Introduction
Ovarian cancer is one of the leading causes of
mortality among women with gynecological malignancies worldwide,
primarily due to its asymptomatic nature in the early stages, which
typically leads to a late diagnosis (1). The introduction of targeted therapies
represents a major breakthrough in the treatment of ovarian cancer,
as it has the potential to improve the survival rates and quality
of life of patients (2). Among
these targeted therapies, angiogenesis inhibitors and poly
(ADP-ribose) polymerase inhibitors (PARPi) have played a leading
role, targeting specific molecular pathways implicated in cancer
progression and DNA repair, respectively (3,4).
Different angiogenesis inhibitors and PARPi have been utilized due
to their distinct mechanisms of action and varying effectiveness in
targeting specific pathways involved in ovarian cancer progression
(5). For example, anti-vascular
endothelial growth factor (VEGF) agents such as bevacizumab
specifically target the VEGF pathway and inhibit the formation of
new blood vessels essential for tumor growth, while tyrosine kinase
inhibitors (TKIs) such as anlotinib offer a broader inhibition of
angiogenesis by targeting multiple receptors (6). These differences may account for the
variability in clinical outcomes observed for combinations with
PARPi, which impair the DNA repair mechanisms of cancer cells.
Understanding these mechanisms provides a rationale for the
selection of particular combinations based on the unique clinical
and molecular context of each patient.
Angiogenesis plays a pivotal role in tumor growth
and metastasis, as it facilitates the supply of nutrients and
oxygen to the tumor (7).
Anti-angiogenic therapies, particularly those targeting the VEGF
pathway, have shown promise in the inhibition of tumor
vascularization (8). Conversely,
PARPi exploit the concept of synthetic lethality to selectively
kill cancer cells with homologous recombination deficiencies by
further impairing their DNA repair capabilities (9). This approach has been demonstrated to
be particularly effective in BRCA-mutated ovarian cancer, in which
the DNA repair mechanisms are already compromised (10).
Mutations of the BRCA1 and BRCA2 genes are known to
impair homologous recombination, a critical DNA repair pathway.
Patients with these mutations are more susceptible to DNA damage,
making them particularly responsive to PARPi therapy, which further
disrupts the already weakened DNA repair mechanisms. Therefore, the
presence of BRCA mutations has become an important predictive
biomarker for the efficacy of PARPi, which can be used to guide
personalized treatment approaches in ovarian cancer (11).
Recent research has posited the potential for
synergistic effects when angiogenesis inhibitors and PARPi are
combined. The rationale is that inhibiting angiogenesis while
simultaneously blocking DNA repair pathways may lead to enhanced
tumor regression (12). However,
the evidence to date has been mixed, with a significant
heterogeneity in outcomes across studies, which has prompted the
suggestion that a more comprehensive analysis is necessary to
obtain an improved understanding of the efficacy and safety of
these combination therapies (13).
Patient-specific factors, such as BRCA mutation
status, have emerged as crucial determinants of the response to
these treatments (14). A recent
systematic review and meta-analysis by Wei et al (15) focused on the overall efficacy and
safety of PARP inhibitors combined with antiangiogenic agents in
ovarian cancer and analyzed the general effects of such
combinations. By contrast, the aim of the present study was to
investigate the efficacy of different types of antiangiogenic
agents when used in combination with PARP inhibitors. Furthermore,
the impact of BRCA mutation status on the effectiveness of these
combined treatments were explored. By addressing these two aspects,
the present study provides a more comprehensive and personalized
understanding of how the type of antiangiogenic agent and the BRCA
mutation status both influence treatment outcomes in ovarian
cancer. Through this approach, it is hoped to obtain a granular
understanding of treatment efficacy and safety profiles, ultimately
guiding clinical decision-making and contributing to personalized
medicine strategies.
Materials and methods
Literature inclusion and exclusion
criteria
Inclusion criteria
The inclusion criteria were as follows: i) Study
design: Randomized controlled trials (RCTs) and single-arm trials
on the combined treatment of ovarian cancer with anti-angiogenic
agents combined with PARPi. The language was limited to English.
ii) Study subjects: Patients with a confirmed diagnosis of ovarian
cancer were included without restrictions on race or age. iii)
Interventions: In the control group, if included, the patients
received monotherapy with anti-angiogenic agents or PARPi, and in
the experimental group, patients received combination therapy using
anti-angiogenic agents and PARPi. iv) Outcome measures: The
objective response rate (ORR), disease control rate (DCR), median
progression-free survival (mPFS) and incidence of adverse events
(grade >3) were assessed.
Exclusion criteria
The exclusion criteria were as follows: i) Case
reports, review articles and duplicates of previously published
studies. ii) Studies on animals and basic research. iii) Literature
not meeting the inclusion criteria. iv) Studies with flawed
research designs or treatment measures unrelated to the experiment.
v) Literature without valid information and data.
Literature search
The literature search was conducted using Embase
(https://www.embase.com/), PubMed (https://pubmed.ncbi.nlm.nih.gov/) and The
Cochrane Library (https://www.cochranelibrary.com/) databases. The
publication dates searched for were from database inception until
February 2024. The search terms were a combination of MeSH terms
and entry terms. The search terms included: (((((((((((‘ovarian
neoplasms’[Mesh]) OR (ovarian neoplasm [Title/Abstract])) OR (ovary
neoplasms[Title/Abstract])) OR (ovary neoplasm [Title/Abstract]))
OR (ovary cancer[Title/Abstract])) OR (ovary
cancers[Title/Abstract])) OR (ovarian cancer [Title/Abstract])) OR
(ovarian cancers[Title/Abstract])) OR (cancer of
ovary[Title/Abstract])) OR (cancer of the ovary[Title/Abstract]))
AND ((((niraparib[Title/Abstract]) OR (olaparib[Title/Abstract]))
OR (veliparib[Title/Abstract])) OR (rucaparib[Title/Abstract])))
AND (((((anlotinib [Title/Abstract]) OR
(cediranib[Title/Abstract])) OR (bevacizumab [Title/Abstract])) OR
(sorafenib[Title/Abstract])) OR (apatinib[Title/Abstract])).
Data extraction
Two researchers independently conducted the
screening and data extraction based on the inclusion and exclusion
criteria. In case of discrepancies between the two researchers,
disagreements were resolved through discussion or, if needed, with
the judgement of the third researcher.
Literature quality assessment
Two independent researchers used the Methodological
Index for Non-Randomized Studies (MINORS) (16) to evaluate the quality of evidence
for each study. This index includes 12 items, each with a mean
score of 0–2, giving a maximum total of 24 points. The studies are
categorized by score as ‘moderate quality’, defined as a score of
9–16, and ‘high quality’, defined as a score of 17–24. For RCTs,
Review manager 5.3 software risk assessment tool was used to
evaluate the included literature according to random sequence
generation, allocation concealment, blinding, whether research
results were blinded to review, completeness of outcome data,
selection of reported research outcomes and other biases. The
meta-analysis was performed in accordance with the guidelines of
the Preferred Reporting Items for Systematic Reviews and
Meta-analysis statement (17).
Data synthesis and statistical
analysis
Data were analyzed using STATA 15.1 (StataCorp LLC)
(18). The forest plots generated
in this analysis visually represent the summary of individual study
results, including key parameters such as the relative risk (RR),
hazard ratio (HR) and effect size (ES), and the associated 95%
confidence interval (95% CI). I2 was used to evaluate
heterogeneity. If the test yielded P<0.1 and
I2>50%, significant heterogeneity was indicated. If
heterogeneity was indicated, whether any specific study was the
source of the heterogeneity was identified using sensitivity
analysis. In the sensitivity analysis, every trial was excluded
individually and a combined analysis of the remaining trials was
performed. Additionally, the contribution of each study to the
pooled result was indicated by its %weight, which reflects the
influence of the study based on its sample size and variability.
Studies with larger sample sizes or lower variability provide a
greater contribution to the overall estimate; thus, they contribute
more heavily to the meta-analysis. A random-effects model was used
for the pooling effect in all meta-analyses. Publication bias was
analyzed using funnel plots and Egger's test, with P>0.05
indicating no publication bias.
