Introduction
Prostate cancer is the second-leading cause of
cancer-related deaths in men worldwide (1). For early-stage prostate cancer,
laparoscopic radical prostatectomy can provide effective treatment
with the benefits of it being less expensive and minimally invasive
(2), requiring only a small
incision, and allowing for quick recovery after the operation. For
recurrent or metastatic prostate cancer, radical surgery is not a
suitable option. At the same time, androgen deprivation therapy is
the current standard-of-care treatment (3), aimed at reducing the level of
androgens and inhibiting their activity to delay the progression of
prostate cancer. However, many patients with prostate cancer may
gradually become unresponsive or resistant to androgen blocking,
leading to the development of castration-resistant prostate cancer
(CRPC), which is associated with high morbidity and mortality
(4). CRPC is particularly
challenging to treat due to its natural or acquired drug
resistance, resulting in a poor prognosis (3). Therefore, it is necessary to elucidate
new approaches to treat patients with advanced prostate cancer
effectively.
Numerous studies have reported that Polygonatum
sibiricum polysaccharides (PSP) possess pharmacological
properties and biological activities (5), making them widely used in treatments
of diabetes mellitus and its complications (6,7), as
well as for their hypoplipidemic (8), anti-atherosclerosis (8,9),
anti-osteoporosis (10) and
anticancer (11,12) effects. Among these, the potential
antitumor effects of PSP have attracted scientific interest due to
its low toxicity and lack of side effects (11–14). A
previous study reported that PSP notably inhibited the growth of
prostate cancer-associated fibroblasts and enhanced the
effectiveness of cancer therapy (13). Another study reported that PSP
induced apoptosis in HepG2 cells and arrested the cell cycle at the
G1 phase, indicating potential antitumor effects (14). Additionally, several studies have
suggested that PSP can hold human gastric cancer HGC-27 cells,
esophageal cancer ECA-109 cells and colorectal cancer HCT-8 cells
in the S stage, thereby promoting their apoptosis (5,15).
Furthermore, studies have reported that the
phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT)
signaling pathway is a key regulator of the cell cycle (16). Additionally, research has identified
the PI3K/AKT signaling pathway as a significant player in prostate
cancer tumorigenesis and therapy, influencing apoptosis,
proliferation, metastasis and invasion of prostate cells (17–21).
The present study aimed to assess the effects of PSP
on human prostate cancer PC-3 cells and elucidate the underlying
mechanism involved.
Materials and methods
Cell culture and reagents
Human prostate cancer PC-3 cells were purchased from
Procell Life Science & Technology Co., Ltd. The cells were
cultured in Ham's F-12K media (Wuhan Pricella Biotechnology Co.,
Ltd.; Cat. CM0185) supplemented with 10% fetal bovine serum (FBS)
(Gibco. Cat. 26140-079), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. PSP was purchased from Shanghai Yuanye
Biotechnology Co., Ltd. with a purity of 70%. The compounds were
dissolved in PBS at a concentration of 20 mg/ml to prepare stock
solution, which was stored at −20°C for in vitro studies.
Before each assay, the stock solution was diluted with the
medium.
Cell Counting kit-8 (CCK-8) assay
The effect of PSP on cell proliferation was assessed
using the CCK-8 assay. Cells were seeded into 96-well tissue
culture plates at a density of 1×104 cells/well and
incubated at 37°C with PSP for 24, 48, 72, 96 or 120 h. Untreated
cells served as the control. Following the treatment period, 10 µl
CCK-8 reagent (Fluorescence, cat. no. DCM7122) was added to each
well and then incubated for an additional 4 h at 37°C. The
absorbance at 450 nm was measured using a microplate reader. Cell
viability was determined by the following formula: Cell viability
(%)=[(As-Ab)/(Ac-Ab)]
×100%, where As is the absorbance of PSP-treated groups,
Ac is the absorbance of 0 µg/ml PSP-treated groups, and
Ab is the absorbance of the blank group.
Flow cytometry
Based on the results of the CCK-8 assay, PC-3 cells
were plated into 6-well plates at a density of 2×105
cells/well and treated with several concentrations of PSP solution
(0, 250, 500 µg/ml, 1, 2 and 4 mg/ml) at 37°C for 72 h. The
apoptosis rate of PC-3 cells was evaluated using the Annexin
V-FITC/PI Apoptosis Detection Kit according to the manufacturer's
instructions (Beijing 4A Biotech Co., Ltd. Cat. FXP018). Cultured
cells were collected, washed with cold PBS and resuspended in a
binding buffer. To this buffer, 5 µl Annexin V-FITC and 5 µl PI
were added, followed by incubation at room temperature for 15 min
in the dark. Annexin V-FITC binds to phosphatidylserine on the
outer membrane of apoptotic cells, whilst PI penetrates and stains
cells with compromised membrane integrity, binding to and labeling
DNA. Data collection was performed using a flow cytometer
(BeamCyte-1026; Bidaiko Biotechnology (Suzhou) Co.,Ltd, and the
data were analyzed using FlowJo software 10.8.1 (BD
Biosciences).
For the cell cycle assay, PC-3 cells were
trypsinized, washed with cold PBS and fixed with 70% ethanol at 4°C
overnight. The fixed cells were collected, resuspended in cold PBS
and stained with PI. After staining, the cells were incubated for
30 min at 37°C in the dark. Data were collected using a flow
cytometer (BeamCyte-1026; Bidaiko Biotechnology (Suzhou) Co., Ltd)
and the data were analyzed with FlowJo software 10.8.1 (BD
Biosciences).
Transwell invasion and migration
assay
A Transwell assay was used to analyze cell invasion
and/or migration. 2×105 cells treated with PSP (0, 500
µg/ml, 1, 2 and 4 mg/ml) were suspended in an FBS-free medium and
4×104 cells seeded into the upper chamber of 24-well
plates, which were either coated with Matrigel at 37°C for 24 h
(for invasion assays) or uncoated (for migration assays). The lower
chamber was filled with a medium containing 10% FBS as a
chemoattractant. After a 24-h incubation at 37°C, non-invaded or
non-migrated cells on the upper surface of the membranes were
carefully removed using cotton swabs, whilst cells that had
transverse the membrane were fixed with 75% carbinol for 10 min at
room temperature and stained using 0.1% Giemsa (Beyotime, C0133)
for 10 min at room temperature. The stained cells were then counted
under an invert light microscope (Leica DM3000).
Wound healing assay
PC-3 cells were seeded in 12-well microplates at a
density of 1.5×105 cells/well and incubated at 37°C for
24 h (confluence reached 90–100%). Confluent monolayers were
scratched with 10 µl tips. After washing with PBS, the cells were
cultured at 37°C in low serum medium (0.5% FBS) with different
concentrations of PSP (0, 500 µg/ml, 1, 2 and 4 mg/ml). Images of
scratched areas were captured by fluorescence microscope at 0, 24,
48 and 72 h of incubation in the dark. Image J v1.8.0 software
(National Institutes of Health) was used to analyze the cell
migration distance. The migration inhibition rate was expressed as
the % scratch closure change according to the following formula:
Scratch closure change
(%)=[(At0-Atc)/At0] ×100%, where
At0 is the scratch area at time 0 and Atc is
the corresponding scratch area at 24, 48 and 72 h.
Western blot
A total of 2×105 Cells treated with PSP
for 72 h were harvested and lysed using RIPA buffer (Solarbio life
sciences, Cat. R0010), and protein concentrations were measured
using a BCA quantification kit. The lysates including 50 µg total
proteins were then separated using 10% SDS-PAGE and transferred
onto PVDF membranes (cat. no. ISEQ00010; MilliporeSigma; Merck
KGaA), then blocked with 5% BSA (Solarbio life sciences, Cat.
