Introduction
Lung cancer has a high incidence and is the leading
cause of cancer associated mortality worldwide (1). Histologically, lung cancer can be
divided into small cell lung cancer and non-small cell lung cancer
(NSCLC) and NSCLC patients possess a higher opportunity of surgical
resection (1). Furthermore, >50%
of patients with lung cancer are diagnosed at locally advanced or
metastatic stages (2,3). Although there have been advances in
the understanding of the pathophysiology and the development of
novel treatments (4), curative
treatments for advanced lung cancer are limited and a diagnosis of
advanced lung cancer is predictive of a poor clinical outcome. For
patients with early-stage lung cancer, complete resection remains
the most reliable treatment option and is currently the only option
that is potentially curative. In terms of resection margin status,
a complete resection is referred to as R0, while microscopic and
macroscopic residual tumors are referred to as R1 and R2
resections, respectively (5).
However, complete resection of early-stage lung
cancer does not guarantee a curative response. It is reported that
30–55% of patients with early-stage lung cancer and R0 resection
may experience disease recurrence and succumb to lung cancer
(2,6). Although neoadjuvant and adjuvant
chemotherapy may improve the outcome of patients with lung cancer,
the benefits in five-year survival rates are limited (7,8).
Currently, numerous novel agents, including immunotherapies, such
as nivolumab, durvalumab, and pembrolizumab as well as targeted
therapies, such as osimertinib and alectinib, are approved by U.S.
Food and Drug Administration and European Medicines Agency for the
treatment of patients with early-stage lung cancer (9–11).
However, previous studies only include early-stage non-small cell
lung cancer (NSCLC) patients with R0 resection (9–11). To
the best of the authors' knowledge, the predicted outcomes and
appropriate management of early-stage NSCLC patients with non-R0
resection is still to be elucidated.
Between 3–7% of patients with early-stage lung
cancer experience incomplete resection of lung tumors (12,13).
There is a positive association between the risk of incomplete
resection and the increasing tumor and/or node stage (12). Additionally, patients with R1 and R2
resection margin status have a notably reduced survival time
compared with patients with R0 resections (12). According to current treatment
guidelines (5,14), the treatment options for patients
with incomplete resection include resection and radiotherapy,
either alone or in combination with chemotherapy. For patients with
advanced NSCLC, nine driver mutations have been recommended to be
assessed and corresponding targeted therapies should be prescribed
according to the results. Of them, epidermal growth factor
receptor (EGFR) mutation is the most common genetic
alteration (5). The role of driver
gene mutation assessment and the corresponding targeted therapies,
such as EGFR-tyrosine kinase inhibitor (TKI), for example,
osimertinib, for non-R0 resected EGFR-mutant NSCLC patients,
is currently unclear. Therefore, the present study investigated the
characteristics, outcomes and prognostic factors of patients with
early-stage NSCLC and non-R0 resection, and focused on driver gene
mutation detection and driver gene-targeted therapy.
Patients and methods
Patient criteria
The present study was retrospective and included
patients with lung cancer that were diagnosed and treated at
Taichung Veterans General Hospital (Taichung, Taiwan) between
August 2011 and December 2020. Included patients were required to
have: i) Pathologically confirmed NSCLC; ii) a resectable disease
prior to operation; iii) history of surgical resection of lung
tumor and/or mediastinal lymph node dissection; iv) non-R0
resection status; v) a precise history of diagnosis; vi) received
all the treatments; and vii) survival follow-up data. Patients were
excluded if they had: i) Small cell lung carcinoma; ii) other
active malignancies; or iii) incomplete data records.
The present study was approved by the Institutional
Review Board (IRB) of Taichung Veterans General Hospital (IRB nos.
CF12019 and CF20175; Taichung, Taiwan). Written informed consent
for clinical data records and genetic testing was obtained from all
patients.
Data records for analysis
Clinical data used for analyses included the age,
sex, smoking status, Eastern Cooperative Oncology Group performance
status (ECOG PS), histological type, driver gene mutation status,
tumor stage, operation types, resection margin status, pathological
features, history of radiotherapy and antineoplastic treatment, and
the survival follow-up data of the patients. Lung cancer tumor,
node and metastases staging was conducted according to the 8th
edition of the American Joint Committee on Cancer staging system
(15).
Driver gene mutation status
Patients with available tumor specimens were tested
for mutations in six driver genes, which included EGFR, Kirsten
rat sarcoma viral oncogene homolog (KRAS), v-raf
murine sarcoma viral oncogene homolog B (BRAF), human
epidermal growth factor receptor 2 (HER2), anaplastic
lymphoma kinase (ALK) and ROS proto-oncogene 1
(ROS1). EGFR, KRAS, BRAF and HER2 mutations were
tested using matrix-assisted laser desorption ionization-time of
flight mass spectrometry (MALDI-TOF MS), which has been validated
as a standard method to detect these driver gene mutations in our
previous studies and implemented in Taiwan clinical practice
(16–20). Genomic DNA (gDNA) was extracted for
serial biochemical reactions. For gDNA extraction, QIAamp DNA
formalin-fixed paraffin-embedded (FFPE) Tissue Kit (cat. no. 56404;
Qiagen GmbH) was used according to the manufacturer's instructions.
