Introduction
Ovarian cancer is the third most common
gynecological malignancy worldwide and the fifth leading cause of
cancer-related death among women (1), with ~200,000 cases diagnosed annually
worldwide (2). A large proportion
of patients with ovarian cancer are diagnosed at late stages of the
disease, due to the lack of symptoms during early stages of the
disease. This is associated with a notable reduction in the 5-year
survival rate, which decreases from 93.5% for patients with stage I
cancer to <30% for patients with stage IV cancer (3,4).
Current available treatments for ovarian cancer include surgery,
chemotherapy and radiotherapy. However, these strategies have
clinical limitations, including the late stage at the time of
discovery, the loss of surgery opportunities and that chemotherapy
can lead to drug resistance (5).
Therefore, the discovery of new therapeutic targets in ovarian
cancer is needed to improve the treatment outcomes for
patients.
Previous studies have demonstrated that cancer cells
can rewire their cellular metabolism to support the demands of
tumorigenesis, cell growth and survival, cellular communication and
cancer metastasis (6–9). The Warburg effect is a process of
metabolic reprogramming in cancer cells, which describes the
preference of cancer cells to metabolize glucose anaerobically
instead of aerobically (10–15).
Disorders relating to tryptophan (Trp) metabolism have gained
interest as a potential therapeutic target in ovarian cancer.
Previous studies have reported that Trp metabolism is overactive in
a certain types of tumors, such as gliomas (16), liver cancer (17) and cervical cancer (18), which promotes a malignant phenotype
and inhibition of tumor immunity. Trp is an essential amino acid
and Trp metabolism can lead to the production of serotonin and
metabolites through the kynurenine (Kyn) pathway (19). In the Kyn pathway, kynurenine is
produced by tryptophan metabolism, which serves important roles in
promoting the pathogenesis of cancer (20,21).
Studies have demonstrated the impact of the Kyn pathway on the
tumor microenvironment and its biological effects on tumor
immunological responses (22,23),
where Kyn serves as a key functional molecule that can activate the
signaling of ligand-activated transcription factor AHR and
transduce tumor immune escape (24). By contrast, PD-L1 is an immune
checkpoint that is expressed in cancer cells (25) and its expression is modulated by
cytokines, including TNF-α, VEGF and IFN (26). Although PD-L1 blockade therapy has
shown promise in clinical practice, the underlying mechanism of
this is currently unclear. Therefore, the reduction of Kyn
expression levels may be an important strategy for the treatment of
ovarian cancer.
In vitro and in vivo assays were
performed to investigate the role of amino acid transporters in
ovarian cancer cells. In addition, the clinical samples were
analyzed by metabonomics. The present study aimed to facilitate the
discovery of novel therapeutic targets and treatment strategies for
the treatment of ovarian cancer.
Materials and methods
Tissue and plasma samples
Ovarian tissue samples and plasma were obtained from
a biorepository at the Zhejiang Cancer Hospital (Hangzhou, China)
and the patients admitted from 1st January 2016 to 31st December
2017. Patients and healthy donors that were enrolled in the present
study provided written informed consent. All patients received
standard clinical treatment. Patients and healthy donors were aged
between 19–89 years. The present study was performed
retrospectively and was approved by the Ethics Committee of the
Zhejiang Cancer Hospital (approval no. IRB-2021-315; Hangzhou,
China).
Tissue and plasma samples were collected during
surgery from patients that were diagnosed with either advanced
serous ovarian cancer or benign cysts (tissue, Table I; plasma, Table II). Tissue samples were stored at
−80°C following sample collection. Plasma was immediately separated
from blood samples after collection by centrifugation at 1,200 × g
for 10 min at 4°C, which was then aliquoted and stored at −80°C.
Patient-derived cells (PDCs) were obtained from a patient with
ovarian cancer (female, 65 years old) during surgery in Zhejiang
Cancer Hospital on 8th August 2017. These generation of these PDCs
were described in our previous study (2) and approved by the Ethics Committee of
the Zhejiang Cancer Hospital [approval no. (2015)-1-7; Hangzhou,
China]. The expression levels of the genes identified in ovarian
cancer classed according to tumor grade were analyzed using the
University of ALabama at Birmingham CANcer data analysis Portal
software (http://ualcan.path.uab.edu/index.html).
 | Table I.Baseline characteristics of patient
with tumors and benign cysts of included tissue samples. |
Table I.
Baseline characteristics of patient
with tumors and benign cysts of included tissue samples.
| A, Benign
cases |
|---|
|
|---|
| Characteristic | No. of patients,
n |
|---|
| Age, years |
|
|
<60 | 25 |
|
≥60 | 15 |
|
| B, Tumor
cases |
|
|
Characteristic | No. of patients,
n |
|
| Age, years |
|
|
<60 | 48 |
|
≥60 | 29 |
| Menarche age,
years |
|
|
<15 | 30 |
|
≥15 | 47 |
| Menopause |
|
|
Yes | 65 |
| No | 12 |
| Histology |
|
|
Non-serous | 3 |
|
Serous | 74 |
| Clinical stage |
|
|
I–II | 4 |
|
III–IV | 72 |
|
Missing | 1 |
| Alcohol
consumption |
|
|
Yes | 0 |
| No | 77 |
| Smoking status |
|
|
Yes | 0 |
| No | 77 |
| Family history of
ovarian cancer |
|
|
Yes | 27 |
| No | 50 |
 | Table II.Baseline characteristics of the
healthy patients and patients with cancer of included serum
samples. |
Table II.
Baseline characteristics of the
healthy patients and patients with cancer of included serum
samples.
| A, Healthy
cases |
|---|
|
|---|
| Characteristic | No. of patients,
n |
|---|
| Age, years |
|
|
<60 | 44 |
|
≥60 | 10 |
|
| B, Tumor
cases |
|
|
Characteristic | No. of patients,
n |
|
| Age, years |
|
|
<60 | 142 |
|
≥60 | 60 |
| Menarche age,
years |
|
|
<15 | 50 |
|
≥15 | 151 |
|
Missing | 1 |
| Menopause |
|
|
Yes | 134 |
| No | 68 |
| Histology |
|
|
Non-serous | 50 |
|
Serous | 152 |
| Clinical stage |
|
|
I–II | 21 |
|
III–IV | 174 |
|
Missing | 7 |
| Alcohol
consumption |
|
|
Yes | 0 |
| No | 202 |
| Smoking status |
|
|
Yes | 0 |
| No | 202 |
| Family history of
ovarian cancer |
|
|
Yes | 6 |
| No | 196 |
Cell lines and chemicals
The ovarian cancer cell lines SKOV3, ID8 and ES-2
(short tandem repeat profiling certified) were purchased from the
American Type Culture Collection. The cells were cultured in an
incubator at 37°C and 5% CO2. SKOV3 cells were cultured
in MyCoy's 5A medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin (Beijing Solarbio Science &
Technology Co., Ltd.). MyCoy's 5A medium without Trp (Wuhan Boster
Biological Technology, Ltd.) was used to culture cells for Trp
starvation experiments. ES-2 and ID8 cells were cultured in Roswell
Park Memorial Institute 1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin-streptomycin. All cell lines were tested using the Cell
Culture Contamination Detection Kit (Thermo Fisher Scientific,
Inc.) to ensure that the cells were negative for mycoplasma
contamination.
L-Trp and L-Kyn were purchased from MedChem Express.
BCH (TargetMol; cat. no. T11820; Shanghai Topscience Co., Ltd.) and
JPH203 (TargetMol; cat. no. TQ0081; Shanghai Topscience Co., Ltd.)
were purchased from TargetMol Chemicals Inc. 13C-Trp was
purchased from Sigma-Aldrich (Merck KGaA).
Cell transfections
Lentiviruses encoding the short hairpin (sh)RNA
targeting human SLC7A5 and an shRNA scramble sequence (negative
control) were purchased from OBiO Technology (Shanghai) Corp.,
(Table III). The lentivirus
containing the mouse shSLC7A5 and shRNA scramble sequence (negative
control) were purchased from Shanghai Genechem Co., Ltd. Briefly,
the shRNA target human SLC7A5 was cloned into a
pSLenti-U6-shRNA-CMV-EGFP-F2A-Puro-WPRE vector and the shRNA target
mouse SLC7A5 was cloned into a hU6-MCS-CBh-gcGFP-IRES-puromycin
vector, resulting in a lenti-shSLC7A5 construct for knockdown. The
MOI used to infect SKOV3 and ID8 cells were 10, with a transduction
duration of 24 h. After 24 h of transfection, the cells were washed
with D-PBS and replaced with complete medium. During this period,
cells were treated with 2 µg/ml puromycin for 5 days to establish
stable knockdown cells, and then maintained in culture with 1 µg/ml
puromycin. Detection of knockdown efficiency was determined by
western blotting.
 | Table III.The sequences of SLC7A5 shRNA and
negative controls. |
Table III.
The sequences of SLC7A5 shRNA and
negative controls.
| A, Human |
|---|
|
|---|
| shRNA | Sequence
(5′-3′) |
|---|
| Negative
control |
CCTAAGGTTAAGTCGCCCTCG |
| SLC7A5 shRNA1 |
GGAAGGGTGATGTGTCCAA |
| SLC7A5 shRNA2 |
CCAATCTAGATCCCAACTT |
|
| B,
Mouse |
|
| Primer | Sequence
(5′-3′) |
|
| Negative
control |
TTCTCCGAACGTGTCACGT |
| SLC7A5 shRNA |
CCTATTTCACTACCCTCTCTA |
Tissue metabolic profiling
In total, tissue samples from 77 patients diagnosed
with advanced serous ovarian cancer and 40 patients diagnosed with
benign cysts were included in the present tissue-based metabolic
profiling analysis. To process tissue samples, ~10 mg of frozen
tissue was mixed with 400 µl of ice-cold methanol for 5 min. Each
tissue sample was homogenized and centrifuged at 16,200 × g for 15
min at 4°C. An aliquot of 200 µl of the supernatant was mixed with
200 µl of water and freeze-dried. Reconstruction was achieved using
80 µl 25% acetonitrile and mixing for 1 min. The sample was then
centrifuged at 16,200 × g for 15 min at 4°C and 10 µl of
supernatant used for LC-MS analysis. The LC-MS conditions were
similar to those previously described (27). In brief, an Ultimate 3000
Ultra-High-Performance LC System (Dionex; Thermo Fisher Scientific,
Inc.) coupled to a Q Exactive Orbitrap mass spectrometer (Thermo
Fisher Scientific, Inc.) was used for the analysis. The separation
was conducted on an ACQUITY UPLC HSS T3 column (Waters Corporation)
that had an internal diameter of 2.1×100.0 mm and particle size of
1.8 mm at 35°C. The mobile phase consisted of acetonitrile (A) and
water containing 0.1% formic acid (B), with a flow rate set at 0.3
ml/min. A gradient elution was employed, starting with 2% A from 0
to 1 min, followed by a gradient from 2 to 100% A from 1 to 10 min.