Results
Literature search results
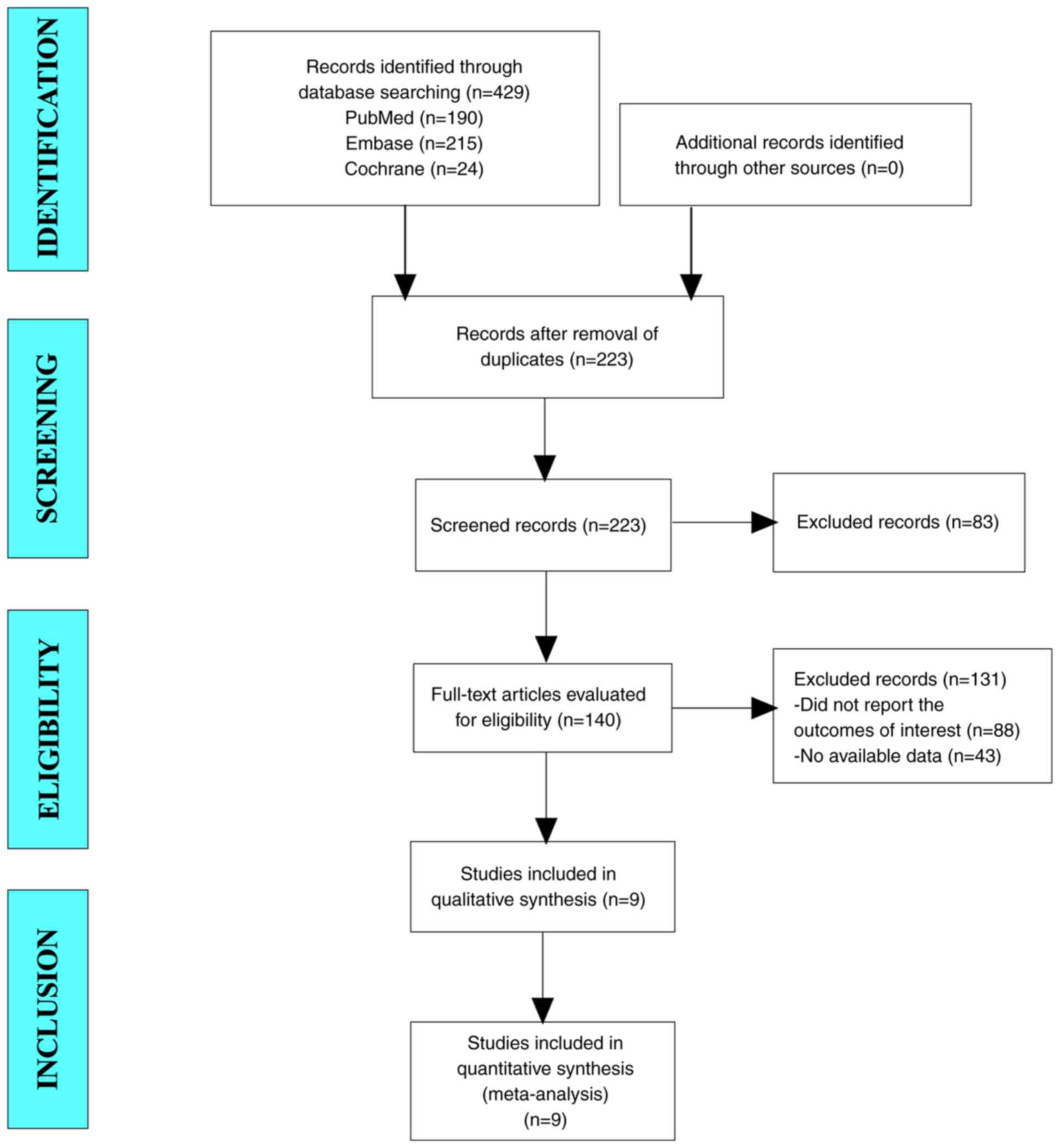
A total of 429 articles were initially identified
for potential inclusion in the present study. After the exclusion
of duplicate studies, 223 articles remained. After reading the
titles and abstracts, a total of 140 articles were selected for
further evaluation. Ultimately, 9 studies were integrated into the
meta-analysis (Fig. 1) (19–27).
Baseline characteristics and quality
assessment of the included studies
The 9 studies included in the study were conducted
in various countries, namely the USA, Korea, Canada, China and
Germany. They comprised 5 single-arm trials and 4 RCTs, with sample
sizes ranging from 16 to 537 participants in the combination
therapy groups. The interventions investigated were cediranib plus
olaparib, anlotinib plus niraparib, and bevacizumab plus niraparib
or olaparib, which reflect a diverse approach to the targeting of
ovarian cancer. Age data, where available, indicate the
participants ranged broadly from their 30 to 80s. However, 5 of the
studies omitted specific data on BRCA mutation status, suggesting a
gap in the genetic characterization of study populations. Quality
assessment scores calculated using the MINORS criteria indicate
that the included studies are of moderate to high quality, with
scores between 17 and 19 (Table
I).
 | Table I.Baseline characteristics and quality
assessment of the included studies. |
Table I.
Baseline characteristics and quality
assessment of the included studies.
| First author,
year | NCT registration
no. | Country | Study design | Sample size | Age, years, median
(range) | Treatments | BRCA mutation,
status n with/n without | MINORS score | (Refs.) |
|---|
| Lee et al,
2022 | NCT02889900 | USA | Single-arm | 60 | 64.5 (42–80) | Cediranib +
olaparib | - | 19 | (19) |
| Lee et al,
2022 | NCT03699449 | Korea | Single-arm | 16 | 58.0 (47–76) | Cediranib +
olaparib | - | 18 | (20) |
| Lheureux et
al, 2020 | NCT02681237 | Canada | Single-arm | 34 | - | Cediranib +