Briefly, gDNA from three sections of 10 µm thick FFPE was
extraction following deparaffination, lysing, heating, washing and
eluting. The Typlex/iPlex PRO kit (cat. no. 10217; Agena
Bioscience, Inc.) was utilized for biochemical reactions according
to the manufacturer's instructions for the MassARRAY®
kit system (cat. no. 10411; Agena Bioscience, Inc.). Briefly, PCR
was used to amplify the region containing EGFR mutations,
and then single nucleotide extension was performed using detection
probes, followed by MALTI-TOF MS analysis. EGFR mutations
could be distinguished from wild-type genes due to the mass
difference of an incorporated single nucleotide. For PCR
amplification, a final volume of 5 µl reaction mixture containing
10 ng gDNA, 0.5 units HotStar Taq polymerase (cat. no. 203203;
Qiagen GmbH), 500 mM dNTPs, 100 nM forward primers, 100 nM reverse
primers, 1X of HotStar buffer (diluted from 10X) and 1.625 mM
MgCl2 was used. The following thermocycling conditions
were used for PCR: Initial denaturation at 94°C for 15 min,
followed by 45 touch-down amplification cycles consisting of 15
cycles of 94°C for 20 sec, annealing at 61°C for 30 sec and 72°C
for 60 sec with another 30 cycles of 94°C for 20 sec, annealing at
57°C for 30 sec and 72°C for 60 sec. Shrimp alkaline phosphatase
(SAP) treatment for dNTP neutralization was carried out as follows:
0.5 units SAP with 1X SAP buffer (diluted from 10X concentrated)
were prepared in a final 2 µl mixture. It was added into the PCR
product for 40 min at 37°C incubation and then inactivated at 80°C
for 5 min. The last step was to probe for single nucleotide
extensions using a Typlex™/iPlex PRO kit (cat. no. 10217; Agena
Bioscience, Inc.) containing 0.0205 µl Sequnase, 0.1 µl termination
mix, 0.2 µl 10X Typlex buffer and multiplex extension primers
(Table SI) at a final
concentration of 7–14 µM in a 2 µl reagent mix. The following
thermocycling conditions were used: 94°C for 30 sec, followed by a
40-cycle extension reaction (each cycle contained five rounds of
94°C for 20 sec, 80°C for 5 sec, and 60°C for 5 sec). After
SpectroClean Resin clean up (using 6 mg resin for each reaction in
the 384-well plate and rotating at room temperature for 20 min to
eliminate salt contamination), samples were loaded onto a
SpectroCHIP® matrix (Agena Bioscience, Inc.) using a
Nanodispenser and then analyzed using Autoflex®
MALDI-TOF MS (Bruker Corporation). Data were collected and analyzed
using the Typer4 software (Ver. 4.0.53; Agena Bioscience, Inc.).
The PCR primers and probes used in the present study are provided
in Table SI and the representative
EGFR mutation spectra detected using MALDI-TOF MS are shown
in Fig. S1. The ALK fusion
mutation was assessed using a fully automated VENTANA ALK (D5F3)
CDx Assay (cat. no. 790-4796, Roche Diagnostics, Ltd.) for
immunohistochemical staining (IHC) using the pre-diluted anti-ALK
(D5F3) rabbit monoclonal primary antibody (cat. no. 3633; Cell
Signaling Technology, Inc.). Based on the diagnostic procedure
suggested by VENTA ALK (D5F3) CDx Assay, the biopsy was fixed by
10% formalin at room temperature overnight. After water resin and
dehydration by gradient alcohol from 75–100%, the biopsy was
embedded in paraffin. A 4 µm thick FFPE section was required for
automatic IHC assay. The ROS1 fusion mutation was
investigated using fluorescent in situ hybridization as
previously described (Fig. S2)
(16–18,21).
All the aforementioned methods for driver gene mutation detection
were included in the written informed consent approved by the IRB
of Taichung Veterans General Hospital.
Statistical analysis
Univariate analyses of the association between the
status of tumor progression and the characteristics of patients
were carried out using Fisher's exact test and logistic regression
model. The Kaplan-Meier method was used to analyze the survival
time of patients. Differences in survival time were analyzed using
the log-rank test. The logistic regression model and Cox
proportional hazard model were used for multivariate analyses of
the prognostic factors of disease progression status and survival
outcomes. Each variable was independently subjected to analysis
using the logistic regression model and Cox proportional hazard
model, followed by selection of statistically significant variables
for subsequent analysis. To evaluate survival outcomes,
progression-free survival (PFS) was defined as the length of time
from operation to disease progression or mortality due to any
cause, overall survival (OS) was defined as the length of time from
operation to mortality due to any cause and post-progression
survival was defined as the length of time from documented tumor
progression to mortality due to any cause among patients that
experienced disease progression. Driver gene-targeted treatment
indicated patients receiving targeted therapy corresponding to the
driver gene mutations detected; for instance, EGFR-TKI for
EGFR mutation and ALK inhibitor for ALK fusion,
respectively (5). All statistical
analyses were carried out using SPSS (version 15.0; SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients and demographic data
Between August 2011 and December 2020, 2,162
patients with lung cancer underwent surgical resection for curative
purposes. Of them, a total of 65 patients (3.0%) had non-R0
resection and were included in the present study for analysis
(Table I). The median follow-up
time was 36.2 months (95% CI, 14.3–58.0). The median age was 64
years (range, 35–87). Of the patient cohort, 24 patients were
female (36.9%) and 27 patients were non-smokers (41.5%). Baseline
ECOG PS was 0–1 in 61 patients (93.8%). Adenocarcinoma (60.0%) and
squamous cell carcinoma (23.1%) were the most common histological
types. In terms of types of operation, 2 (3.1%), 50 (76.9%) and 13
(20.0%) patients underwent pneumonectomy, lobectomy and wedge
resection, respectively. Mediastinal lymph node dissection was
performed in 61 patients (93.8%). A total of 42 patients (64.6%)
had received adjuvant chemotherapy, while 26 patients (40.0%) had
received adjuvant radiotherapy.
 | Table I.Patient characteristics and
demographic data. |
Table I.
Patient characteristics and
demographic data.
| Characteristic | No. of patients
(n=65) |
|---|
| Median age, years
(range) | 64 (35–87) |
| Sex, n (%) |
|
|
Female | 24 (36.9) |
|
Male | 41 (63.1) |
| Smoking status, n
(%) |
|
|
Non-smokers | 27 (41.5) |
|
Smokers | 38 (38.5) |
| ECOG PS, n (%) |
|
|
0-1 | 61 (93.8) |
| 2 | 4 (6.2) |
| Histological types,
n (%) |
|
|
Adenocarcinoma | 39 (60.0) |
|
Squamous cell carcinoma | 15 (23.1) |
|
Adenosquamous cell
carcinoma | 6 (9.2) |
|
Othersa | 5 (7.7) |
| Pathological stage,
n (%) |
|
|
0-I | 16 (24.6) |
| II | 15 (23.1) |
|
IIIA-B | 34 (52.3) |
| Operation type, n
(%) |
|
|
Pneumonectomy | 2 (3.1) |
|
Lobectomy | 50 (76.9) |
| Wedge
resection | 13 (20.0) |
| Lymph node
dissection, n (%) |
|
|
Yes | 61 (93.8) |
| No | 4 (6.2) |
| Driver gene
mutation status, n (%) |
|
|
Yesb | 22 (33.8) |
| No or
unknown | 43 (55.2) |
| Adjuvant
chemotherapy, n (%) |
|
|
Yes | 42 (64.6) |
| No | 23 (35.4) |
| Adjuvant
radiotherapy, n (%) |
|
|
Yes | 26 (40.0) |
| No | 39 (60.0) |
Pathological features and disease
progression patterns
An analysis of the pathological features and disease
progression patterns are presented in Table II. Regarding the resection margin
status, 60 patients (92.3%) had R1 status, while 5 patients (7.7%)
had R2 status. The involved surgical margins were predominantly in
the parenchymal margin (33 patients; 50.8%) and bronchial margin
(21 patients; 32.3%). Of the patient cohort, 6 patients (9.2%)
exhibited positive surgical margins in the vascular areas. The
involvement of the bronchial and vascular margin was identified in
5 patients (7.7%; Table SII).