This was held at 100% A for 3 min. Subsequently, the mobile phase
was adjusted from 100 to 2% A from 13 to 13.1 min, and then
maintained at 2% A from 13.1 to 16 min. The spray voltage values
were set to 3.5 and 2.5 kV in positive and negative modes,
respectively. The capillary temperatures were set to 320°C for
positive mode and 350°C for negative mode. Sheath gas flow rates
were adjusted to 35 and 40 Arb for positive and negative modes,
respectively. The S-Lens RF level was maintained at 55 in both
modes. Full mass scans were conducted across a range of m/z
70–1,000, with automatic gain control set to 3×106 for
both modes. For MS/MS spectrum acquisition, data-dependent
acquisition (DDA) mode was employed, covering a range of m/z
70–1,000, and utilizing stepped normalized collision energies of
10, 20 and 40. The resolutions for the full mass scan and DDA were
70,000 and 17,500, respectively.
The R package XCMS (version 3.8.2; Posit Software)
was used for peak detection, retention-time alignment, peak
matching and correction. The R package statTarget (version 1.32.0;
Posit Software) was used to filter for noisy ions that: i) Were not
detected in >80% of all samples in any group; and ii) had an RSD
of >30% in quality control (QC) samples. The k-nearest neighbors
method was performed for missing values imputation, and then the
QC-based random forest signal correction was used to correct the
influence of the signal shift during LC-MS analysis. The R package,
ropls (version 1.14.1; Posit Software), which included principal
component analysis and partial least-squares discriminant analysis
(PLSDA), was used for multivariate analysis. Metabolic features
with a variable importance in the projection score (VIP) >1,
Benjamini-Hochberg adjusted P-value (false discovery rate) <0.05
and |log2 fold change|>2 were defined as differential features.
Metabolite annotation was performed through matching the spectra
(MS/MS) from the metabolite mass spectral (METLIN; http://metlin.scripps.edu), the Human Metabolome
Database (HMDB; http://www.hmdb.ca/) and an in-house
spectral database. The annotated metabolites were further enriched
in metabolic pathways using MetaboAnalyst (https://www.metaboanalyst.ca) software.
Plasma metabolic profiling
A total of 100 µl of plasma was pipetted into an
Eppendorf tube, an equal volume of 5% (v/v) perchloric acid
solution was added and the solution was mixed thoroughly. The
mixture stood at room temperature for 10 min to fully precipitate
the proteins in the plasma. After which, the mixture was
centrifuged at 10,000 × g for 10 min at 4°C. Finally, 10 µl of the
supernatant was aspirated for injection analysis. A DIONEX UltiMate
3000 (Thermo Fisher Scientific, Inc.) with a Thermo Hypersil GOLD
column (100×2.1 mm, 1.9 µm) was used for chromatographic
separation. The flow rate was set at 0.2 ml/min, and the column
oven temperature was set at 30°C. Mobile phase A was 15 mM sodium
acetate buffer at pH 4.0, and mobile phase B was acetonitrile. A
linear gradient was applied, in which percentage of B began in 0%
and increased to 6% at 4 min, to 80% at 6 min and kept for 9 min,
then returned to 0% at 10 min and held until 13 min. Samples were
kept at 4°C. The data were analyzed by Xcalibur (version 2.1.0;
Thermo Fisher Scientific, Inc.).
Clonogenic assay
SKOV3 cells (500–1,000) were seeded in 6 cm dishes
in culture medium containing 500 µM BCH or 100 µM JPH203 for
clonogenic survival analysis. After 48 h, the cells were washed
twice with complete culture medium to remove BCH and JPH203. Cells
were then cultured in drug-free medium for 10–14 days, stained with
0.1% crystal violet for 15 min at room temperature and counted
manually. Colonies consisting of >50 cells were considered
viable and scored using a light microscope.
Cell cycle analysis
Cell cycle analysis was performed the cell cycle kit
(cat. no CCS012; Multi Sciences (LIANKE) Biotech, Co., Ltd.).
Harvested SKOV3 cells were washed with PBS, 1 ml of DNA holding
solution and 10 µl of permeabilization solution was added and the
sample was vortexed for 5 sec. The samples was incubated at room
temperature in the dark for 30 min. The samples were analyzed by
flow cytometry (Beckman CytoFLEX; Beckman Coulter, Inc.) and the
data were analyzed using Cytomics™ FC500 software
(Beckman Coulter, Inc.).
Immunohistochemistry (IHC) and
immunofluorescence
The tissue were fixed with 4% formaldehyde for 24 h
at room temperature and immunohistochemical staining was performed
on 4-µm-thick formalin-fixed paraffin-embedded (FFPE) tumor
samples. FFPE sections were dewaxed with xylene for 5 min and
placed in 100, 95 and 75% ethanol at room temperature for hydrate
for 5 min each time. Antigen retrieval was performed by heating the
sections to 95°C in citrate buffer (pH 6.0; cat. no. C1010; Beijing
Solarbio Science & Technology Co., Ltd.) for 40 min. The
sections were treated with 0.1% Triton 100 at room temperature for
10 min to break the membrane. The sections were washed twice with
PBS for 5 min at room temperature. The sections were blocked with
5% goat serum (cat. no. SL038; Beijing Solarbio Science &
Technology Co., Ltd.) for 30 min at room temperature. Tissues were
incubated with primary antibodies for 1 h at room temperature,
followed by incubation with Dako EnVision + System HRP-labeled
polymer for 5 min at room temperature (Dako; Agilent Technologies,
Inc.). Tissue samples were counterstained with hematoxylin at room
temperature for 2 min, and then dehydrated with different
concentrations of alcohol (75, 95 and 100%) for 5 min each time.
Finally, the sections were, placed onto coverslips and imaged under
an upright microscope. The antibodies used were: Anti-aryl
hydrocarbon receptor (AHR; 1:500; cat. no. 67785-1-Ig; Wuhan
Sanying Biotechnology), anti-SLC7A5 (1:200; cat. no. 28670-1-AP;
Wuhan Sanying Biotechnology), anti-indoleamine 2,3-dioxygenase 1
(IDO1; 1:100; cat. no. 13268-1-AP; Wuhan Sanying Biotechnology) and
arylformamidase (AFMID; 1:100; cat. no. 19533-1-AP; Wuhan Sanying
Biotechnology). The tissue samples were manually categorized as:
Negative, -; weak, +; medium, ++; and strong, +++, based on the
degree of positive immunostaining staining.
Immunofluorescence staining was performed using
8-chamber slides. SKOV3cells were fixed with 4% paraformaldehyde
for 15 min at room temperature, then washed three times with PBS
for 5 min each time, permeabilized with 0.5% Triton-100 (Beijing
Solarbio Science & Technology Co., Ltd.) and blocked with 5%
BSA (Beijing Solarbio Science & Technology Co., Ltd.) for 1 h
at room temperature. Cells were incubated with primary antibodies
overnight at 4°C, followed by incubation with fluorescent secondary
antibodies at room temperature for 1 h at room temperature. Nuclear
counterstaining was performed using DAPI (Beijing Solarbio Science
& Technology Co., Ltd.) at room temperature for 10 min. The
primary antibodies used were: Anti-AHR (1:500; cat. no. 67785-1-Ig;
Proteintech Group, Inc.) and anti-programmed death-ligand 1 (PD-L1;
1:300; cat. no. 66248-1-Ig; Proteintech Group, Inc.). The
fluorescent secondary antibodies used were: iFluor™ 488
conjugated goat anti-mouse IgG goat polyclonal antibodies (1:1,000;
cat no. HA1125; HUABIO) and iFluor™ 594 conjugated goat
anti-mouse IgG Goat polyclonal antibodies (1:1,000; cat. no.
HA1126; HUABIO).
Measurement of 13C-Trp and
13C-Kyn levels
SKOV3 parental cells and SLC7A5-shRNA knockdown
SKOV3 cells were cultured in Trp-depleted MyCoy's 5A medium for 48
h. The cells were washed twice with the same medium before the
addition of 1 µM 13C-Trp and cells were harvested with a
cell scraper following 5 min of incubation. Metabolites were
extracted in 80% cold methanol and the extract was treated in
vacuum freeze dryer for 6 h (−80°C, 5 bar) to obtain the dry
pellets. The dried pellets were used for cell-based LC-MS analysis
as previously described (27).
Chromatin
immunoprecipitation-quantitative PCR (ChIP-qPCR)
ChIP-qPCR was performed using a ChIP Kit (cat. no.
56383S; CST Biological Reagents Co., Ltd.) according to the
manufacturer's instructions. Briefly, SKOV3 cells (1×107
cells) were cross-linked with 1% formaldehyde for 10 min at room
temperature. Fixed cells were lysed by 1X ChIP Sonication Cell
lysis Buffer on ice for 10 min. The cells were centrifuged at 5,000
× g for 5 min at 4°C. The supernatant was removed and cells were
resuspended in ice-cold ChIP Sonication Nuclear lysis Buffer and
incubated on ice for 10 min. The DNA of the sample was broken by a
non-contact ultrasonic crusher (Covaris M220; Covaris, LLC) under
the following ultrasonic conditions: PIP (75), duty factor (5%),
CPB (200), treatment time (4 min), setpoint temperature (7°C). Then
the lysates were incubated with anti-AHR antibodies (1:50; cat. no.
83200s; CST Biological Reagents Co., Ltd.) at 4°C overnight. After
antibody incubation, ChIP-Grade Protein G magnetic beads were added
and the sample was incubated for 2 h at 4°C. After which, the
magnetic beads were washed with cold PBS three times, for 5 min
each. Proteinase K was added and incubated for 2 h at 65°C to
obtain the crude extract of DNA. After which, five times the volume
of DNA Binding buffer was added and mixed gently, and the DNA
sample was transferred to the DNA spin column at 4°C for 17,000 × g
centrifugation for 1 min. The DNA column was washed with Wash
Buffer, and the liquid was discarded after 17,000 × g
centrifugation at 4°C for 1 min. Finally, DNA Elution Buffer was
added to obtain purified DNA. The purified DNA were amplified by
qPCR. The primers targeting the PD-L1 promoter were designed using
Primer Premier (version 5; Premier Biosoft International) software
(Table IV; Appendix S1). The master reaction mix was
as follows: 6 µl nuclease-free H2O, 2 µl primers (5 µM),
10 µl SYBR Green Master Mix (2X) and 2 µl DNA. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 3 min,
denaturing at 95°C for 15 sec, and annealing and extension at 60°C
for 60 sec, denaturing, annealing and extension were repeated for a
total of 40 cycles. The 2−ΔΔCq formula (28) was used to calculate the relative
expression levels of the target gene.
 | Table IV.The sequences of primers used for
quantitative-PCR analysis of gene expression. |
Table IV.
