|
1
|
Dexter JM, Brubaker LA, Bitler BG, Goff
BA, Menon U, Moore KN, Sundaram KM, Walsh CS, Guntupalli SR and
Behbakht K: Ovarian cancer think tank: An overview of the current
status of ovarian cancer screening and recommendations for future
directions. Gynecol Oncol Rep. 53:1013762024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson EM, Eskander RN and Binder PS:
Recent therapeutic advances in gynecologic oncology: A review.
Cancers (Basel). 16:7702024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirschl N, Leveque W, Granitto J, Sammarco
V, Fontillas M and Penson RT: PARP inhibitors: Strategic use and
optimal management in ovarian cancer. Cancers (Basel). 16:9322024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldlust IS, Guidice E and Lee JM: PARP
inhibitors in ovarian cancer. Semin Oncol. 51:45–57. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Min T, Lee SH and Lee S: Angiogenesis and
apoptosis: Data comparison of similar microenvironments in the
corpus luteum and tumors. Animals (Basel). 14:11182024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Q and Zhang YH: Flavonoids with
anti-angiogenesis function in cancer. Molecules. 29:15702024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ratnaparkhi R, Javellana M, Jewell A and
Spoozak L: Evaluation of homologous recombination deficiency in
ovarian cancer. Curr Treat Options Oncol. 25:237–260. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collet L, Hanvic B, Turinetto M, Treilleux
I, Chopin N, Le Saux I and Ray-Coquard I: BRCA1/2 alterations and
reversion mutations in the area of PARP inhibitors in high grade
ovarian cancer: State of the art and forthcoming challenges. Front
Oncol. 14:13544272024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dellino M, D'Amato A, Battista G, Cormio
G, Vimercati A, Loizzi V, Lagana AS, Damiani GR, Favilli A, Gerli