Angiolymphatic invasion was revealed in 43 patients (66.2%),
perineural invasion was revealed in 23 patients (35.4%), extranodal
involvement was revealed in 18 patients (27.7%) and spread through
air space (STAS) invasion was revealed in 12 patients (18.5%). In
terms of visceral pleural invasion status, 33 (50.8%), 14 (21.5%),
9 (13.8%) and 9 (13.8%) patients were revealed to be PL0, PL1, PL2
and PL3, respectively (Table
II).
 | Table II.Pathological features and disease
progression patterns of patients with early-stage non-small cell
lung cancer and non-R0 resection after surgery. |
Table II.
Pathological features and disease
progression patterns of patients with early-stage non-small cell
lung cancer and non-R0 resection after surgery.
| Pathological
features | No. of patients
(n=65) |
|---|
| Resection margin, n
(%) |
|
| R1 | 60 (92.3) |
| R2 | 5 (7.7) |
| Angiolymphatic
invasion, n (%) |
|
|
Yes | 43 (66.2) |
| No | 22 (33.8) |
| Perineural
invasion, n (%) |
|
|
Yes | 23 (35.4) |
| No | 42 (64.6) |
| Extranodal
involvement, n (%) |
|
|
Yes | 18 (27.7) |
| No | 47 (72.3) |
| Spread through air
space, n (%) |
|
|
Yes | 12 (18.5) |
| No | 7 (10.8) |
|
Unknown | 46 (70.8) |
| Visceral pleural, n
(%) |
|
|
PL0 | 33 (50.8) |
|
PL1 | 14 (21.5) |
|
PL2 | 9 (13.8) |
|
PL3 | 9 (13.8) |
| Disease
progression, n (%) |
|
| No | 23 (35.4) |
|
Yes | 39 (60.0 |
|
Intrathoracic | 17 (26.2) |
|
Extrathoracic | 22 (33.8) |
|
Mortalitya | 3 (4.6) |
The pathological stages were reported as 0-I, II and
IIIA-B in 16 (24.6%), 15 (23.1%) and 34 (52.3%) patients,
respectively (Table I). Regarding
driver gene mutation status, 21 patients harbored an EGFR
mutation, while 1 patient harbored an ALK fusion. The
EGFR mutation spectrum included 10 patients with an exon 19
deletion, 7 patients with an exon 21 L858R mutation, 2 patients
with an exon 18 G719X mutation, 1 patient with an exon 20 insertion
and 1 patient with an exon 19 deletion plus an exon 20 T790M
compound mutation. The driver gene mutation status of the remaining
43 patients (55.2%) was negative or unknown (Table I). In total, 39 patients (60.0%) had
disease progression and 3 patients (4.6%) died. Among patients with
disease progression and patients who succumbed, a total of 20
(47.6%) patients received driver-gene targeted therapy following
disease progression.
Association between patient
characteristics and disease progression
Results of a univariate analysis of the association
between patient characteristics and disease progression are shown
in Table III. Disease progression
was more likely in patients with pathological stage II–IIIB
compared with patients with stage 0-I (73.5 vs. 37.5%,
respectively; P=0.015). Positive angiolymphatic invasion was
associated with an increased risk of disease progression (76.7 vs.
40.9%, respectively; P=0.006). The risk of disease progression for
patients with positive perineural invasion and STAS was markedly
increased. Rates of disease progression were similar between
different histological types. Furthermore, patients with known
driver gene mutations had a significantly increased risk of disease
progression compared with patients with a negative or unknown
status (95.5 vs. 48.8%, respectively; P<0.001).
 | Table III.Univariate analysis of the
association between the characteristics of patients and disease
progression. |
Table III.
Univariate analysis of the
association between the characteristics of patients and disease
progression.
| Characteristic | No. of disease
progression or mortality |
P-valuea | OR (95% CI) |
P-valueb |
|---|
| Age, n (%) |
| >0.999 |
| 0.615 |
| <65
years | 21 (61.8) |
| Reference |
|
| ≥65
years | 21 (67.7) |
| 1.30
(0.47–3.61) |
|
| Sex, n (%) |
| >0.999 |
| 0.791 |
|
Female | 16 (66.7) |
| Reference |
|
|
Male | 26 (63.4) |
| 0.87
(0.30–2.50) |
|
| Smoking status, n
(%) |
| >0.999 |
| 0.814 |
|
Non-smoker | 17 (63.0) |
| Reference |
|
|
Smoker | 25 (65.8) |
| 1.13
(0.40–3.16) |
|
| ECOG PS, n (%) |
| 0.178 |
| 0.999 |
|
0-1 | 38 (62.3) |
| Reference |
|
| 2 | 4 (100.0) |
| 977787404.9
(0.00-NE) |
|
| Histological types,
n (%) |
| >0.999 |
| 0.916 |
|
Adenocarcinoma | 25 (64.1) |
| Reference |
|
|
Others | 17 (65.3) |
| 1.06
(0.37–2.99) |
|
| Pathological stage,
n (%) |
| 0.015 |
| 0.012 |
| Stage
0-I | 6 (37.5) |
| Reference |
|
| Stage
II–IIIB | 36 (73.5) |
| 4.61
(1.40–15.15) |
|
| Operation type, n
(%) |
| 0.049 |
| 0.034 |
|
Lobectomy or
pneumonectomy | 37 (71.2) |
| Reference |
|
| Wedge
resection | 5 (38.5) |
| 0.25
(0.07–0.90) |
|
| Angiolymphatic
invasion, n (%) |
| 0.006 |
| 0.006 |
|
Yes | 33 (76.7) |
| Reference |
|
| No | 9 (40.9) |
| 0.21
(0.07–0.63) |
|
| Perineural
invasion, n (%) |
| 0.109 |
| 0.094 |
|
Yes | 18 (78.3) |
| Reference |
|
| No | 24 (57.1) |
| 0.37
(0.12–1.19) |
|
| Extranodal
invasion, n (%) |
| 0.565 |
| 0.430 |
|
Yes | 13 (72.2) |
| Reference |
|
| No | 29 (61.7) |
| 0.62
(0.19–2.03) |
|
| Spread through air
space, n (%) |
| 0.082 |
|
|
|
Yes | 7 (58.3) |
| Reference |
|
| No | 2 (28.6) |
| 0.29
(0.04–2.11) | 0.220 |
|
Unknown | 33 (71.7) |
| 1.18
(0.49–6.75) | 0.375 |
| Pleural
involvement, n (%) |
| 0.606 |
| 0.493 |
|
Yes | 22 (62.5) |
| Reference |
|
| No | 20 (60.6) |
| 0.70
(0.25–3.89) |
|
| Adjuvant
chemotherapy, n (%) |
| 0.057 |
| 0.039 |
|
Yes | 31 (73.8) |
| Reference |
|
| No | 11 (47.8) |
| 0.33
(0.04–0.95) |
|
| Adjuvant
radiotherapy, n (%) |
| 0.602 |
| 0.526 |
|
Yes | 18 (69.2) |
| Reference |
|
| No | 24 (61.5) |
| 0.71
(0.25–2.04) |
|
| Driver gene
mutation status, n (%) |
| <0.001 |
| 0.004 |
|
Yes | 21 (95.5) |
| Reference |
|
| No or
unknown | 21 (48.8) |
| 0.05
(0.01–0.37) |
|
An increased risk of disease progression was
revealed for patients that underwent lobectomy or pneumonectomy,
compared with patients that underwent wedge resection (71.2 vs.