The sequences of primers used for
quantitative-PCR analysis of gene expression.
| Primer | Sequence
(5′-3′) |
|---|
| PD-L1-P1 | F:
GAATAGGAAGTGGTGGTA |
|
| R:
TGGACGAAATAGATGGAG |
| PD-L1-P2 | F:
AAAATGAATGGCTGAAGG |
|
| R:
AAAGTTGCTGATGGGAAT |
| PD-L1-P3 | F:
GCTCTGAAGCCAGTTGTT |
|
| R:
CTGCAATGCCCTCTGATA |
| PD-L1-P4 | F:
CAACTTCGGGAACTTTGG |
|
| R:
CTTGATTTGGCAGGAGCA |
| PD-L1-P5 | F:
AAGGAAAGGCAAACAACG |
|
| R:
AAGTGATCCGCCAAAGTG |
| PD-L1-P6 | F:
GCCCATTCACTAACCCAA |
|
| R:
CCTGATATTCTGCCACCC |
| PD-L1-P7 | F:
TCAGATGTTGGCTTGTTG |
|
| R:
TTTCACCGGGAAGAGTTT |
Immunoblotting
Whole cell lysates were prepared using RIPA Lysis
Buffer (Beyotime Institute of Biotechnology) containing 1 mM
phenylmethylsulfonylfluoride. Cytoplasmic and nuclear fractions
were isolated using an NE-PER™ kit (Thermo Fisher
Scientific, Inc.). A BCA kit (Beyotime Institute of Biotechnology)
was used to determine the protein concentration. Next, 30 µg of
protein lysate from each sample was loaded into each lane of a 10%
SDS-PAGE gel, electrophoresed and transferred to a PVDF membrane.
The membranes were blocked with 5% non-fat milk for 1 h at room
temperature and washed three times at room temperature with TBST
for 10 min each time. After which, the samples incubated with the
primary antibodies overnight at 4°C. The next day, the membranes
were washed three times at room temperature with TBST for 10 min
and incubated with the respective secondary antibodies at room
temperature for 1 h and detected using an ECL reagent (cat. no.
FD8000; Fdbio Science) with the ChemiDOC™ XRS + (Bio-Rad
Laboratories, Inc.). The primary antibodies used were: Anti-AHR
(1:1,000 dilution; cat. no. 67785-1-Ig; Proteintech Group, Inc.),
anti-SLC7A5 (1:1,000; cat. no. 28670-1-AP; Proteintech Group,
Inc.), anti-PD-L1 (1:1,000; cat. no. 66248-1-Ig; Proteintech Group,
Inc.), anti-histone H3 (1:10,000, cat. no. 68345-1-Ig; Proteintech
Group, Inc.), anti-tubulin (1:5,000; cat. no. 11224-1-AP;
Proteintech Group, Inc.) and anti-GAPDH (1:5,000; cat. no.
10494-1-AP; Proteintech Group, Inc.). Tubulin was used as the
cytoplasmic reference, histone H3 as the nuclear reference and
GAPDH as the whole cell reference. The secondary antibodies were as
follows: Anti-rabbit IgG (1:3,000; cat. no. 7074; CST Biological
Reagents Co., Ltd.) and anti-mouse IgG (1:3,000; cat. no. 7076; CST
Biological Reagents Co., Ltd.)
T cell co-culture with ovarian cancer
cells
CD3+ T cells were magnetically isolated
from peripheral blood mononuclear cells (PBMCs) through
Ficoll-Paque density gradient centrifugation of peripheral blood
derived from healthy adult donors. Briefly, 10 ml of peripheral
blood was diluted with PBS (containing 0.5% BSA) in a 1:1 ratio.
Next, 5 ml of Ficoll-Paque PLUS was added into a new centrifuge
tube and diluted blood cells were added to the upper layer of the
tube. The sample was centrifuged at 800 × g for 20 min at room
temperature to separate the PBMCs. CD3+ MicroBeads human
(Miltenyi Biotec GmbH), LS columns (Miltenyi Biotec GmbH) and a
MACS®MultiStand separator (Miltenyi Biotec GmbH) were
used to magnetically separate CD3+ cells from PBMCs
according to the manufacturer's protocol. Once CD3+ T
cells were isolated from PBMCs, they were activated using 0.5 µg/ml
anti-CD3 (cat. no. 555336; Becton, Dickinson and Company), 5 µg/ml
anti-CD28 (cat. no. 555725, Becton, Dickinson and Company) and 100
U/ml IL-2 (cat. no. 200-02-10UG; PeproTech, Inc.) antibodies for 48
h (37°C, 5% CO2). Preactivated T cells were then
co-cultured with SKOV3 cells at a ratio of 5:1 for 48 h. Hoechst
33324 (Sigma-Aldrich; Merck KGaA) staining for 10 min at room
temperature was performed to observe T cell localization. Changes
in cell morphology were detected using fluorescence microscopic
imaging.
CCK-8 assay
Cell survival rates were measured using a CCK-8
assay (Beyotime Institute of Biotechnology). Preactivated T cells
and SKOV3 cells were co-cultured in 96-well plates at a ratio of
5:1 for 48 h, after which the supernatant was discarded and 100 µl
of culture medium containing 10% CCK-8 (Beyotime Institute of
Biotechnology) was added and incubated for 2 h. The absorbance was
measured at 450 nm using a microplate reader (Varioskan Flash;
Thermo Fisher Scientific, Inc.).
In vivo tumorigenicity
A total of 16 female BALB/c nude mice (age, 4 weeks;
body weight, 16–18 g) were purchased from the Shanghai Laboratory
Animal Center. Animals were maintained in pathogen-free facilities
at the Zhejiang Cancer Hospital, under a controlled environment
(temperature, 23±1°C; humidity, 50±5%; 12/12 h light/dark cycle),
provided with standard laboratory chow and free access to purified
water. All experiments were conducted in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals and approved by the Zhejiang Cancer Hospital
Laboratory Animal Ethics Committee (approval no. 2021-05-003;
Hangzhou, China). Parental SKOV3 cells or SKOV3 cells transfected
with shSLC7A5 were injected into the left flank of mice, at a
concentration of 5×106 cells in 100 µl in PBS/Matrigel
mixed in a 1:1 ratio. The tumor diameters were measured every 3
days for 3 weeks. Tumor length was not permitted to exceed 10 µm
during this time period. The mice were then anesthetized using an
intraperitoneal (i.p.) injection of sodium pentobarbital (50
mg/kg), sacrificed by cervical dislocation and the tumors were
isolated. Euthanasia was confirmed by the lack of movement
including respiration and heartbeat.
To investigate the efficacy of immunotherapy, a
total of 20 female C57BL/6 mice (age, 4 weeks; body weight, 16–18
g) were purchased from the Shanghai Laboratory Animal Center.
Parental ID8 cells or ID8 cells stably expressing shSLC7A5 were
injected into the left flank of mice, at a concentration of
2×106 cells in 100 µl in PBS/Matrigel mixed in a 1:1
ratio. PD-1 monoclonal antibodies were diluted with normal saline
(1:200; cat. no. BE0146; BioXCell) and 10 mg/kg injected (i.p.)
twice per week. The tumor diameter was measured every 5 days and
the tumor volumes were calculated using the formula: (Length ×
width2)/2. Tumor length was not permitted to exceed 10
mm. After 5 weeks, the mice were anesthetized using sodium
pentobarbital (50 mg/kg, i.p.), sacrificed by cervical dislocation
and the tumors were isolated.
Statistical analysis
Statistical analyses were performed using SPSS
software 18.0 (IBM Corp.) and GraphPad software 9.0 (Dotmatics)
software. Comparison analysis was performed using with an unpaired
Student's t-test between two groups or a one-way ANOVA with
Dunnett's post hoc test on >2 groups. Kaplan-Meier curves were
used to identify associations between metabolites and PFS using
median split and log-rank tests. Cox proportional hazards
regression analysis was performed for analysis of metabolites. All
statistical tests were two-sided and P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased expression levels of Trp
importers and pathway enzymes in ovarian cancer
To investigate the regulatory roles of the Trp
metabolism pathway in the context of increased Kyn expression
levels demonstrated in patients with ovarian cancer (29), changes in the protein and enzyme
expression levels that are involved in Trp uptake and/or
Trp-catabolic processes were examined. Analysis using the Cancer
Genome Atlas (TCGA; http://gepia.cancer-pku.cn/) database demonstrated
increased expression levels of SLC7A5 (Fig. 1A), IDO1 (Fig. 1B) and AFMID (Fig. 1C) in tissue samples from patients
with ovarian cancer compared with healthy tissue samples. High mRNA
expression levels of SLC7A5 (Fig.
1D), IDO1 (Fig. 1E) and AFMID
(Fig. 1F) were associated with
tumor stage, although only the expression levels of SLC7A5 were
associated with tumor stage variation. IHC staining demonstrated
increased protein expression levels of SLC7A5, IDO1 and AFMID in
tissue samples from patients with ovarian cancer compared with
benign ovarian cysts (Fig. 1G). The
expression of SLC7A5 was positive in most tumor tissues, but
negative in only a few benign cysts (Fig. 1H). Therefore, SLC7A5 was chosen for
further investigation in the present study. SKOV3 and ES-2 cells
treated with the SLC7A5 inhibitors BCH (500 µM) and JPH203 (100 µM)
demonstrated significantly decreased Trp and Kyn expression levels
compared with control cells, through LC-MS analyses (Fig. 1I and J). Tracking experiments using
13C-Trp and 13C-Kyn in SKOV3 control and
SLC7A5-shRNA cells showed that knockdown of SLC7A5 expression
levels led to a significant decrease in cellular 13C-Trp
and 13C-Kyn expression levels compared with control
cells (Fig. 1K and L).