SW3015) at room temperature for 1 h. The membranes were incubated
overnight at 4°C with primary antibodies (Affinity Biosciences,
Ltd.) diluted at 1:1,000, including those targeting Akt (cat.
AF6261), p-Akt (Cat. AF0016), PI3K (Cat. AF6241), p-PI3K (Cat.
AF3241), caspase-3 (Cat. no. AF6311), P65 (Cat. AF5006), p-P65
(Cat. AF2006) and β-actin (Cat. AF7018). After being washed thrice
with 1×PBS-T (1% Tween-20), the membranes were incubated with
HRP-conjugated sheep anti-rabbit secondary antibodies (1:3,000,
Medical Discovery Leader, MD912565) at room temperature for 1 h.
Finally, the protein bands were visualized using enhanced
chemiluminescence. The intensity of the bands was semi-quantified
by the ChemiDoc MP Chemiluminescence Imaging System with Image Lab
software 6.0.1 (Bio-Rad Laboratories, Inc.). β-actin was used as
internal control.
Reverse transcription-quantitative
PCR
2×105 PC-3 cells were treated with
different concentrations of PSP (0, 500 µg/ml, 1, 2, 4 mg/ml) at
37°C for 72 h, after which total RNA was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA
concentration was determined using a NanoDrop™ 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). A total of 1 µg
RNA was then reverse transcribed into cDNA using the SuperRT cDNA
Synthesis Kit (Jiangsu CoWin Biotech Co., Ltd. cat. CW0741m), with
β-actin serving as the internal control, the condition for reverse
transcription are incubated at 42°C for 30–50 min and at 85 ° C for
5 min. The expression levels of multidrug resistance-1 (MDR1) mRNA
were assessed using the UltraSYBR Mixture (Low ROX; Jiangsu CoWin
Biotech Co., Ltd.; cat. CW2601M). The data were normalized with
β-actin using the following formula: Relative mRNA
expression=2−ΔΔCq (22),
where Cq is the cycle threshold. The primer sequences used were as
follows: MDR1 (forward), 5′-TGCTCAAGTTAAAGGGGCTA-3′ and (reverse),
5′-CAGTGTTAGTTGCCAACCAT-3′; and β-actin (forward),
5′-TCCTCCTGAGCGCAAGTACTCC-3′ and (reverse),
5′-CATACTCCTGCTTGCTGATCCAC-3′. The thermocycling conditions were as
follows: Pre-denatured at 95°C for 5 min, 40 cycles at 95°C for 10
sec, 58°C for 20 sec and 72°C for 20 sec.
Immunocytochemistry
PC-3 cells were seeded into 6-well plates with
sterile slides at a density of 2×105 cells/well, treated
with several concentrations of PSP solution (0 and 500 µg/ml and 1,
2, 4 mg/ml) at 37°C for 72 h, fixed with pre-cooled 4%
paraformaldehyde at room temperature for 20 min, and then blocked
with 10% goat serum (Beyotime, C0265) for 30 min at 37°C. The
slides were subsequently stained with MDR1/P-glycoprotein (P-gp)
primary antibodies (1:1,000; Affinity Biosciences, Ltd.; Cat.
AF5185) overnight at 4°C. Following this, the slides were incubated
with HRP-conjugated secondary anti-rabbit IgG (1:5,000, MDL
Biotech; cat. no. MD912565) at 37°C for 40 min. Slides were
visualized using DAB, counterstained with hematoxylin at room
temperature for 5 min and observed under a light microscope (Leica
DM2000 LED).
Statistical analysis
All experiments were performed in triplicate. Data
were analyzed using SPSS 19.0 statistical software (IBM Corp.) and
are expressed as the mean ± standard deviation. One-way analysis of
variance was used to compare data among groups when they had a
normal distribution and homogeneous variance, and Tukey's was used
for the post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PSP inhibits the proliferation of PC-3
cells
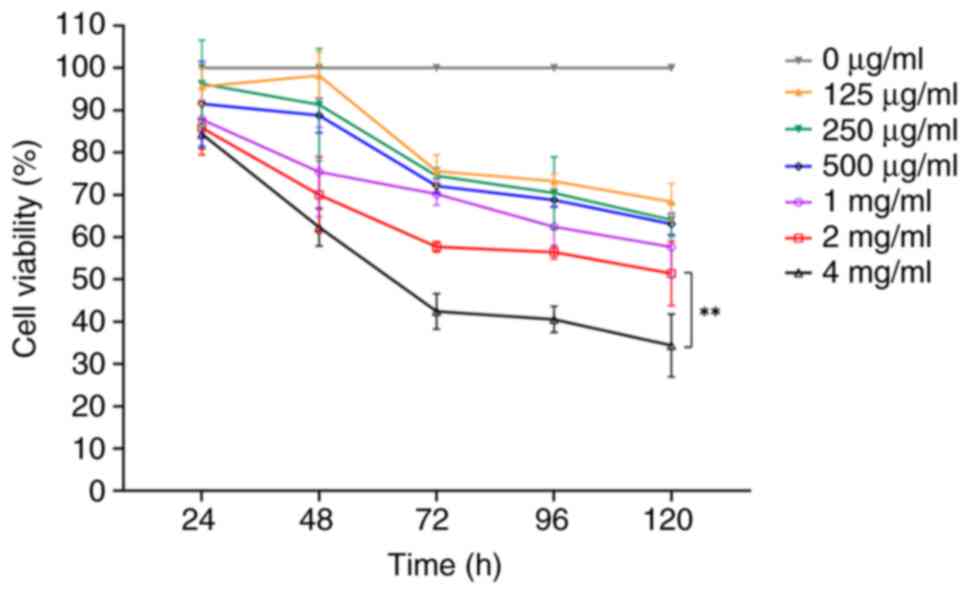
The CCK-8 assay was used to determine whether PSP
inhibits the proliferation of PC-3 cells at varying concentrations.
PC-3 cells were treated with different concentrations of PSP (0,
125, 250, 500 µg/ml, 1, 2 and 4 mg/ml) for 24, 48, 72, 96 and 120
h. The results demonstrated there was no significant inhibition
observed across the concentrations at 24 h. However, at 48 h,
inhibition increased with higher concentrations of PSP,
particularly at 4 mg/ml. By 72 and 96 h, significant inhibition was
observed at concentrations of 2 and 4 mg/ml (Fig. 1). These results indicate that PSP
effectively inhibits the proliferation of PC-3 cells.