38.5%, respectively; P=0.049). There was also a numerically higher
risk of disease progression in patients who received adjuvant
chemotherapy compared with individuals who did not (P=0.057). The
risk of disease progression was similar between patients that
received adjuvant radiotherapy and patients that did not
(P=0.602).
There was a significant association between the
tumor stages and patient treatments. Compared with patients with
stage 0-I tumors, an increased number of patients with stage
II–IIIB tumors underwent lobectomy or pneumonectomy (89.8 vs.
50.0%, respectively; P=0.002) and adjuvant chemotherapy (77.6 vs.
25.0%, respectively; P<0.001), which demonstrated the
association of tumor stages on the treatment decision. Furthermore,
stage II–IIIB tumors were associated with an increased risk of
angiolymphatic invasion (81.6 vs. 18.8%, respectively; P<0.001).
However, the rate of positive driver gene mutations was not
significantly different between patients with stage II–IIIB and 0-I
tumors (36.7 vs. 25.5%, respectively; P=0.546).
Survival outcomes and impact of driver
gene mutation status
The impact of driver gene mutation status on PFS and
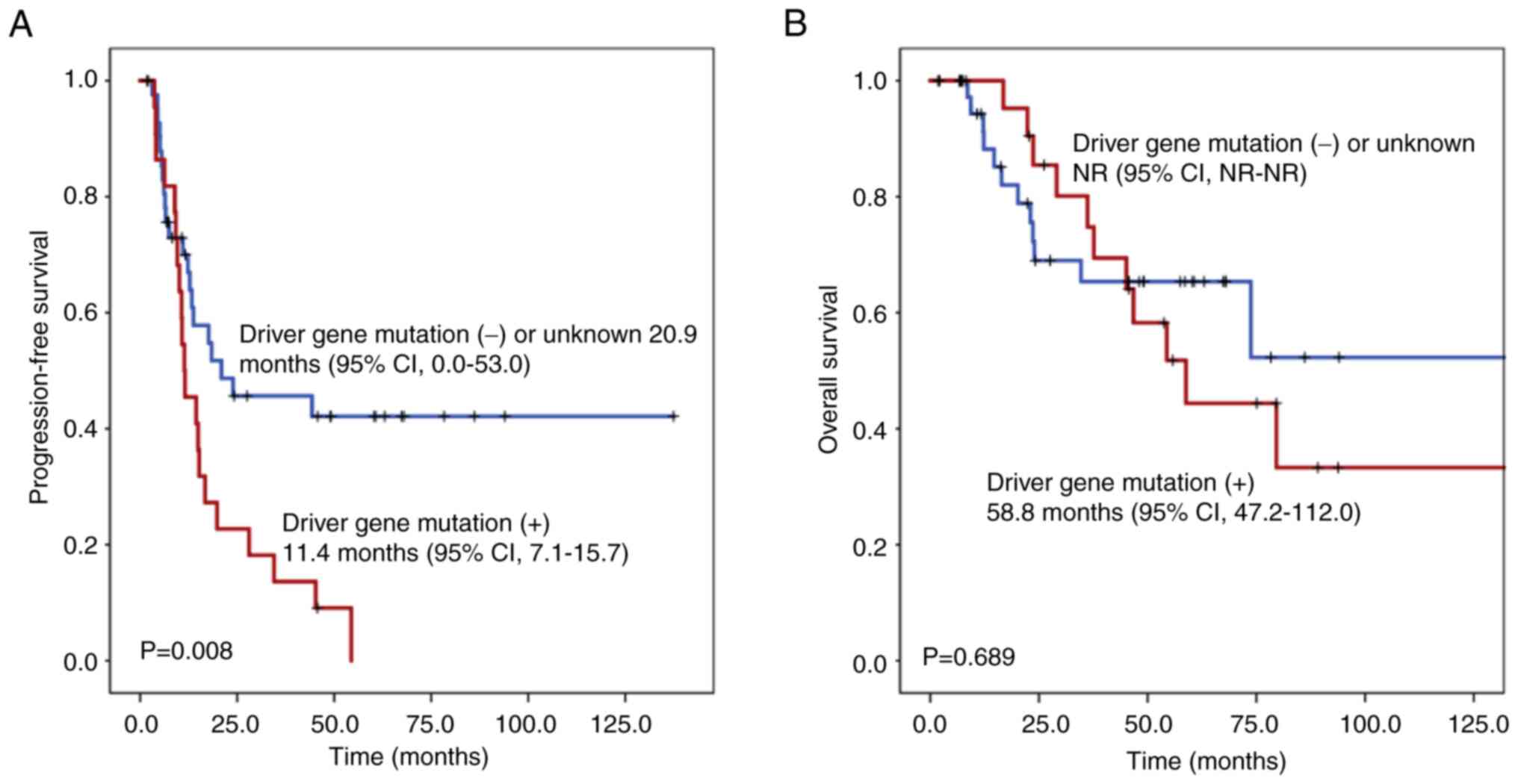
OS of the overall study cohort was assessed (Fig. 1). Patients harboring known driver
gene mutations (11.4 months; 95% CI, 7.1–15.7) were associated with
a significantly shorter PFS compared with patients with negative or
unknown driver gene mutation status (20.9 months; 95% CI, 0.0–53.0;
P=0.008). The OS was not reached for patients with negative or
unknown driver gene mutation status compared with patients with
known driver gene mutations (58.8 months; 95% CI, 47.2–112.0;
P=0.689).