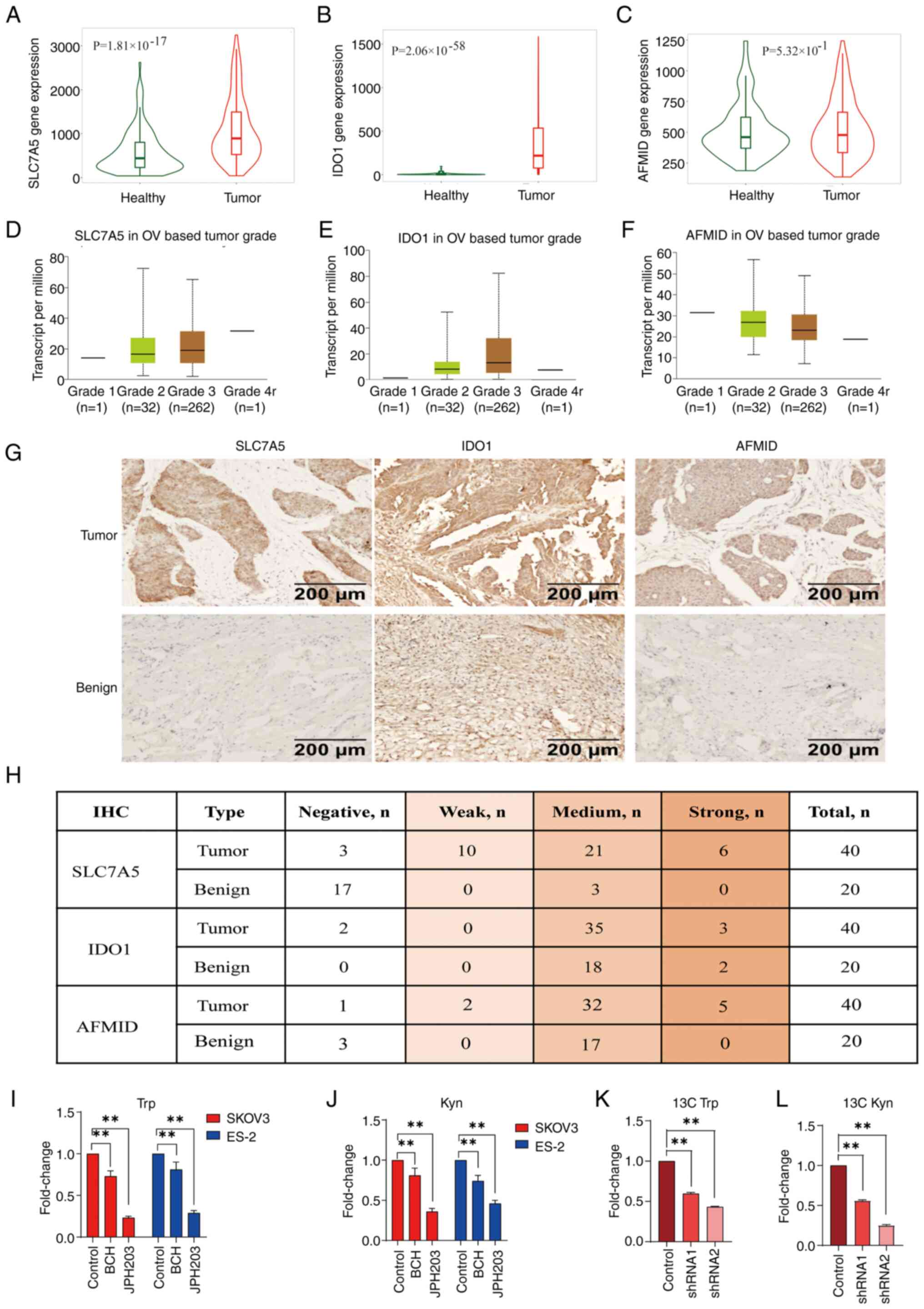 | Figure 1.Increased expression levels of Trp
importers and enzymes in the Trp pathway in ovarian cancer. Violin
plots of mRNA expression levels of (A) SLC7A5, (B) IDO1 and (C)
AFMID in tissue samples from patients with ovarian cancer compared
with healthy tissues from The Cancer Genome Atlas ovarian cancer
database. The mRNA expression levels of (D) SLC7A5, (E) IDO1 and
(F) AFMID were expressed in different tumor stages. (G)
Representative images of IHC staining of SLC7A5, IDO1 and AFMID in
ovarian cancer tissue samples (n=40) and benign cysts (n=20). (H)
Quantification of IHC staining of SLC7A5, IDO1 and AFMID in tissue
samples from patients with ovarian cancer (n=40) and benign cysts
(n=20). LC-MS quantification of cellular (I) Trp and (J) Kyn
expression levels in SKOV3 and ES-2 cells treated with BCH (500 µM)
or JPH203 (100 µM) for 48 h. LC-MS/MS quantification of cellular
(K) 13C-Trp and (L) 13C-Kyn in SKOV3 cells
transfected with either control shRNA or shRNA targeting SLC7A5.
**P<0.01; n=3. Data are presented as mean ± SD. shRNA, short
hairpin RNA; IHC, immunohistochemistry; LC-MS/MS, liquid
chromatography-tandem mass spectrometry; LC-MS, liquid
chromatography-mass spectrometry; OV, ovarian; Trp, tryptophan;
Kyn, kynurenine; SLC7A5, solute carrier family 7 member 5; IDO1,
indoleamine 2,3-dioxygenase 1; AFMID, arylformamidase. |
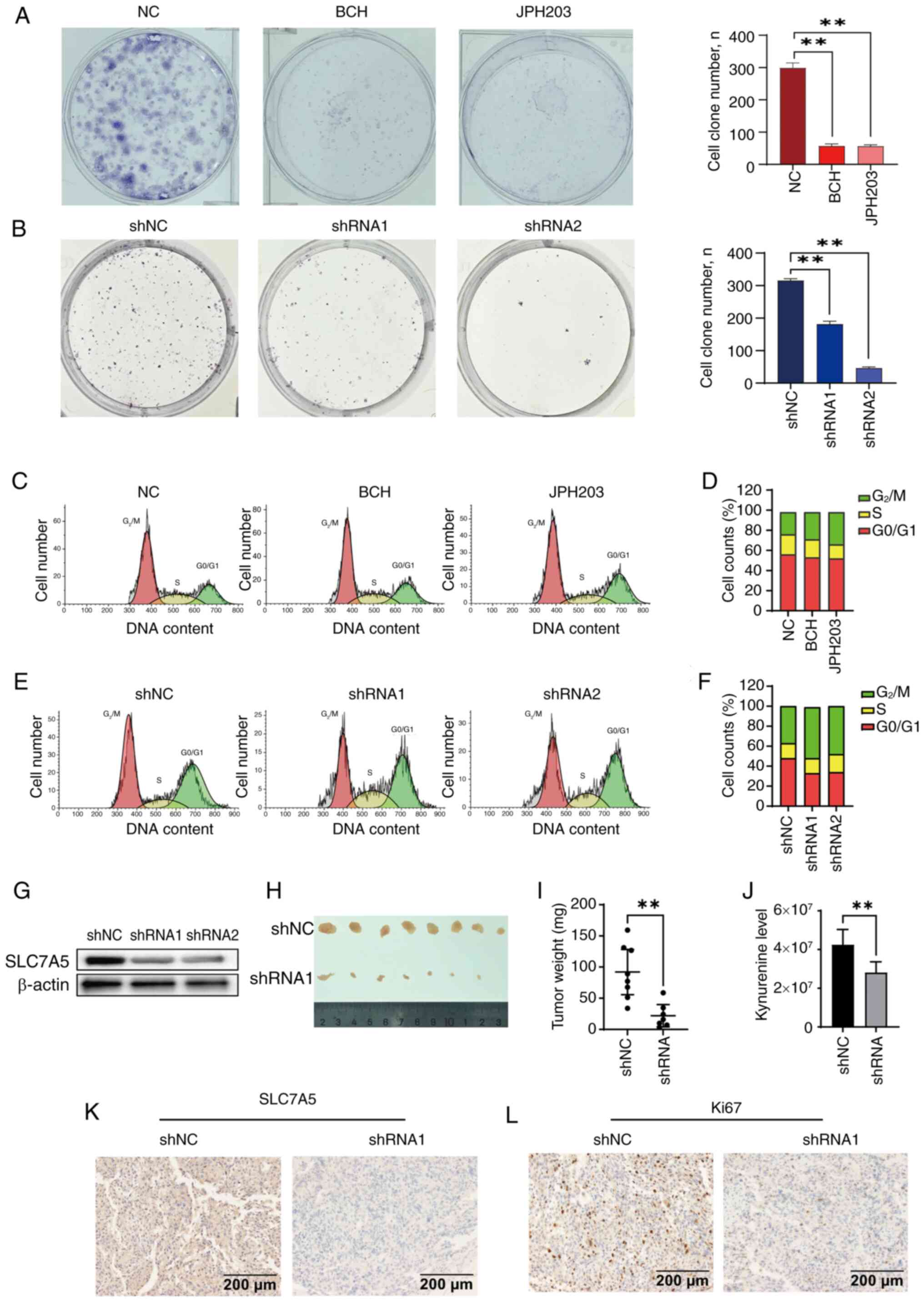
SLC7A5 exhibited anti-ovarian cancer
activity in vitro and in vivo
The antitumor effects of SLC7A5 in ovarian cancer
tissues were tested in vitro and in vivo. SKOV3 cells
were treated with either BCH (500 µM) or JPH203 (100 µM), which
both significantly inhibited clonogenesis in SKOV3 cells (Fig. 2A). SLC7A5 knockdown in SKOV3 cells
similarly demonstrated inhibition of clonogenesis (Fig. 2B). Flow cytometry showed that
treatment with the SLC7A5 inhibitors, BCH (500 µM) or JPH203 (100
µM), were associated with G2/M phase arrest in SKOV3
cells (Fig. 2C and D) and in SKOV3
shSLC7A5 cells (Fig. 2E and F).
SLC7A5 expression levels were notably decreased using the SLC7A5
shRNA in SKOV3 cells (Fig. 2G).
The in vivo tumor model demonstrated a
significant decrease in tumorigenesis in the SLC7A5-shRNA
engineered SKOV3 ×enograft tumors (shSLC7A5) group compared with
control mice with the parental SKOV3 ×enograft shNC) group
(Fig. 2H and I). Kyn expression
levels were decreased in the serum of the shSLC7A5 group compared
with that in the shNC group (Fig.
2J). IHC results demonstrated decreased SLC7A5 expression
levels in the shSLC7A5 group compared with the shNC group (Fig. 2K). Silencing of SLC7A5 expression
levels in SKOV3 cells was associated with decreased Ki67 expression
levels in the shSLC7A5 group compared with the shNC group (Fig. 2L).