olaparib | - | 17 | (21) |
| Liu et al,
2022 | NCT02446600 | USA | RCT | 189a/189b | - | Cediranib +
olaparib/olaparib | 45/144a, 45/144b | - | (22) |
| Liu et al,
2022 | NCT04376073 | China | Single-arm | 40 | 54.0 (37–69) | Anlotinib +
niraparib | 5/35 | 18 | (23) |
| Liu et al,
2014 | NCT01116648 | USA | RCT | 44a/46b | 57.8
(41.9–85.6)a/ 58.1
(32.7–81.9)b | Cediranib +
olaparib/plaparib | 23/21a, 24/22b | - | (24) |
| Mirza et al,
2019 | NCT02354131 | USA | RCT | 48a/49b | 67
(59–70)a/66
(58–70)b | Bevacizumab +
niraparib/niraparib | 15/33a, 18/31b | - | (25) |
| Hardesty et
al, 2022 | NCT03326193 | USA | Single-arm | 105 | 60.0 (54–67) | Bevacizumab +
niraparib | - | 18 | (26) |
| Ray-Coquard et
al, 2023 | NCT02477644 | Germany | RCT | 537a/269b | 61
(32–87)a/60
(26–85)b | Bevacizumab +
olaparib/bevacizumab | - | - | (27) |
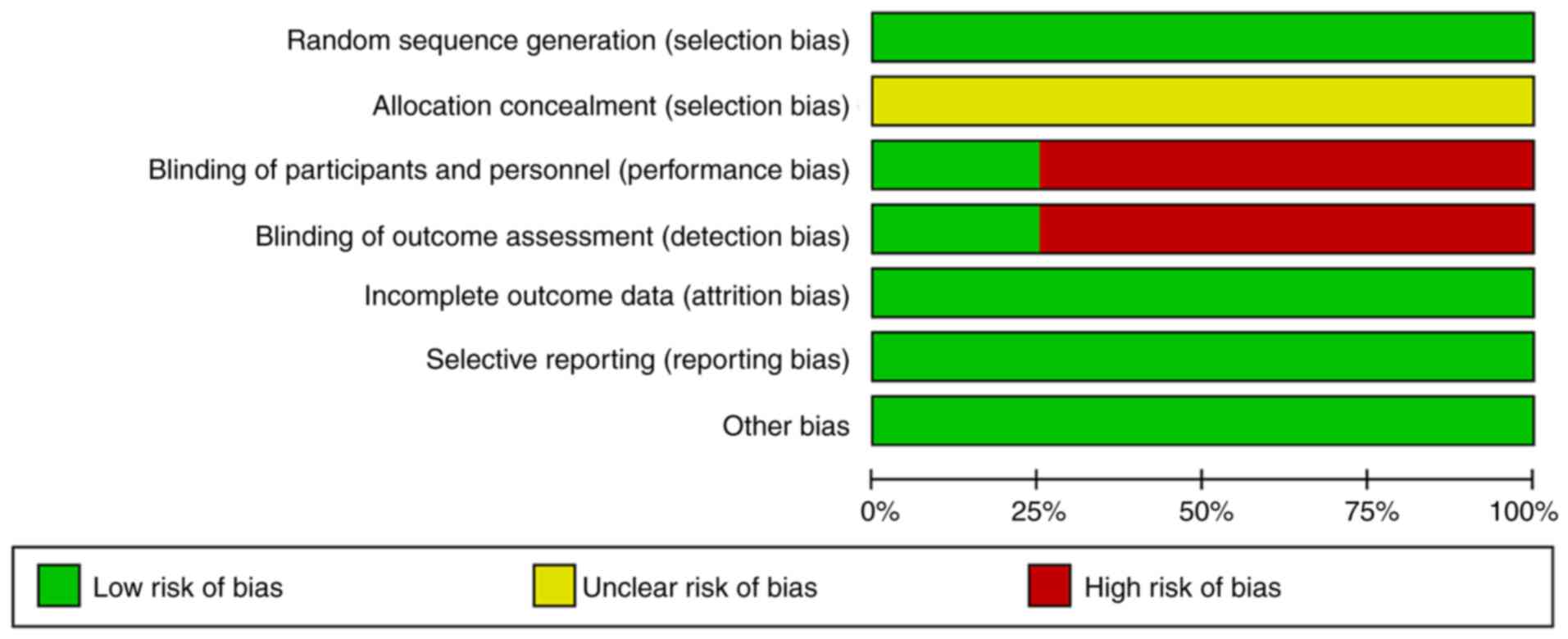
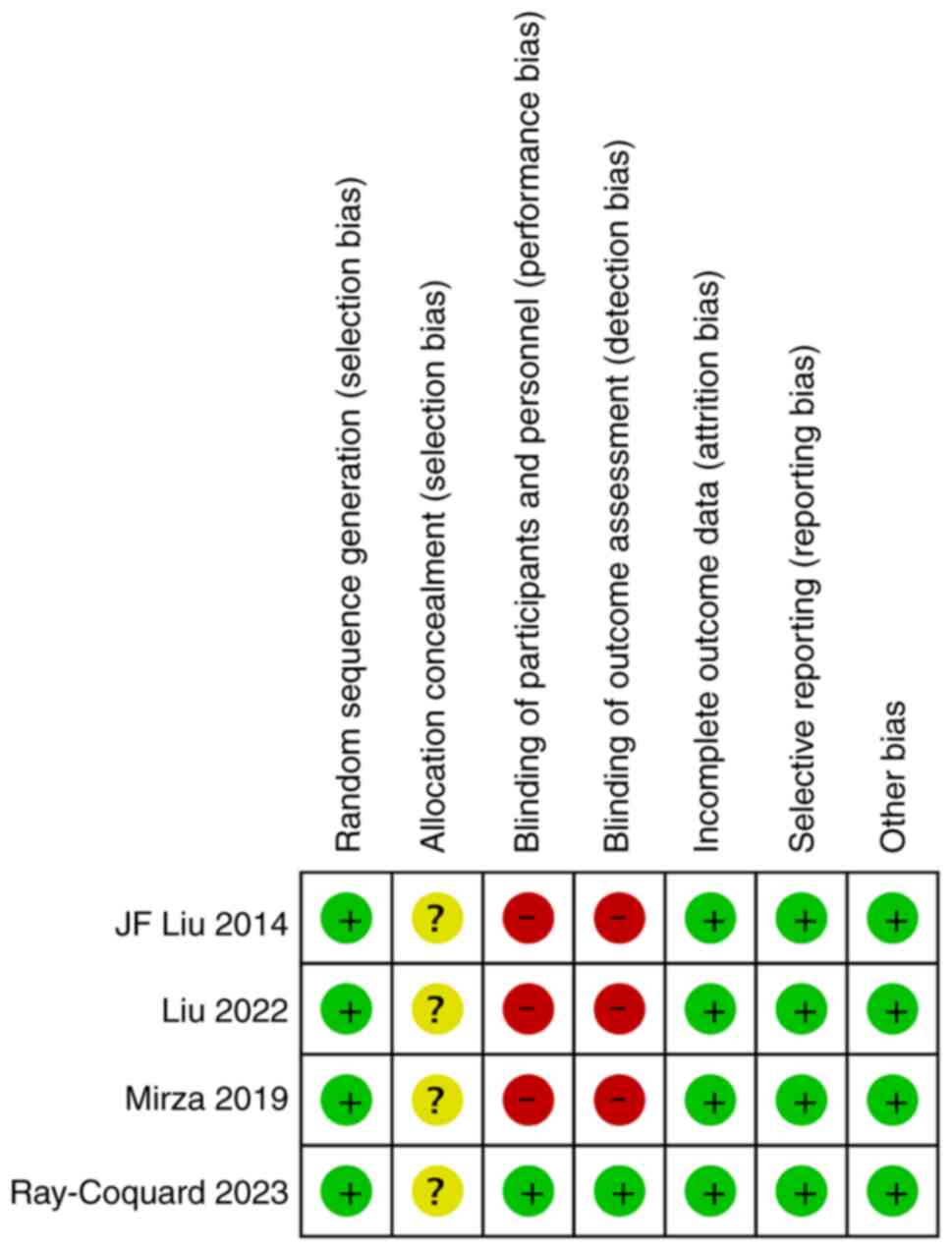
The findings of quality assessment showed that the 4
RCT studies included in this review used random sequence
generation. However, none of the studies described allocation
concealment, and only one study used a double-blinding method
(Figs. 2 and 3).
Meta-analysis results
ORR
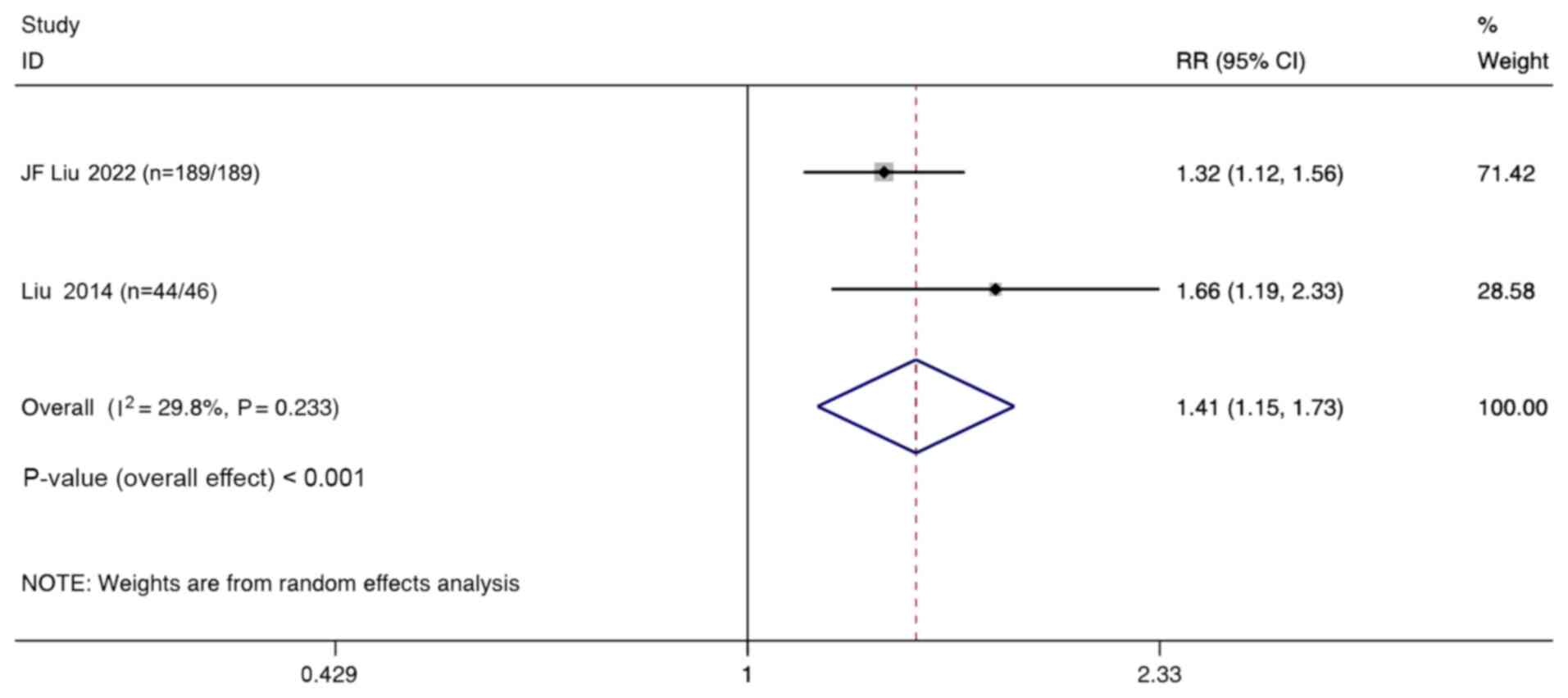
Two studies compared the ORR of combination therapy
with monotherapy in the treatment of ovarian cancer. Meta-analysis
of the aggregated results using a random-effect model indicates
that the ORR in the combination therapy group was significantly
higher than that in the monotherapy group (RR, 1.41; 95% CI,
1.15–1.73; P<0.001; Fig. 4).
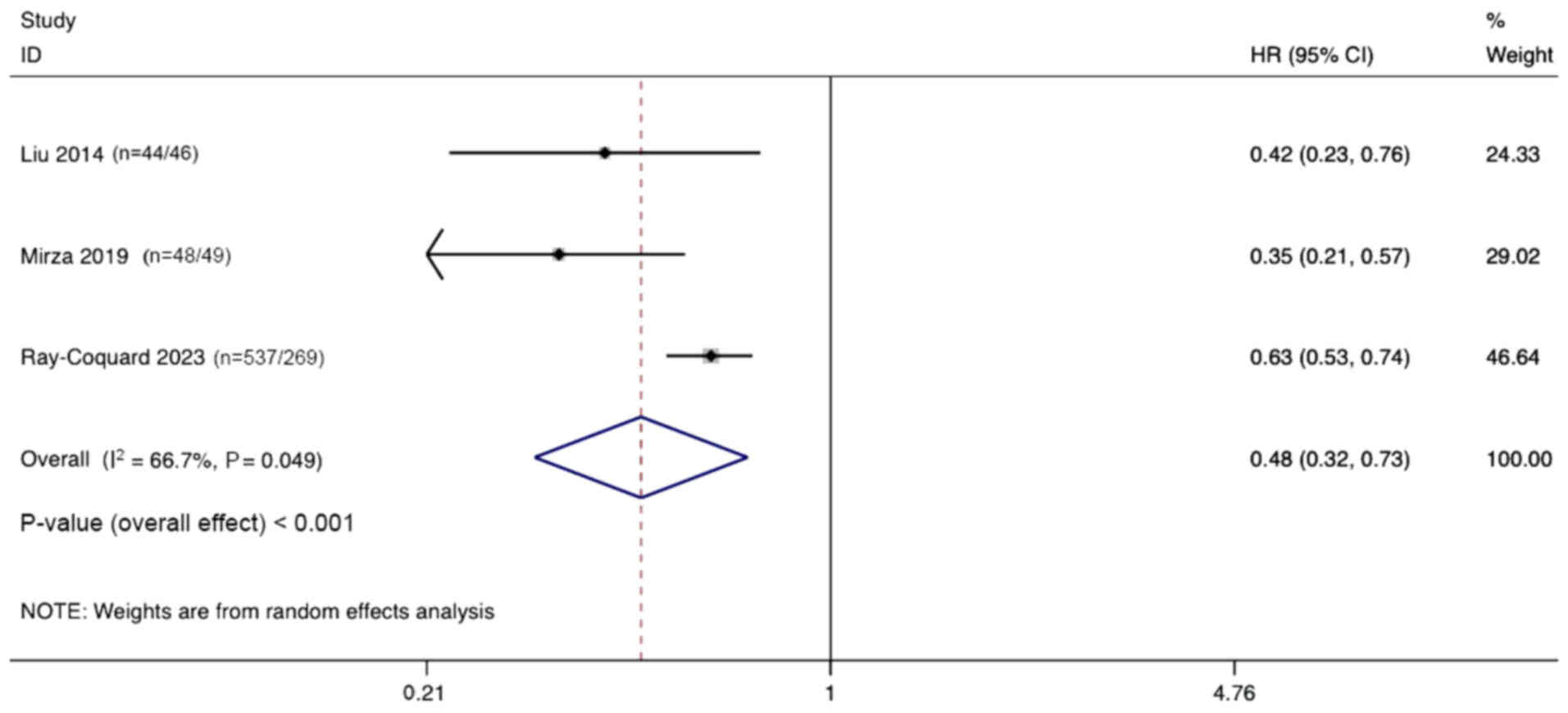
HR of PFS
Three studies documented the differences in PFS
between combination therapy and monotherapy in the treatment of
ovarian cancer. Significant heterogeneity was observed
(I2, 66.7%, P=0.049), and a random effects model was
used. The pooled results indicate that combination therapy
significantly prolongs PFS for ovarian cancer treatment compared
with monotherapy (HR, 0.48; 95% CI, 0.32–0.73; P<0.001; Fig. 5).
