|
|
| (HISCL-5000) |
|
|
|
|
| Mak et
al, | n=100 | n=185 | N/A | Chemilumine- | M2BPGi | High baseline
M2BPGi (≥1.15) is | AUC=0.64 | (11) |
| 2019 | HBV-related | CHB |
| scent enzyme- |
| predictive for
developing HCC in | P=0.0370 |
|
|
| HCC |
|
| linked |
| CHB on entecavir
therapy |
|
|
|
| Median FU |
|
| immunoassay |
|
|
|
|
|
| time: 7.1 y |
|
| (HISCL-5000) |
|
|
|
|
| Tseng et
al, | n=899 CHB | N/A | n=384 | Chemilumine- | M2BPGi | High baseline
M2BPGi (≥1.73) | P<0.001 | (12) |
| 2020 | n=64 HCC |
| CHB | scent enzyme- |
| is predictive for
developing HCC |
|
|
|
| Median FU |
| n=36 HCC | linked |
| in CHB on entecavir
therapy |
|
|
|
| time: 7 y |
| HBV- | immunoassay |
|
|
|
|
|
|
|
| related | (HISCL-5000) |
|
|
|
|
| Kawaguchi, | n=141 CHB | N/A | N/A | Chemilumine- |
WFA+-M2BP | High
WFA+-M2BP (≥0.8) during | M2BPGi
recurrence | (14) |
| 2018 | n=17 + 71 |
|
| scent enzyme- |
| NA treatment is
predictive | AUC=0.71
P=0.0110 |
|
|
| HCC FU- |
|
| linked |
| for HCC recurrence
and combi | M2BPGi +
HBcrAg |
|
|
| time: 10 y |
|
| immunoassay |
| (≥1.05) with HBcrAG
(≥3) is | development
P<0.0001 |
|
|
|
|
|
| (HISCL-5000) |
| predictive for
HCC |
|
|
|
|
|
|
|
|
| development in
CHB |
|
|
| Lin et al,
2018 | n=921 HCV | n=799 | N/A | Chemilumine- |
WFA+-M2BP | High baseline
WFA+-M2BP | 1 year model:
AUC=0.91 | (21) |
|
| n=122 | HCV |
| scent enzyme- |
| (≥1.5) and in
combination with + | M2BPGi:
AUC=0.87 |
|
|
| Median FU- |
|
| linked |
| AFP + age + sex
+ALT + AST/ | AFP: AUC=0.82 |
|
|
| time: 21.7 y |
|
| immunoassay |
| ALT ratio are
predictive for | M2BPGi overall |
|
|
|
|
|
| (HISCL-5000) |
| developing HCC
intreatment- | AUC=0.76 |
|
|
|
|
|
|
|
| naïve HCV |
|
|
| Yamasaki et
al, | n=707 HCV | N/A | N/A | Chemilumine- |
WFA+-M2BP | High baseline
WFA+-M2BP | P<0.001 3
years | (22) |
| 2014 | n =110 HCC |
|
| scent enzyme- |
| (≥4.0) is
predictive for | M2BPGi:
AUC=0.83 |
|
|
| Mean FU- |
|
| linked |
| developing HCC in
HCV | AFP: AUC=0.77 |
|
|
| time: 8.2 y |
|
| immunoassay |
| (53% antiviral
therapy) | 5 years
M2BPGi: |
|
|
|
|
|
| (HISCL-5000) |
|
| AUC=0.86 AFP: |
|
|
|
|
|
|
|
|
| AUC=0.80 |
|
|
|
|
|
|
|
|
| 7 years |
|
|
|
|
|
|
|
|
| M2BPGi:
AUC=0.82 |
|
|
|
|
|
|
|
|
| AFP: AUC=0.80 |
|
| Sasaki et
al, | n=238 HCV | N/A | N/A | Chemilumine- |
WFA+-M2BP | High
WFA+-M2BP (>2) at SVR | P<0.0001 3
years | (25) |
| 2015 | n=16 HCC |
|
| scent enzyme- |
| is predictive for
developing | M2BPGi: |
|
|
| Median FU |
|
| linked |
| HCC in HCV after
IFN therapy | AUC=0.91 AFP: |
|
|
| time: 9.1 y |
|
| immunoassay |
|
| AUC=0.88 |
|
|
|
|
|
| (HISCL-5000) |
|
| 5 years
M2BPGi: |
|
|
|
|
|
|
|
|
| AUC=0.81 AFP: |
|
|
|
|
|
|
|
|
| AUC=0.78 |
|
|
|
|
|
|
|
|
| 10 years
M2BPGi: |
|
|
|
|
|
|
|
|
| AUC=0.71 |
|
|
|
|
|
|
|
|
| AFP: AUC=0.63 |
|
| Nagata et
al, | n=119 HCV | N/A | N/A | Chemilumine- | M2BPGi | High M2BPGi (≥2.2)
at SVR is | M2BPGi:
AUC=0.97 | (26) |
| 2016 | n=8 HCC |
|
| scent enzyme- |
| predictive for
developing HCC | AFP: AUC=0.75 |
|
|
| Mean FU- |
|
| linked |
| in HCV after
therapy | P=0.0007 |
|
|
| time: 7.1 y |
|
| immunoassay |
|
|
|
|
|
|
|
|
| (HISCL-5000) |
|
|
|
|
| Matsumae et
al, | n=786 HCV | N/A | n=262 of | ELISA | TSP-2 AFT = TSP-2
+ | High TSP-2
(>86.95 ng/µl) at | TSP-2: AUC=0.70
AFT: | (30) |
| 2023 | n=24 HCC |
| 786 HCV |
| FIB-4 + AFP | SVR is predictive
for developing | AUC=0.83 AFP: |
|
|
| Median FU- |
|
|
|
| HCC in HCV after
DAAs therapy | AUC=0.72
P<0.0001 |
|
|
| time: 41.5 m |
|
|
|
|
|
|
|
| Asazawa et
al, | n=19 TNM I | N/A | N/A | Lectin ELISA | Fuc-Hpt elevation
rate = change in | High Fuc-Hpt
elevation rate | Fuc-Hpt elevation
rate | (31) |
| 2015 | (<2 cm) FU |
|
|
| Fuc-Hpt values at 2
time points | (>498.2%) is
predictive for | AUC=0.75 |
|
|
| time: 10 y |
|
|
| during 10-year
FU) | developing HCC in
LC |
|
|
HCC prediction in chronic liver
disease
In whole serum, a high baseline GlycoCirrhoTest,
calculated as the logarithmic ratio of the bigalacto
core-α-1,6-fucosylated bisecting biantennary glycan NA2FB to the