Kyn increased nuclear AHR and PD-L1
expression levels in ovarian cancer cells
Analysis using the TCGA database showed a
significant correlation between SLC7A5 with AHR and PD-L1
expression levels in ovarian cancer (Fig. 3A). IHC analysis of AHR expression
levels in ovarian cancer and benign ovarian cyst tissue samples
showed increased AHR staining in the nuclei of ovarian cancer cells
when compared with ovarian benign cyst tissue samples (Fig. 3B).
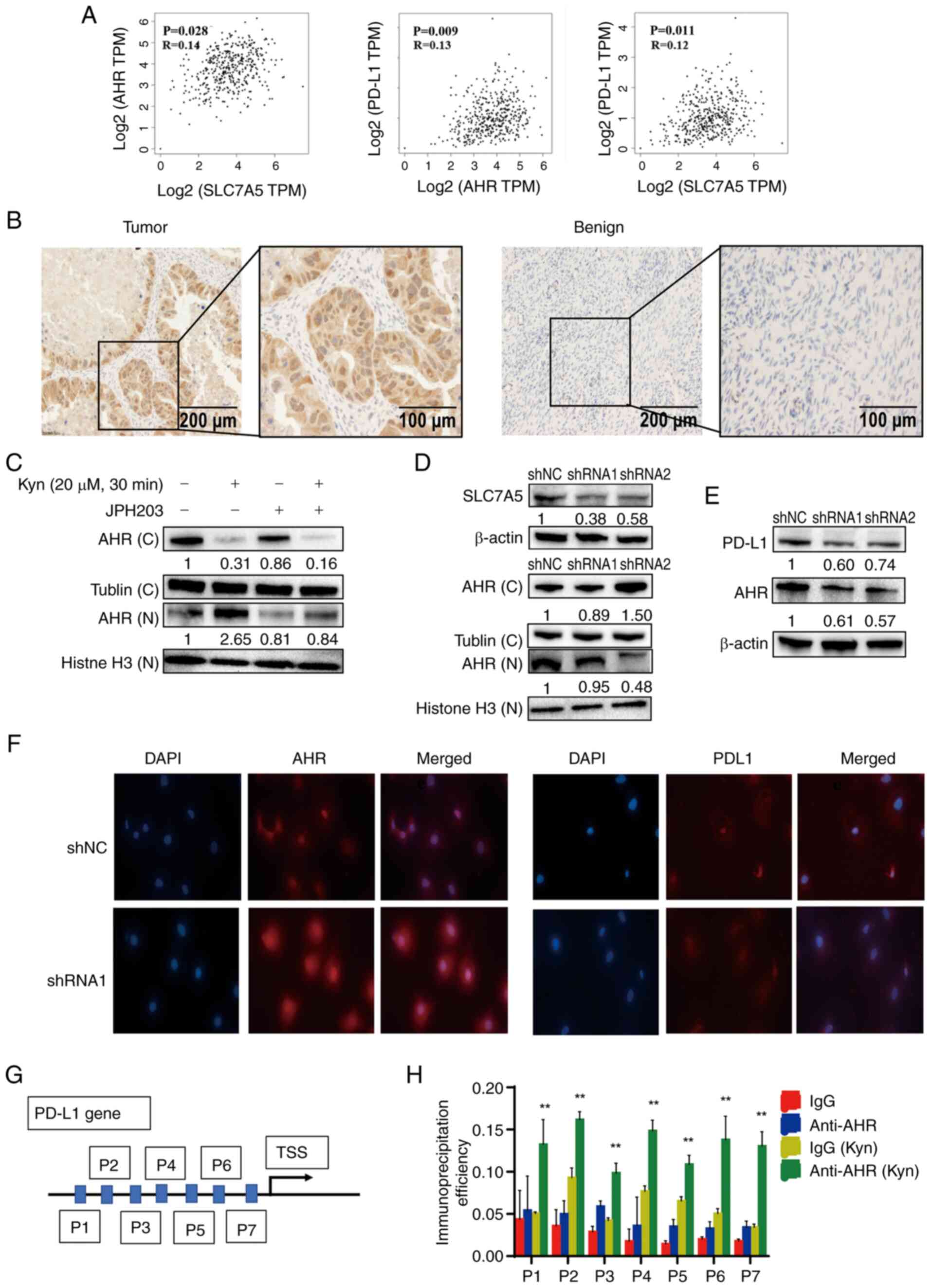 | Figure 3.SLC7A5 affected Trp metabolism and
PD-L1 expression levels. (A) Correlation between SLC7A5 with AHR
and PD-L1 in human ovarian cancer, obtained from The Cancer Genome
Atlas database. (B) Immunohistochemistry staining of AHR in ovarian
cancer and benign ovarian cysts. (C) The expression of nuclear and
cytoplasmic AHR from SKOV3 cells treated with 20 µM Kyn and SLC7A5
inhibitor JPH203 by western blotting. (D) The expression of nuclear
and cytoplasmic AHR from SKOV3 cells transfected with sh-SLC7A5 or
control by western blotting. (E) The expression of PD-L1 and AHR
from SKOV3 cells transfected with control shRNA or shSLC7A5 by
western blotting. (F) The immunofluorescence localization of AHR
(red) and PD-L1 (red) in control or shSLC7A5 SKOV3 cells. Nuclei
were counterstained with DAPI (blue). (G) The schematic diagram of
predicted AHR binding sites in the PD-L1 promoter. (H)
Chromatin-immunoprecipitation analysis of AHR binding to the PD-L1
promoter in SKOV3 cells treated with Kyn. Samples were normalized
to the amount of input DNA, **P<0.01, n=3. Data are presented as
mean ± SD. TPM, transcripts per million; TSS, transcription start
site; shRNA, short hairpin RNA; C, cytoplasmic fraction; N, nuclear
fraction; Trp, tryptophan; Kyn, kynurenine; SLC7A5, solute carrier
family 7 member 5; AHR, aryl hydrocarbon receptor; PD-LI,
programmed death ligand 1. |
The effects of cellular Kyn levels on the
localization and expression of AHR and PD-L1 in PDC and SKOV3
ovarian cancer cells were examined through the manipulation of Kyn
concentrations in the cell culture medium and were determined by
immunofluorescence and immunoblotting, respectively. The addition
of 20 µM Kyn to the culture medium 30 min prior to cell collection
significantly decreased the cytoplasmic AHR protein expression
levels and increased nuclear AHR protein expression levels in SKOV3
and PDC cells, which indicated that Kyn could promote AHR
translocation to the nucleus (Fig. S1A
and B). Compared with control cells, the addition of 20 µM Kyn
upregulated the protein expression levels of PD-L1 and AHR in SKOV3
and PDC cells (Fig. S1C and D).
Immunofluorescence staining showed increased nuclear staining of
AHR after cells were treated with 20 µM Kyn compared with control
cells (Fig. S1E).
The ability of Kyn to increase the nuclear
translocation of AHR was significantly decreased when the cells
were co-treated with JPH203 (Fig.
3C). Silencing of SLC7A5 in shSLC7A5 SKOV3 cells significantly
decreased the nuclear AHR protein expression levels and the total
protein expression levels of AHR and PD-L1 in SKOV3 cells compared
with the shNC group, as demonstrated through immunoblotting
(Fig. 3D and E). Immunofluorescent
staining showed decreased nuclear translocation of AHR upon Kyn
treatment in shSLC7A5 SKOV3 cells compared with the shNC SKOV3
cells (Fig. 3F). ChIP-qPCR
demonstrated that 20 µM Kyn activated the binding of AHR to the
PD-L1 promoter, which significantly upregulated PD-L1 expression
levels compared with untreated SKOV3 cells (Fig. 3G and H). These results indicated
that SLC7A5 may serve as an important regulator of the innate
immune response in cancer cells.
Kyn inhibited the inhibitory
interaction of T cells on SKOV3
To investigate if Kyn could affect the cytotoxic
activity of T cells on ovarian cancer cells, SKOV3 cells were
co-cultured with preactivated T cells in cell culture media with or
without Kyn and the cell survival rate was assessed. These results
showed that reactivated T cells aggregated at the edges of tumor
cells. Furthermore, lysed SKOV3 cells and the reduced proliferation
of SKOV3 cells in the co-culture system were observed. By contrast,
T cell function in the co-culture system supplemented with Kyn was
reduced compared with that in the control group (Fig. S2A). Kyn supplementation
significantly decreased the cell survival rate of SKOV3 cells by
~27.8% compared with control cells without Kyn supplementation
(Fig. S2B).
SLC7A5 increased the effects of PD-1
immunotherapy in ovarian cancer
The immune regulatory functions of SLC7A5 were
examined in vivo. C57BL/6 mice were subcutaneously injected
with either ID8 cells treated with control shRNA or shSLC7A5 and
treated with PD-1 therapy. Tumors with shSLC7A5-treated ID8 cells
showed significantly decreased tumorigenesis (Fig. 4A and B) and increased efficacy of
anti-PD-1 antibody treatment (Fig.
4C). The results of IHC showed that compared with the shNC
group, the expression of Ki67 and PDL1 in shRNA group decreased,
while the expression of CD3 increased (Fig. 4D).