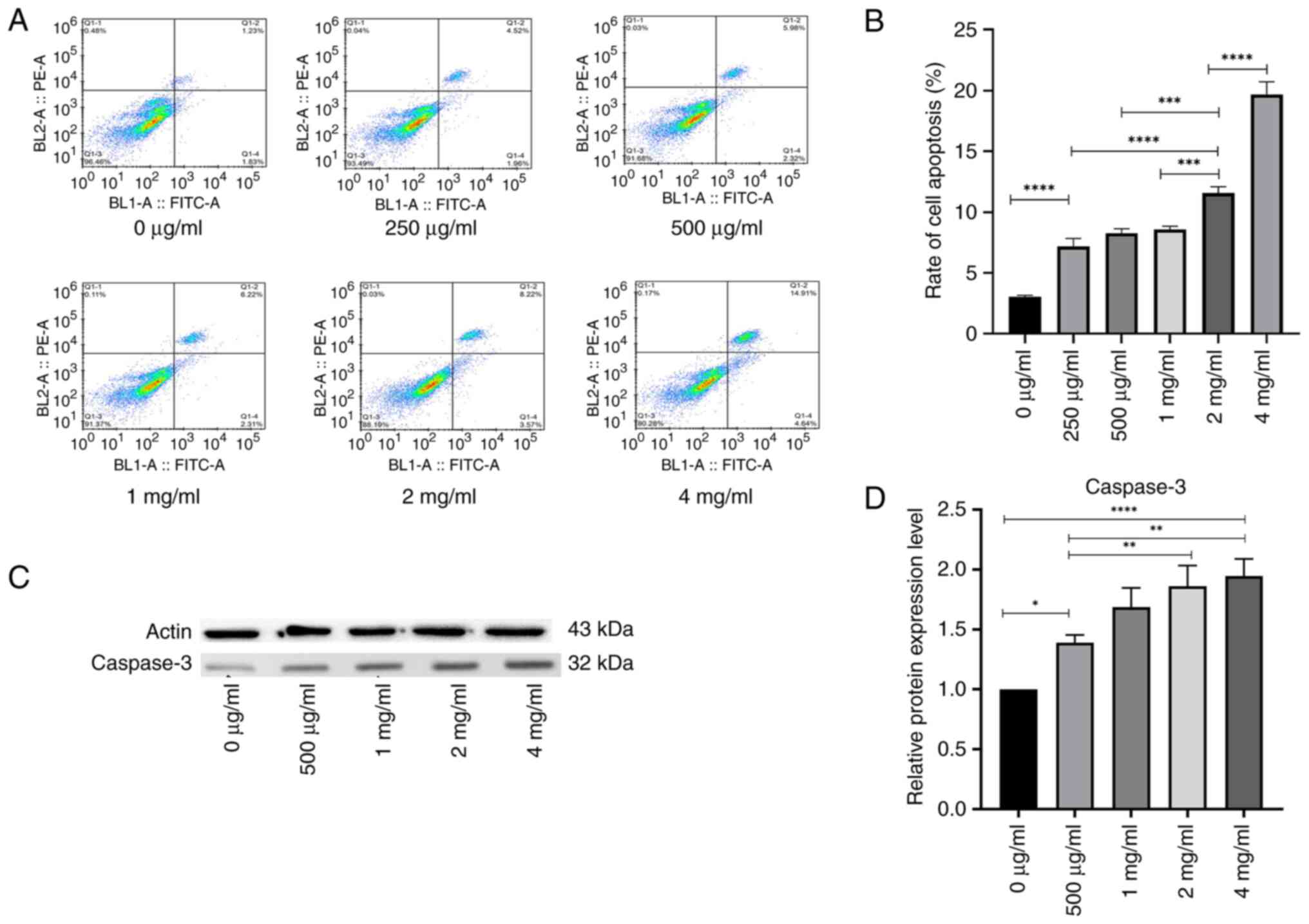
PSP induces apoptosis in PC-3 cells
and arrests the cell cycle in the S phase
The PC-3 cells were treated with different
concentrations of PSP for 72 h, after which the apoptosis rate was
analyzed using flow cytometry. The results demonstrated that the
apoptosis rate in the PSP-treated groups was significantly higher
than in the blank control group, and this increase was
concentration-dependent (P=0.001; Fig.
2A and B).
As activated caspase-3 is a specific marker of
apoptosis (14), the present study
evaluated whether its activation contributed to PSP-induced
apoptosis. Caspase-3 activity was assessed using western blot
analysis following the treatment of PC-3 cells with PSP for 72 h.
PSP concentrations of 0, 500 µg/ml, 1, 2 and 4 mg/ml were selected
based on the results of the apoptosis assay. The results indicated
a concentration-dependent increase in caspase-3 activity (Fig. 2C and D), suggesting the involvement
of the intrinsic apoptotic pathway in PSP-induced apoptosis in PC-3
cells.
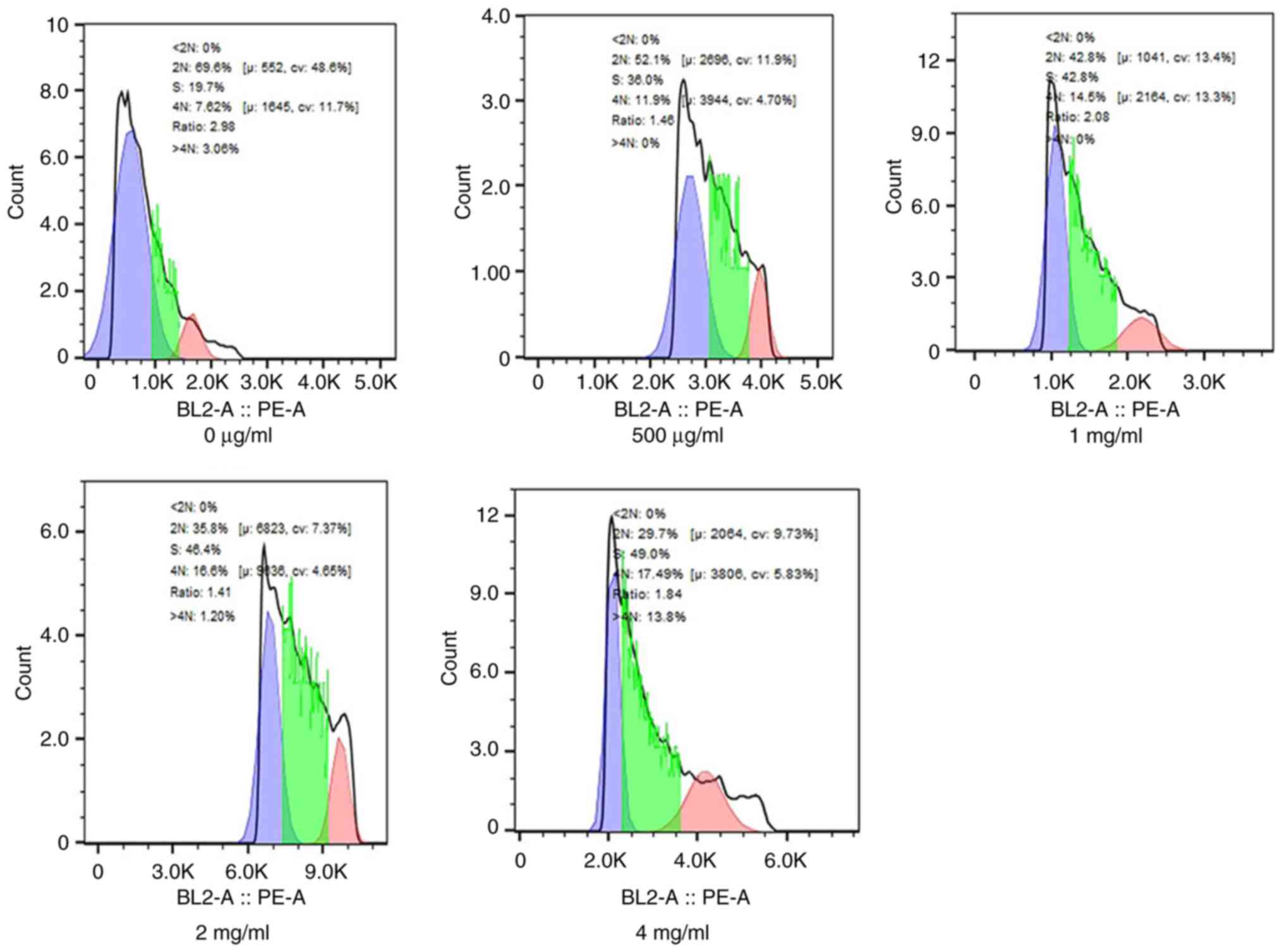
Cell cycle analysis revealed a marked increase in
the % cells in the S phase after 72 h of PSP treatment compared
with the blank control. Specifically, at a concentration of 4
mg/ml, PSP significantly increased the portion of PC-3 cells in the
S phase from 19.7 to 49.0% (Fig.