S, et al: Reproductive outcomes in women with BRCA 1/2 germline
mutations: A retrospective observational study and literature
review. Open Med (Wars). 19:202499992024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hockings H and Miller RE: The role of PARP
inhibitor combination therapy in ovarian cancer. Ther Adv Med
Oncol. 15:175883592311731832023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi XF, Gao RL, Sun L, Wu ZX, Zhang SL,
Huang LT, Han CB and Ma JT: Dual antitumor immunomodulatory effects
of PARP inhibitor on the tumor microenvironment: A counterbalance
between anti-tumor and pro-tumor. Biomed Pharmacother.
163:1147702023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szentmartoni G, Muhl D, Csanda R, Szasz
AM, Herold Z and Dank M: Predictive value and therapeutic
significance of somatic BRCA mutation in solid tumors.
Biomedicines. 12:5932024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, He L, Liu T, Guo T, Xie C, Jia J,
Lin Y, Liu J and Fan J: Efficacy and safety of PARP inhibitors
combined with antiangiogenic agents in the maintenance treatment of
ovarian cancer: A systematic review and meta-analysis with trial
sequential analysis of randomized controlled trials. Front
Pharmacol. 15:13720772024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernardo WM: PRISMA statement and
PROSPERO. Int Braz J Urol. 43:383–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo LL, Cheng TP, Feng LX, Feng J and Li
XY: Incidence and risk factors for deep infection after primary
shoulder arthroplasty: A meta-analysis. Eur Rev Med Pharmacol Sci.
26:4606–4613. 2022.PubMed/NCBI
|
|
19
|
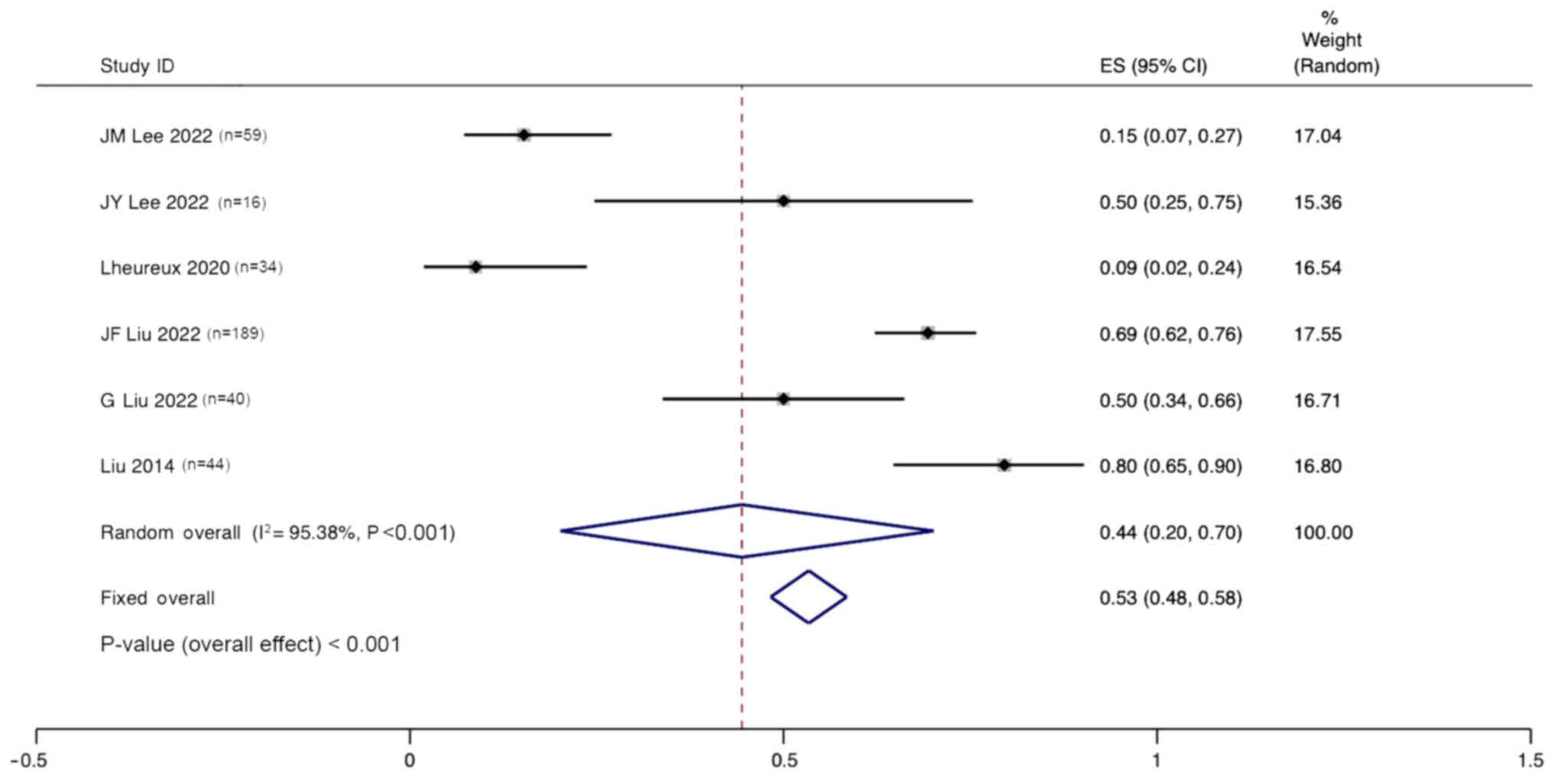
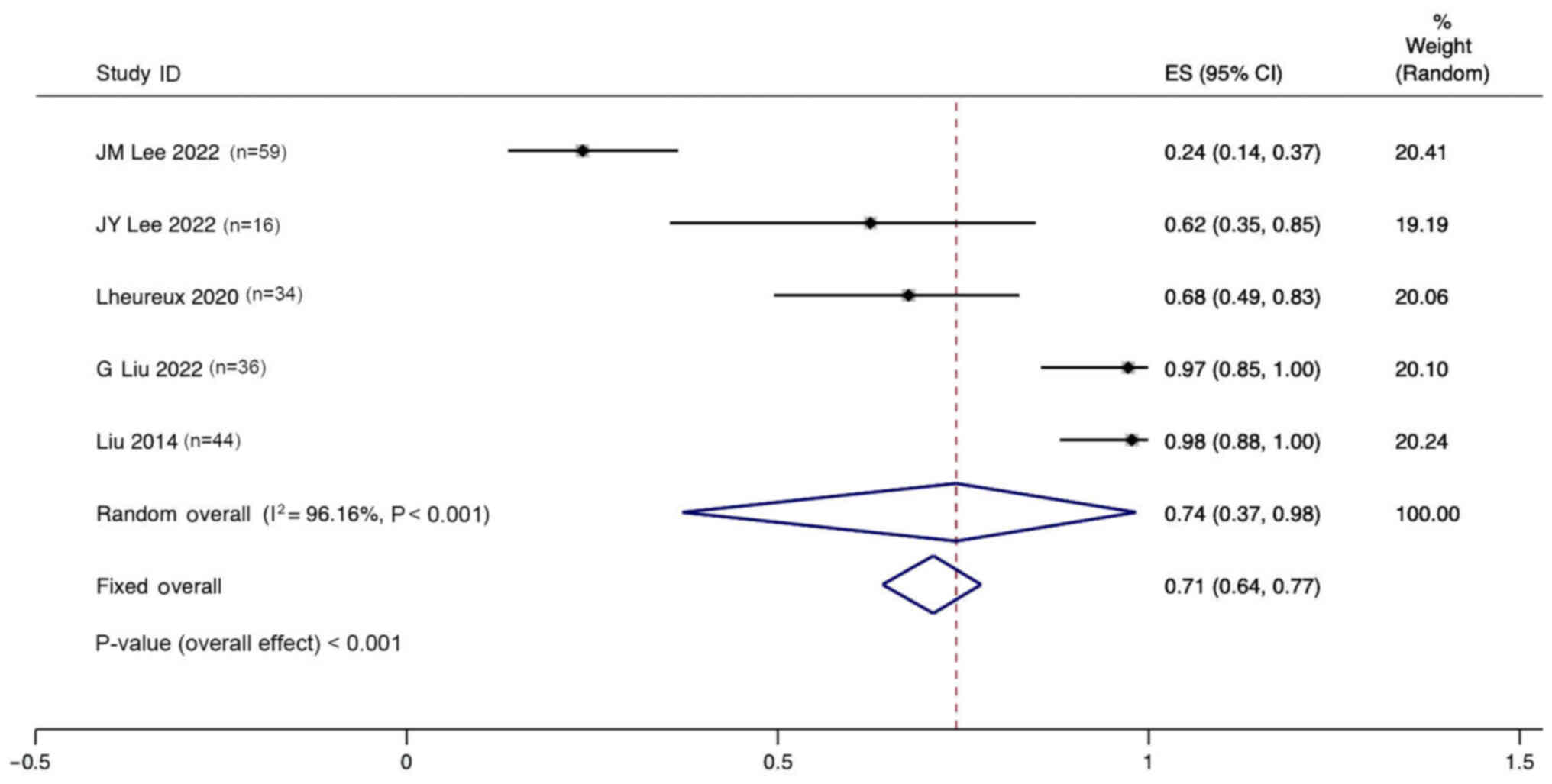
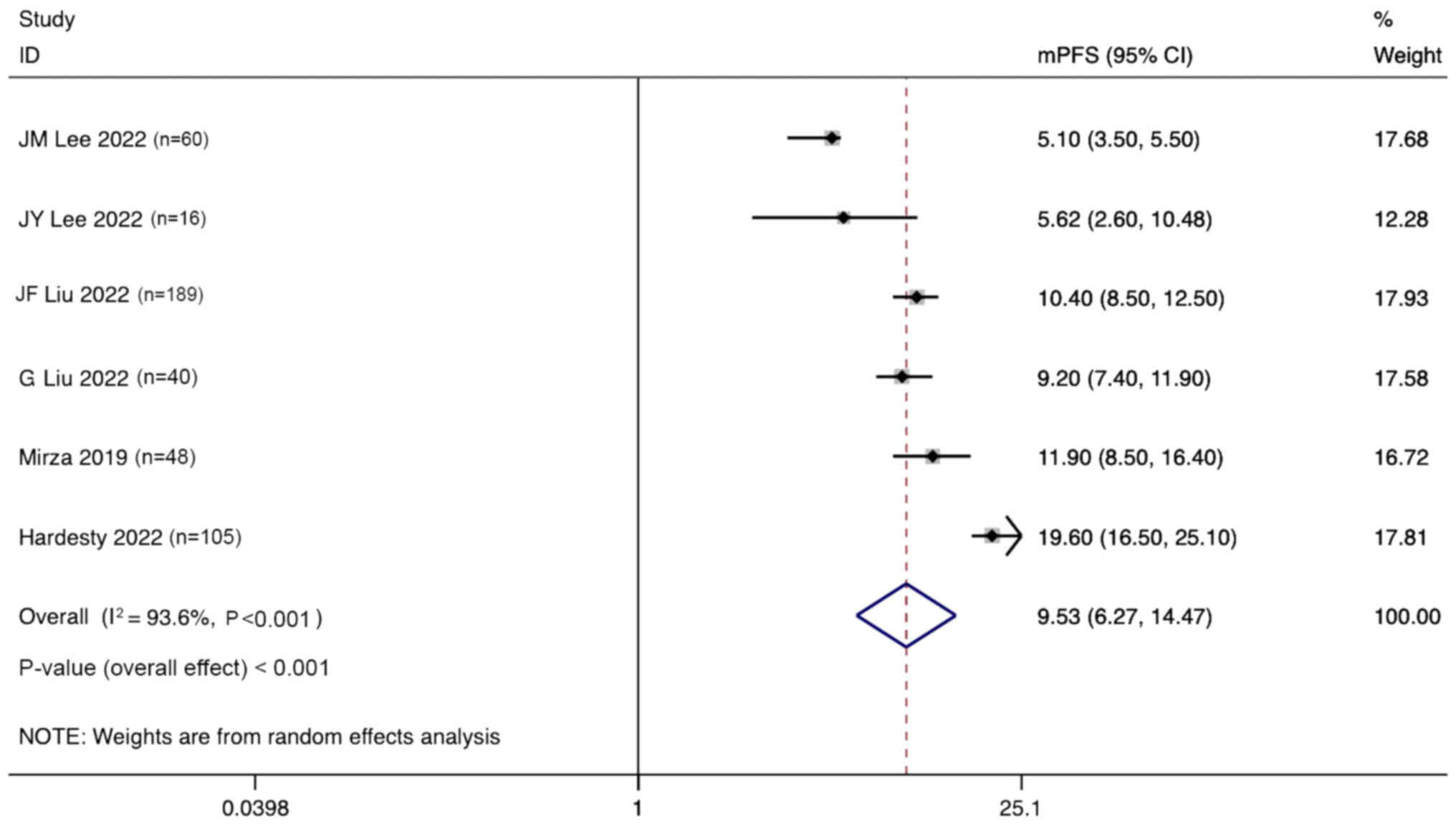
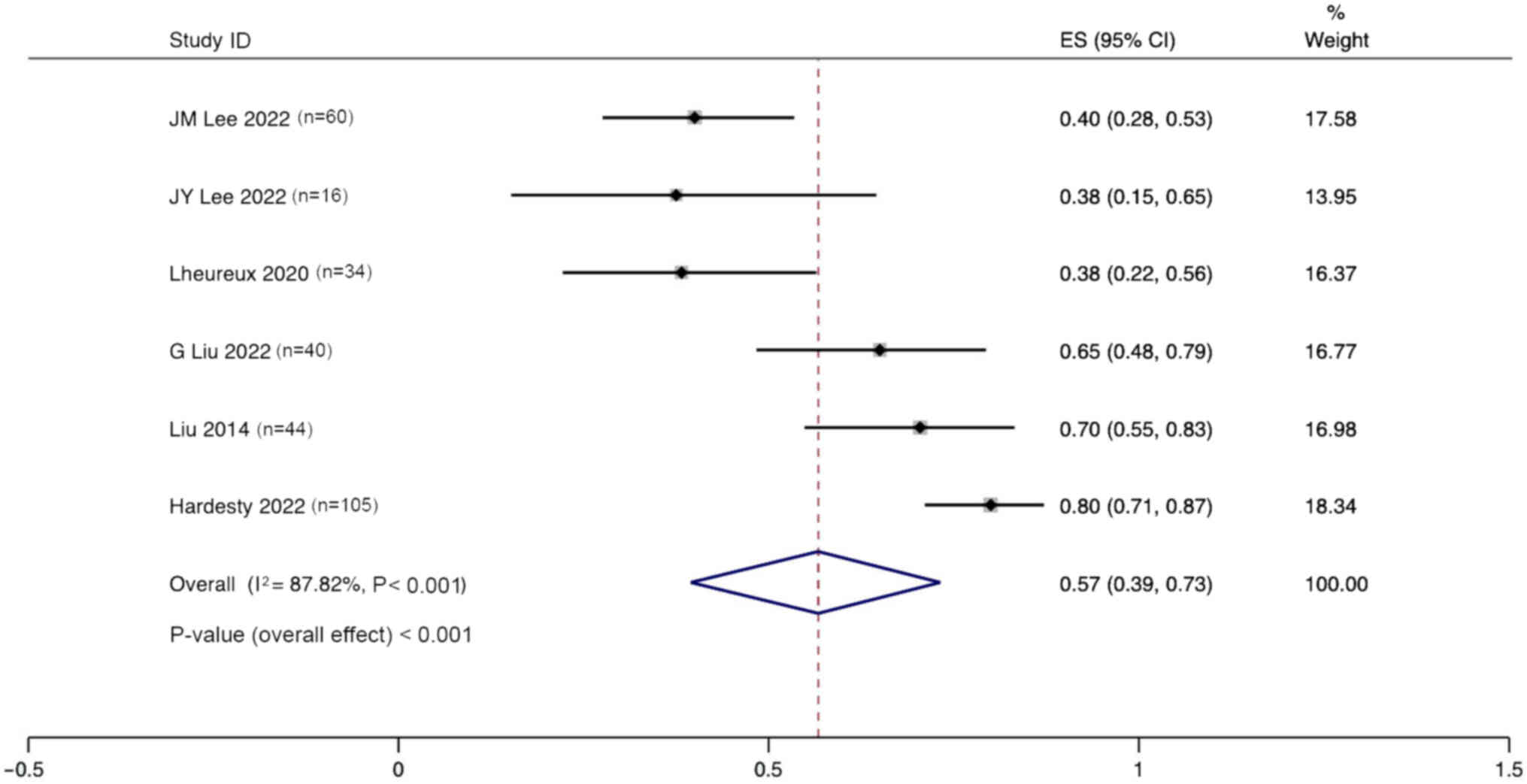
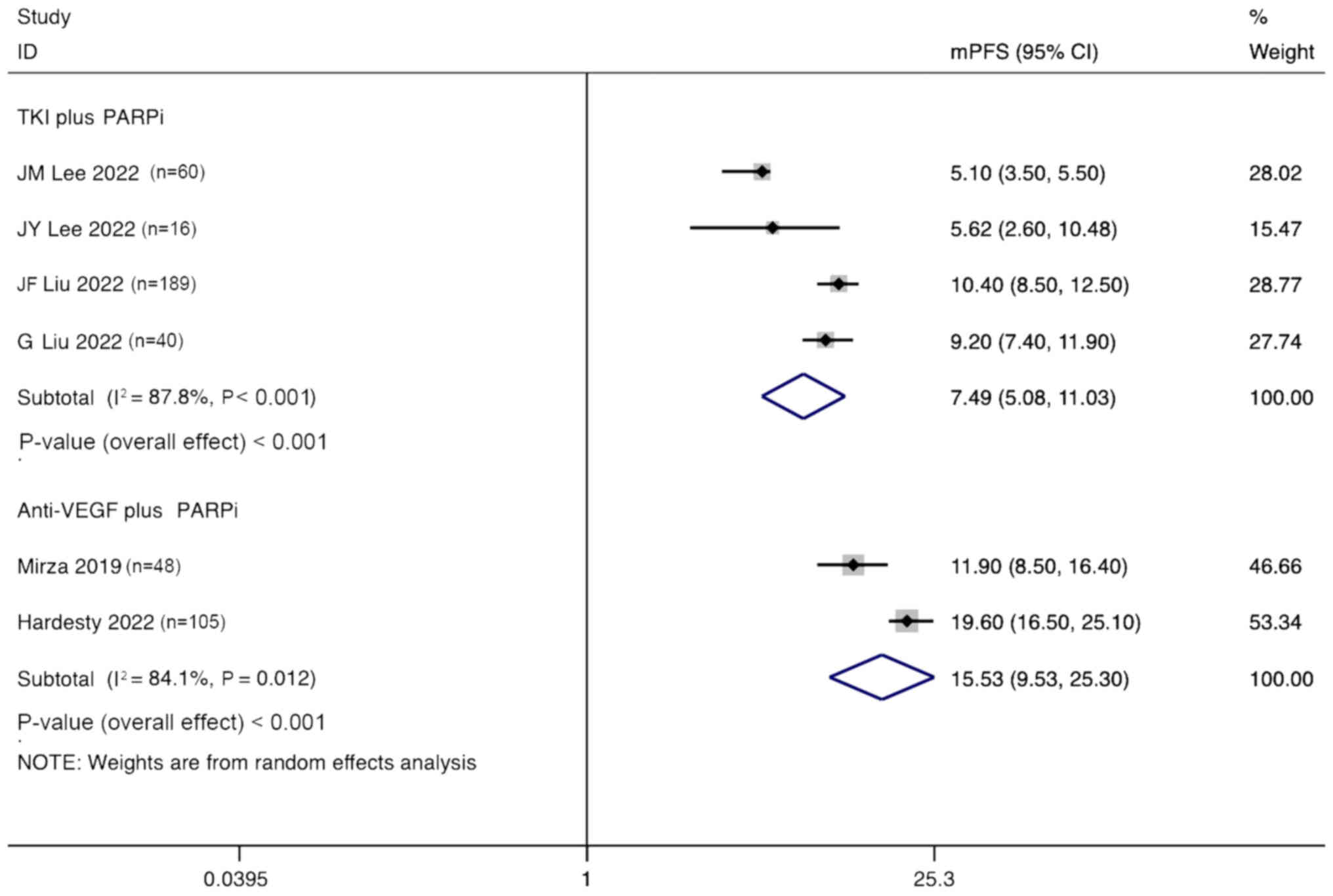
Lee JM, Moore RG, Ghamande S, Park MS,
Diaz JP, Chapman J, Kendrick J, Slomovitz BM, Tewari KS, Lowe ES,
et al: Cediranib in combination with olaparib in patients without a
germline BRCA1/2 mutation and with recurrent platinum-resistant
ovarian cancer: Phase IIb CONCERTO trial. Clin Cancer Res.
28:4186–4193. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JY, Kim BG, Kim JW, Lee JB, Park E,
Joung JG, Kim S, Choi CH and Kim HS; Korean Gynecologic Oncology
Group (KGOG) investigators, : Biomarker-guided targeted therapy in
platinum-resistant ovarian cancer (AMBITION; KGOG 3045): A
multicentre, open-label, five-arm, uncontrolled, umbrella trial. J
Gynecol Oncol. 33:e452022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lheureux S, Oaknin A, Garg S, Bruce JP,
Madariaga A, Dhani NC, Bowering V, White J, Accardi S, Tan Q, et
al: EVOLVE: A multicenter open-label single-arm clinical and
translational phase II trial of cediranib plus olaparib for ovarian
cancer after PARP inhibition progression. Clin Cancer Res.