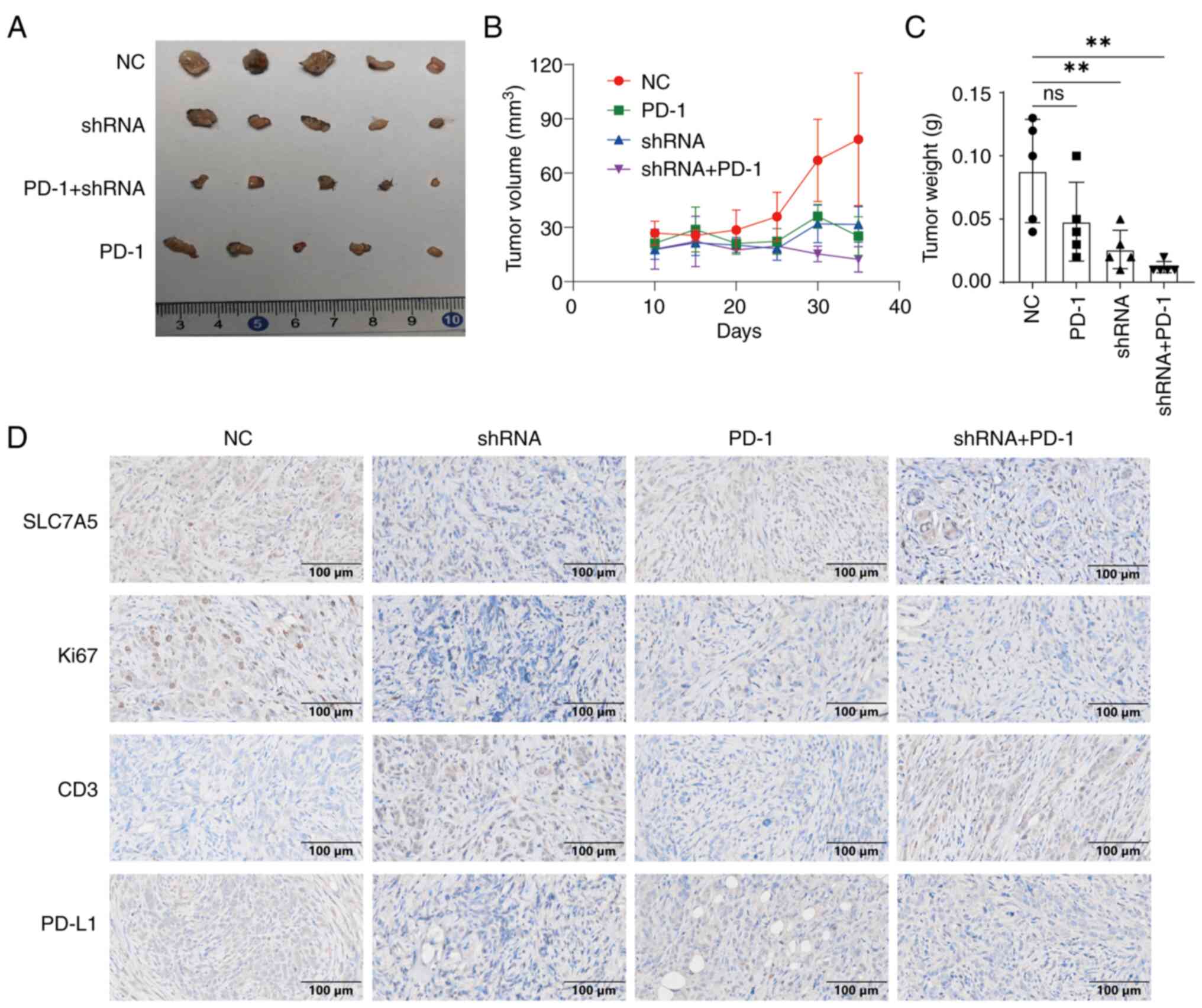 | Figure 4.Knockdown of SLC7A5 increased PD-1
immunotherapy efficacy in ovarian cancer. (A) The expression of
SLC7A5 from ID8 cells transfected with control shRNA or shSLC7A5 by
western blotting. Representative images of tumor from four groups
of C57BL/6 mice inoculated with either control shRNA ID8 cells,
shSLC7A5 SKOV3, PD-1 monotherapy or shSLC7A5 ID8 cells combined
with PD-1 therapy. Comparison of the (B) tumor volume and (C)
weight of all groups. (D) The immunohistochemical staining of
SLC7A5, Ki67, CD3 and PD-L1 protein in tumor from all four groups.
**P<0.01, n=5. Data are presented as mean ± SD. shRNA, short
hairpin RNA; NC, negative control; ns, not significant; SLC7A5,
solute carrier family 7 member 5; PD-1, programmed death-1; PD-LI,
programmed death ligand 1. |
Disordered Trp metabolism and Trp as a
novel prognostic marker and therapeutic target in ovarian
cancer
To evaluate the association between Trp metabolism
and ovarian cancer, a total of 117 tissue samples were obtained
from patients, which included 77 tissue samples of advanced serous
ovarian cancer and 40 of benign cysts. PLSDA score plots with ion
features from LC-MS analysis demonstrated a notable separation
between malignant and benign ovarian tissues (Fig. 5A). Differential ion features were
defined as VIP >1, |log2Fold change|>0.585 and P<0.05. In
the positive mode, 1,181 ion features were downregulated and 1,531
metabolites were upregulated, while 985 ion features were
downregulated and 1,309 metabolites were upregulated in the
negative mode (Fig. 5B). Through
matching the MS1 and MS2 spectra with the reference metabolites
from the HMDB and METLIN databases, 35 differential metabolites
were annotated (Fig. 5C).
Metabolomic Enrichment Analysis was performed using MetaboAnalyst
and the top five enriched pathways were: Trp, methylhistidine,
arginine and proline, glycine and serine and pyrimidine metabolic
pathways (Fig. 5D). As the most
enriched pathway, the Trp metabolic pathway had a total of six
metabolites, which included Kyn, Trp, 5-hydroxyindoleaceyic acid,
formylkynurenine, flavin adenine dinucleotide (FAD) and
S-adenosylhomocysteine (Figs. 5E;
S3A-E). The MS spectra of Kyn,
Trp, 5-hydroxyindoleaceyic acid, formylkynurenine, FAD and
S-adenosylhomocysteine were generated (Fig. S1K-P).
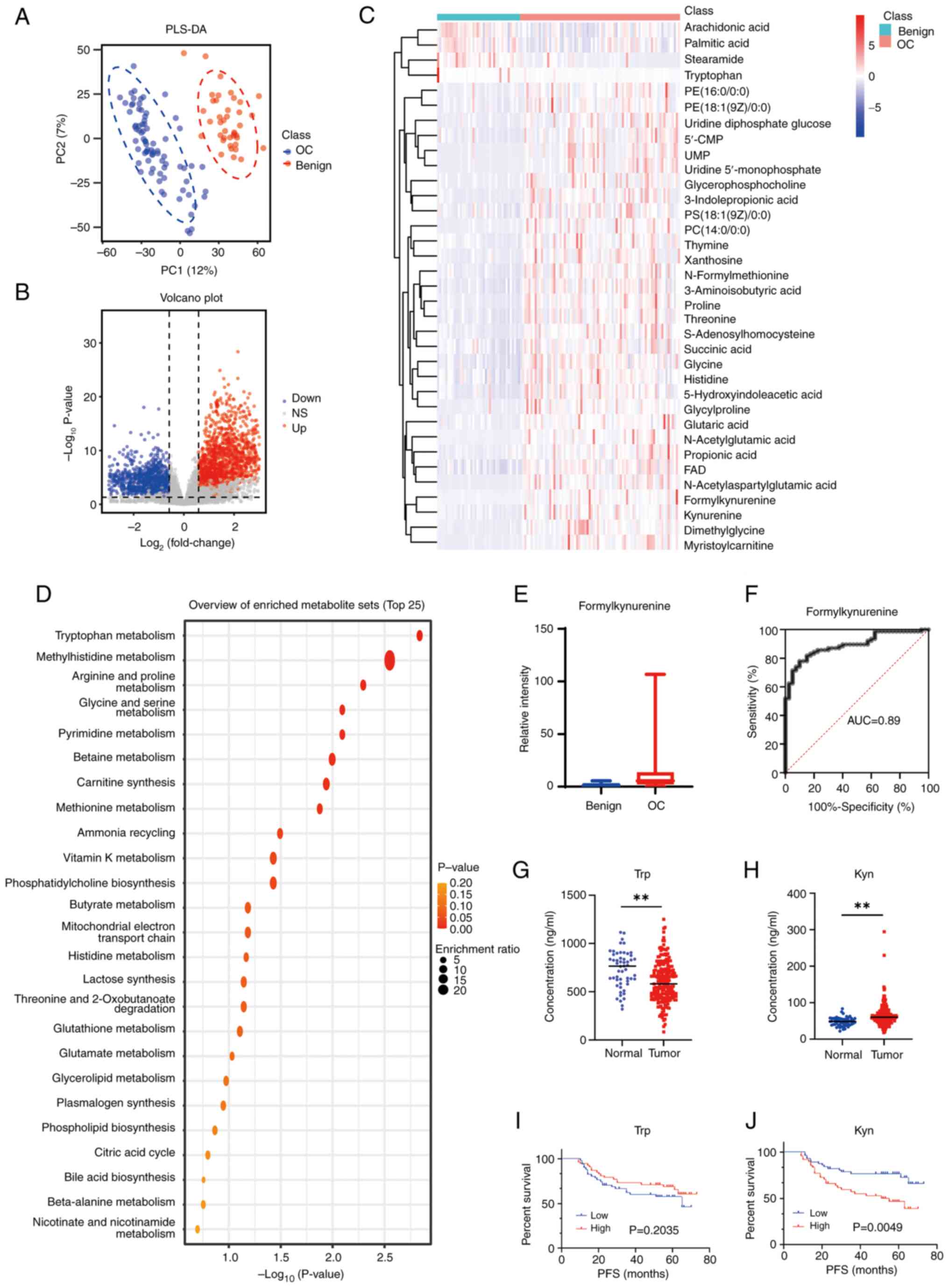 | Figure 5.Metabolic differences in ovarian
cancer and benign cysts. (A) Positive ion score plot derived from
partial least squares discriminant analysis among OC and benign
tissues. (B) Differential positive ion features were defined
between OC and benign tissues, variable importance in the
projection score >1, fold change >1.5, fold change <0.667
and P<0.05. (C) Heatmap analysis with 35 differential
metabolites demonstrated a separation in metabolic pattern between
OC and benign tissues. (D) Pathway analysis of 35 differential
metabolites with the 35 most significant metabolism pathways. (E)
The levels of formylkynurenine in ovarian cancer and benign
tissues. (F) The receiver operating characteristic curve of the
formylkynurenine for OC. The levels of (G) Trp and (H) Kyn in
plasma of patients with OC (n=202) and healthy individuals (n=54).
**P<0.01. Data are presented as mean ± SD. The PFS of patients
with high and low (I) Trp and (J) Kyn level. PC1, principal
component 1; PC2, principal component 2; PLS-DA, principal
component analysis and partial least-squares-discriminant analysis;
AUC, area under the curve; OC, ovarian cancer; NS, not significant;
PFS, progression-free survival; Trp, tryptophan; Kyn,
kynurenine. |
Among the five metabolite hits in the Trp pathway,
the difference in multiplicity of formylkynurenine was the largest
(Fold Change=9.24). The receiver operating characteristic (ROC)
curve analysis showed the area under the curve (AUC) value of
formylkynurenine to be 0.89 and the optimal cut-off value was 3.009
(Fig. 5F). The AUC values of Kyn,
Trp, 5-hydroxyindoleaceyic acid, formylkynurenine, FAD and
S-adenosylhomocysteine additionally were analyzed (Fig. S3F-J). Using reagent grade L-Trp and
L-Kyn as standards, LC-MS analysis was performed to measure Trp and
Kyn expression levels in the plasma specimens. Significantly
decreased Trp and increased Kyn expression levels were detected in
the plasma of patients with ovarian cancer compared with plasma
samples from healthy donors (Fig. 5G
and H). Follow-up information from 104 of the aforementioned
patients were obtained. There were 51 cases with a Trp expression
levels <600.79 and 53 cases with a Trp expression levels
>600.79. Furthermore, there were 56 cases with a Kyn expression
levels <62.97 and 48 cases with a Kyn expression levels
>62.97. PFS analyses demonstrated that patients with lower Kyn
expression levels detected in the plasma had longer PFS compared
with those with high Kyn expression levels. However, no correlation
between PFS and Trp expression levels was demonstrated (Fig. 5I and J).