3).
These results indicate that PSP significantly
promotes apoptosis in PC-3 cells and arrests the cell cycle in the
S phase. Furthermore, PSP appears to induce apoptosis of PC-3 cells
in vitro through the activation of the caspase-3
pathway.
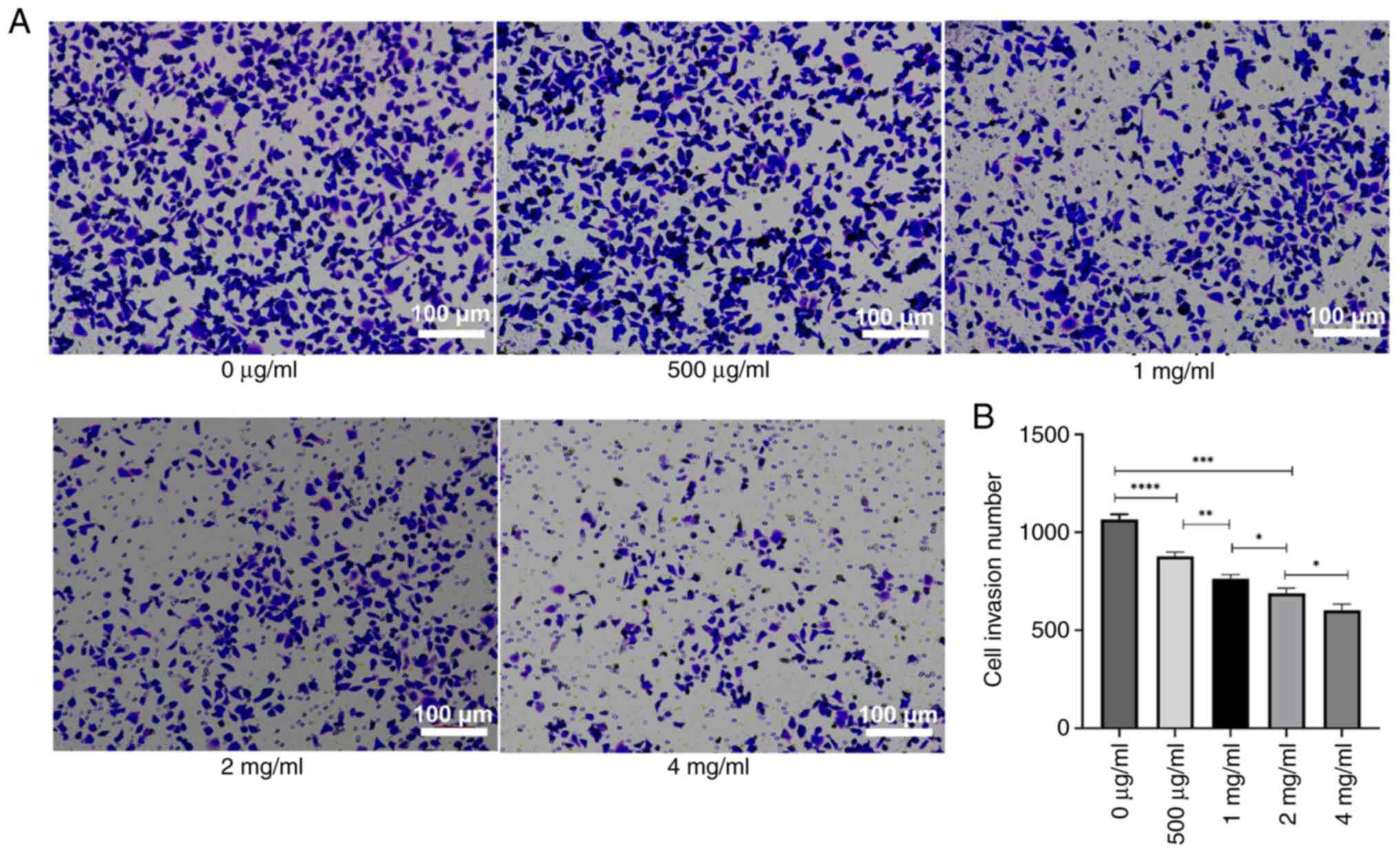
PSP inhibits the migration and
invasion of PC-3 cells
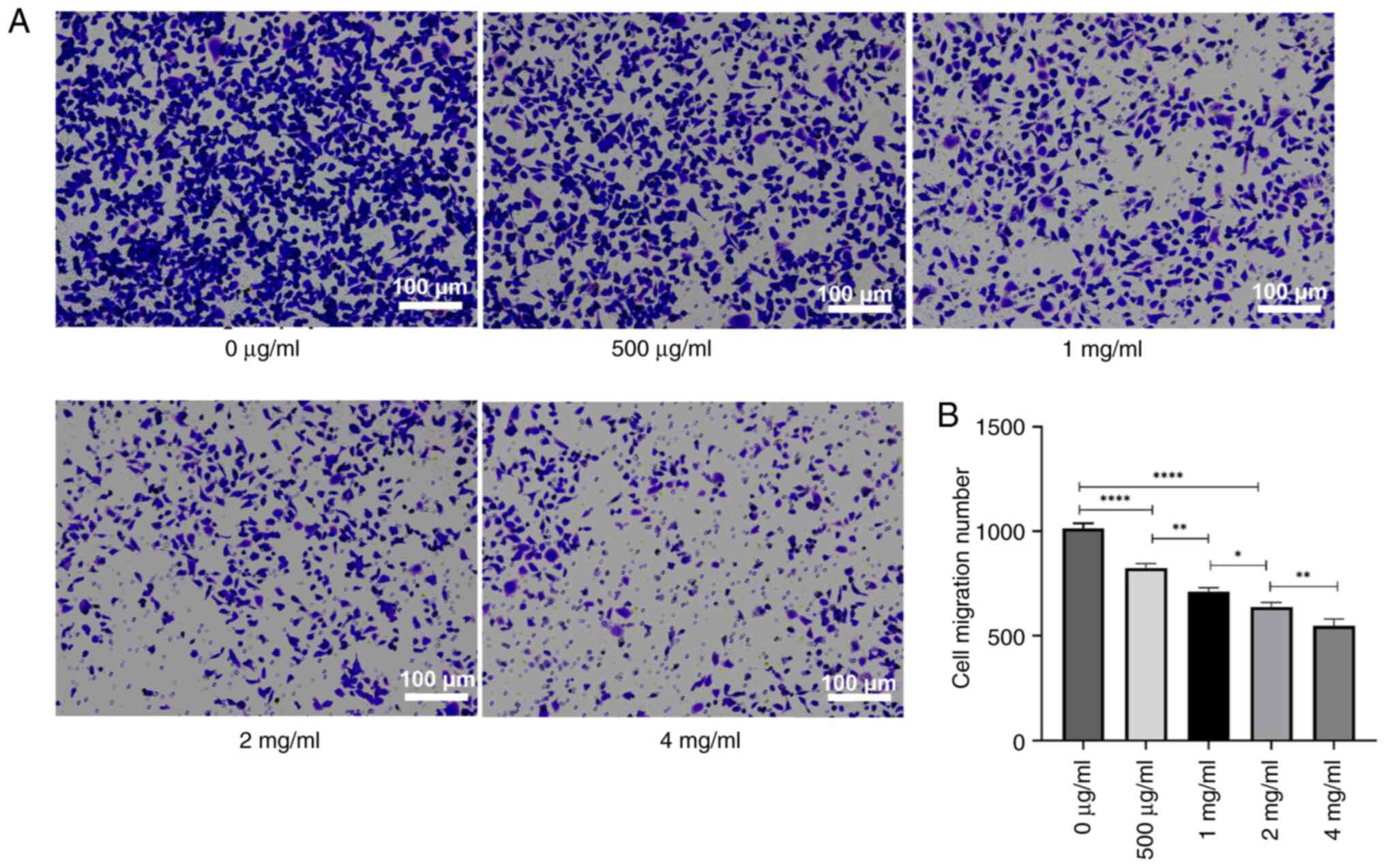
To assess the impact of PSP on the invasion and
migration of PC-3 cells, the cells were treated with several
concentrations of PSP for 72 h, and invasion and migration were
measured using a Transwell assay. The results revealed a
significant reduction in both invasion and migration cell numbers
with increasing PSP concentrations, particularly at 2 and 4 mg/ml
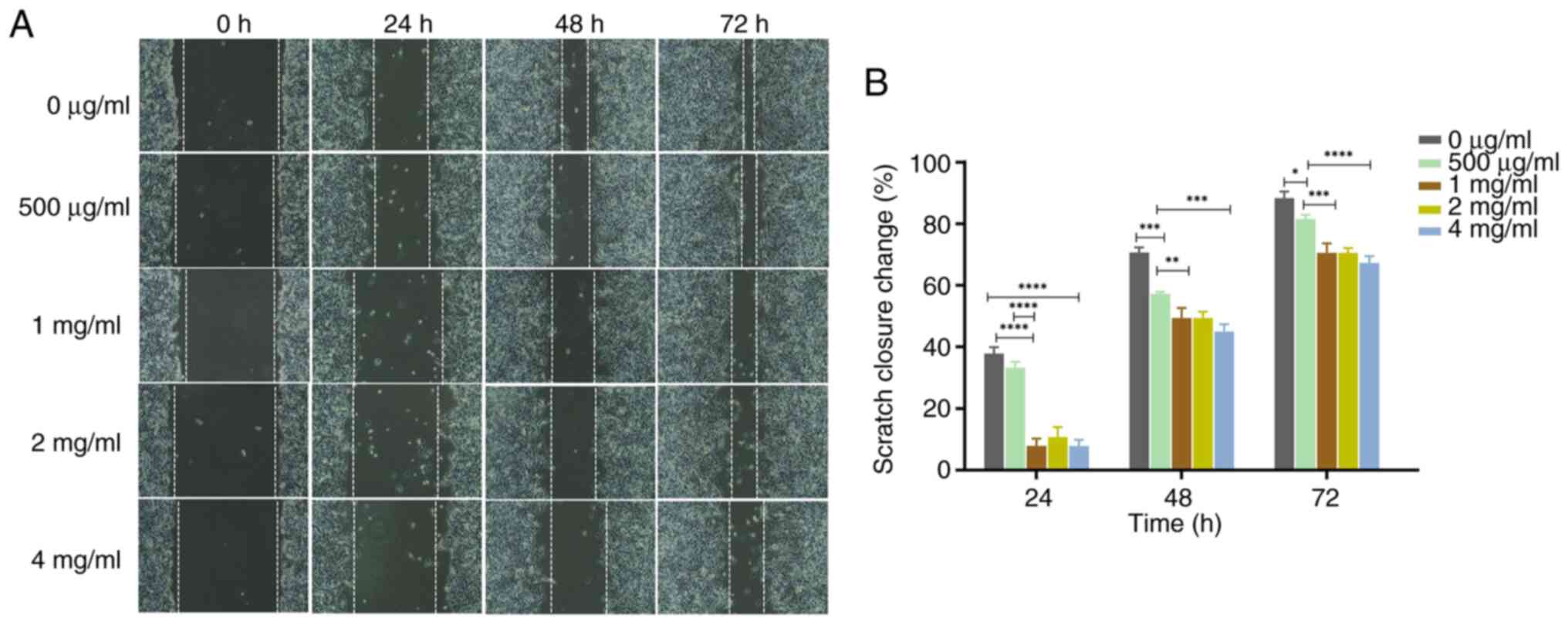
compared to concentration of 0 µg/ml (Figs. 4 and 5). Wound healing assays also demonstrated
that the wound healing of PC-3 cells was inhibited by PSP in a
dose-dependent manner (Fig. 6A).
Furthermore, the scratch closure change indicated that PSP could
significantly prevent wound healing, especially at concentrations