26:4206–4215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
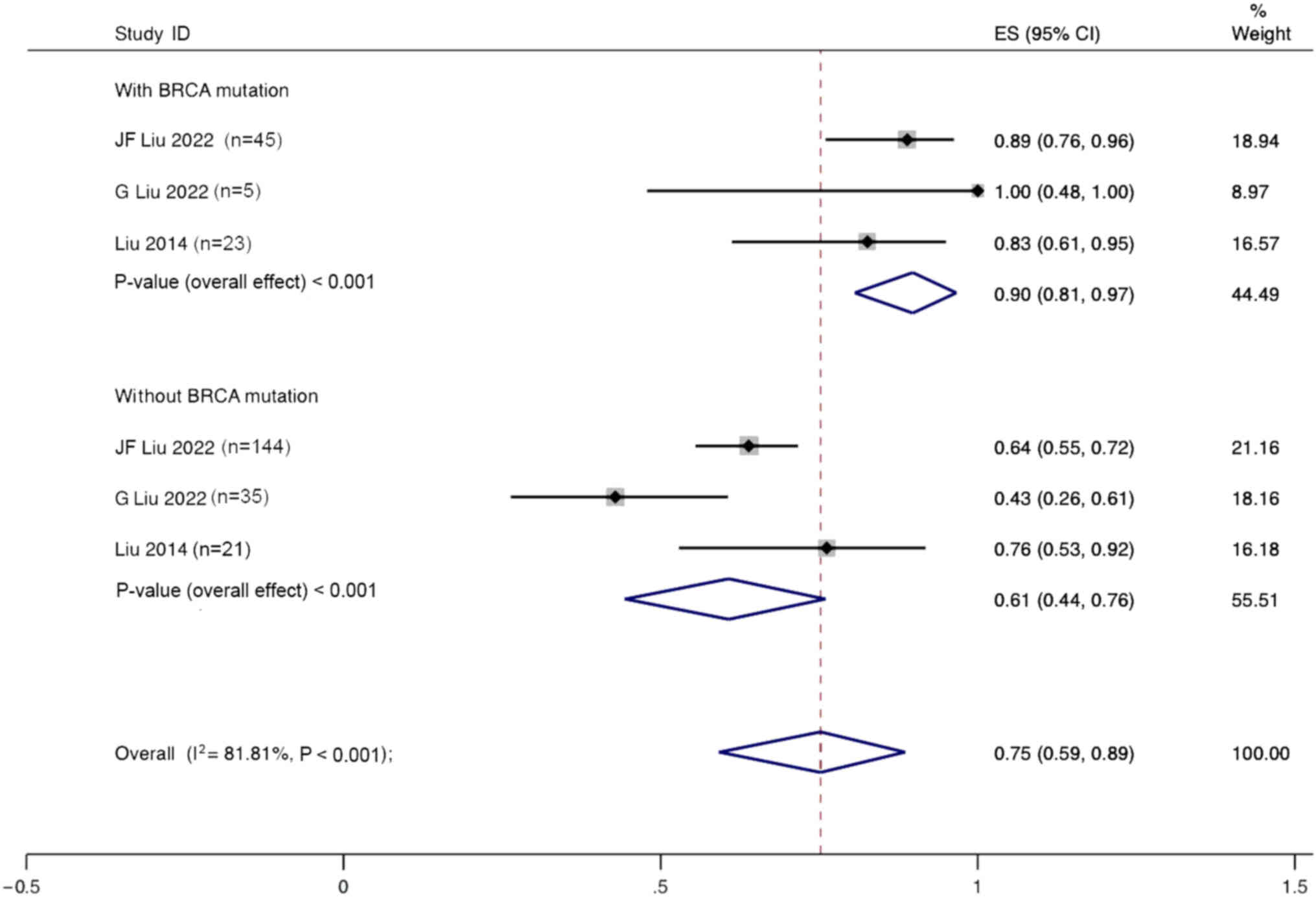
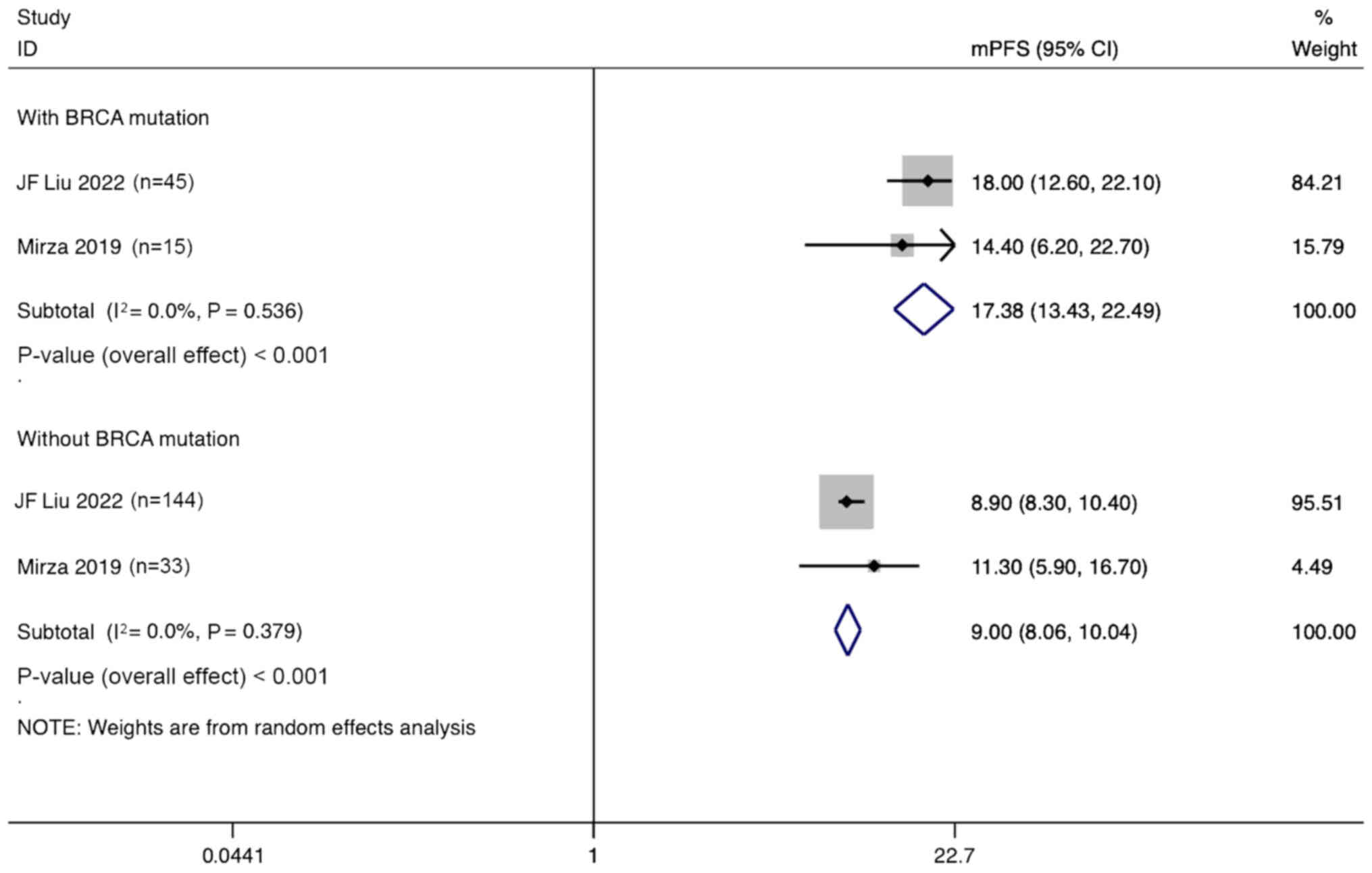
Liu JF, Brady MF, Matulonis UA, Miller A,
Kohn EC, Swisher EM, Cella D, Tew WP, Cloven NG, Muller CY, et al:
Olaparib with or without cediranib versus platinum-based
chemotherapy in recurrent platinum-sensitive ovarian cancer
(NRG-GY004): A randomized, open-label, phase III trial. J Clin
Oncol. 40:2138–2147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu G, Feng Y, Li J, Deng T, Yin A, Yan L,
Zheng M, Xiong Y, Li J, Huang Y, et al: A novel combination of
niraparib and anlotinib in platinum-resistant ovarian cancer:
Efficacy and safety results from the phase II, multi-center ANNIE
study. EClinicalMedicine. 54:1017672022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
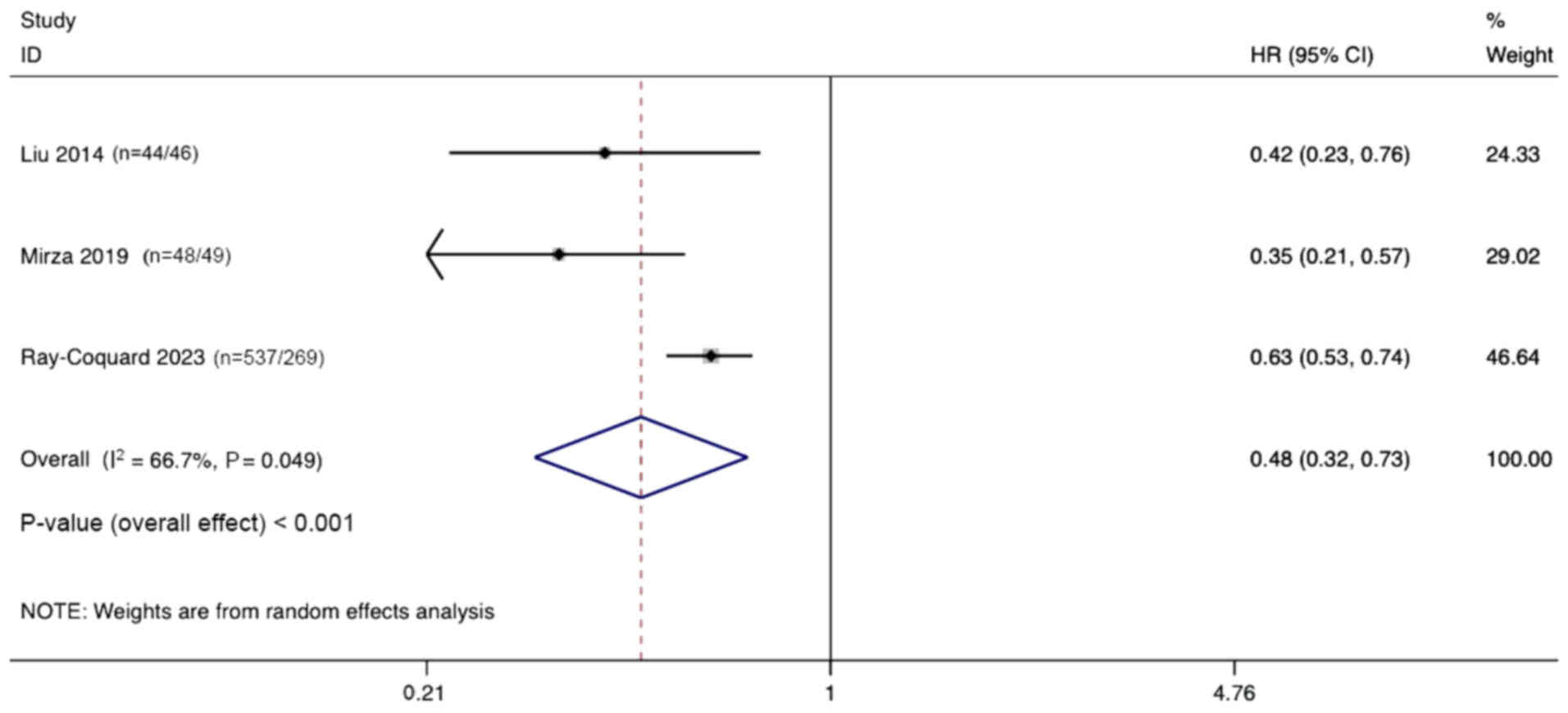
Liu JF, Barry WT, Birrer M, Lee JM,
Buckanovich RJ, Fleming GF, Rimel B, Buss MK, Nattam S, Hurteau J,
et al: Combination cediranib and olaparib versus olaparib alone for
women with recurrent platinum-sensitive ovarian cancer: A
randomised phase 2 study. Lancet Oncol. 15:1207–1214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mirza MR, Lundqvist EA, Birrer MJ, dePont
