Introduction
Lung cancer ranks among the most lethal and most
prevalent oncological diseases worldwide. According to the latest
‘GLOBOCAN 2022’ review of global cancer statistics, it stands as
the second most commonly diagnosed type of cancer, trailing only
breast cancer. In 2022 alone, almost 2.5 million new cases of lung
cancer were diagnosed. Notably, lung cancer holds the grim
distinction of being the primary cause of mortality among patients
afflicted by malignant diseases, claiming the lives of 1.8 million
individuals in 2022. Typically, it manifests in individuals aged
≥70, emerging as the leading cause of oncological fatalities among
those aged ≥40 (1,2).
The success of lung cancer treatment is dependent
upon various factors, including the clinical characteristics of
patients, the tumor histological type, the outcomes of predictive
biomarker testing, and effective communications between
pathologists, radiologists and oncologists. Over the past decade,
substantial strides have been made in therapeutic development,
mainly through identifying and utilizing predictive biomarkers
(3).
Invasive non-mucinous adenocarcinoma is the most
common type of lung cancer. It comprises malignant epithelial cells
whose morphology or immunohistochemical phenotype suggest glandular
differentiation, and thus it does not meet the criteria for any
other type of adenocarcinoma (4).
Anaplastic lymphoma kinase (ALK) rearrangement
encompasses a group of gene mutations encoding the transmembrane
receptor tyrosine kinase, belonging to the insulin receptor protein
superfamily. To date, >20 rearrangement partners of the ALK gene
have been identified, with the most prevalent occurring in
non-small cell lung cancer (NSCLC): an intra-chromosomal inversion
of the short arm of chromosome 2, resulting in the fusion of the
2p21 gene locus of the echinoderm microtubule-associated
protein-like protein 4 (EML4) gene and 2p23 ALK genes (5). Among the genomic alterations observed
in NSCLC, the ALK rearrangement is a targetable alteration for
therapy, providing a therapeutic response that extends patient
survival (6,7). While the histological type of the
majority of lung cancers with ALK rearrangement is adenocarcinoma,
the studies available to date exploring the detailed
histomorphological and cytomorphological characteristics of these
samples are limited and have yielded contradictory results
(8–12).
Epidermal growth factor receptor (EGFR) belongs to
the family of tyrosine kinase protein receptors whose mutation
leads to the uncontrolled proliferation of malignant cells, their
invasion, metastatic spread, the inhibition of apoptosis, as well
as tumor angiogenesis, it plays a leading role in tumor
carcinogenesis and progression. These somatic mutations mainly
target exons 18–21 of the gene encoding part of the tyrosine kinase
domain of EGFR. Among the most well-known and frequently occurring
mutations are deletions in exon 19 and substitutions in exon 21,
particularly the L858R substitution, which collectively account for
80–90% of all EGFR mutations in NSCLC (13). According to certain studies, the
histopathological subtype of adenocarcinoma predicts prognosis and
mutational status (14–16).
The scarcity of available data in the literature
regarding the association between the microscopic morphology of
adenocarcinoma and the status of biomarkers available in Serbia
underscores the necessity for research in this area. Furthermore,
the present study aimed to provide valuable insight into the
morphology of primary lung adenocarcinomas, which may aid in the
typing of NSCLC, particularly in cases where only cytological
smears are available.
Materials and methods
Study design
The present retrospective study analyzed
histological and cytological material from the internal tissue bank
obtained from patients diagnosed with lung adenocarcinoma between
1st January 2016 and 31st December 2023 at
the Institute for Pulmonary Diseases of Vojvodina (Sremska
Kamenica, Serbia). The study with research methodology including
the use of the external controls for the analysis, was approved by
the Institutional Professional and Ethics Committee of the
Institute for Pulmonary Diseases of Vojvodina (approval nos.
25-VIII/10 and 24-VII/10). A total of 101 patients were included in
the study (mean age, 63.48 and 32 to 84 years old, respectively.
The sex distribution of the patients was nearly balanced, with a
slight predominance of males (52.48 vs. 47.52%). Patients were
divided into the ALK, EGFR and negative groups. The clinical
features analyzed included sex, age, smoking status and disease
stage. The data were collected from the patients' medical
records.