Discussion
Tumor cells undergo metabolic reprogramming, a
process where they alter their energy-producing pathways and
nutrient utilization to sustain their rapid and uncontrolled growth
(30). The present study indicated
that ovarian cancer cells have an increased ability to take up Trp
and process it through the Kyn pathway. It was demonstrated that
the Trp transporter SLC7A5 served vital roles in Trp uptake and
could inhibit the proliferation of ovarian cancer cells through
regulation of the cell cycle. It was additionally demonstrated that
the transcription factor AHR could be activated by Kyn to increase
PD-L1 expression levels.
A rewired cellular metabolism is considered to be a
notable hallmark of cancer. Abnormalities in Trp metabolism have
been detected in certain types of cancers (including liver cancer,
myeloma and breast cancer) and are recognized as important
microenvironmental factors that can influence the immune responses
of the tumor (31). Previous
studies have demonstrated activation of the catabolic Kyn pathway
in certain types of tumors and the potential for targeting Kyn
catabolism for cancer therapy (32). The present study demonstrated that
patients with ovarian cancer with low plasma Kyn expression levels
had a longer PFS compared with those with high Kyn expression
levels. Therefore, the reduction of Kyn expression levels may
potentially be an important strategy for the treatment of patients
with ovarian cancer. Catabolism of the Kyn pathway involves several
key enzymes, such as IDO1, IDO2, Trp 2,3-dioxygenase (TDO) and
AFMID (33). However, a number of
IDO1 inhibitors have failed in previous preclinical and clinical
trials (34,35). A potential explanation is that when
IDO1 is inhibited in tumor cells, TDO activity compensates for Trp
metabolism and promotes Kyn production (36). The present study showed that high
expression levels of SLC7A5 in ovarian cancer and inhibition of
SLC7A5 can effectively decrease the Kyn content. In addition, the
T-cell immune cytotoxic function was decreased in the
Kyn-supplemented co-culture system compared with the control group.
Thus, targeting the proteins upstream of Kyn metabolism could
potentially be an alternative therapeutic treatment for patients
with ovarian cancer.
SLC7A5 is an L-type amino acid transporter that
functions together with SLC3A2 as a heterodimeric complex at the
plasma membrane (37,38). SLC7A5 overexpression has been
detected in a number of types of cancer (including breast cancer
and lung cancer) (39,40). It has been demonstrated that high
SLC7A5 mRNA levels correlate with poor clinical outcomes in a
number of types of cancer, such as ovarian cancer and gastric
cancer (41,42). Previous retrospective studies have
also reported that SLC7A5 overexpression is associated with shorter
patient survival in a number of types of cancer, including breast
(43), lung (44) and prostate cancers (45). The present study demonstrated that
high expression levels of SLC7A5 in ovarian cancer, and inhibition
of its activity, could significantly inhibit tumor proliferation,
primarily though arrest of the G2/M phase of the cell
cycle. In addition, it was reported that high expression levels of
SLC7A5 are present in activated lymphocytes (46,47),
which suggested that SLC7A5 expression may also contribute to
immunity or immunological responses. However, the underlying
mechanism of this process is currently unclear. Kyn is reported to
be associated with ligand-activated AHR in transducing tumor immune
escape (24). AHR is a
transcription factor that regulates the expression of immune
checkpoint-related proteins, and thus serves an important role in
carcinogenesis and cancer development (48,49).
In the present study, inhibition of SLC7A5 transport activity or
SLC7A5 expression levels blocked AHR nuclear translocation and
decreased PD-L1 expression levels in ovarian cancer cells in
vitro. The present study additionally examined the in
vivo biological effects of SLC7A5 expression levels on the
enhancement of PD-1 immunotherapy, which suggested that SLC7A5
could potentially serve as a therapeutic target for ovarian cancer
immunotherapy.
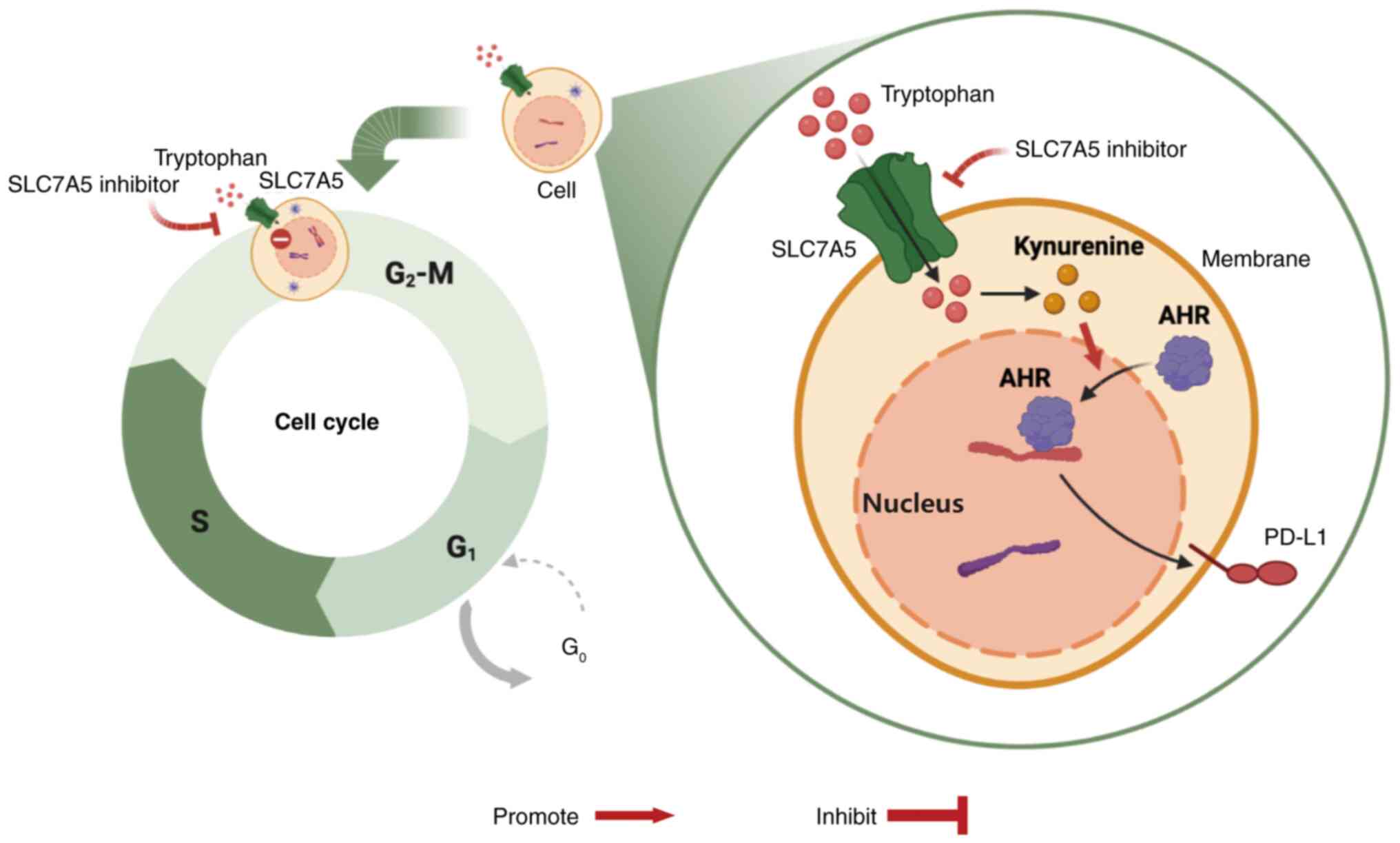
In conclusion, the present study demonstrated the
importance of the SLC7A5-Kyn-AHR-PD-L1 signaling pathway in ovarian
cancer cells. The impact of targeting SLC7A5 in ovarian cancer on
cell survival and innate immunological response (Fig. 6) provides novel insights into the
diagnosis and treatment of ovarian cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the Zhejiang Provincial
Medicine and Health Science Fund (grant no. 2019RC025), the Natural
Science Foundation of Zhejiang Province (grant no. LQ12H16015) and
the National Natural Science Foundation of China Grant (grant no.
82003188).
Availability of data and materials
The data generated in the present study may be found
in the OMIX (China National Center for Bioinformation/Beijing
Institute of Genomics, Chinese Academy of Sciences) database under
accession no. OMIX007054 or at the following URL: https://ngdc.cncb.ac.cn/omix/release/OMIX007054.
Authors' contributions
RJ, LG and BJ designed this study. RJ, YS, ZC and LY
analyzed the data. RJ performed the experiments with contributions
from the DW, CP and JF. RJ wrote the manuscript. RJ and LG confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All patient donors signed written informed consent
forms. Ethical approval was obtained from the Ethics Committee of
Zhejiang Cancer Hospital (approval no. IRB-2021-315; Hangzhou,
China). Patient-derived cells were obtained from an patient with
ovarian cancer during surgery in Zhejiang Cancer Hospital who
provided signed informed consent forms, as approved by the Ethics
Committee of the Zhejiang Cancer Hospital [approval no. (2015)-1-7;
Hangzhou, China]. This study was conducted in accordance with the
Declaration of Helsinki. All animal experiments were conducted in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals and were approved by the
Zhejiang Cancer Hospital Laboratory Animal Ethics Committee
(approval no. 2021-05-003; Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oza AM, Pierce A, Lau A, Kurian N, Parr G,
Lao-Sirieix SH, Ah-See MLW, Dean E and Loembé B: DUETTE: A
randomized phase II study to assess a second maintenance treatment
with olaparib (ola) or ola+ceralasertib (cer), in patients (pts)
with platinum-sensitive relapsed (PSR) epithelial ovarian cancer
who have previously received PARP inhibitor maintenance treatment
(NCT04239014). J Clin Oncol. 38 (15 Suppl):TPS61042020. View Article : Google Scholar
|
|
2
|
Ruibin J, Bo J, Danying W, Jianguo F and
Linhui G: Cardamonin induces G2/M phase arrest and apoptosis
through inhibition of NF-κB and mTOR pathways in ovarian cancer.