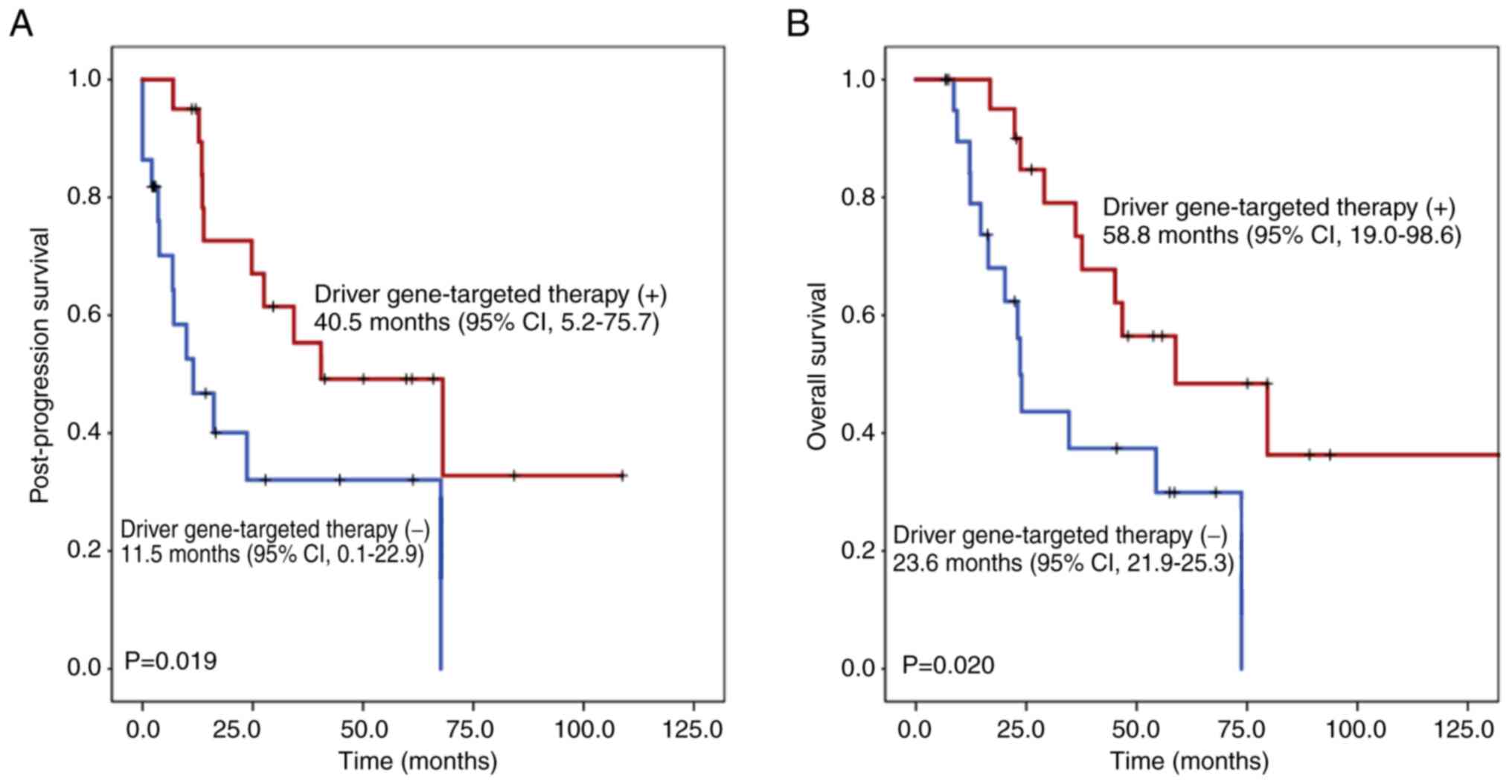
The influence of driver gene-targeted therapy on
post-progression survival and OS of the cohort of patients that had
disease progression was also evaluated (Fig. 2). Patients that received driver
gene-targeted therapy were associated with a significantly longer
post-progression survival (40.5 months; 95% CI, 5.2–75.7) compared
with patients that did not receive driver gene-targeted therapy
(11.5 months; 95% CI, 0.1–22.9; P=0.019). Additionally, patients
that received driver gene-targeted therapy were associated with a
significantly longer OS (58.8 months; 95% CI, 19.0–98.6) compared
with patients that did not receive driver gene-targeted therapy
(23.6 months; 95% CI, 21.9–25.3; P=0.020).
Multivariate analysis of disease
progression and survival outcomes
Results of the multivariate analyses are summarized
in Table IV. Stage II–IIIB tumor
stage was associated with a significantly increased risk of disease
progression (adjusted OR, 4.95; 95% CI, 1.12–22.22; P=0.035). The
presence of a driver gene mutation was also independently
associated with a significantly increased risk of disease
progression (adjusted OR, 24.08; 95% CI, 2.77–209.01; P=0.004).
 | Table IV.Multivariate analysis of disease
progression, progression-free survival and post-progression
survival. |
Table IV.