Molecular analyses
A representative paraffin block was selected for the
immunohistochemical analysis of ALK rearrangement based on the
hematoxylin and eosin (H&E)-stained section. The block was
subsequently cut into histological sections that were 4-µm-thick.
Appendices removed during appendectomies were used as external
control tissue (Fig. S1). Paraffin
samples were melted onto the slides overnight at 53°C. The
following day, the slides were labeled and placed in the Benchmark,
Ventana Roche machine using an anti-ALK antibody (Rabbit Monoclonal
D5F3; ready-to-use; Roche Tissue Diagnostics; cat. no.
790-4794/06679072001) and operated according to the manufacturer's
protocol. The antibody for the analysis was incubated for 16
minutes at a temperature of 36°C.
In all patients included in the study, the
qualitative detection and identification of mutations in exons 18,
19, 20 and 21 of the EGFR gene were determined using the real-time
PCR Cobas EGFR Mutation Test v2 (cat. no. P/N 07248563190) after
DNA isolation with the Cobas DNA Sample Preparation kit. The entire
process of amplification, detection, and validation of the samples
was conducted using the Cobas 4800 software on the Cobas z 480
analyzer following the manufacturer's protocol (Roche
Diagnostics).
All samples for ALK and EGFR testing were selected
based on the number of viable tumor cells. The degree of
differentiation and the Ki67 proliferative index were not
considered in the selection of test samples and, therefore, did not
influence the study's results.
Sampling and processing of
material
All histological material was obtained through
bronchoscopic methods, including bronchial biopsy, transbronchial
biopsy and catheter biopsy. Tissues were fixed in 10% neutral
formalin for 12–18 h (room temperature), dehydrated in increasing
ethanol concentrations (70, 80, 96 and 100%), embedded in paraffin,
and cut into 4-µm thick sections using a rotary microtome (Leica
Microsystems GmbH). All sections were stained with H&E
(Bio-Optica). After rehydration, the sections were stained with
hematoxylin for 1 min. The slides were then rinsed in running tap
water, followed by differentiation in an acid-alcohol solution to
remove excess stain. In the next step, the sections were
counterstained with eosin for 1.5 min. The slides were briefly
rinsed in water to remove excess eosin and then rehydrated through
a graded series of alcohols.
Material for cytopathological analysis was collected
via thoracentesis, percutaneous lymph node fine needle aspiration
and bronchoscopic methods, including transbronchial fine needle
aspiration, brush biopsy and catheter biopsy. Conventional
cytological smears were prepared and stained using the
May-Grunwald-Giemsa method. The entire histological and cytological
material was evaluated using a light microscope (Leica,
DM2500).
Cytomorphological features
Cytomorphological features encompass qualitative
characteristics of cellular arrangements observed on cytology
smears. The following parameters were analyzed: Size of cell
clusters, the size of nuclei, nuclear atypia, visibility of
nucleoli, the presence of intracytoplasmic vacuoles, signet ring
cells, and necrosis. The method of parameter estimation is outlined
in Table I.
 | Table I.The method of estimation for
cytomorphological features. |
Table I.
The method of estimation for
cytomorphological features.
| Cytomorphological
features |
| Categories |
|
|---|
| Size of
clusters | ≤200 µm | >200 µm, ≤400
µm | >400 µm |
| Size of
nucleia | <3X
lymphocyte | 3–5×lymphocyte | <5X
lymphocyte |
| Nuclear atypia | Moderate | Severe |
|
| Nucleolar
visibility | Visible | Not visible |
|
| Intra-cytoplasmic
vacuoles | <20% | ≥20% |
|
| Signet ring
cells | <5% | ≥5% |
|
| Necrosis | Absent | Moderate | Abundant |
Histomorphological features
The analyzed histomorphological characteristics
included cell arrangement, the presence of cribriform arrangement,
the amount of stroma, nucleus size, degree of nuclear atypia, the
visibility of nuclei, the presence of intracytoplasmic vacuoles,
the presence of signet ring cells, the presence of inflammatory
infiltrate and the presence of necrosis. The method of parameter
estimation is detailed in Table
II. In the present study, two experienced cytopathologists and
a pathology resident evaluated both types of sample cases.