of 2 and 4 mg/ml compared to 0 µg/ml, at 24, 48 and 72 h (Fig. 6B). These results indicate that PSP
could inhibit the invasion and migration of PC-3 cells.
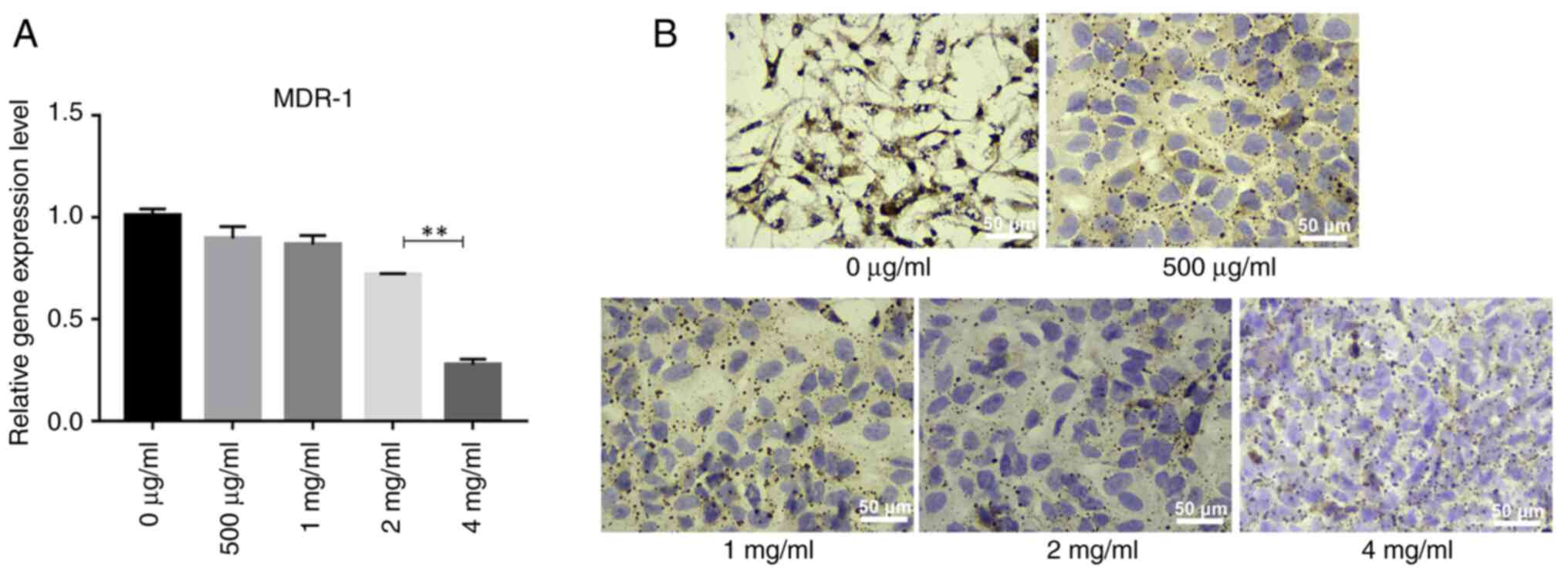
PSP decreases the expression of the
MDR-1 gene and its encoded protein, P-gp, in PC-3 cells
Elevated expression of MDR-1 is known to be
associated with hormone-independent prostate cancer, making it
crucial to identify effective drugs to reverse multidrug resistance
(MDR) and enhance the efficacy of prostate cancer chemotherapy
(23,24). As PC-3 cells are
androgen-independent, the present study used PC-3 cells as an MDR
model to assess the effects of PSP on the MDR-1 gene and P-gp
protein expression. This was evaluated using reverse
transcription-quantitative PCR and immunocytochemistry. The results
indicated that MDR-1 gene expression decreased gradually with
increasing PSP concentrations, with the most significant reduction
observed at 4 mg/ml, where MDR-1 gene expression was significantly
lower compared with at other concentrations (Fig. 7A). Similarly, the expression of P-gp
also decreased gradually with higher PSP concentrations,
particularly at 4 mg/ml (Fig. 7B).