triantennary glycan NA3 and GlycoHCCRiskScore (based upon six
altered glycans) was investigated as a predictor for developing HCC
in cirrhotic patients (9). Both
glycotests outperformed AFP (AUC=0.56) at a COI value of 5.75
ng/ml, with no significant difference between the GlycoCirrhoTest
(AUC=0.71) and GlycoHCCRiskScore (AUC=0.73) themselves.
Furthermore, particular glycoproteins associated
with HCC development were investigated, such as Mac-2 binding
protein, thrombospondin-2 (TSP-2) and fucosylated haptoglobin. The
role of the serum Mac-2 binding protein glycosylation isomer or
Wisteria floribunda agglutinin-positive Mac-2 binding protein
(M2BPGi or WFA+-M2BP), a well-known glycomarker of
hepatic fibrosis, was studied in 19 articles. Eighteen of them
investigated viral HCC aetiology, such as chronic hepatitis B (CHB)
virus (10–20) and hepatitis C virus (HCV) infection
(21–27), while only a single research group
considered other underlying liver diseases (28). In terms of outcome measures, the
majority addressed the impact of baseline
M2BPGi/WFA+-M2BP value in the development of HCC
(10,12,13,15,16,21,22),
and the remainder aligned pre- or post-treatment values with
overall survival (23,24,27),
recurrence (14,20,27,28) or
death (24). Remarkably, all
studies used the same chemiluminescent enzyme-linked immunoassay by
a two-step sandwich method to analyse the
M2BPGi/WFA+-M2BP concentration in the serum (29).
Viral hepatitis remains a significant global health
issue, exposing patients at increased risk of developing cirrhosis,
hepatic decompensation and eventually HCC. Highly successful
antiviral medications have reduced liver inflammation and
suppressed virus replication; nevertheless, these agents can only
minimise but not prevent the risk of developing HCC. Even after
long-term viral suppression, this liver cancer type can occur
(12,17,25).
As such, HCC risk prediction has become imperative in the clinical
monitoring and disease management of CHB and HCV patients. In the
past few years, the significance of M2BPGi/WFA+-M2BP as
a feasible predictive marker for the development of HCC in viral
hepatitis has been explored. Elevated baseline values of this
glycoprotein were able to discriminate CHB individuals at high risk
for HCC from those at low risk before treatment (10–13),
during treatment with nucleoside analogues (NA) (14) such as Entecavir, and in
treatment-naive patients (both cirrhotic and non-cirrhotic)
(11,13,15,16).
The same results were obtained when M2BPGi was assessed as an
independent predictor during viral remission following NA treatment
(17,18), even in those with low AFP
(P<0.001) (19). This prognostic
potential was also achieved in the hepatitis C population, in which
both the baseline value (in mostly untreated patients) (21–23)
and the post-therapeutic value after direct-acting antivirals
(DAAs) or interferon (IFN) at the time of virological elimination
(24–26), were considered to predict HCC
development.
Subsequently, six studies compared
M2BPGi/WFA+-M2BP directly to AFP and their combination
in predicting the development of HBV- and HCV-related HCC. They
showed that baseline M2BPGi and the combination with AFP (model,
AUC=0.81) was superior to AFP alone for long-term prediction until
five years (M2BPGi, AUC=0.74) (15)
and ten years (M2BPGi, AUC=0.84) (16) in treatment-naive CHB. Equivalently,
baseline WFA-M2BP (AUC=0.87) and the combination with AFP
(AUC=0.91) served as a better marker for short-term prediction
within the first year in treatment-naive HCV (21). At time of sustained viral response
(SVR) after antivirals in HCV, M2BPGi/WFA+-M2BP with COI
above 2 was able to predict the development of HCC, with an AUC of
0.97 [26], even up to ten years after treatment (AUC=0.71)
(25). Similarly, elevated TSP-2
level - a glycoprotein produced by the fibrotic liver - in HCV
patients who achieved SVR with DAAs was found to have a function as
a biomarker for the development of HCC (AUC=0.70), which further
improved (AUC=0.83) when combined with AFP and the Fibrosis-4
(FIB-4) index (30).