Aging (Albany NY). 12:25730–25743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruibin J, Bo J, Danying W, Chihong Z,
Jianguo F and Linhui G: Therapy effects of wogonin on ovarian
cancer cells. Biomed Res Int. 2017:93815132017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De La Franier B and Thompson M: Early
stage detection and screening of ovarian cancer: A research
opportunity and significant challenge for biosensor technology.
Biosens Bioelectron. 135:71–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang R, Chen Z, Ni M, Li X, Ying H, Fen
J, Wan D, Peng C, Zhou W and Gu L: A traditional gynecological
medicine inhibits ovarian cancer progression and eliminates cancer
stem cells via the LRPPRC-OXPHOS axis. J Transl Med. 21:5042023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai Y, Wang Z, Guo S, Lin C, Yao H, Yang
Q, Wang Y, Yu X, He X, Sun W, et al: Detection, mechanisms, and
therapeutic implications of oncometabolites. Trends Endocrinol
Metab. 34:849–861. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang K, Wang X, Song C, He Z, Wang R, Xu
Y, Jiang G, Wan Y, Mei J and Mao W: The role of lipid metabolic
reprogramming in tumor microenvironment. Theranostics.
13:1774–1808. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Wu X, Chen HN and Wang K: Amino
acid metabolic reprogramming in tumor metastatic colonization.
Front Oncol. 13:11231922023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng Y, Yao Y, Ge T, Ge S, Jia R, Song X
and Zhuang A: Amino acid metabolism reprogramming: Shedding new
light on T cell anti-tumor immunity. J Exp Clin Cancer Res.
42:2912023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dang CV: Links between metabolism and
cancer. Genes Dev. 26:877–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daye D and Wellen KE: Metabolic
reprogramming in cancer: Unraveling the role of glutamine in
tumorigenesis. Semin Cell Dev Biol. 23:362–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao Z, Dai Z and Locasale JW: Metabolic
landscape of the tumor microenvironment at single cell resolution.
Nat Commun. 10:37632019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedrich M, Sankowski R, Bunse L, Kilian
M, Green E, Guevara CR, Pusch S, Poschet G, Sanghvi K, Hahn M, et
al: Tryptophan metabolism drives dynamic immunosuppressive myeloid
states in IDH-mutant gliomas. Nat Cancer. 2:723–740. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Wen L, Bao Y, Tang Z, Zhao J,
Zhang X, Wei T, Zhang J, Ma T, Zhang Q, et al: Gut flora
disequilibrium promotes the initiation of liver cancer by
modulating tryptophan metabolism and up-regulating SREBP2. Proc
Natl Acad Sci a USA. 119:e22038941192022. View Article : Google Scholar
|
|
18
|
Venancio PA, Consolaro MEL, Derchain SF,
Boccardo E, Villa LL, Maria-Engler SS, Campa A and Discacciati MG:
Indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase
expression in HPV infection, SILs, and cervical cancer. Cancer
Cytopathol. 127:586–597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ala M: Tryptophan metabolites modulate
inflammatory bowel disease and colorectal cancer by affecting
immune system. Int Rev Immunol. 41:326–345. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Austin CJD and Rendina LM: Targeting key
dioxygenases in tryptophan-kynurenine metabolism for
immunomodulation and cancer chemotherapy. Drug Discov Today.
20:609–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SH, Mahendran R, Tham SM, Thamboo TP,
Chionh BJ, Lim YX, Tsang WC, Wu QH, Chia JY, Tay MHW, et al:
Tryptophan-kynurenine ratio as a biomarker of bladder cancer. BJU
Int. 127:445–453. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greene LI, Bruno TC, Christenson JL,
D'Alessandro A, Culp-Hill R, Torkko K, Borges VF, Slansky JE and
Richer JK: A role for tryptophan-2,3-dioxygenase in CD8 T-cell
suppression and evidence of tryptophan catabolism in breast cancer
patient plasma. Mol Cancer Res. 17:131–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin R, Zhao C, Wang CJ, Xu W, Zhao JY, Lin
Y, Yuan YY, Lin PC, Li Y, Zhao S and Huang Y: Tryptophan
potentiates CD8+ T cells against cancer cells by TRIP12
tryptophanylation and surface PD-1 downregulation. J Immunother
Cancer. 9:e0028402021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheong JE and Sun L: Targeting the
IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy-challenges and
opportunities. Trends Pharmacol Sci. 39:307–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nambirajan A, Malgulwar PB, Sharma A,
Boorgula MT, Doddamani R, Singh M, Suri V, Sarkar C and Sharma MC:
Clinicopathological evaluation of PD-L1 expression and cytotoxic
T-lymphocyte infiltrates across intracranial molecular subgroups of
ependymomas: Are these tumors potential candidates for immune
check-point blockade? Brain Tumor Pathol. 36:152–161. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu B, Song M, Dong Q, Xiang G, Li J, Ma X
and Wei F: UBR5 promotes tumor immune evasion through enhancing
IFN-γ-induced PDL1 transcription in triple negative breast cancer.
Theranostics. 12:5086–5102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, Gao Y, Huang X, Yao Y, Chen K,
Zeng S and Mao W: Tissue-based metabolomics reveals metabolic
biomarkers and potential therapeutic targets for esophageal
squamous cell carcinoma. J Pharm Biomed Anal. 197:1139372021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gostner JM, Obermayr E, Braicu IE, Concin
N, Mahner S, Vanderstichele A, Sehouli J, Vergote I, Fuchs D and
Zeillinger R: Immunobiochemical pathways of neopterin formation and
tryptophan breakdown via indoleamine 2,3-dioxygenase correlate with
circulating tumor cells in ovarian cancer patients-A study of the
OVCAD consortium. Gynecol Oncol. 149:371–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Platten M, Wick W and Van den Eynde BJ:
Tryptophan catabolism in cancer: Beyond IDO and tryptophan
depletion. Cancer Res. 72:5435–5440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mor A, Tankiewicz-Kwedlo A and Pawlak D:
Kynurenines as a novel target for the treatment of malignancies.
Pharmaceuticals (Basel). 14:6062021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia R and Conacci Sorrell M:
Investigating the tryptophan-metabolizing enzyme AFMID
(arylformamidase) in colon cancer. FASEB J. 36:2022. View Article : Google Scholar
|
|
34
|
Van den Eynde BJ, van Baren N and Baurain
JF: Is there a clinical future for IDO1 inhibitors after the
failure of epacadostat in melanoma? Ann Rev Cancer Biol. 4:241–256.
2020. View Article : Google Scholar
|
|
35
|
Chen S, Tan J and Zhang A: The ups, downs
and new trends of IDO1 inhibitors. Bioorg Chem. 110:1048152021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim C, Lee NK, Kim JS, Kim WR, Kim DH, Kim
DJ, Oh JS, Chang SK, Kim JW and Chon HJ: An oral dual inhibitor of
IDO and TDO enhances anti-cancer immunity and synergizes with
immune checkpoint blockade. Ann Oncol. 29:viii4162018. View Article : Google Scholar
|
|
37
|
Yanagida O, Kanai Y, Chairoungdua A, Kim
DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, et
al: Human L-type amino acid transporter 1 (LAT1): Characterization
of function and expression in tumor cell lines. Biochim Biophys
Acta. 1514:291–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan R, Zhao X, Lei J and Zhou Q: Structure
of the human LAT1-4F2hc heteromeric amino acid transporter complex.
Nature. 568:127–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Q, Wang J, Shi Y, Zha X and Wang S:
Bioinformatics prediction and in vivo verification identify SLC7A5
as immune infiltration related biomarker in breast cancer. Cancer
Manag Res. 14:2545–2559. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Ma G, Liu J, Zheng H, Huang G, Song
Q, Pang Z and Du J: SLC7A5 is a lung adenocarcinoma-specific
prognostic biomarker and participates in forming immunosuppressive
tumor microenvironment. Heliyon. 8:e108662022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saito Y and Soga T: Amino acid
transporters as emerging therapeutic targets in cancer. Cancer Sci.
112:2958–2965. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ding K, Tan S, Huang X, Wang X, Li X, Fan
R, Zhu Y, Lobie PE, Wang W and Wu Z: GSE1 predicts poor survival
outcome in gastric cancer patients by SLC7A5 enhancement of tumor
growth and metastasis. J Biol Chem. 293:3949–3964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shennan DB and Thomson J: Inhibition of
system L (LAT1/CD98hc) reduces the growth of cultured human breast
cancer cells. Oncol Rep. 20:885–889. 2008.PubMed/NCBI
|
|
44
|
Rajasinghe LD, Hutchings M and Gupta SV:
Delta-tocotrienol modulates glutamine dependence by inhibiting
ASCT2 and LAT1 transporters in non-small cell lung cancer (NSCLC)
cells: A metabolomic approach. Metabolites. 9:502019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu M, Sakamoto S, Matsushima J, Kimura T,
Ueda T, Mizokami A, Kanai Y and Ichikawa T: Up-regulation of LAT1
during Antiandrogen therapy contributes to progression in prostate
cancer cells. J Urol. 195:1588–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hayashi K, Kaminuma O, Nishimura T, Saeki
M, Matsuoka K, Hiroi T, Jutabha P, Iwata Y, Sugiura K, Owada T, et
al: LAT1-specific inhibitor is effective against T cell-mediated
allergic skin inflammation. Allergy. 75:463–467. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cibrian D, Saiz ML, de la Fuente H,
Sánchez-Díaz R, Moreno-Gonzalo O, Jorge I, Ferrarini A, Vázquez J,
Punzón C, Fresno M, et al: CD69 controls the uptake of L-tryptophan
through LAT1-CD98 and AhR-dependent secretion of IL-22 in
psoriasis. Nat Immunol. 17:985–996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Budhwar S, Bahl C, Sharma S, Singh N and
Behera D: Role of sequence variations in AhR gene towards
modulating smoking induced lung cancer susceptibility in north
indian population: A multiple interaction analysis. Curr Genomics.
19:313–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Helou DG, Shafiei-Jahani P, Hurrell BP,
Painter JD, Quach C, Howard E and Akbari O: LAIR-1 acts as an
immune checkpoint on activated ILC2s and regulates the induction of
airway hyperreactivity. J Allergy Clin Immunol. 149:223–236.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|