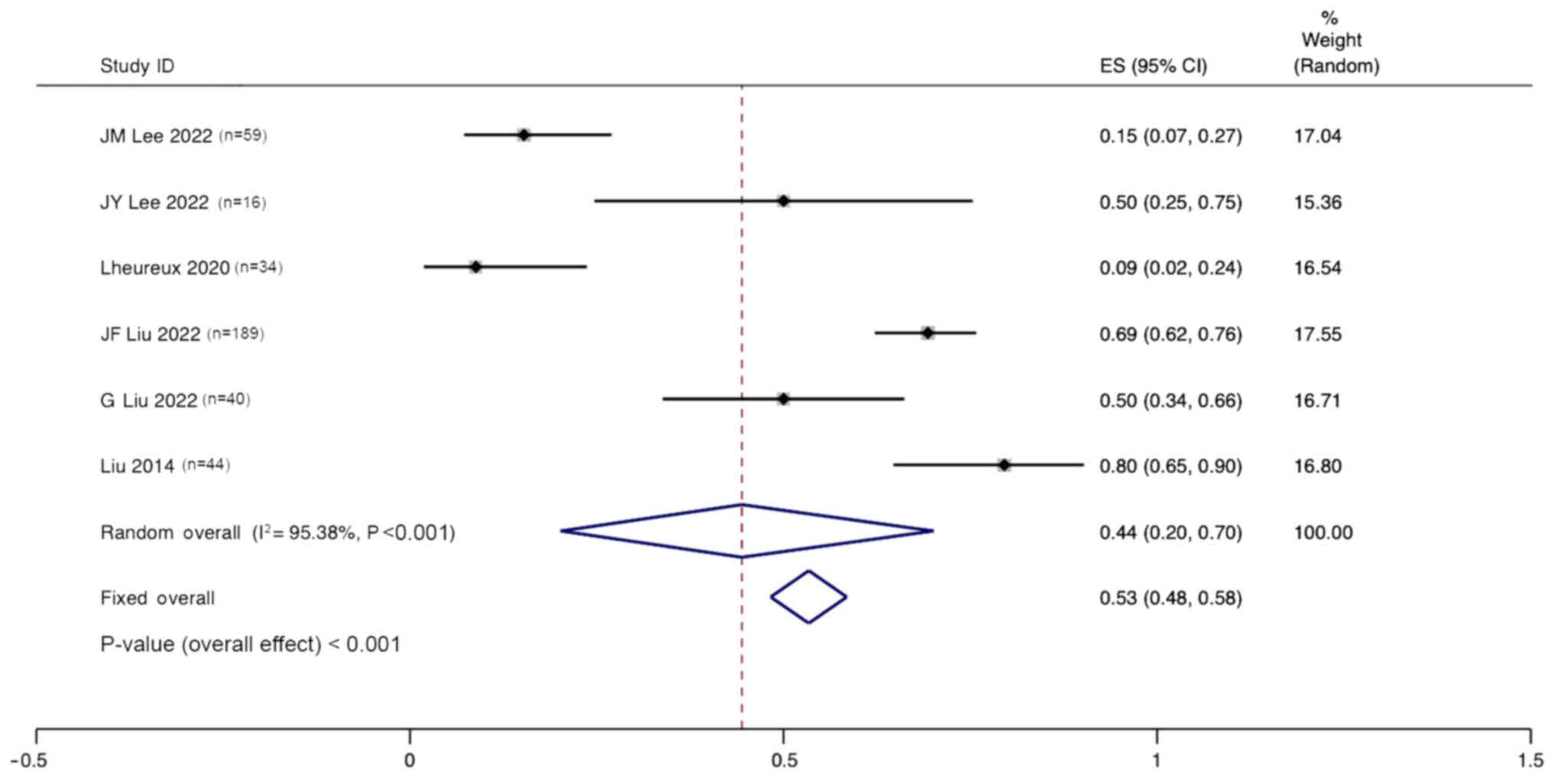
ORR of combination therapy
Six studies reported the ORR for the treatment of
ovarian cancer with angiogenesis inhibitors combined with PARPi.
Significant heterogeneity was observed (I2, 95.38%;
P<0.001), and a random effects model was used. The pooled
results indicate that the ORR for the treatment of ovarian cancer
with anti-angiogenic agents combined with PARPi was 44% (95% CI,
20–70%; Fig. 6).
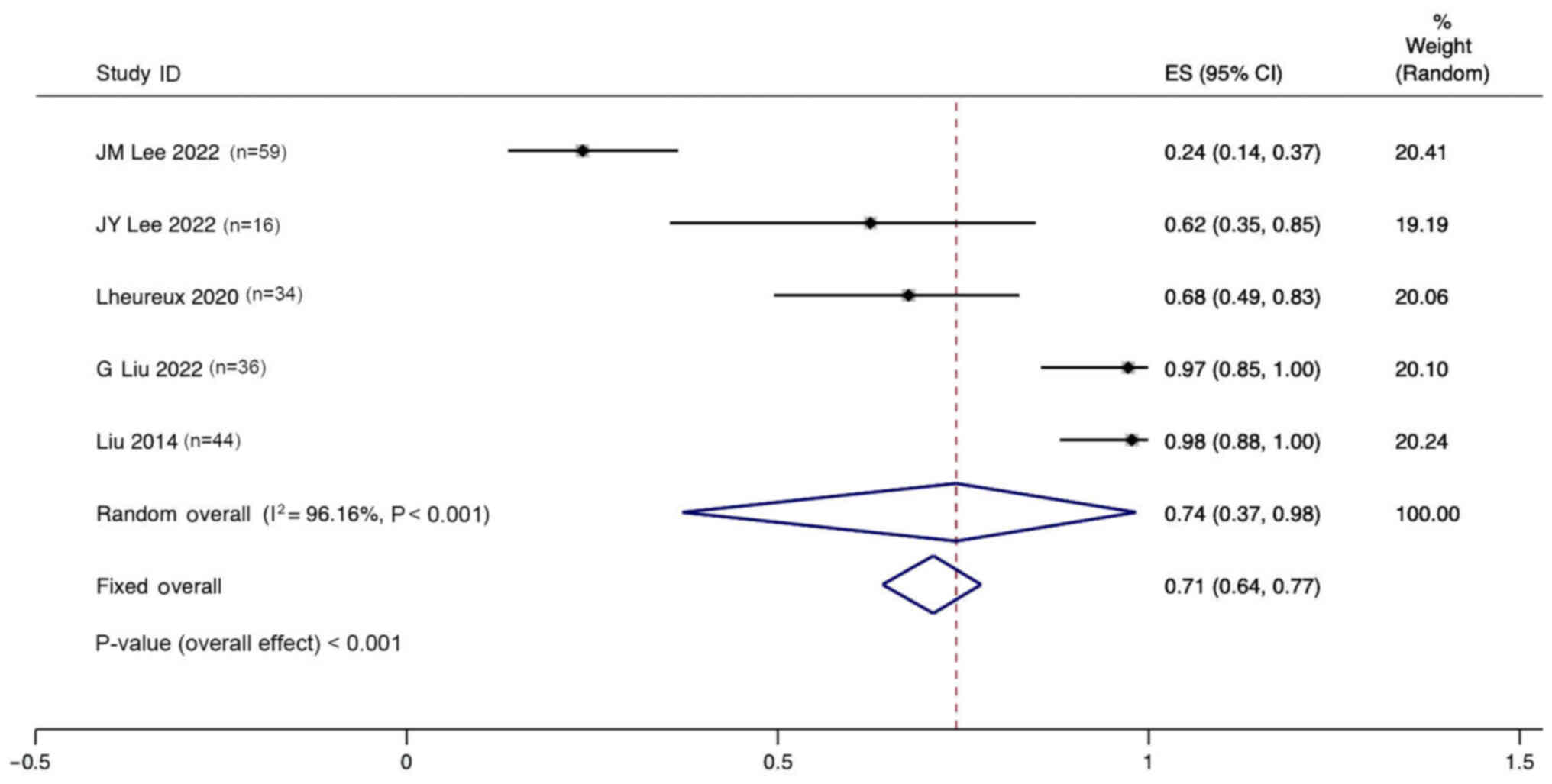
DCR of combination therapy
Five studies reported the DCR for the treatment of
ovarian cancer with angiogenesis inhibitors combined with PARPi.