Finally, Asazawa et al (31) determined the elevation rate of
fucosylated haptoglobin (Fuc-Hpt) as the difference in Fuc-Hpt
levels at two time points over a ten-year follow-up period to
predict the development of HCC in cirrhotic patients (AUC=0.75) and
found that an increase of more than 498.2% had 100% specificity for
HCC occurrence.
Survival prediction in HCC
The Car-G risk score (32) was constructed to predict survival in
AFP-negative HCC patients based on a whole serum biomarker panel of
13 N-glycan abundances using logistic regression. After HCC
resection, 30% of high-risk patients with a baseline Car-G risk
score of more than 1.80 had shorter overall survival (Log Rank,
P=0.025), and 35% had lower recurrence-free survival (Log Rank,
P=0.046) than low-risk patients.
The role of the glycosylated AFP specifically
garnered attention in prognostic biomarker research, just as it is
valued in diagnostic settings. In 1998, Aoyagi et al
(33) defined the fucosylation
index (FI) of AFP as the percentage of the lectin-reactive fraction
of AFP (AFP-L3) to total AFP when assessing overall survival in HCC
patients receiving transarterial locoregional treatment based on
baseline FI, AFP and their combination. They elucidated that
patients with FI scores >18% and AFP concentrations >200
ng/ml were at risk for low overall survival; in terms of separating
high-risk and low-risk individuals, the combination of FI and AFP
concentration excelled the individual indices (Log Rank, P=0.0003).
Similarly, a more recent study (34) demonstrated that baseline AFP-L3
prognostic performance was successful, even in patients with low
AFP serum concentrations (≤20 ng/ml). The COI of AFP-L3 was 10% for
AFP above 20 ng/ml, and the five-year overall survival of HCC
patients with an AFP-L3 level above this COI was 28.3% lower than
those with an AFP-L3 level below 10% (Log Rank, P=0.001). Five-year
recurrence-free survival was not substantially different between
both groups in a subcohort of 129 HCC patients treated with
curative RFA.
Toyoda et al (28) and Fujiyoshi et al (27) questioned whether
WFA+-M2BP could act as a potential marker for the
assessment of survival after curative resection and displayed that
high baseline levels were able to predict low overall survival (Log
Rank, P=0.013, COI >4.615) (27)
or survival rate (Log Rank, P=0.0187, COI >3) (28). Similar results were seen in
treatment-naive HCV patients or patients who did not achieve SVR
after IFN therapy (23).
Subsequently, an association was found between higher M2BPGi levels
(cut-off 1.8–2.2) after DAA-induced SVR and mortality in HCV (Log
Rank, P=0.02) (24). In addition,
low overall survival (Log Rank, P=0.010, AUC=0.69) and high
carcinogenesis rate (Log Rank, P<0.006, AUC=0.76) in HCV-related
cirrhosis have been linked to augmented serum levels of
WFA+-colony stimulating factor 1 receptor
(WFA+-CSF1R, COI ≥310 ng/ml) and WFA+-CSF1R%
(COI ≥35%), respectively (35). The
comparison for both outcomes was made with AFP and AFP-L3, but no
significant results could be retained for both markers. The
Kaplan-Meier technique with the Log Rank test was used to conduct
survival studies. Corresponding P-values may also be found in
Tables SIII and SIV.
Recurrence prediction in HCC
In whole serum, Fang et al (36) displayed that the logarithm of the
ratio of the branching α-1,3-fucosylated triantennary glycan NA3Fb
to the single monogalacto core-α-1,6-fucosylated biantennary glycan
NG1A2F and the Cscore C performed better in monitoring HBV-related
HCC recurrence after surgical resection, regarding the appearance
of vascular invasion. As to AFP at COI of 200 ng/ml (AUC=0.61), the
specificity of the log ratio improved by 16% compared to the same
sensitivity level, with an AUC reaching 0.706 (Cscore C=[(–0.3829 ×
NG1A2F) + (0.4214 × NA3Fb) + 0.9539], AUC=0.703).
Towards research on predictive glycoproteins, three
research teams revealed that an increased level of
WFA+-M2BP before curative resection (14,20,29) or
RFA (combined with or without transarterial chemoembolisation
[TACE]) (14) in viral hepatitis or
other aetiologies (28) was
associated with a high recurrence rate of HCC. Furthermore, three
other glycoproteins are reported to play a potential role in
predicting relapse after HCC treatment, namely fetuin-A (FetA)
(37), immunoglobulin G (IgG)
(38) and mucin 1 (MUC1) (39). Two studies questioned whether the
risk for recurrence and low survival rates following curative liver
resection in HBV-related HCC patients could be related to
alterations in the glycomarker concentration. High preoperative
fucosylated FetA (Fuc-FetA, COI >1.105) and IgG (IgG-L3%, COI
>28%) levels were shown to be predictive of low recurrence-free
survival (P=0.018) [37] and overall survival (P=0.023), whereas a
postoperative increase in IgG-L3% predicted HCC recurrence
(P=0.003) (38). Tamaki et
al (39) disclosed that
elevated serum Wisteria floribunda agglutinin-positive sialylated
mucin 1 (WFA+-sialylated MUC1, COI ≥900 µl/ml) levels
could indicate a high recurrence rate (P=0.020) and less fortunate
type of recurrence (P=0.020) in RFA-cured early-stage HCC
patients.