Disagreements in estimations were reanalyzed, after which a joint
decision was made on the result.
 | Table II.The method of estimation for
histomorphological features. |
Table II.
The method of estimation for
histomorphological features.
| Histomorphological
features |
| Categories |
|---|
| Predominant
arrangement | Lepidic |
Papillary/acinar |
Solid/micropapillary |
| Cribriform
pattern | No | Yes |
|
| Stroma | Poor | Moderate | Abundant |
| Size of
nucleia | <3X
lymphocyte | 3-5X
lymphocyte | <5X
lymphocyte |
| Nuclear atypia | Moderate | Severe |
|
| Nucleoli
visibility | Visible | Not visible |
|
| Intra-cytoplasmic
vacuoles | No | Yes |
|
| Signet ring
cells | No | Yes |
|
| Inflammatory
infiltrate | Poor | Moderate | Abundant |
| Necrosis | Absent | Moderate | Abundant |
The inflammatory infiltrate was classified into
three categories. The infiltrate was considered poor when the tumor
was infiltrated with scant inflammatory cells, typically <5% of
the tumor area, with sparse lymphocytes and/or neutrophils
scattered across the stroma with minimal clustering. It was
considered moderate when inflammatory cells comprised 5–30% of the
tumor area, with more noticeable clusters of lymphocytes,
neutrophils and occasional plasma cells within the stroma and
surrounding tumor cells. Abundant inflammatory infiltrate was
defined by dense and widespread inflammatory infiltrate, occupying
>30% of the tumor area, with prominent clusters of lymphocytes,
neutrophils and plasma cells throughout the stroma and within the
tumor.
The presence of a partially preserved structure of
tumor cells with remains of their outlines in a foci of necrotic
tissue was considered a sign of tumor necrosis. Necrosis was
assessed as moderate when the sample contained viable primary tumor
cells with small foci of necrosis comprising <30% of the sample.
Necrosis was classified as abundant when it occupied >30% of the
sample or when the sample predominantly consisted of necrotic foci,
with viable tumor cells present in only small amounts, complicating
the interpretation. The presence of necrotic masses of
unrecognizable cells mixed with purulent exudate is considered
necrosis of inflammatory/infectious etiology, and such cases are
not categorized as tumor necrosis (10,17–20).
The size of the nuclei is expressed as the size of
the lymphocyte. Nuclear atypia was scored as moderate or severe
nuclear atypia. The specimen was categorized as moderate nuclear
atypia when nuclei were uniform in size and shape, with mild
irregularity of the nuclear membrane and homogenous or fine
granular chromatin pattern. The specimen was categorized as severe
nuclear atypia in cases with varied sizes of nuclei with bizarre
shapes and coarse chromatin patterns.
Statistical analysis
Statistical analysis was performed using JASP
0.18.3.0 software (https://jasp-stats.org/). The difference in the
frequency of cyto- and histomorphological features relative to the
type of mutation was assessed using the Chi-squared test or
Fisher's exact test, depending on the conditions met for each
analysis. The same statistical method was also used to examine
tumor morphology relative to the other clinical parameters. Cyto-
and histomorphological features were analyzed as predictors using
binary logistic regression, with the mutation status as the
criterion. The analysis was performed using the default settings in
JASP, applying a logit link function. Predictor variables were
entered using the forced entry method. Tolerance values >0.2 and
variance inflation factor (VIF) values <5 were used as criteria
to confirm the absence of multicollinearity. Model validation was
conducted through cross-validation using the k-Nearest Neighbors
test. Predictor significance was evaluated using Wald's chi-square
tests, and odds ratios (ORs) were calculated with corresponding 95%
confidence intervals (CIs). The results are presented tabularly and
graphically, with P<0.05 considered to indicate a statistically
significant difference.