Significant heterogeneity was observed (I2, 96.16%;
P<0.001), and a random effects model was used. The pooled
results indicate that the DCR for the treatment of ovarian cancer
with anti-angiogenic agents combined with PARPi was 74% (95% CI,
37–98%; Fig. 7).
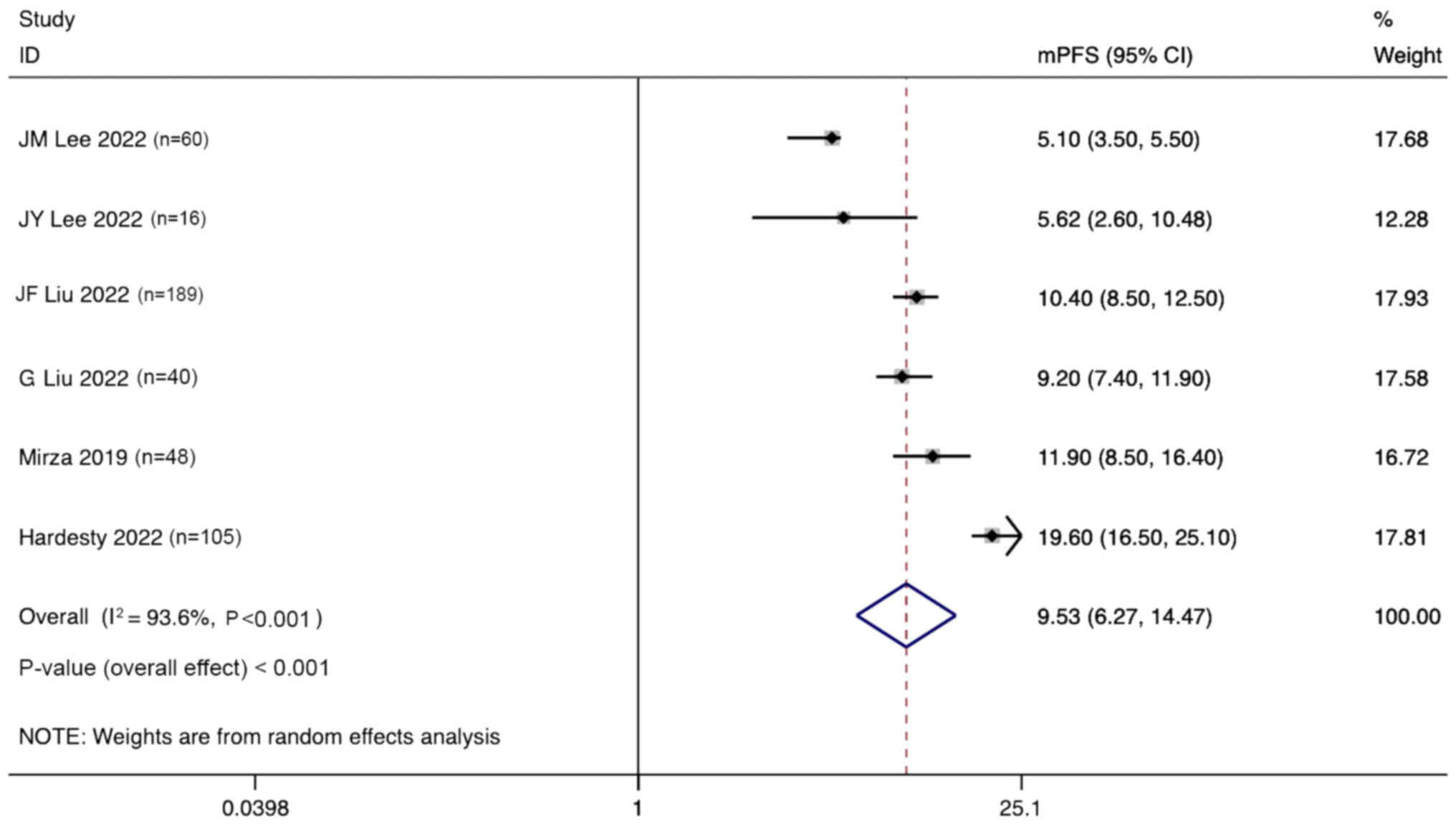
mPFS
Six studies reported the mPFS for the treatment of
ovarian cancer with angiogenesis inhibitors combined with PARPi.
Significant heterogeneity was observed (I2, 93.6%;
P<0.001), and a random effects model was used. The pooled
results indicate that the mPFS for the treatment of ovarian cancer
with anti-angiogenic agents combined with PARPi was 9.53 months
(95% CI, 6.27–14.47 months; Fig.
8).
Incidence of adverse events (grade
>3)
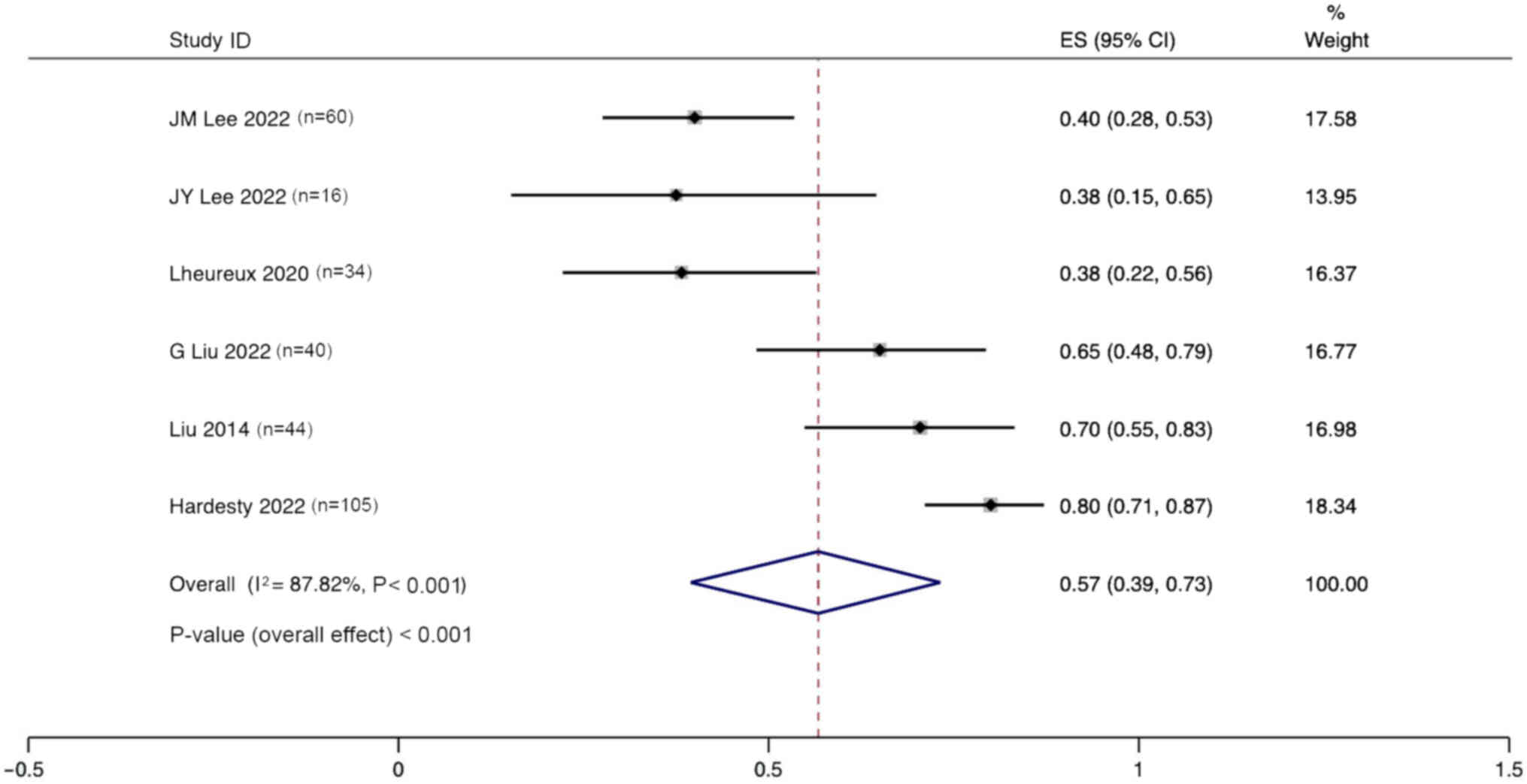
Six studies reported the incidence of adverse events
(grade >3) after the treatment of ovarian cancer with
angiogenesis inhibitors combined with PARPi. Significant
heterogeneity was observed (I2, 87.42%; P<0.001), and
a random effects model was used. The pooled results indicate that
the incidence of adverse events after the treatment of ovarian
cancer with anti-angiogenic agents combined with PARPi was 57% (95%
CI, 39–73%; Fig. 9).