Discussion
In this systematic review, we demonstrated that
serum N-glycomics might be a valuable biomarker for predicting de
novo HCC development in chronic liver disease or recurrence after
treatment. Within the current shift towards personalised medicine,
the prediction of disease progression and response to therapy is
undeniable. To date, routinely performed liver biopsy has been
discarded as the gold standard for diagnosis of HCC recurrence due
to its invasiveness, sampling error and associated complication
risks (13,40). Surveillance imaging at a
well-defined interval, with or without serum AFP, is generally
recommended (41). However, these
methods do not always seem adequate for early HCC detection and
disease monitoring. Although imaging can be sensitive for detecting
a lesion, a certain tumour load is required before it becomes
apparent, implying we are continuously falling behind. Similarly,
the historically used marker AFP in diagnosing early HCC (with
traditional COI of 20 ng/ml) is known for its poor sensitivity
(ranging from 39 to 64%) with limited specificity (ranging from 76
to 97%), meaning that it can be a false positive in non-malignant
conditions with active hepatocyte regeneration or false negative in
the presence of HCC (4,42). In analogy with other malignant
tumours, such as soft tissue sarcomas (43), recent reports have uncovered
inflammatory parameters that may influence tumour aggressiveness
and outcomes, such as neutrophil-to-lymphocyte ratio (NLR),
platelet-to-lymphocyte ratio (PLR), C-reactive protein (CRP) and
cytokines (44). However, the
biomarkers often remain specific within a given setting (e.g.,
immunotherapy) and they have not been widely implemented clinically
to date. The major unmet need in HCC management remains the lack of
validated and clinically feasible noninvasive biomarkers with
appropriate sensitivity and specificity, providing prompt
information concerning HCC progression and prognosis compared to
conventional biomarkers and allowing treatment decisions to be
guided more effectively.
Serum N-glycomics might be able to meet this demand
since it is known that alterations in the serum protein glycomic
profile reflect a disbalance of the hepatocyte homeostasis, where
the glycan product is mostly generated. Glycosylation, the most
prominent posttranslational modification of proteins, depends on
the expression of glycosyltransferases, which are dynamically
regulated depending on the cell state. These glycome alterations
disrupt the control of cell adhesion, migration and proliferation,
resulting in pathological processes that lead to cancer development
(7). The metastatic potential of
tumour cells, for example, has been linked to increased sialylation
of cell surface glycoproteins, occurring as a result of the
addition of terminal sialic acids to newly formed branches in the
synthesis of multi-antennary glycans (↑ GlcNAc-transferase V).
Other known glycomic changes linked to HCC include aberrant outer
arm fucosylation of highly branched N-glycans (↑
α-1,3-fucosyl-transferase), synthesis of AFP-L3 (↑
α-1,6-fucosyl-transferase) and upregulated GlcNAc-transferase III,
resulting in increased formation of bisecting GlcNAc (7,9,45).
Fucose can be conjugated to N-glycans controlled by different
fucosyltransferases (FUTs) in various ways. It has been displayed
that α-1,6 core fucosylation of N-glycan GlcNAc residues, produced
by FUT8, has a key function in modulating growth factor signalling
pathways promoting tumourigenesis, notably those mediated by TGFβR,
EGFR, VEGFR and c-Met1 (45–47).
FUT3 to FUT7 and FUT9 to FUT11 facilitate α-1,3/4 branch
fucosylation, acknowledged for generating a number of Lewis
antigens and enhancing metastatic ability. In particular,
E-selectin, expressed on endothelial cells, is bound by increased
sialyl-Lewis X (sLe X) in cancer cells. Thus, the capacity of
circulating tumour cells to extravasate from the vessels into
neighbouring tissues may be improved (47,48).
We investigated 30 reports studying serum
N-glycomics as omics-based prognostic biomarkers for the
development and recurrence of, and overall survival in, HCC. Recent
technology breakthroughs have resulted in multi-omics data,
representing the biological heterogeneity of HCC and their
potential as biomarkers. Proteomics research has focused, among
many others, on the tumour markers AFP-L3, DCP and M2BPGi. An early
stage of liver cancer is frequently diagnosed using the proportion
of AFP-L3 to total AFP (AFP-L3%), which is believed to be an
HCC-specific glycoprotein (32–34).
Together with DCP, it is incorporated in the BALAD-2 prognostic
model, which has been confirmed as an effective model for
predicting survival in HCC patients by an international study
(49). Both glycomarkers also seem
to have prognostic value for survival after treatment (50) and waitlist dropout among HCC
patients awaiting liver transplantation (51). Consequently, elevated levels of DCP,
a non-functional prothrombin precursor linked to tumour
angiogenesis and vascular proliferation, were considered effective
for HCC prognosis in general (20,28,33,34).