Multivariate analysis of disease
progression, progression-free survival and post-progression
survival.
| A, Disease
progression |
|---|
|
|---|
| Factor | Adjusted OR/HR | 95% CI |
P-valuea |
|---|
| Stage
II–IIIB vs. 0-I | 4.95 | 1.12–22.22 | 0.035 |
| Driver
gene mutation status, yes vs. no or unknown | 24.08 | 2.77–209.01 | 0.004 |
|
| B,
Progression-free survival |
|
| Factor | Adjusted
OR/HR | 95% CI |
P-valueb |
|
| ECOG PS
2 vs. 0–1 | 3.49 | 1.10–11.03 | 0.033 |
| Stage
II–IIIB vs. 0-I | 2.55 | 1.06–6.17 | 0.037 |
| Driver
gene mutation status, yes vs. no or unknown | 3.28 | 1.55–6.94 | 0.002 |
|
| C,
Post-progression survival |
|
| Factor | Adjusted
OR/HR | 95% CI |
P-valueb |
|
| Driver
gene-targeted therapy, yes vs. no | 0.38 | 0.16–0.91 | 0.030 |
Reduced PFS times were associated with an ECOG PS of
two (adjusted HR, 3.49; 95% CI, 1.10–11.03; P=0.033), stage II–IIIB
tumors (adjusted HR, 2.55; 95% CI, 1.06–6.17; P=0.037) and the
presence of a driver gene mutation (adjusted HR, 3.28; 95% CI,
1.55–6.94; P=0.002).
The patients that underwent driver gene-targeted
therapy due to disease progression were associated with a
significantly longer post-progression survival time (adjusted HR,
0.38; 95% CI, 0.16–0.91; P=0.030).
Discussion
The outcomes for patients with lung cancer have
improved over the past number of decades, which is attributed to
the advances in novel treatments and the early diagnosis of lung
cancer. The current strategy for treatment is personalized therapy,
which particularly benefits patients with advanced stage lung
cancer. In such a scenario, both pathological classification and
biomarker assessment serve important roles in the decision of
treatment regimens (4,22,23).
Low dose computed tomographic screening both increases the
identification of lung cancer cases and reduces mortality due to
lung cancer among high-risk populations (24,25). A
retrospective study from Taiwan also suggests a positive
association between the diagnostic shift from late to early-stage
and improved outcomes for patients with lung cancer (26). For patients diagnosed with
early-stage lung cancer, complete resection is the mainstream form
of therapy as it provides the opportunity to cure this disease.
However, a small proportion of patients may have an incomplete lung
tumor resection, which is hypothesized to both increase the risk of
disease progression and worsen patient outcomes (12). The appropriate treatment of these
patients is currently unclear. The present study revealed the
characteristics and outcomes of a cohort of patients (obtained over
a 10-year period) with early-stage NSCLC and non-R0 resection, and
investigated the importance of driver gene mutation detection and
driver gene-targeted therapy.
The treatment options for patients that have
incomplete resection include re-resection and adjuvant chemotherapy
and radiotherapy (5,14). However, the evidence to prescribe
these treatments is limited as previous studies indicate
inconsistent results (5,14,26).
Osarogiagbon et al (27)
analyzed the National Cancer Database from 2004 to 2011, and reveal
that 4.7% of 112,998 patients with NSCLC had incomplete resection.
Of the 4.7% of patients, adjuvant chemotherapy increases the 5-year
survival rate across all stages, although radiotherapy is
associated with reduced 5-year survival rate in patients diagnosed
with stage I disease. A population-based cohort study carried out
in the Netherlands, including 427 patients with incompletely
resected lung cancer out of a total of 8,528 patients that
underwent surgical treatment between 2015 and 2018, suggests that
adjuvant chemotherapy, but not radiotherapy, may improve OS
(28). By contrast, a study by Park
et al (29) reports that
chemotherapy does not affect the disease progression pattern or
survival time of patients following the incomplete resection of
NSCLC. However, to the best of the authors' knowledge, previous
studies have not analyzed the impact of driver gene mutation status
and the role of targeted therapy.
EGFR mutations and ALK fusions are
prognostic factors of higher disease recurrence rates after surgery
for patients with early-stage lung cancer. A study by Ito et
al (30), including 877
patients with resected lung cancer, evaluated the prognostic impact
of EGFR mutations and suggests that the presence of
EGFR mutations are associated with an increased 5-year
recurrence rate and a decreased 5-year recurrence-free survival,
compared with healthy patients. Additionally, a previous study by
Park et al (31), including
659 patients with resected NSCLC, also reveals that EGFR
mutations are associated with an increased risk of recurrence and
distant metastasis. Similarly, previous studies by Fujibayashi
et al (32) and Shin et
al (33) both suggest a reduced
recurrence-free survival in patients with resected stage IA lung
adenocarcinoma with an ALK fusion. However, all these
previous studies evaluated patients with complete resection;
therefore, this may suggest different outcomes for patients with
non-R0 resection, as these patients do not have a cancer-free
status after surgery. Without prompt treatment, some patients with
EGFR mutations may experience a hyper-progressive disease,
which may lead to a rapid tumor progression and a shorter survival
time (34). In the present study,
EGFR mutations were the most common driver gene mutations
revealed. Following an adjustment for clinicopathological features
of the patients, the presence of driver gene mutations remained an
independent predictor of both an increased risk of disease
progression and a decreased PFS. However, in patients experiencing
disease progression, driver-gene targeted therapy improved
post-progression survival time, which may explain the similar OS
between patients with and without driver gene mutations.
In the present study, patients that received
lobectomy or pneumonectomy, as well as patients that underwent
adjuvant chemotherapy were associated with an increased risk of
disease progression, following incomplete resection of the lung
tumor. However, tumor stage is the most important factor when
determining postoperative treatment (5). Current guidelines suggest adjuvant
chemotherapy for patients with stage II disease or higher, and that
sub-lobar resection may be only suitable for patients with a single
lung tumor that is >2 cm (5,14). A
positive association between the tumor stage and therapeutic
options was aforementioned in the present study; hence, an
increased risk of progression may be attributed to tumor stage, but
not the postoperative treatments. However, there was not a
significant effect of tumor stage on driver gene mutation status,
which supported the independent role of driver gene mutation status
in predicting disease progression.
The Lung Cancer Mutation Consortium study
prospectively enrolled patients with advanced stage lung
adenocarcinoma and assessed 10 driver gene mutations (34). The results demonstrate that patients
harboring driver gene mutations with corresponding targeted therapy
have notably improved outcomes (35). Regarding patients with completely
resected early-stage NSCLC, ADAURA (11) and ALINA (9) clinical trials both suggest adjuvant
osimertinib and alectinib may increase the progression-free
survival time of patients with EGFR mutations and ALK
fusions, respectively (9,11). Currently, early initiation of
molecular testing and biomarker assessment are recommended by
clinical practice guidelines (36).