Results
Clinical characteristics
The clinical characteristics of the patients are
summarized in Table III. Sex
distribution in the ALK group was uniform, while the EGFR group had
a higher proportion of female patients (57.14 vs. 42.86%). In the
negative group, male patients comprised almost 70% of the cohort.
Statistically significant differences in sex distribution were
observed between the EGFR and negative groups (χ2=4.439;
P<0.05; data not shown). By contrast, no significant differences
were noted between the EGFR and ALK group (χ2=0.345;
P>0.05; data not shown) or between ALK and negative group
(χ2=1.919; P>0.05; data not shown). The mean age of
the patients was 64 years, with no statistically significant
differences in age observed between the groups. Notably, smoking
habits differed significantly between the groups: The patients in
the EGFR group were significantly more likely to be non-smokers
compared with those in the negative and ALK groups (P<0.001 and
P<0.05, respectively). Furthermore, non-smokers and ex-smokers
were significantly more prevalent in the ALK group than in the
negative group (χ2=6.679; P<0.05). Conversely,
patients in the ALK group were significantly more likely to be
smokers compared with those in the EGFR group. There was an
approximately equal distribution between the two most common EGFR
mutations. The majority of patients had stage IV of the disease. As
regards treatment, the majority of patients with confirmed ALK or
EGFR mutations received onco-specific treatment. In the group of
ALK-positive patients, 82.14% received onco-specific therapy, with
alectinib being the most commonly administered, accounting for 80%
of these cases. In the EGFR-positive group, 80.96% of patients
received onco-specific therapy, with afatinib and gefitinib being
the most frequently used drugs. At the time of diagnosis,
osimertinib was either unavailable in Serbia or accessible only
through clinical trials. Consequently, only 4.76% of patients
received osimertinib as a first-line treatment, while an additional
9.52% (4 patients) received it as a second-line treatment following
the detection of T790M mutation resistance upon retesting. In the
negative group, 61.29% of patients received chemotherapy with or
without radiotherapy, while 22.58% received palliative care.
 | Table III.Patient's clinical
characteristics. |
Table III.
Patient's clinical
characteristics.
| Clinical
characteristics | ALK | EGFR | Negative | Total | Test value | P-value |
|---|
| Total | 28 | 42 | 31 | 101 |
|
|
| Sex |
|
|
|
|
|
|
|
Male | 14 (50%) | 18 (42.86%) | 21 (67.74%) | 53 (52.48%) | 4.524 | 0.104 |