According to a recent review (52),
DCP has a specificity of 81 to 98% and a sensitivity of 48 to 62%
as a predictive marker in HCC, identifying advanced-stage
individuals who may benefit from (first-line) sorafenib treatment
and playing a prognostic role in the detection of portal vein
invasion. Alternatively, the extensively glycosylated form of Mac-2
binding protein (M2BP), so-called M2BPGi or WFA+- M2BP, garnered a
lot of attention as a novel biomarker of hepatic fibrosis
progression, one of the strongest predictors of HCC development
(28). Briefly, M2BPGi serves as a
messenger for the activation of hepatic stellate cells throughout
the advancement of fibrosis by triggering sinusoidal cell
dysfunction. Since the sugar chain structure of M2BP would alter in
response to the evolution of fibrosis, the potential of M2BPGi to
predict HCC may be ascribed to its pertinent ability to distinguish
different fibrotic stages. Moreover, the increased serum level of
M2BPGi reflects underlying hepatocellular carcinogenesis, given its
properties of activating the mammalian target of rapamycin (mTOR)
signalling pathway by binding galectin-3. Besides being an indirect
marker for hepatic fibrosis, serum M2BPGi could indicate early HCC
detection during disease monitoring. Nevertheless, M2BPGi levels
may not be used alone in HCC prediction, as elevated levels may
also be seen in chronic cardio-pulmonary diseases (13,15,26,53).
Since hepatic fibrosis progression is considered to
be one of the strongest predictors of HCC development, the
investigated glycomics-based biomarkers were often compared to
other indirect biochemical markers like the FIB-4 index and
aspartate aminotransferase-to-platelet ratio index (APRI) (9,10,14,16,19,26,29,35).
It is known that these evaluation methods are hampered by limited
specificity in distinguishing fibrosis state, which was also
confirmed in this systematic review. Individual laboratory values,
whether or not linked to demographic characteristics like age and
gender, are frequently used in prognostic HCC models to increase
predictive accuracy. For example, we see that albumin and bilirubin
are included in the BALAD-2 score (49), while platelet count is taken into
account in the PAGE-B score (46).
Not surprisingly, many glycomics studies in this systematic review
also include (one of) these factors within their uni- or
multivariate analysis.
Despite the potential use of serum N-glycomics as
predictive biomarkers in HCC, various limitations must be
acknowledged. First, specific study population groups were selected
regarding aetiology in many of the included articles. Most studies
considered only Asian patients with viral aetiology, making it
difficult to extrapolate these results to the overall HCC
population. Although viral hepatitis continues to be the leading
cause of HCC globally, alcohol abuse and nonalcoholic
steatohepatitis (NASH) are increasingly responsible for the rise in
HCC incidence in Western countries. Second, most of the
glycoproteins investigated are markers for fibrosis and, by
extension, cirrhosis. It is unclear whether these indicators are
equally effective in predicting the development of HCC in
individuals who lack a cirrhotic background. Third, due to
insufficient sample sizes and often retrospective study design, the
test performances might be overestimated, and selection bias might
be present. Further studies involving all aetiologies and expanding
their sample sizes are required to resolve these issues. Fourth,
not all research included statistical comparisons with ‘a gold
standard’ like AFP, making it challenging to assess the benefit of
the new biomarker across studies and obscuring its significance.
More comprehensive studies are necessary to confirm the clinical
application of N-glycan markers in this predictive setting.
Finally, heterogeneity among the reports was too extended for a
meta-analysis to be performed. Statistical analysis was considered
on the subdomain related to the glycoprotein M2BPGi, since most
articles investigated this glycoprotein. Of the eighteen
M2BPGi-related publications there was access to hazard ratios (or
the data from which the ratios could be calculated), seven articles
focused on univariate analysis and eleven on multivariate analysis
- with only seven articles correcting for the same variable in the
latter. Consequently, the numbers and power were too small to
conduct a proper meta-analysis. Moreover, a conscious decision was
made not to concentrate the research on specific glycoproteins, as
the purpose of this systematic review is to provide all available
data on predictive glycomics-based biomarkers in HCC.
To the best of our knowledge, no systematic review
have been performed on this subject. In addition to the latest
comprehensive analyses of non-invasive biomarkers for HCC screening
(3) and immunotherapy (44), this review offers an outline of the
updated knowledge regarding glycomics-based biomarkers in HCC,
which could encourage current interest in the epigenetics of cancer
and personalized medicine. Therefore, our findings may contribute
to the research field as they provide a fresh perspective on the
present state of expertise on glycomics in HCC.
In conclusion, it is generally accepted that early
detection of HCC lesions can significantly improve long-term
survival, and several HCC biomarkers have been studied for this
purpose. However, none have obtained broad clinical usage, except
for AFP, the current HCC biomarker that is approved on a global
scale. The significant false positive rate of AFP in LC and the
inadequate sensitivity in detecting early-stage HCC imply that new
biomarkers are necessary. Aberrant N-glycosylation of serum
proteins is known to contribute to HCC development. In particular,
increased levels of M2BPGi may reflect underlying hepatocellular
carcinogenesis. Aberrant N-glycosylation of serum proteins may
ultimately have value as predictive biomarkers for the development,
recurrence and survival of HCC. However, more research with a
refined study design and patient selection is essential to validate
this.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This project was supported by a grant from Kom op tegen Kanker
(grant no. STI.VLK.2020.0003.01). XV received a translational
clinical mandate from the Stichting tegen Kanker.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NS, XV, HVV, LM and NC conceptualized the study and
developed the theoretical framework. NS, EB and NP designed and
constructed the methodology of the study, with extensive support
from SL, LG, AG, SR and LD. NS, EB and XV curated data for the
study (study selection was performed by NS and XV; full text
selection by NS and EB; data extraction by NS and EB). NS wrote the
original draft of the study. XV, LM, NC and HVV provided general
supervision, project administration and funding acquisition. NS and
XV confirm the authenticity of all the raw data. All authors
discussed the results, and have read and approved the final version
of the manuscript.