Subgroup analyses
ORR of combination therapy
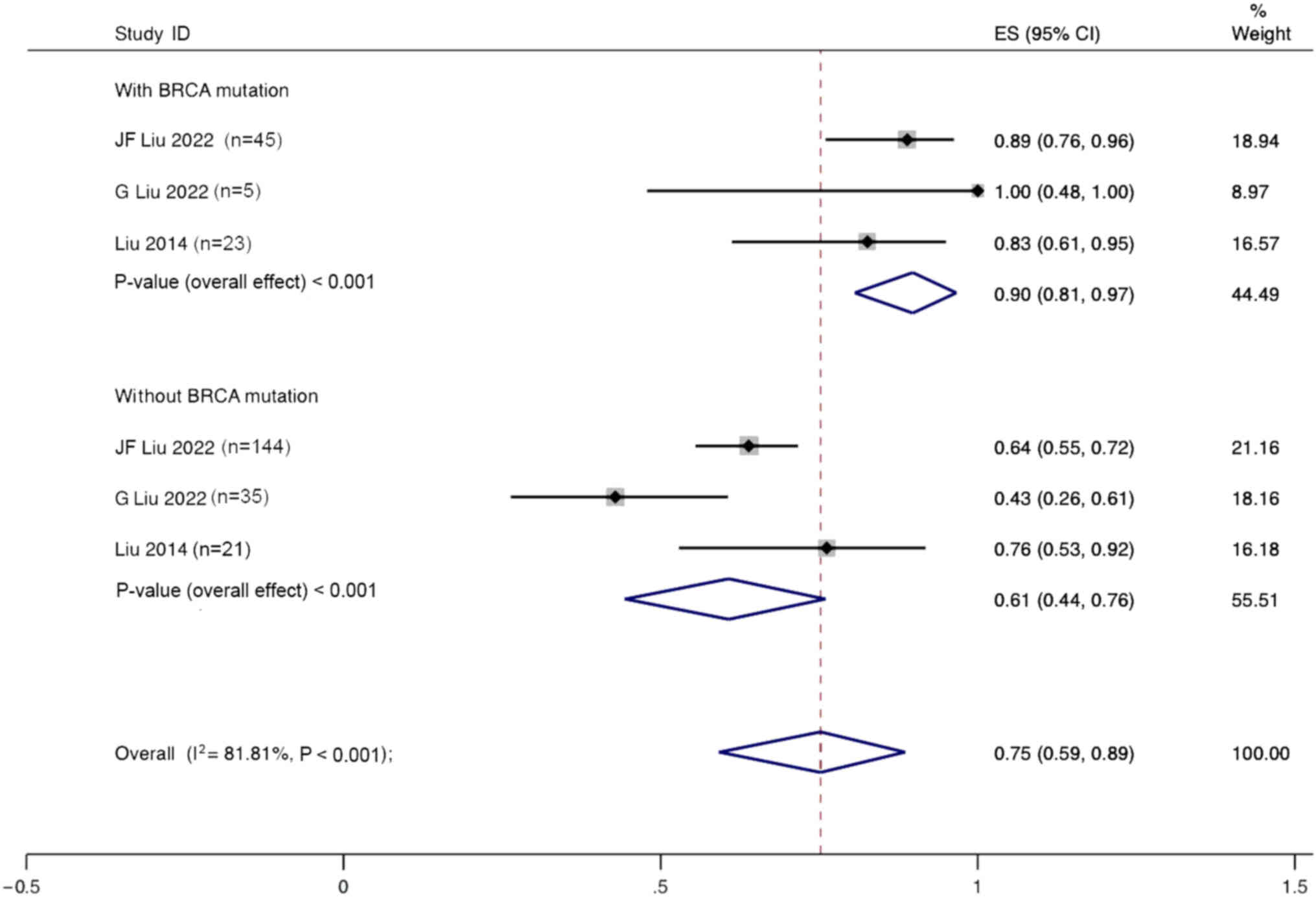
Subgroup analysis based on BRCA mutation status
using a random effects model showed that the ORR for patients with
ovarian cancer and BRCA mutations treated with angiogenesis
inhibitors combined with PARPi was 90% (95% CI, 81–97%), whereas
the ORR for patients without BRCA mutations was only 61% (95% CI,
44–76%) (Fig. 10).
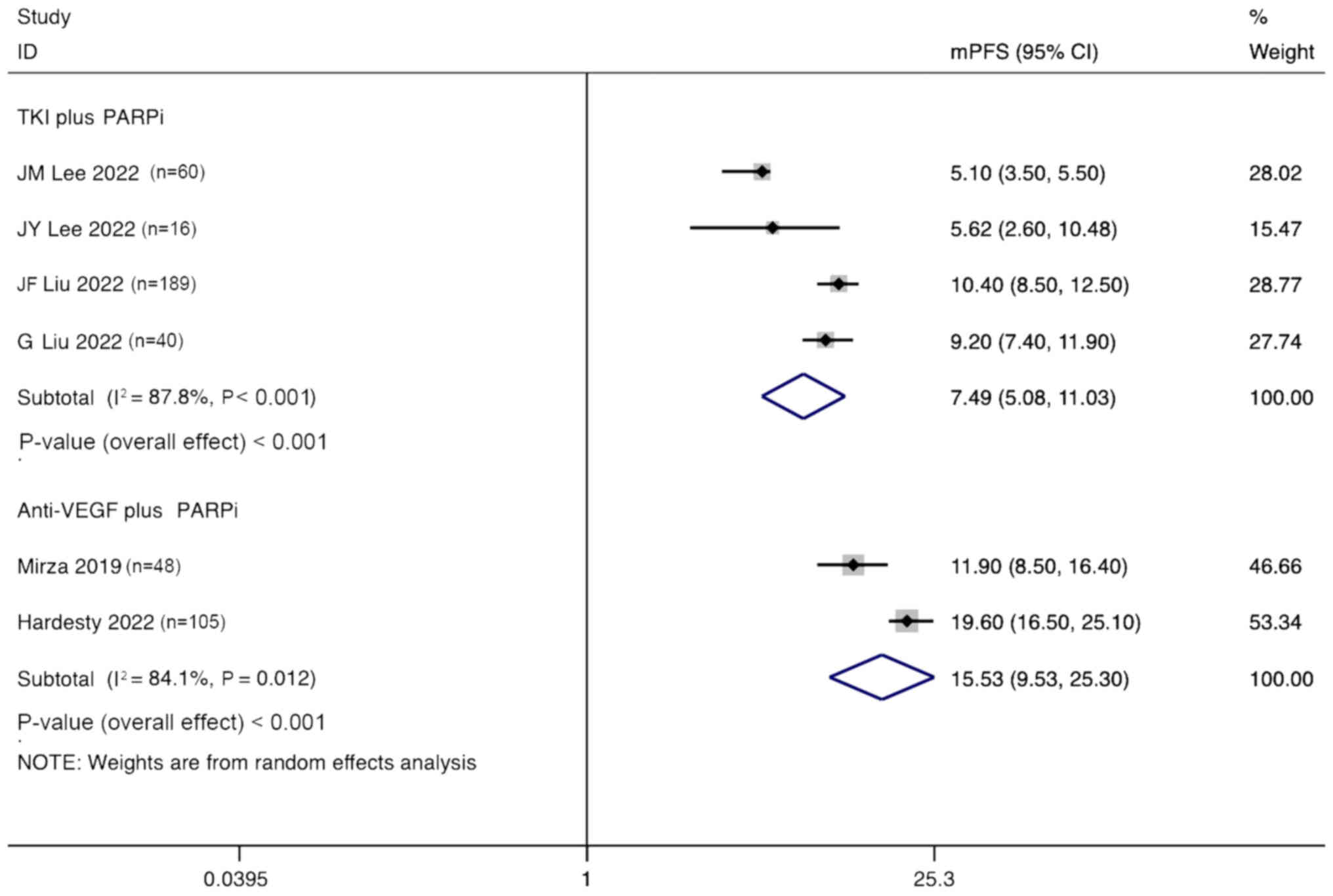
mPFS
A random effects model was used to perform a
subgroup analysis, based on the type of angiogenesis inhibitor used
for the combination therapy of patients with ovarian cancer. The
analysis showed that the mPFS for patients treated with TKIs
combined with PARPi was 7.49 months (95% CI, 5.08–11.03 months),
while the mPFS for patients treated with anti-VEGF agents combined
with PARPi was 15.53 months (95% CI, 9.53–25.30 months) (Fig. 11).