These findings suggest that PSP may have the potential to reverse
MDR in PC-3 cells.
Reduced expression of p-P65, p-PI3K
and p-AKT in PC-3 cells after treatment with PSP
To assess the potential mechanisms through which PSP
affects the biological behavior of prostate cancer cells, western
blot analysis was performed on proteins involved in the PI3K-AKT
and NF-kB signaling pathways after PC-3 cells were treated with
several concentrations of PSP for 72 h. The results revealed no
significant differences in the levels of total AKT, PI3K and P65
among the groups. However, the phosphorylated forms, p-P65, p-PI3K
and p-AKT, demonstrated a concentration-dependent decrease, with
the most pronounced reduction observed at 4 mg/ml. At this
concentration, the levels of p-P65, p-PI3K and p-AKT were
significantly lower compared with at other concentrations (Fig. 8).
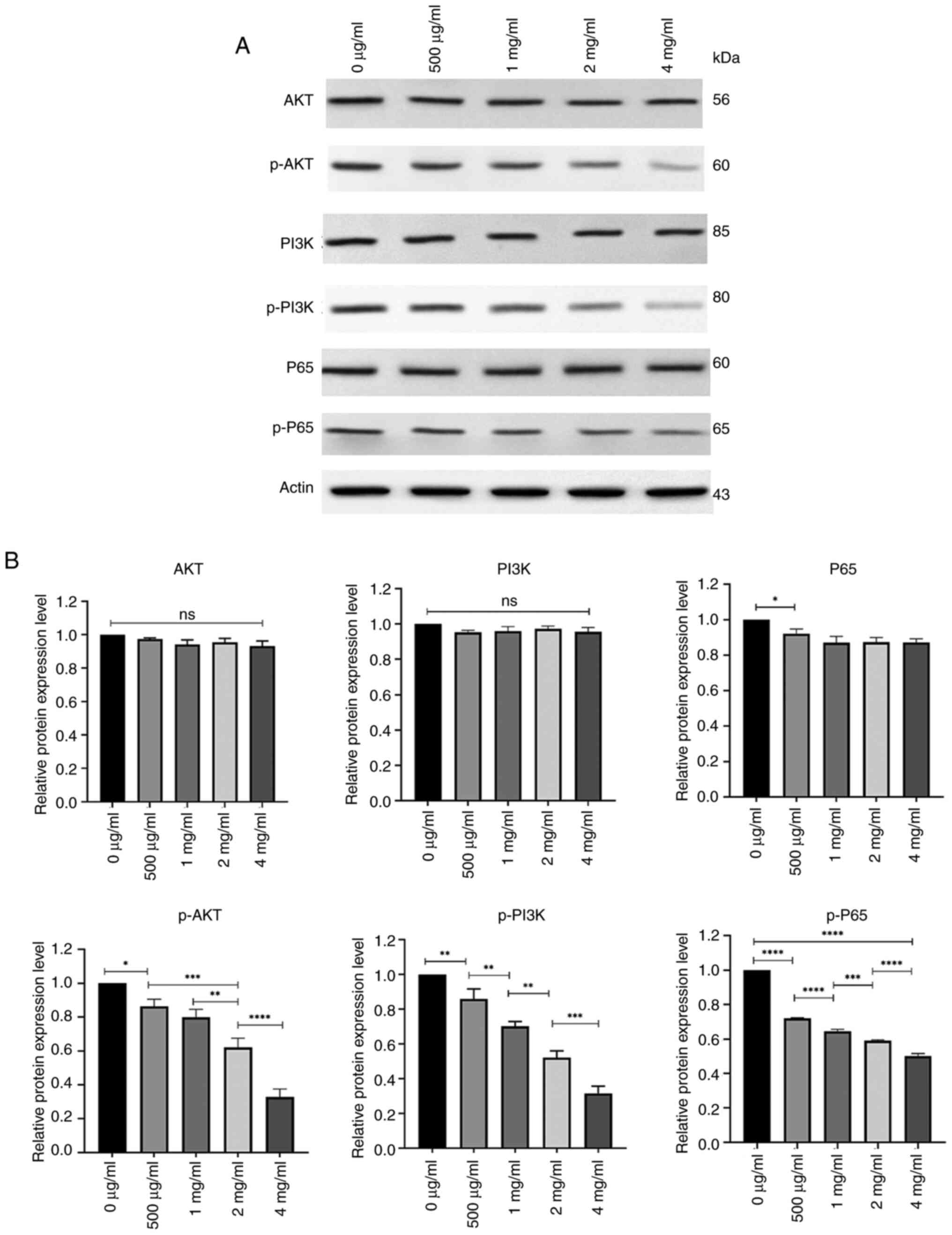 | Figure 8.Expression of AKT, p-AKT, PI3K,
p-PI3K, P65 and p-P65 in PSP-treated PC-3 cells. (A) PC-3 cells
were treated with different concentrations of PSP for 72 h,
harvested and lysed with RIPA. The lysates were subjected to
western blot analysis to assess the expression of Akt, p-AKT, PI3K,
p-PI3K, P65 and p-P65. (B) Semi-quantification of protein levels
was performed, revealing that the levels of p-P65, p-PI3K, p-AKT
decreased in a concentration-dependent manner, especially at a
concentration of 4 mg/ml. The samples were derived from the same
experiment, and the blots were processed in parallel. *P<0.05;
**P<0.01; ***P<0.001; ****P<0.0001. PSP, Polygonatum
sibiricum; ns, not significant. |
Discussion
PSP is gaining increasing popularity for its
potential applications in functional foods and medicine (25), especially in cancer treatment. The
present study assessed the effects of PSP on prostate cancer PC-3
cells and evaluated its potential mechanisms of action. It was
demonstrated that PSP inhibits the proliferation of PC-3 cells and
induces apoptosis. These results align with a previous study that
reported the inhibitory effects of PSP on HepG2 cells and its
ability to induce apoptosis in those cells (14). However, the results of the present
study revealed that PSP induces cell cycle arrest in the S phase of
PC-3 cells, whereas the previous study reported PSP-induced arrest
in the G1 phase of HepG2 cells. This difference may be attributed
to the distinct characteristics of cell types. Other studies have
reported that PSP can induce cell cycle arrest in the G0/G1 phase
in mouse hepatoma H22 cells, whilst it causes arrest in the S phase
in human esophageal cancer ECA-109 cells, human gastric cancer
HGC-27 cells and human colorectal cancer HCT-8 cells (5,15),
thereby promoting apoptosis.