|
Female | 14 (50%) | 24 (57.14%) | 10 (32.26%) | 48 (47.52%) |
|
|
| Age, years |
|
|
|
|
|
|
| Mean ±
SD | 62.39±10.74 | 65.52±8.75 | 61.68±7.28 | 63.48±9.03 | F 1.930 | 0.151 |
|
Median | 63 | 66 | 62 | 64 |
|
|
|
Minimum | 32 | 46 | 49 | 32 |
|
|
|
Maximum | 83 | 84 | 79 | 84 |
|
|
| Smoking
history |
|
|
|
|
|
|
|
Non-smokers | 6 (21.43%) | 21 (50%) | 2 (6.45%) | 29 (28.71%) | 30.105 | <0.001 |
|
Ex-smokers | 6 (21.43%) | 11 (26.19%) | 2 (6.45%) | 19 (18.81%) |
|
|
|
Smokers | 16 (57.14%) | 10 (23.81%) | 27 (87.10%) | 53 (52.48%) |
|
|
| Mutation type |
|
|
|
|
|
|
| Exon
19 | N/A | 22 (52.38%) | N/A | N/A | N/A | N/A |
| Exon
21 | N/A | 20 (47.62%) | N/A | N/A |
|
|
| Stage |
|
|
|
|
|
|
| IB | 0 | 1 (2.38%) | 0 | 1 (0.99%) | 27.635 | 0.035 |
|
IIA | 0 | 1 (2.38%) | 1 (3.23%) | 2 (1,98%) |
|
|
|
IIB | 1 (3.59%) | 1 (2.38%) | 1 (3.23%) | 3 (2.97%) |
|
|
|
IIIA | 0 | 3 (7.14%) | 1 (3.23%) | 4 (3.96%) |
|
|
|
IIIB | 9 (32.14%) | 5 (11.91%) | 4 (12.90%) | 18 (17.82%) |
|
|
|
IIIC | 0 | 3 (7.15%) | 1 (3.23%) | 4 (3.96%) |
|
|
|
IVA | 18 (64.29%) | 14 (33.33%) | 12 (38.70%) | 44 (43.56%) |
|
|
|
IVB | 0 | 14 (33.33%) | 11 (35.48%) | 25 (24.75%) |
|
|
| Treatment | Crizotinib | Afatinib | ChT ±
RTb |
| N/A | N/A |
|
| 3 (10.71%) | 16 (38.10%) | 19 (61.29%) |
|
| Alectinib | Gefitinib | Palliative
care |
|
|
| 17 (60.72%) | 11 (26.19%) | 7 (22,58%) |
|
|
|
|
| Brigatinib | Erlotinib | Dieda |
|
| 3 (10.71%) | 5 (11.90%) | 2 (6,45%) |
|
|
|
|
| Chemotherapy | Osimertinib | No data |
|
| 1 (3.57%) | 2 (4.76%) | 3 (9,68%) |
|
|
|
|
| No data | Palliative
care |
|
| 4 (14.29%) | 1 (2.38%) |
|
|
|
|
|
|
| Dieda |
|
|
| 3 (7.14%) |
|
|
|
|
|
|
| No data |
|
|
| 4 (9.52%) |
|
|
|
|
Cytomorphological features
The present study examined the differences in the
frequency of cytomorphological features of lung adenocarcinoma
between the groups (Table IV).
There were no statistically significant differences in the
frequency of different sizes of cell clusters, size of the nuclei,
nuclear atypia and visibility of nuclei between the groups.
Although intracytoplasmic vacuoles and signet ring cells were more
frequently present in the ALK group, this difference was not
statistically significant (P>0.05). The only statistically
significant difference between the groups was observed regarding
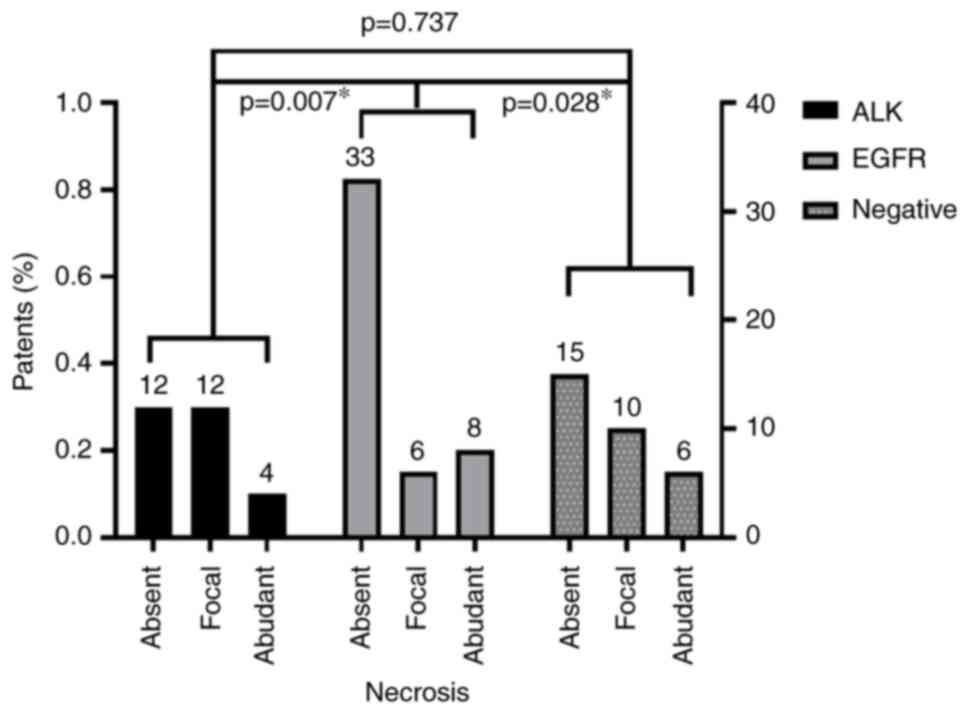
necrosis. Necrosis was significantly prevalent in the ALK and
negative group samples than in the EGFR group (P<0.05; Fig. 1).
 | Table IV.Frequency of cytomorphological
features of lung adenocarcinoma in smears in relation to mutation
status. |
Table IV.