Ethics approval and consent for
participation
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singal AG, Pillai A and Tiro J: Early
detection, curative treatment, and survival rates for
hepatocellular carcinoma surveillance in patients with cirrhosis: A
meta-analysis. PLoS Med. 11:e10016242014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parikh ND, Tayob N and Singal AG:
Blood-based biomarkers for hepatocellular carcinoma screening:
Approaching the end of the ultrasound era? J Hepatol. 78:207–216.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao YY, Takahashi M, Gu JG, Miyoshi E,
Matsumoto A, Kitazume S and Taniguchi N: Functional roles of
N-glycans in cell signaling and cell adhesion in cancer. Cancer
Sci. 99:1304–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor M and Drickamer K: Introduction to
Glycobiology. Oxford (England): Oxford University Press; pp.
250–256. 2011
|
|
8
|
Taniguchi N and Kizuka Y: Glycans and
cancer: Role of N-glycans in cancer biomarker, progression and
metastasis, and therapeutics. Adv Cancer Res. 126:11–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verhelst X, Vanderschaeghe D, Castéra L,
Raes T, Geerts A, Francoz C, Colman R, Durand F, Callewaert N and
Van Vlierberghe H: A glycomics-based test predicts the development
of hepatocellular carcinoma in cirrhosis. Clin Cancer Res.
23:2750–2758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mak LY, To WP, Wong DK, Fung J, Liu F,
Seto WK, Lai CL and Yuen MF: Serum Mac-2 binding protein
glycosylation isomer level predicts hepatocellular carcinoma
development in E-negative chronic hepatitis B patients. World J
Gastroenterol. 25:1398–1408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mak LY, Ko M, To E, Wong DK, Ma JH, Hui
TL, Seto WK, Fung J, Lai CL and Yuen MF: Serum Mac-2-binding
protein glycosylation isomer and risk of hepatocellular carcinoma
in entecavir-treated chronic hepatitis B patients. J Gastroenterol
Hepatol. 34:1817–1823. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tseng TC, Peng CY, Hsu YC, Su TH, Wang CC,
Liu CJ, Yang HC, Yang WT, Lin CH, Yu ML, et al: Baseline Mac-2
binding protein glycosylation isomer level stratifies risks of
hepatocellular carcinoma in chronic hepatitis b patients with oral
antiviral therapy. Liver Cancer. 9:207–220. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heo JY, Kim SU, Kim BK, Park JY, Kim DY,
Ahn SH, Park YN, Ahn SS, Han KH and Kim HS: Use of Wisteria
Floribunda Agglutinin-Positive Human Mac-2 Binding Protein in
Assessing Risk of Hepatocellular Carcinoma Due to Hepatitis B
Virus. Medicine (Baltimore). 95:e33282016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawaguchi K, Honda M, Otha H, Terashima T,
Shimakami T, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi
E, et al: Serum Wisteria floribunda agglutinin-positive Mac-2
binding protein predicts hepatocellular carcinoma incidence and
recurrence in nucleos(t)ide analogue therapy for chronic hepatitis
B. J Gastroenterol. 53:740–751. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Hu HH, Lee MH, Korenaga M, Jen CL,
Batrla-Utermann R, Lu SN, Wang LY, Mizokami M, Chen CJ and Yang HI:
Serum Levels of M2BPGi as short-term predictors of hepatocellular
carcinoma in untreated chronic hepatitis B patients. Sci Rep.
7:143522017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jun T, Hsu YC, Ogawa S, Huang YT, Yeh ML,
Tseng CH, Huang CF, Tai CM, Dai CY, Huang JF, et al: Mac-2 binding
protein glycosylation isomer as a hepatocellular carcinoma marker
in patients with chronic hepatitis B or C Infection. Hepatol
Commun. 3:493–503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su TH, Peng CY, Tseng TC, Yang HC, Liu CJ,
Liu CH, Chen PJ, Chen DS and Kao JH: Serum Mac-2-Binding protein
glycosylation isomer at virological remission predicts
hepatocellular carcinoma and death in chronic hepatitis B-Related
cirrhosis. J Infect Dis. 221:589–597. 2020.PubMed/NCBI
|
|
18
|
Murata A, Amano N, Sato S, Tsuzura H,
Tomishima K, Sato S, Matsumoto K, Shimada Y, Iijima K and Genda T:
On-treatment Serum Mac-2 binding protein glycosylation isomer
(M2BPGi) Level and risk of hepatocellular carcinoma development in
patients with chronic hepatitis B during Nucleot(s)ide analogue
therapy. Int J Mol Sci. 21:20512020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shinkai N, Nojima M, Lio E, Matsunami K,
Toyoda H, Murakami S, Inoue T, Ogawa S, Kumada T and Tanaka Y: High
levels of serum Mac-2-binding protein glycosylation isomer (M2BPGi)
predict the development of hepatocellular carcinoma in hepatitis B
patients treated with nucleot(s)ide analogues. J Gastroenterol.