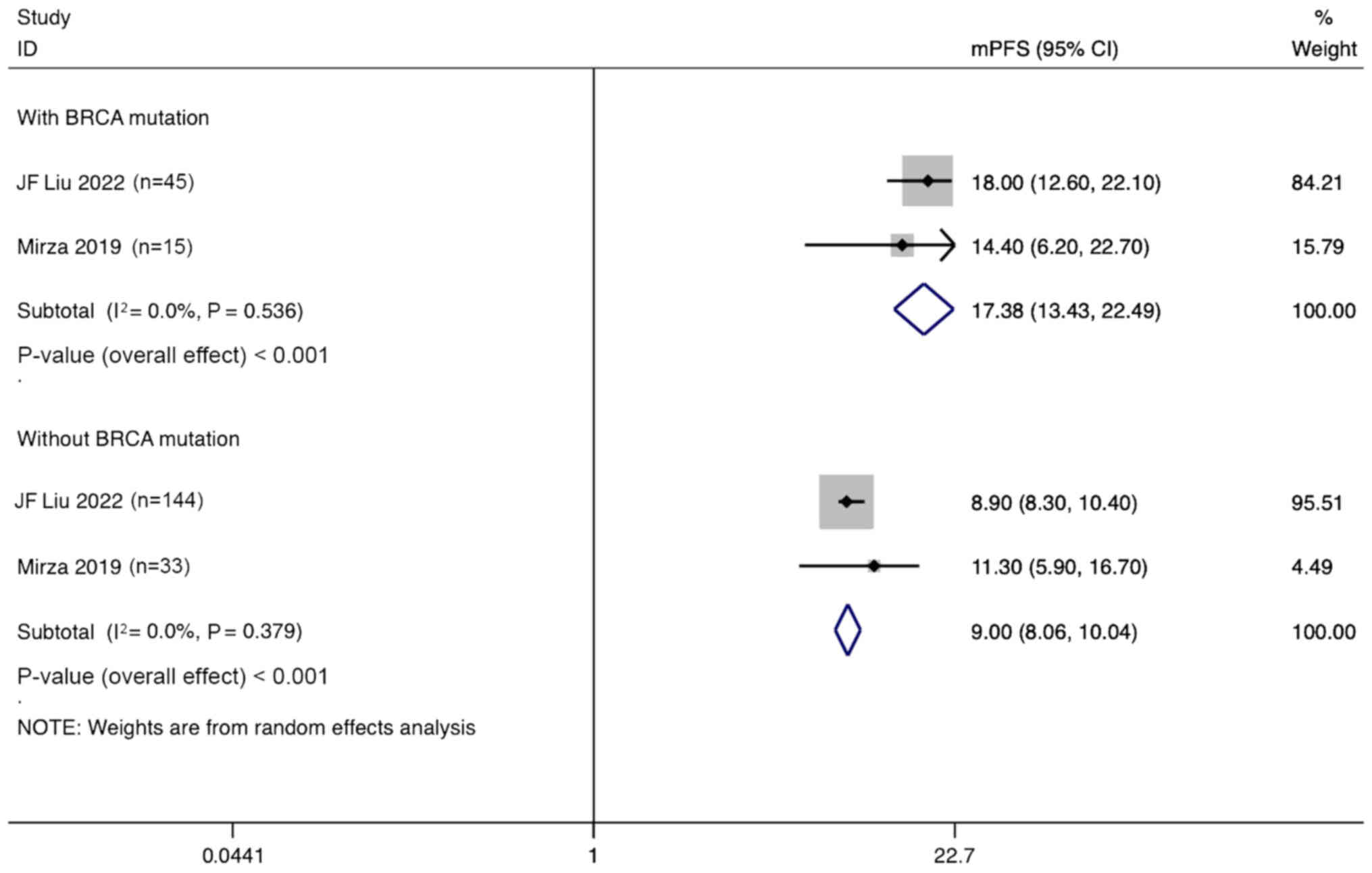
Additionally, a subgroup analysis based on the
presence of BRCA mutations showed that ovarian cancer patients with
BRCA mutations treated with angiogenesis inhibitors combined with
PARPi had a mPFS of 17.38 months (95% CI, 13.43–22.49 months). By
contrast, the mPFS for patients without BRCA mutations was only
9.00 months (95% CI, 8.06–10.04 months) (Fig. 12).
Sensitivity analysis
The sensitivity analysis indicated that no study had
a great influence on the results, suggesting that the results of
the present study are reliable and stable (Fig. S1, Fig.
S2, Fig. S3, Fig. S4, Fig.
S5).
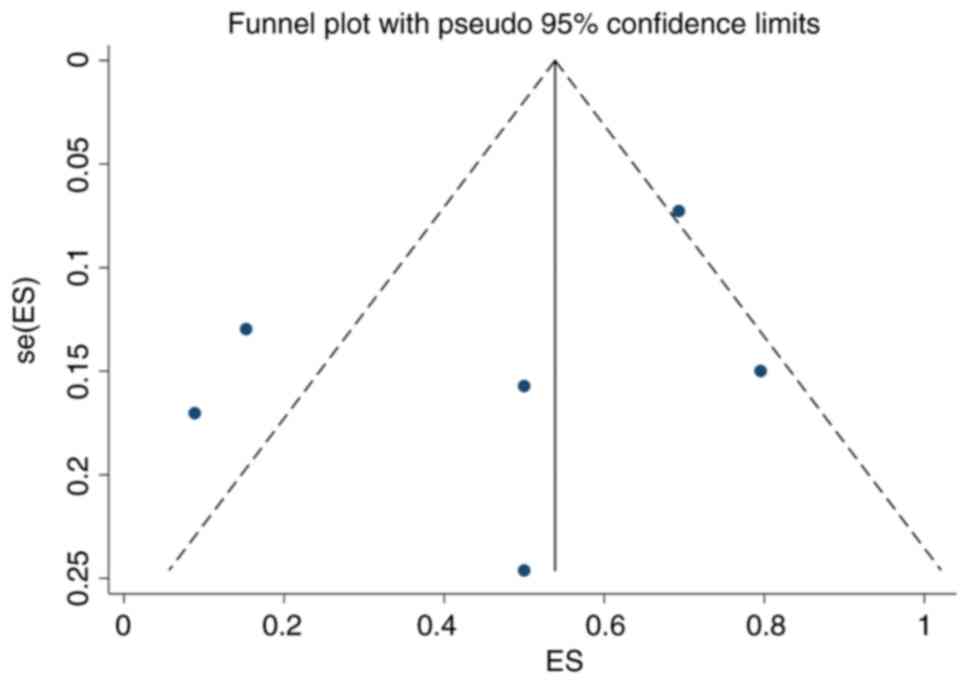
Publication bias
A funnel plot was constructed for the present
meta-analysis (Fig. 13). It is
largely symmetrical, with P=0.330 from Egger's test, indicating
that there was no significant publication bias in the present
meta-analysis.
Discussion
The present meta-analysis provides a critical
evaluation of the efficacy and safety of different angiogenesis
inhibitors combined with PARPi in the treatment of ovarian cancer.
The results demonstrate a significant improvement in ORR and mPFS
when combination therapy is employed compared with monotherapy.
Notably, the subgroup analysis revealed distinct differences in
efficacy between TKIs and anti-VEGF agents when combined with
PARPi, highlighting the complexity involved in the optimization of
combination therapy for patients with ovarian cancer.
Combination therapy had a pooled ORR of 44%, and
induced a marked improvement in mPFS, underscoring the therapeutic
potential of this strategy. Such findings align with previous
research suggesting that the simultaneous inhibition of
angiogenesis and DNA repair pathways can synergistically hinder
tumor growth (28,29). In present study, the superior
performance of anti-VEGF agents over TKIs in the treatment of
ovarian cancer, as indicated by a mPFS of 15.53 months compared
with 7.49 months, respectively, suggests that the specificity of
anti-VEGF agents in targeting the VEGF pathway may confer a more
potent anti-angiogenic effect, thereby enhancing the effectiveness
of PARPi. Moreover, the pronounced benefit in ORR and mPFS observed
in patients with BRCA mutations compared with those without BRCA
mutations when treated with anti-VEGF agents and PARPi emphasizes
the importance of genetic profiling in tailoring treatment
strategies. This supports the notion that individuals with inherent
DNA repair deficiencies are more susceptible to treatments
targeting DNA repair mechanisms, a concept that is gaining traction
in personalized oncology (30). The
clinical translation of these insights necessitates a nuanced
approach, underpinned by the principles of precision medicine. The
differential treatment outcomes observed indicate that it is
imperative to adopt a more individualized approach to treatment
planning. This entails leveraging the comprehensive genetic and
molecular profiling of tumors to discern patient-specific
vulnerabilities that these combination therapies can exploit
(31). For instance, the distinct
advantage observed in patients with BRCA mutations when treated
with this combination therapy underscores the importance of genetic
markers in predicting therapeutic success (32). This stratification not only aids in
the identification of candidates likely to derive the most benefit
but also in the tailoring of treatment regimens to mitigate
potential adverse effects, thus optimizing patient outcomes.
Moreover, the variance in efficacy between different classes of
angiogenesis inhibitors when used in conjunction with PARPi
suggests a pivotal area for future clinical research. It beckons
the design of clinical trials aimed at elucidating the underlying
mechanisms of this differential response, which could, in turn,
inform the development of more effective combination strategies
(33). Such endeavors would not
only contribute to the refinement of current treatment paradigms
but also assist in the identification of novel therapeutic targets
within these pathways.
Despite the promising findings of the present
meta-analysis, the marked heterogeneity observed across the studies
underscores the complexity of ovarian cancer treatment. This
variability highlights that personalized approaches, involving the
consideration of genetic and molecular tumor profiles, are
necessary to optimize therapeutic outcomes. Furthermore, the 57%
incidence of adverse events (grade >3) in patients treated with
combination therapy indicates that cautious patient management is
necessary and underscores the necessity for ongoing research into
the mitigation of treatment-related toxicities.