Frequency of cytomorphological
features of lung adenocarcinoma in smears in relation to mutation
status.
| Cytomorphological
features | ALK (%) | EGFR (%) | Negative (%) | Test | P-value |
|---|
| Size of
clusters |
|
|
|
|
|
| ≤200
µm | 23 (82.14) | 35 (83.33) | 23 (74.20) | Fisher's=3.202 | 0.539 |
| >200
µm, ≤400 µm | 3 (10.72) | 6 (14.29) | 4 (12.90) |
|
|
| >400
µm | 2 (7.14) | 1 (2.38) | 4 (12.90) |
|
|
| Size of nuclei |
|
|
|
|
|
| <3
lymphocyte | 4 (14.29) | 6 (14.29) | 6 (19.35) |
χ2=2.821 | 0.588 |
| 3–5
lymphocyte | 15 (53.57) | 16 (38.09) | 15 (48.39) |
|
|
| >5
lymphocyte | 9 (32.14) | 20 (47.62) | 10 (32.56) |
|
|
| Nuclear atypia |
|
|
|
|
|
|
Moderate | 11 (39.29) | 18 (42.86) | 16 (53.33) |
χ2=1.289 | 0.525 |
|
Severe | 17 (60.71) | 24 (57.14) | 14 (46.67) |
|
|
| Nucleoli
visibility |
|
|
|
|
|
|
Visible | 23 (82.14) | 35 (83.33) | 25 (80.65) |
χ2=0.088 | 0.957 |
| Not
visible | 5 (17.86) | 7 (16.67) | 6 (19.35) |
|
|
| Intracytoplasmic
vacuoles |
|
|
|
|
|
|
<20% | 14 (50) | 27 (64.29) | 19 (61.29) |
χ2=1.487 | 0.475 |
|
≥20% | 14 (50) | 15 (35.71) | 12 (38.71) |
|
|
| Signet ring
cells |
|
|
|
|
|
|
<5% | 24 (85.71) | 38 (90.48) | 29 (93.55) | Fisher's=1.053 | 0.641 |
|
≥5% | 4 (14.29) | 4 (9.52) | 2 (6.45) |
|
|
| Necrosis |
|
|
|
|
|
|
Absent | 12 (42.86) | 33 (78.57) | 15 (48.39) | Fisher's=
11.962 | 0.015a |
|
Moderate | 12 (42.86) | 6 (14.29) | 10 (32.26) |
|
|
|
Abundant | 4 (14.28) | 3 (7.14) | 6 (19.35) |
|
|
Histomorphological features
The histomorphological features of patients are
presented in Table V. Statistically
significant differences in the tissue arrangement of adenocarcinoma
among the groups were observed (P<0.01). In the ALK group, no
samples exhibited lepidic or micropapillary arrangements. By
contrast, these arrangements were present in 16.67 and 9.51% of the
EGFR group, respectively, and in 9.68 and 3.12% of the negative
group, respectively. Conversely, papillary arrangement was detected
in 21.43% of the ALK-positive adenocarcinomas, while no such
arrangement was observed in the EGFR and negative groups.
 | Table V.Frequency of histomorphological
features of lung adenocarcinoma from tissue samples in relation to
mutation status. |
Table V.