53:883–889. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HS, Kim SU, Kim BK, Park JY, Kim DY,
Ahn SH, Han KH, Park YN, Han DH, Kim KS, et al: Serum Wisteria
floribunda agglutinin-positive human Mac-2 binding protein level
predicts recurrence of hepatitis B virus-related hepatocellular
carcinoma after curative resection. Clin Mol Hepatol. 26:33–44.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin YJ, Chang CL, Chen LC, Hu HH, Liu J,
Korenaga M, Huang YH, Jen CL, Su CY, Nishida N, et al: A
glycomarker for short-term prediction of hepatocellular carcinoma:
A longitudinal study with serial measurements. Clin Trans
Gastroenterol. 9:1832018. View Article : Google Scholar
|
|
22
|
Yamasaki K, Tateyama M, Abiru S, Komori A,
Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y, et
al: Elevated serum levels of wisteria floribunda
agglutinin-positive human Mac-2 binding protein predict the
development of hepatocellular carcinoma in hepatitis C patients.
Hepatology. 60:1563–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue T, Tsuzuki Y, Lio E, Shinkai N,
Matsunami K, Fujiwara K, Matsuura K, Nojiri S and Tanaka Y:
Clinical evaluation of hepatocarcinogenesis and outcome using a
novel glycobiomarker wisteria floribunda agglutinin-positive Mac-2
Binding Protein (WFA+-M2BP) in Chronic Hepatitis C with Advanced
Fibrosis. Jpn J Infect Dis. 71:177–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakagawa M, Nawa N, Takeichi E, Shimizu T,
Tsuchiya J, Sato A, Miyoshi M, Kawai-Kitahata F, Murakawa M, Nitta
S, et al: Mac-2 binding protein glycosylation isomer as a novel
predictive biomarker for patient survival after hepatitis C virus
eradication by DAAs. J Gastroenterol. 55:990–999. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sasaki R, Yamasaki K, Abiru S, Komori A,
Nagaoka S, Saeki A, Hashimoto S, Bekki S, Kugiyama Y, Kuno A, et
al: Serum wisteria floribunda agglutinin-positive Mac-2 binding
protein values predict the development of hepatocellular carcinoma
among patients with chronic hepatitis c after sustained virological
response. PLoS One. 10:e01290532015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagata H, Nakagawa M, Nishimura-Sakurai Y,
Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Goto F, Otani S,
Kawai-Kitahata F, et al: Serial measurement of Wisteria floribunda
agglutinin positive Mac-2-binding protein is useful for predicting
liver fibrosis and the development of hepatocellular carcinoma in
chronic hepatitis C patients treated with IFN-based and IFN-free
therapy. Hepatol Int. 10:956–964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujiyoshi M, Kuno A, Gotoh M, Fukai M,
Yokoo H, Kamachi H, Kamiyama T, Korenaga M, Mizokami M, Narimatsu
H, et al: Clinicopathological characteristics and diagnostic
performance of Wisteria floribunda agglutinin positive
Mac-2-binding protein as a preoperative serum marker of liver
fibrosis in hepatocellular carcinoma. J Gastroenterol.
50:1134–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toyoda H, Kumada T, Tada T, Kaneoka Y,
Maeda A, Korenaga M, Mizokami M and Narimatsu H: Serum WFA+-M2BP
levels as a prognostic factor in patients with early hepatocellular
carcinoma undergoing curative resection. Liver int. 36:293–301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mak LY, Wong DK, Cheung KS, Seto WK, Lai
CL and Yuen MF: Role of serum M2BPGi levels on diagnosing
significant liver fibrosis and cirrhosis in treated patients with
chronic hepatitis B virus infection. Clin Transl Gastroenterol.
9:1632018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsumae T, Kodama T, Tahata Y, Myojin Y,
Doi A, Nishio A, Yamada R, Nozaki Y, Oshita M, Hiramatsu N, et al:
Thrombospondin-2 as a Predictive Biomarker for Hepatocellular
Carcinoma after Hepatitis C Virus Elimination by Direct-Acting
Antiviral. Cancers (Basel). 15:4632023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asazawa H, Kamada Y, Takeda Y, Takamatsu
S, Shinzaki S, Kim Y, Nezu R, Kuzushita N, Mita E, Kato M and
Miyoshi E: Serum fucosylated haptoglobin in chronic liver diseases
as a potential biomarker of hepatocellular carcinoma development.