Additionally, the present study demonstrated that
PSP inhibits the invasion and migration of PC-3 cells in a
dose-dependent manner. This finding is consistent with a previous
study that demonstrated the dose-dependently inhibition of
migration, invasion and epithelial-to-mesenchymal transition of
liver cancer cells by PSP. The study indicated that PSP
dose-dependently reduced the activation of the Toll-like receptor 4
(TLR4)/signal transducer and activator of transcription 3 (STAT3)
and noncanonical nuclear factor-κB (NF-κB) signaling pathways. PSP
inhibits liver cancer primarily by targeting and eliminating the
TLR4/STAT3 pathway (12). Another
study assessed the inhibitory effect of anlotinib on
cisplatin-resistant ovarian cancer cells and reported that the
ERK1/2/PLK2 signaling axis mediated the impact of alnotinib on the
proliferation and migration of ovarian cancer cell lines (26). This suggests that different
signaling pathways may be involved in the same inhibitory effects
of drugs on tumor cells simultaneously.
The present also explored the potential mechanisms
behind the PSP-mediated apoptosis, migration and invasion of
prostate cancer cells. A study assessing the association between
AKT/PKB expression and the Gleason pattern in human prostate cancer
reported that AKT was upregulated in prostate cancer and that its
expression was associated with tumor progression (27). Another study which involved
detecting Akt expression through immunohistochemical staining of
paraffin-embedded tissue, reported that the staining intensity for
phosphor-Akt (p-AKT) was markedly higher in Gleason grades 8–10
compared with that in prostatic intraepithelial neoplasia and other
low grades of prostate cancer (28). This indicates that Akt and its
related signaling pathway may serve a critical role in the
progression of prostate cancer. Therefore, in the present study,
the expression levels of AKT and p-AKT were assessed after treating
PC-3 cells with different doses of PSP. The results demonstrated
that whilst AKT expression remained consistent across the different
dose groups, p-AKT expression decreased with the increasing doses
of PSP. Protein kinase AKT is the dominant key effector in the PI3K
signaling pathway (29). Following
this, the present study evaluated the expression levels of PI3K and
p-PI3K. Similar to AKT, it was revealed that PI3K expression showed
no significant variation across the different dose groups, whilst
p-PI3K expression decreased with increasing doses of PSP.
Therefore, we hypothesize that the PI3K/Akt pathway may serve a
role in regulating the initiation and progression of prostate
cancer and that PSP could inhibit the phosphorylation of AKT and
PI3K, thereby blocking prostate cancer cell proliferation,
migration and invasion. This is similar to the findings from a
previous study, which reported that palmitic acid inhibited the key
molecules in the PI3K/Akt pathway, effectively blocking prostate
cancer proliferation and metastasis (30). Additionally, another study reported
that the phosphorylation of NF-κB p65 at ser536 serves a critical
role in promoting prostate cancer oncogenesis (31). The study also highlighted the
synergistic activities of NF-κB and AKT signaling in promoting
prostate cancer tumorigenesis (31). In the present study, it was also
demonstrated that the expression of P65 in prostate cancer cells
remained unchanged after PSP treatment, whilst phosphorylated P65
decreased with increasing doses of PSP. This suggests that PSP may
inhibit the phosphorylation of P65, thereby blocking the
proliferation, migration and invasion of prostate cancer cells.
Therefore, we hypothesize that PSP could exert its inhibitory
effects on prostate cancer cell proliferation, invasion and
migration by regulating both the PI3K/Akt and NF-κB signaling
pathways. These findings align with a previous study that suggested
a synergistic anticancer effect of salinomycin combined with
cabazitaxel by simultaneously downregulating the Wnt, NF-κB and AKT
signaling pathways (32). Another
study reported the functional link between PI3K-AKT and NF-κB
pathways in modulating anti-apoptotic and MDR effects, including
the expression of the MDR1 gene, in AML HL-60 cells (23).
In addition, MDR in cancer cells markedly hinders
the therapeutic efficacy of treatments (24). There is increasing interest in
developing several therapeutic regimens, including inhibitory
drugs, to overcome MDR (33,34).
Studies have reported that natural products can target multiple
targets, making them valuable in addressing drug resistance from
different perspectives (35,36).
The present study demonstrated that PSP, a traditional Chinese
medicine, can decrease expression of the MDR-1 gene and its encoded
protein, P-gp, which may help reverse MDR in PC-3 cells. This
effect could be linked to the broader anti-cancer properties of
PSP.
In summary, the present study is the first, to the
best of our knowledge, to demonstrate that PSP can inhibit the
proliferation, invasion and migration of PC-3 cells in
vitro, as well as reverse MDR in these cells. The underlying
mechanism may involve the simultaneous regulation of the PI3K/Akt
and NF-κB signaling pathways. However, the present study is limited
by the fact that the effects of PSP were only assessed using PC-3
cells. It remains unclear whether these findings apply to other
prostate cancer cell lines, such as LNCap or DU145, or benign
prostate hyperplasia cell lines. Future research should evaluate
the effects of PSP on a broader range of cell lines and further
investigation in animal models should be performed to deepen the
understanding of its potential therapeutic benefits. However, the
findings of the present study suggest the antitumor potential of
PSP for prostate cancer by targeting the PI3K/Akt and NF-κB
pathways.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Natural Science Foundation
of Hebei Province (grant no. H2021405012) and the Basic Scientific
Research Business of Hebei North University (grant no.
JYT2022005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GZ and YZ confirm the authenticity of all the raw
data. GZ and YZ conceived the study, performed experiments and
wrote the manuscript. YT, SD, XZ and XL designed the experiments.
XW, CL, MA and SD performed experiments. YT, SD and CXL analyzed
data. XZ, SD wrote the manuscript. MA, YZ, XW and YT reviewed the
manuscript. MA, GZ and YZ constructed figures. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Silk N, Reich J, Sinha R, Chawla S, Geary
K and Zhang D: The effects of resveratrol on prostate cancer
through targeting the tumor microenvironment. J Xenobiot. 11:16–32.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Penezić L, Kuliš T, Hudolin T, Zekulić T,
Saić H and Kaštelan Ž: Laparoscopic radical prostatectomy: Single
center case series. Acta Clin Croat. 61 (Suppl 3):S15–S20.
2022.PubMed/NCBI
|
|
3
|
Stoykova GE and Schlaepfer IR: Lipid
metabolism and endocrine resistance in prostate cancer, and new
opportunities for therapy. Int J Mol Sci. 20:26262019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai M, Song XL, Li XA, Chen M, Guo J, Yang
DH, Chen Z and Zhao SC: Current therapy and drug resistance in
metastatic castration-resistant prostate cancer. Drug Resist Updat.