Although the appropriate treatment for disease progression
following incomplete lung cancer resection has not yet been
established, numerous patients with this condition may require
systemic treatment, for example, chemotherapy. In the present
patient cohort, the assessment of driver gene mutations and the use
of corresponding targeted therapy could improve patient outcomes
and improve the survival time. The data from the present study
suggested that driver gene-targeted therapy was independently
associated with an improved post-progression survival. Therefore,
due to the increased risk of disease progression, early
comprehensive analysis of driver gene mutation status for patients
with incomplete resection of lung cancer may be suggested.
In addition to chemotherapy, immunotherapy and
targeted therapy (in either a neoadjuvant or adjuvant setting) both
improve the outcomes of patients with early-stage resectable NSCLC
(9–11). However, these studies evaluated
patients with complete resection of lung tumors. Theoretically,
immunotherapy and targeted therapy may potentially benefit patients
with non-R0 resection lung cancer. However, prospective studies are
still required in order to establish the appropriate treatment for
these patients in the future.
A major limitation of the present study was that it
was retrospective, which may have led to bias as the operations
included, and the data collected from patients were not planned
ahead of time. With the recent advances in surgical techniques
(37), incomplete resection only
occurs in a small subset of patients (12). The present study involved a cohort
from a 10-year period from in Taichung Veterans General Hospital
(Taichung, Taiwan), with a 3-year follow-up. Although the data were
collected retrospectively, the present study attempted to ensure
the validity of the characteristics, diagnosis as well as treatment
course of each patient, genetic alterations and the outcome
evaluation. Compared with previous studies (27–29),
the importance of driver gene mutation detection and driver
gene-targeted therapy was further investigated. In the era of
precision medicine for lung cancer, sorting patients according to
both their clinicopathological features and results of biomarker
assessment remains important in order to prescribe personalized
treatment, and may also be beneficial for patients with
incompletely resected lung cancer (5). Further studies are needed to
investigate why patients with driver gene mutations have an
increased risk of disease progression and to evaluate whether
targeted therapy as treatment following a non-R0 resection could
improve the outcomes of patients. In addition to the possibility of
bias, it is difficult to establish a cause-and-effect relationship
between the intervention and patient outcome in a retrospective
study. Therefore, future prospective studies using a larger cohort
may provide an improved algorithm in the biomarker assessment and
management of patients with lung cancer and incomplete
resection.
Although the presence of driver gene mutations were
associated with an increased risk of disease progression and a
reduced PFS, patients that had disease progression may benefit from
driver-gene targeted therapy. Therefore, the results of present
study suggested an earlier comprehensive analysis of the driver
gene mutation status for patients with incomplete resection of lung
cancer in order to identify at-risk individuals and apply the
corresponding targeted therapy promptly when they experience
disease progression.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in Mendeley Data at the following URL:
https://data.mendeley.com/datasets/v4jxnb9gt3/1.
Authors' contributions
PYS and JST confirm the authenticity of all the raw
data. JST designed this manuscript. PYS, CYC, CHL, YWH, YHH, KHH,
JST, GCC and TYY conducted the study and analyzed the data. PYS
wrote the manuscript and JST guided the writing. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Taichung Veterans General Hospital (IRB nos.
CF12019 and CF20175). Written informed consent for clinical data
records and genetic testing was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALK
|
anaplastic lymphoma kinase
|
|
BRAF
|
v-raf murine sarcoma viral oncogene
homolog B
|
|
CI
|
confidence interval
|
|
EGFR
|
epidermal growth factor receptor
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
PS
|
performance status
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hazard ratio
|
|
KRAS
|
Kirsten rat sarcoma viral oncogene
homolog
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
ROS1
|
ROS proto-oncogene 1
|
|
STAS
|
spread through air space
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 counteries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang YJ, Huang JY, Lin CH and Wang BY:
Survival and Treatment of Lung Cancer in Taiwan between 2010 and
2016. J Clin Med. 10:46752021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Detterbeck FC: The eighth edition TNM
stage classification for lung cancer: What does it mean on main
street? J Thorac Cardiovasc Surg. 155:356–359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang SR, Schultheis AM, Yu H, Mandelker D,
Ladanyi M and Buttner R: Precision medicine in non-small cell lung
cancer: Current applications and future directions. Semin Cancer
Biol. 84:184–198. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NCCN Clinical Practice Guidelines in
Oncology, . Non-Small Cell Lung Cancer. Version 8.2024. August
23–2024
|
|
6
|
Carnio S, Novello S, Papotti M, Loiacono M
and Scagliotti GV: Prognostic and predictive biomarkers in early
stage non-small cell lung cancer: Tumor based approaches including
gene signatures. Transl Lung Cancer Res. 2:372–381. 2013.PubMed/NCBI
|
|
7
|
NSCLC Meta-analysis Collaborative Group, :
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE Collaborative Group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solomon BJ, Ahn JS, Dziadziuszko R,
Barlesi F, Nishio M, Lee DH, Lee JS, Zhong WZ, Horinouchi H, Mao W,
et al: LBA2 ALINA: Efficacy and safety of adjuvant alectinib versus
chemotherapy in patients with early-stage ALK+ non-small cell lung
cancer (NSCLC). Annals of Oncology. 34:S1295–S1296. 2023.
View Article : Google Scholar
|
|
10
|
Ni Y, Lei J, Huang W, Wang J, Guo H, Lv F,
Kang S, Lan K and Jiang T: Systematic review of the perioperative
immunotherapy in patients with non-small cell lung cancer: evidence
mapping and synthesis. Front Oncol. 13:10926632023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsuboi M, Herbst RS, John T, Kato T, Majem
M, Grohé C, Wang J, Goldman JW, Lu S, Su WC, et al: Overall
survival with osimertinib in resected EGFR-Mutated NSCLC. N Engl J
Med. 389:137–147. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edwards JG, Chansky K, Van Schil P,
Nicholson AG, Boubia S, Brambilla E, Donington J, Galateau-Sallé F,
Hoffmann H, Infante M, et al: The IASLC lung cancer staging
project: analysis of resection margin status and proposals for
residual tumor descriptors for non-small cell lung cancer. J Thorac
Oncol. 15:344–359. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasing MJA, Peters M, Moreno AC, Hofman
EFN, Herder GJM, Welvaart PWN, Schramel FMNH, Lodeweges JE, Lin SH,
Verhoeff JJC and van Rossum PSN: Predicting incomplete resection in
non-small cell lung cancer preoperatively: A validated nomogram.