Clin Chem Lab Med. 53:95–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang C, Fang M, Feng H, Liu L, Li Y, Xu
X, Wang H, Wang Y, Tong L, Zhou L and Gao C: N-glycan fingerprint
predicts alpha-fetoprotein negative hepatocellular carcinoma: A
large-scale multicenter study. Int J Cancer. 149:717–727. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aoyagi Y, Isokawa O, Suda T, Watanabe M,
Suzuki Y and Asakura H: The Fucosylation Index of a-Fetoprotein as
a possible prognostic indicator for patients with hepatocellular
carcinoma. Cancer. 83:2076–2082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nouso K, Kobayashi Y, Nakamura S,
Kobayashi S, Takayama H, Toshimori J, Kuwaki K, Hagihara H, Onishi
H, Miyake Y, et al: Prognostic importance of fucosylated
alpha-fetoprotein in hepatocellular carcinoma patients with low
alpha-fetoprotein. J Gastroenterol Hepatol. 26:1195–1200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lio E, Ocho M, Togayachi A, Nojima M, Kuno
A, Ikehara Y, Hasegawa I, Yatsuhashi H, Yamasaki K, Shimada N, et
al: A novel glycobiomarker, Wisteria floribunda agglutinin
macrophage colony-stimulating factor receptor, for predicting
carcinogenesis of liver cirrhosis. Int J Cancer. 138:1462–1471.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang M, Zhao YP, Zhou FG, Lu LG, Qi P,
Wang H, Zhou K, Sun SH, Chen CY and Gao CF: N-glycan based models
improve diagnostic efficacies in hepatitis B virus-related
hepatocellular carcinoma. Int J Cancer. 127:148–159. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Gu X, Fang M, Ji J, Yi C and Gao C:
The diagnostic value of serum fucosylated fetuin A in hepatitis B
virus-related liver diseases. Clin Chem Lab Med. 54:693–701. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yi CH, Weng HL, Zhou FG, Fang M, Ji J,
Cheng C, Wang H, Liebe R, Dooley S and Gao CF: Elevated
core-fucosylated IgG is a new marker for hepatitis B virus-related
hepatocellular carcinoma. Oncoimmunology. 4:e10115032015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tamaki N, Kuno A, Matsuda A, Tsujikawa H,
Yamazaki K, Yasui Y, Tsuchiya K, Nakanishi H, Itakura J, Korenaga
M, et al: Serum wisteria floribunda agglutinin-positive sialylated
mucin 1 as a marker of progenitor/biliary features in
hepatocellular carcinoma. Sci Rep. 7:2442017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuno A, Ikehara Y, Tanaka Y, Ito K,
Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M and
Narimatsu H: A serum ‘sweet-doughnut’ protein facilitates fibrosis
evaluation and therapy assessment in patients with viral hepatitis.
Sci Rep. 3:10652013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim TH, Kim SY, Tang A and Lee JM:
Comparison of international guidelines for noninvasive diagnosis of
hepatocellular carcinoma: 2018 update. Clin Mol Hepatol.
25:245–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korean Liver Cancer Study Group (KLCSG);
National Cancer Center and Korea (NCC), . 2014 Korean Liver Cancer
Study Group-National Cancer Center Korea practice guideline for the
management of hepatocellular carcinoma. Korean J Radiol.
16:465–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T and Akagi M: Characterizing inflammatory markers in highly
aggressive soft tissue sarcomas. Medicine (Baltimore).
101:e306882022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peng X, Gong C, Zhang W and Zhou A:
Advanced development of biomarkers for immunotherapy in
hepatocellular carcinoma. Front Oncol. 12:10910882023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vajaria BN and Patel PS: Glycosylation: A
hallmark of cancer? Glycoconj J. 34:147–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yilma M, Saxena V and Mehta N: Models to
predict development or recurence of hepatocellular carcinoma (HCC)
in patients with advanced hepatic fibrosis. Curr Gastroenterol Rep.
24:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Keeley TS, Yang S and Lau E: The diverse
contributions of fucose linkages in cancer. Cancers (Basel).
11:12412019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin X, Rana K, Ponmudi V and King MR:
Knockdown of fucosyltransferase III disrupts the adhesion of
circulating cancer cells to E-selectin without a ecting
hematopoietic cell adhesion. Carbohydr Res. 345:2334–2342. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Berhane S, Toyoda H, Tada T, Kumada T,
Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP,
Benckert J, et al: Role of the GALAD and BALAD-2 serological models
in diagnosis of hepatocellular carcinoma and prediction of survival
in patients. Clin Gastroenterol Hepatol. 14:875–886.e6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Toyoda H, Kumada T, Kaneoka Y, Osaki Y,
Kimura T, Arimoto A, Oka H, Yamazaki O, Manabe T, Urano F, et al:
Prognostic value of pretreatment levels of tumor markers for
hepatocellular carcinoma on survival after curative treatment of
patients with HCC. J Hepatol. 49:223–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kotwani P, Chan W, Yao F and Mehta N: DCP
and AFP-L3 are complementary to AFP in predicting high-risk explant
features: Results of a prospective study. Clin Gastroenterol
Hepatol. 20:701–703.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Samman BS, Hussein A, Samman RS and
Alharbi AS: Common sensitive diagnostic and prognostic markers in
hepatocellular carcinoma and their clinical significance: A review.
Cureus. 14:e239522022.PubMed/NCBI
|
|
53
|
Witarto AP, Witarto BS, Pramudito SL,
Putra AJE, Nurhadi GM and Maimunah U: Baseline serum Mac-2 binding
protein glycosylation isomer as a predictor of hepatocellular
carcinoma in chronic hepatitis B patients: A systematic review and
meta-analysis. Ann Gastroenterol. 35:627–639. 2022.PubMed/NCBI
|