68:1009622023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui X, Wang S, Cao H, Guo H, Li Y, Xu F,
Zheng M, Xi X and Han C: A Review: The bioactivities and
pharmacological applications of polygonatumsibiricum
polysaccharides. Molecules. 23:11702018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Li H, Pan B, Zhang S, Su X, Sun
W, Zhang T, Zhang Z, Lv S and Cui H: Integrated 16S rRNA sequencing
and untargeted metabolomics analysis to reveal the protective
mechanisms of polygonatum sibiricum polysaccharide on type 2
diabetes mellitus model rats. Curr Drug Metab. 24:270–282. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Lan C, Liao X, Chen D, Song W and
Zhang Q: Polygonatum sibiricum polysaccharide potentially
attenuates diabetic retinal injury in a diabetic rat model. J
Diabetes Investig. 10:915–924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang JX, Wu S, Huang XL, Hu XQ and Zhang
Y: Hypolipidemic Activity and Anti-atherosclerotic Effect of
Polysaccharide of Polygonatum sibiricum in Rabbit Model and Related
Cellular Mechanisms. Evid Based Complement Alternat Med.
2015:3910652015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye G, Zhao Y, Zhu J, Zhang Z, Wang Q,
Jiang X and Wang Z: Synergistic effect of polydatin and polygonatum
sibiricum polysaccharides in combating atherosclerosis via
suppressing TLR4-Mediated NF-κB Activation in ApoE-Deficient Mice.
Evid Based Complement Alternat Med. 2022:38851532022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du L, Nong MN, Zhao JM, Peng XM, Zong SH
and Zeng GF: Polygonatum sibiricum polysaccharide inhibits
osteoporosis by promoting osteoblast formation and blocking
osteoclastogenesis through Wnt/β-catenin signaling pathway. Sci
Rep. 6:322612016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Long T, Liu Z, Shang J, Zhou X, Yu S, Tian
H and Bao Y: Polygonatum sibiricum polysaccharides play anti-cancer
effect through TLR4-MAPK/NF-kappaB signaling pathways. Int J Biol
Macromol. 111:813–821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Guo Y, Lu C, Yu L, Fang C and Li C:
Polygonatum sibiricum polysaccharide inhibited liver cancer in a
simulated tumor microenvironment by eliminating TLR4/STAT3 pathway.
Biol Pharm Bull. 46:1249–1259. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han SY, Hu MH, Qi GY, Ma CX, Wang YY, Ma
FL, Tao N and Qin ZH: Polysaccharides from Polygonatum Inhibit the
Proliferation of Prostate Cancer-Associated Fibroblasts. Asian Pac
J Cancer Prev. 17:3829–3833. 2016.PubMed/NCBI
|
|
14
|
Li M, Liu Y, Zhang H, Liu Y, Wang W, You
S, Hu X, Song M, Wu R and Wu J: Anti-cancer Potential of
Polysaccharide Extracted From Polygonatum sibiricum on HepG2 cells
via cell cycle arrest and apoptosis. Front Nutr. 9:9382902022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Tian LN, Ren ZX and Long ZJ:
Research progress on the structural analysis and functional
activity of polysaccharides. Chin J Exp Tradit Med Fromul.
21:231–234. 2015.(In Chinese).
|
|
16
|
Tewari D, Patni P and Bishayee A, Sah AN
and Bishayee A: Natural products targeting the PI3K-Akt-mTOR
signaling pathway in cancer: A novel therapeutic strategy. Semin
Cancer Biol. 80:1–17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hashemi M, Taheriazam A, Daneii P,
Hassanpour A, Kakavand A, Rezaei S, Hejazi ES, Aboutalebi M,
Gholamrezaie H, Saebfar H, et al: Targeting PI3K/Akt signaling in
prostate cancer therapy. J Cell Commun Signal. 17:423–443. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Wu S, Gu Y, Liang H, He F, Wang X,
He D and Wu K: RASAL2 regulates the cell cycle and cyclin D1
expression through PI3K/AKT signalling in prostate tumorigenesis.
Cell Death Discov. 8:2752022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y and Liang C: TRAIP suppressed
apoptosis and cell cycle to promote prostate cancer proliferation
via TRAF2-PI3K-AKT pathway activation. Int Urol Nephrol.
56:1639–1648. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raja Singh P, Sugantha Priya E,
Balakrishnan S, Arunkumar R, Sharmila G, Rajalakshmi M and
Arunakaran J: Inhibition of cell survival and proliferation by
nimbolide in human androgen-independent prostate cancer (PC-3)
cells: Involvement of the PI3K/Akt pathway. Mol Cell Biochem.
427:69–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davoudi Z, Akbarzadeh A, Rahmatiyamchi M,
Movassaghpour AA, Alipour M, Nejati-Koshki K, Sadeghi Z,
Dariushnejad H and Zarghami N: Molecular Target Therapy of AKT and
NF-kB Signaling Pathways and Multidrug Resistance by Specific Cell
Penetrating Inhibitor Peptides in HL-60 Cells. Asian Pac J Cancer
Prev. 15:4353–4358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duan C, Yu M, Xu J, Li BY, Zhao Y and
Kankala RK: Overcoming Cancer Multi-drug Resistance (MDR): Reasons,
mechanisms, nanotherapeutic solutions, and challenges. Biomed
Pharmacother. 162:1146432023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu D, Tang W, Han C and Nie S: Advances
in Polygonatum sibiricum polysaccharides: Extraction, purification,
structure, biosynthesis, and bioactivity. Front Nutr.
9:10746712022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji Y, Li XY, Qi Y, Zhao J, Zhang W and Qu
P: Anlotinib exerts inhibitory effects against cisplatin-resistant
ovarian cancer in vitro and in vivo. Molecules. 27:88732022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao YD, Grobholz R, Abel U, Trojan L,
Michel MS, Angel P and Mayer D: Increase of AKT/PKB expression
correlates with gleason pattern in human prostate cancer. Int J
Cancer. 107:676–680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malik SN, Brattain M, Ghosh PM, Troyer DA,
Prihoda T, Bedolla R and Kreisberg JI: Immunohistochemical
demonstration of phospho-Akt in high Gleason grade prostate cancer.
Clin Cancer Res. 8:1168–1171. 2002.PubMed/NCBI
|
|
29
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. CurrOpin Cell Biol. 45:62–71.
2017.
|
|
30
|
Zhu S, Jiao W, Xu Y, Hou L, Li H, Shao J,
Zhang X, Wang R and Kong D: Palmitic acid inhibits prostate cancer
cell proliferation and metastasis by suppressing the PI3K/Akt
pathway. Life Sci. 286:1200462021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Shao L, Creighton CJ, Zhang Y,
Xin L, Ittmann M and Wang J: Function of phosphorylation of NF-kB
p65 ser536 in prostate cancer oncogenesis. Oncotarget. 6:6281–6294.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Erdogan S, Serttas R, Turkekul K and
Dibirdik I: The synergistic anticancer effect of salinomycin
combined with cabazitaxel in CD44+ prostate cancer cells
by downregulating wnt, NF-κB and AKT signaling. Mol Biol Rep.
49:4873–4884. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian Y, Lei Y, Wang Y, Lai J, Wang J and
Xia F: Mechanism of multidrug resistance to chemotherapy mediated
by P-glycoprotein (Review). Int J Oncol. 63:1192023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu YX, Jia HR, Duan QY and Wu FG:
Nanomedicines for combating multidrug resistance of cancer. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 13:e17152021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu T, Guo P, He Y, Pi C, Wang Y, Feng X,
Hou Y, Jiang Q, Zhao L and Wei Y: Application of curcumin and its
derivatives in tumor multidrug resistance. Phytother Res.
34:2438–2458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen T, Xiao Z, Liu X, Wang T, Wang Y, Ye
F, Su J, Yao X, Xiong L and Yang DH: Natural products for combating
multidrug resistance in cancer. Pharmacol Res. 202:1070992024.
View Article : Google Scholar : PubMed/NCBI
|