The present study has certain limitations, Firstly,
there is some heterogeneity among the studies, which might be
attributed to the different PARPi used. Future studies are
necessary in which more unified research designs and more
consistent patient cohorts are implemented. Additionally, most of
the included studies focused on short-term treatment effects, such
as the ORR and mPFS, and data on long-term or OS rates, quality of
life and follow-up after treatment are lacking. Therefore, our
understanding of the long-term benefits of these treatment
strategies remains limited. Another limitation of the present study
is the relatively small sample size, as only a small number of
studies met the rigorous inclusion criteria from an initial pool of
140 articles. This may affect the generalizability and consistency
of the findings. Despite this, meta-analytical techniques and
sensitivity analyses were employed to ensure the robustness of the
results, although the small sample size remains a
consideration.
In conclusion, the present study highlights the
significant therapeutic potential of combining angiogenesis
inhibitors with PARPi in the treatment of ovarian cancer. The
findings suggest that this combination therapy can offer enhanced
efficacy, particularly in patients with BRCA mutations, who are
more likely to benefit from the combined effects of these agents.
The comparative analysis between TKIs and anti-VEGF agents reveals
distinct differences in their effectiveness, emphasizing the
importance of selecting the appropriate angiogenesis inhibitor
based on the molecular and genetic characteristics of the
tumor.
The results underscore the critical requirement for
personalized treatment strategies that leverage comprehensive
genetic and molecular profiling to optimize therapeutic outcomes.
While the combination of angiogenesis inhibitors and PARPi shows
promise, further research is necessary to fully understand the
long-term benefits and potential risks of such combinations,
particularly regarding OS. Future studies should also explore the
development of biomarkers to better predict patient response and
refine treatment protocols, which should ultimately advance the
field of ovarian cancer therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XH and JL made substantial contributions to
conception and design. XH wrote the manuscript and JL created the
figures. LG participated in the acquisition of data. XH and LG
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dexter JM, Brubaker LA, Bitler BG, Goff
BA, Menon U, Moore KN, Sundaram KM, Walsh CS, Guntupalli SR and
Behbakht K: Ovarian cancer think tank: An overview of the current
status of ovarian cancer screening and recommendations for future
directions. Gynecol Oncol Rep. 53:1013762024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson EM, Eskander RN and Binder PS:
Recent therapeutic advances in gynecologic oncology: A review.
Cancers (Basel). 16:7702024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirschl N, Leveque W, Granitto J, Sammarco
V, Fontillas M and Penson RT: PARP inhibitors: Strategic use and
optimal management in ovarian cancer. Cancers (Basel). 16:9322024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldlust IS, Guidice E and Lee JM: PARP
inhibitors in ovarian cancer. Semin Oncol. 51:45–57. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Min T, Lee SH and Lee S: Angiogenesis and
apoptosis: Data comparison of similar microenvironments in the
corpus luteum and tumors. Animals (Basel). 14:11182024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Q and Zhang YH: Flavonoids with
anti-angiogenesis function in cancer. Molecules. 29:15702024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ratnaparkhi R, Javellana M, Jewell A and
Spoozak L: Evaluation of homologous recombination deficiency in
ovarian cancer. Curr Treat Options Oncol. 25:237–260. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collet L, Hanvic B, Turinetto M, Treilleux
I, Chopin N, Le Saux I and Ray-Coquard I: BRCA1/2 alterations and
reversion mutations in the area of PARP inhibitors in high grade
ovarian cancer: State of the art and forthcoming challenges. Front
Oncol. 14:13544272024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dellino M, D'Amato A, Battista G, Cormio
G, Vimercati A, Loizzi V, Lagana AS, Damiani GR, Favilli A, Gerli
S, et al: Reproductive outcomes in women with BRCA 1/2 germline
mutations: A retrospective observational study and literature
review. Open Med (Wars). 19:202499992024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hockings H and Miller RE: The role of PARP
inhibitor combination therapy in ovarian cancer. Ther Adv Med
Oncol. 15:175883592311731832023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi XF, Gao RL, Sun L, Wu ZX, Zhang SL,
Huang LT, Han CB and Ma JT: Dual antitumor immunomodulatory effects
of PARP inhibitor on the tumor microenvironment: A counterbalance
between anti-tumor and pro-tumor. Biomed Pharmacother.
163:1147702023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szentmartoni G, Muhl D, Csanda R, Szasz
AM, Herold Z and Dank M: Predictive value and therapeutic
significance of somatic BRCA mutation in solid tumors.
Biomedicines. 12:5932024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, He L, Liu T, Guo T, Xie C, Jia J,
Lin Y, Liu J and Fan J: Efficacy and safety of PARP inhibitors
combined with antiangiogenic agents in the maintenance treatment of
ovarian cancer: A systematic review and meta-analysis with trial
sequential analysis of randomized controlled trials. Front
Pharmacol. 15:13720772024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernardo WM: PRISMA statement and
PROSPERO. Int Braz J Urol. 43:383–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo LL, Cheng TP, Feng LX, Feng J and Li
XY: Incidence and risk factors for deep infection after primary
shoulder arthroplasty: A meta-analysis. Eur Rev Med Pharmacol Sci.
26:4606–4613. 2022.PubMed/NCBI
|
|
19
|
Lee JM, Moore RG, Ghamande S, Park MS,
Diaz JP, Chapman J, Kendrick J, Slomovitz BM, Tewari KS, Lowe ES,
et al: Cediranib in combination with olaparib in patients without a
germline BRCA1/2 mutation and with recurrent platinum-resistant
ovarian cancer: Phase IIb CONCERTO trial. Clin Cancer Res.
28:4186–4193. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JY, Kim BG, Kim JW, Lee JB, Park E,
Joung JG, Kim S, Choi CH and Kim HS; Korean Gynecologic Oncology
Group (KGOG) investigators, : Biomarker-guided targeted therapy in
platinum-resistant ovarian cancer (AMBITION; KGOG 3045): A
multicentre, open-label, five-arm, uncontrolled, umbrella trial. J
Gynecol Oncol. 33:e452022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lheureux S, Oaknin A, Garg S, Bruce JP,
Madariaga A, Dhani NC, Bowering V, White J, Accardi S, Tan Q, et
al: EVOLVE: A multicenter open-label single-arm clinical and
translational phase II trial of cediranib plus olaparib for ovarian
cancer after PARP inhibition progression. Clin Cancer Res.
26:4206–4215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu JF, Brady MF, Matulonis UA, Miller A,
Kohn EC, Swisher EM, Cella D, Tew WP, Cloven NG, Muller CY, et al:
Olaparib with or without cediranib versus platinum-based
chemotherapy in recurrent platinum-sensitive ovarian cancer
(NRG-GY004): A randomized, open-label, phase III trial. J Clin
Oncol. 40:2138–2147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu G, Feng Y, Li J, Deng T, Yin A, Yan L,
Zheng M, Xiong Y, Li J, Huang Y, et al: A novel combination of
niraparib and anlotinib in platinum-resistant ovarian cancer:
Efficacy and safety results from the phase II, multi-center ANNIE
study. EClinicalMedicine. 54:1017672022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu JF, Barry WT, Birrer M, Lee JM,
Buckanovich RJ, Fleming GF, Rimel B, Buss MK, Nattam S, Hurteau J,
et al: Combination cediranib and olaparib versus olaparib alone for
women with recurrent platinum-sensitive ovarian cancer: A
randomised phase 2 study. Lancet Oncol. 15:1207–1214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mirza MR, Lundqvist EA, Birrer MJ, dePont
Christensen R, Nyvang GB, Malander S, Anttila M, Werner TL, Lund B,
Lindahl G, et al: Niraparib plus bevacizumab versus niraparib alone
for platinum-sensitive recurrent ovarian cancer
(NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority
trial. Lancet Oncol. 20:1409–1419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardesty MM, Krivak TC, Wright GS,
Hamilton E, Fleming EL, Belotte J, Keeton EK, Wang P, Gupta D,
Clements A, et al: OVARIO phase II trial of combination niraparib
plus bevacizumab maintenance therapy in advanced ovarian cancer
following first-line platinum-based chemotherapy with bevacizumab.
Gynecol Oncol. 166:219–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ray-Coquard I, Leary A, Pignata S, Cropet
C, González-Martín A, Marth C, Nagao S, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab first-line maintenance
in ovarian cancer: final overall survival results from the
PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 34:681–692. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ge Y, Wang Q, Yao Y, Xin Q, Sun J, Chen W,
Lin Y and Cai X: Framework nucleic acids-based VEGF signaling
activating system for angiogenesis: A dual stimulation strategy.
Adv Sci (Weinh). 11:e23087012024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan Y, Zheng L, Zhang S and Qian B:
Abnormal expression of FOXM1 in carcinogenesis of renal cell
carcinoma: From experimental findings to clinical applications.
Biochem Biophys Res Commun. 692:1492512024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saadi S, Nacer NE, Saari N, Mohammed AS
and Anwar F: The underlying mechanism of nuclear and mitochondrial
DNA damages in triggering cancer incidences: Insights into
proteomic and genomic sciences. J Biotechnol. 383:1–12. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuroda T and Kohno T: Precision medicine
for ovarian clear cell carcinoma based on gene alterations. Int J
Clin Oncol. 25:419–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boussios S, Rassy E, Moschetta M, Ghose A,
Adeleke S, Sanchez E, Sheriff M, Chargari C and Pavlidis N: BRCA
mutations in ovarian and prostate cancer: Bench to bedside. Cancers
(Basel). 14:38882022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozols RF: Future directions in the
treatment of ovarian cancer. Semin Oncol. 29:32–42. 2002.
View Article : Google Scholar : PubMed/NCBI
|