Frequency of histomorphological
features of lung adenocarcinoma from tissue samples in relation to
mutation status.
| Histomorphological
features | ALK | EGFR | Negative | Test | P-value |
|---|
| Arrangement |
|
|
|
|
|
|
Acinar | 17 (60.71%) | 26 (61.91%) | 21 (67,74%) |
Fisher's=19.952 | 0.003b |
|
Lepidic | 0 | 7 (16.67%) | 3 (9,68%) |
|
|
|
Micropapillary | 0 | 4 (9.51%) | 1 (3,23%) |
|
|
|
Papillary | 6 (21.43%) | 0 | 0 |
|
|
|
Solid | 5 (17.86%) | 5 (11.91%) | 6 (19,35%) |
|
|
| Cribriform
pattern |
|
|
|
|
|
|
Yes | 5 (17.86%) | 6 (14.29%) | 7 (22,58%) |
χ2=0.838 | 0.688 |
| No | 23 (82.14%) | 36 (85.71%) | 24 (77,42%) |
|
|
| Stroma |
|
|
|
|
|
|
Poor | 17 (60.71%) | 27 (64.29%) | 23 (74,19%) | Fisher's=2.635 | 0.624 |
|
Moderate | 9 (32.14%) | 10 (23.81%) | 7 (22,58%) |
|
|
|
Abundant | 2 (7.14) | 5 (11.90%) | 1 (3,23%) |
|
|
| Size of nuclei |
|
|
|
|
|
| <3X
lymphocyte | 7 (25%) | 12 (28.57%) | 11 (35,48%) |
χ2=1.211 | 0.876 |
| 3-5X
lymphocyte | 13 (46.43%) | 21 (50%) | 13 (41,94%) |
|
|
| >5X
lymphocyte | 8 (28.57%) | 9 (21.43%) | 7 (22,58%) |
|
|
| Nuclear atypia |
|
|
|
|
|
|