Ann Thorac Surg. 111:1052–1058. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Remon J, Soria JC and Peters S; ESMO
Guidelines Committee. Electronic address, : Early and locally
advanced non-small-cell lung cancer: An update of the ESMO Clinical
Practice Guidelines focusing on diagnosis, staging, systemic and
local therapy. Ann Oncol. 32:1637–1642. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amin MB, Gress DM and Vega LR: AJCC Cancer
Staging Manual. 8th edition. Springer; Heidelberg: 2018
|
|
16
|
Ou WF, Liao PY, Hsu YW, Huang YH, Hsu KH,
Tseng JS, Chang GC and Yang TY: Outcome of thromboembolic events
and its influence on survival time of advanced NSCLC patients
treated with antiangiogenic therapy. Cancer Manag Res.
15:1251–1262. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu KH, Ho CC, Hsia TC, Tseng JS, Su KY,
Wu MF, Chiu KL, Yang TY, Chen KC, Ooi H, et al: Identification of
five driver gene mutations in patients with treatment-naive lung
adenocarcinoma in Taiwan. PLoS One. 10:e01208522015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su KY, Kao JT, Ho BC, Chen HY, Chang GC,
Ho CC and Yu SL: Implementation and Quality Control of Lung Cancer
EGFR Genetic Testing by MALDI-TOF Mass Spectrometry in Taiwan
Clinical Practice. Sci Rep. 6:309442016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su KY, Chen HY, Li KC, Kuo ML, Yang JC,
Chan WK, Ho BC, Chang GC, Shih JY, Yu SL and Yang PC: Pretreatment
epidermal growth factor receptor (EGFR) T790M mutation predicts
shorter EGFR tyrosine kinase inhibitor response duration in
patients with non-small-cell lung cancer. J Clin Oncol. 30:433–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng JS, Yang TY, Tsai CR, Chen KC, Hsu
KH, Tsai MH, Yu SL, Su KY, Chen JJ and Chang GC: Dynamic plasma
EGFR mutation status as a predictor of EGFR-TKI efficacy in
patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol.
10:603–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mescam-Mancini L, Lantuéjoul S,
Moro-Sibilot D, Rouquette I, Souquet PJ, Audigier-Valette C,
Sabourin JC, Decroisette C, Sakhri L, Brambilla E and McLeer-Florin
A: On the relevance of a testing algorithm for the detection of
ROS1-rearranged lung adenocarcinomas. Lung Cancer. 83:168–173.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Oncogene-addicted metastatic non-small-cell lung cancer: ESMO
Clinical Practice Guideline for diagnosis, treatment and follow-up.
Ann Oncol. 34:339–357. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene-addicted metastatic non-small-cell lung cancer:
ESMO Clinical Practice Guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Koning HJ, van der Aalst CM, de Jong
PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C,
Yousaf-Khan U, Horeweg N, et al: Reduced Lung-Cancer Mortality with
Volume CT Screening in a Randomized Trial. N Engl J Med.
382:503–513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Lung Screening Trial Research
Team, . Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan
F, Fagerstrom RM, Gareen IF, Gierada DS, et al: Results of initial
low-dose computed tomographic screening for lung cancer. N Engl J
Med. 368:1980–1991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang CY, Lin YT, Lin LJ, Chang YH, Chen
HY, Wang YP, Shih JY, Yu CJ and Yang PC: Stage shift improves lung
cancer survival: real-world evidence. J Thorac Oncol. 18:47–56.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osarogiagbon RU, Lin CC, Smeltzer MP and
Jemal A: Prevalence, prognostic implications, and survival
modulators of incompletely resected non-small cell lung cancer in
the U.S. National Cancer Data Base. J Thorac Oncol. 11:e5–e16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rasing MJA, Peters M, Aarts MJ, Herder
GJM, van Lindert ASR, Schramel FMNH, van der Meer FS, Verhoeff JJC
and van Rossum PSN: Adjuvant treatment following irradical
resection of stage I–III Non-small cell lung cancer: A
population-based study. Curr Probl Cancer. 46:1007842022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park J, Song SY, Kim SS, Kim SW, Kim WS,
Park SI, Kim DK, Kim YH, Park J, Lee SW, et al: Postoperative
radiation therapy following the incomplete resection of a non-small
cell lung cancer. Radiat Oncol J. 32:70–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito M, Miyata Y, Kushitani K, Ueda D,
Takeshima Y and Okada M: Distribution and prognostic impact of EGFR
and KRAS mutations according to histological subtype and tumor
invasion status in pTis-3N0M0 lung adenocarcinoma. BMC Cancer.
23:2482023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park HK, Choi YD, Yun JS, Song SY, Na KJ,
Yoon JY, Yoon CS, Oh HJ, Kim YC and Oh IJ: Genetic alterations and
risk factors for recurrence in patients with non-small cell lung
cancer who underwent complete surgical resection. Cancers (Basel).
15:56792023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujibayashi Y, Tane S, Kitazume M, Kuroda
S, Kimura K, Kitamura Y and Nishio W: Resected stage I anaplastic
lymphoma kinase-positive lung adenocarcinoma has a negative impact
on recurrence-free survival. Thorac Cancer. 13:1109–1116. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin SH, Lee H, Jeong BH, Choi YS, Shin
MH, Kim S, Han J, Lee KS, Shim YM, Kwon OJ and Kim H: Anaplastic
lymphoma kinase rearrangement in surgically resected stage IA lung
adenocarcinoma. J Thorac Dis. 10:3460–3467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kato S, Goodman A, Walavalkar V,
Barkauskas DA, Sharabi A and Kurzrock R: Hyperprogressors after
immunotherapy: Analysis of genomic alterations associated with
accelerated growth rate. Clin Cancer Res. 23:4242–4250. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kris MG, Johnson BE, Berry LD, Kwiatkowski
DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson
SL, Su PF, et al: Using multiplexed assays of oncogenic drivers in
lung cancers to select targeted drugs. JAMA. 311:1998–2006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Expert Consensus Panel, . Kidane B, Bott
M, Spicer J, Backhus L, Chaft J, Chudgar N, Colson Y, D'Amico TA,
David E, et al: The American Association for Thoracic Surgery
(AATS) 2023 Expert Consensus Document: Staging and
multidisciplinary management of patients with early-stage non-small
cell lung cancer. J Thorac Cardiovasc Surg. 166:637–654. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Udelsman BV and Blasberg JD: Advances in
Surgical Techniques for Lung Cancer. Hematol Oncol Clin North Am.
37:489–497. 2023. View Article : Google Scholar : PubMed/NCBI
|