Christensen R, Nyvang GB, Malander S, Anttila M, Werner TL, Lund B,
Lindahl G, et al: Niraparib plus bevacizumab versus niraparib alone
for platinum-sensitive recurrent ovarian cancer
(NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority
trial. Lancet Oncol. 20:1409–1419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardesty MM, Krivak TC, Wright GS,
Hamilton E, Fleming EL, Belotte J, Keeton EK, Wang P, Gupta D,
Clements A, et al: OVARIO phase II trial of combination niraparib
plus bevacizumab maintenance therapy in advanced ovarian cancer
following first-line platinum-based chemotherapy with bevacizumab.
Gynecol Oncol. 166:219–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ray-Coquard I, Leary A, Pignata S, Cropet
C, González-Martín A, Marth C, Nagao S, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab first-line maintenance
in ovarian cancer: final overall survival results from the
PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 34:681–692. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ge Y, Wang Q, Yao Y, Xin Q, Sun J, Chen W,
Lin Y and Cai X: Framework nucleic acids-based VEGF signaling
activating system for angiogenesis: A dual stimulation strategy.
Adv Sci (Weinh). 11:e23087012024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan Y, Zheng L, Zhang S and Qian B:
Abnormal expression of FOXM1 in carcinogenesis of renal cell
carcinoma: From experimental findings to clinical applications.
Biochem Biophys Res Commun. 692:1492512024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saadi S, Nacer NE, Saari N, Mohammed AS
and Anwar F: The underlying mechanism of nuclear and mitochondrial
DNA damages in triggering cancer incidences: Insights into
proteomic and genomic sciences. J Biotechnol. 383:1–12. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuroda T and Kohno T: Precision medicine
for ovarian clear cell carcinoma based on gene alterations. Int J
Clin Oncol. 25:419–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boussios S, Rassy E, Moschetta M, Ghose A,
Adeleke S, Sanchez E, Sheriff M, Chargari C and Pavlidis N: BRCA
mutations in ovarian and prostate cancer: Bench to bedside. Cancers
(Basel). 14:38882022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozols RF: Future directions in the
treatment of ovarian cancer. Semin Oncol. 29:32–42. 2002.
View Article : Google Scholar : PubMed/NCBI
|