Moderate | 9 (32.14%) | 15 (35.71%) | 16 (51,61%) |
χ2=2.786 | 0.248 |
|
Severe | 19 (67.86%) | 27 (64.29%) | 15 (48,39%) |
|
|
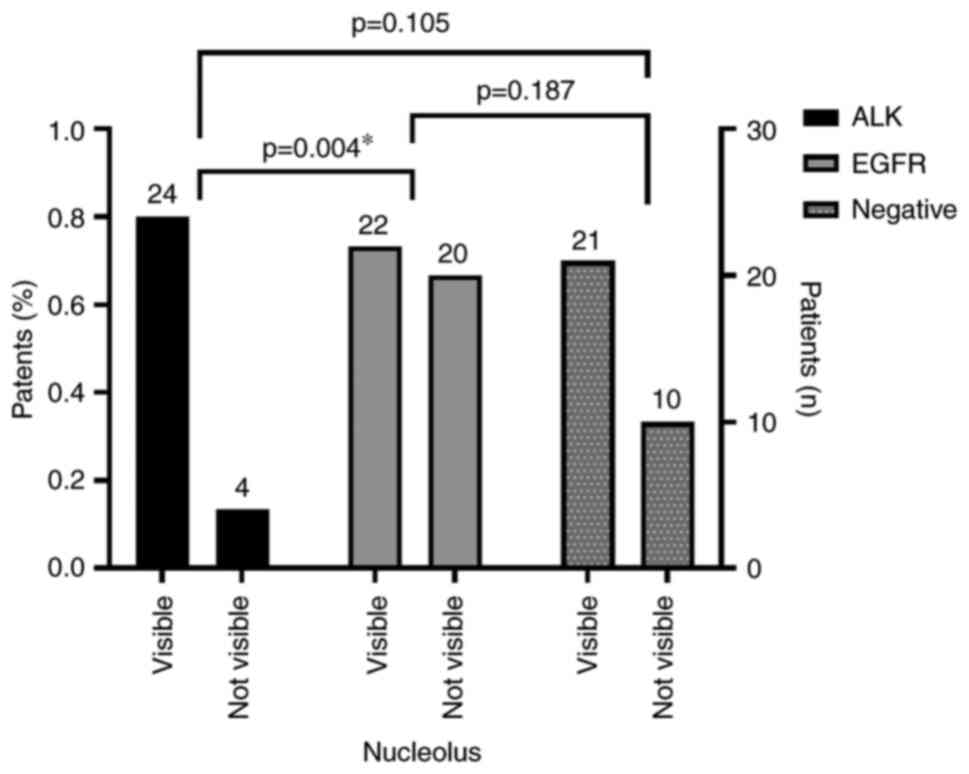
| Nucleoli
visibility |
|
|
|
|
|
|
Visible | 24 (85.71%) | 22 (52.38%) | 21 (67,74%) |
χ2=8.399 | 0.015a |
| Not
visible | 4 (14.29%) | 20 (47.62%) | 10 (32,26%) |
|
|
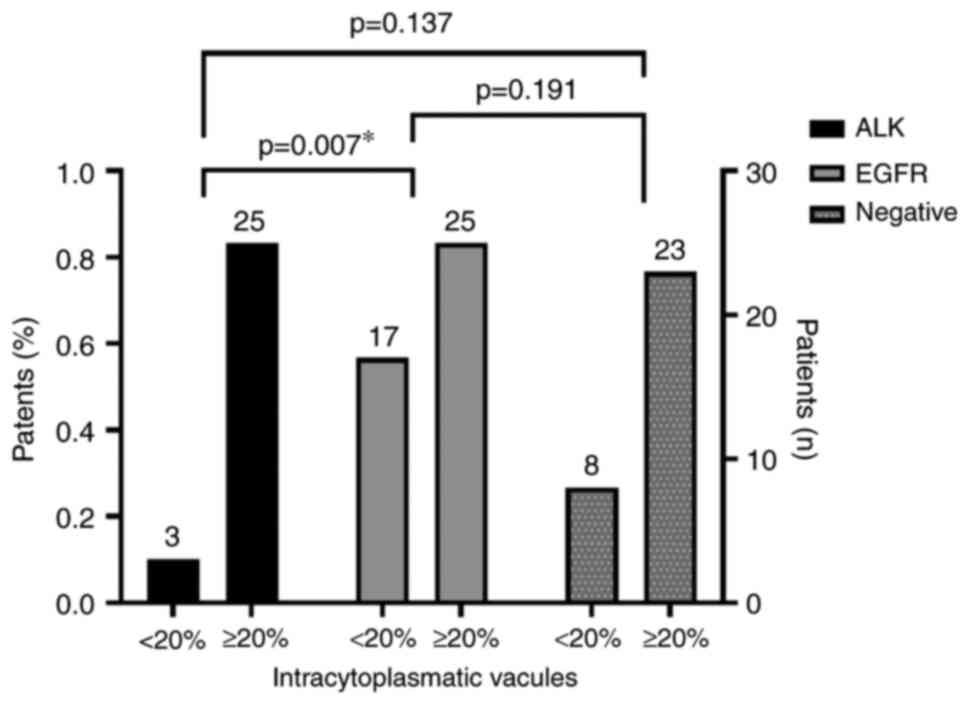
| Intra-cytoplasmic
vacuole |
|
|
|
|
|
|
<20% | 3 (10.71%) | 17 (40.48%) | 8 (25,81%) |
χ2=7.508 | 0.023a |
|
≥20% | 25 (89.29%) | 25 (59.52%) | 23 (74,19%) |
|
|
| Signet ring
cells |
|
|
|
|
|
|
<5% | 15 (53.57%) | 36 (85.71%) | 19 (61,29%) |
χ2=9.511 | 0.008b |
|
≥5% | 13 (46.43%) | 6 (14.29%) | 12 (38,71%) |
|
|
| Inflammatory
infiltrate |
|
|
|
|
|
|
Poor | 18 (64.29%) | 25 (59.52%) | 25 (80,65%) | Fisher's=6.529 | 0.123 |
|
Moderate | 9 (32.14%) | 16 (38.10%) | 4 (12,90%) |
|
|
|
Abundant | 1 (3.57%) | 1 (2.38%) | 2 (6,45%) |
|
|
| Necrosis |
|
|
|
|
|
|
Absent | 26 (92.86%) | 42 (100%) | 27 (87,10%) | Fisher's=6.465 | 0.046a |
|
Moderate | 2 (7.14%) | 0 | 2 (6,45%) |
|
|
|
Abundant | 0 | 0 | 2 (6,45%) |
|
|
Visible nucleoli, the presence of intracytoplasmic
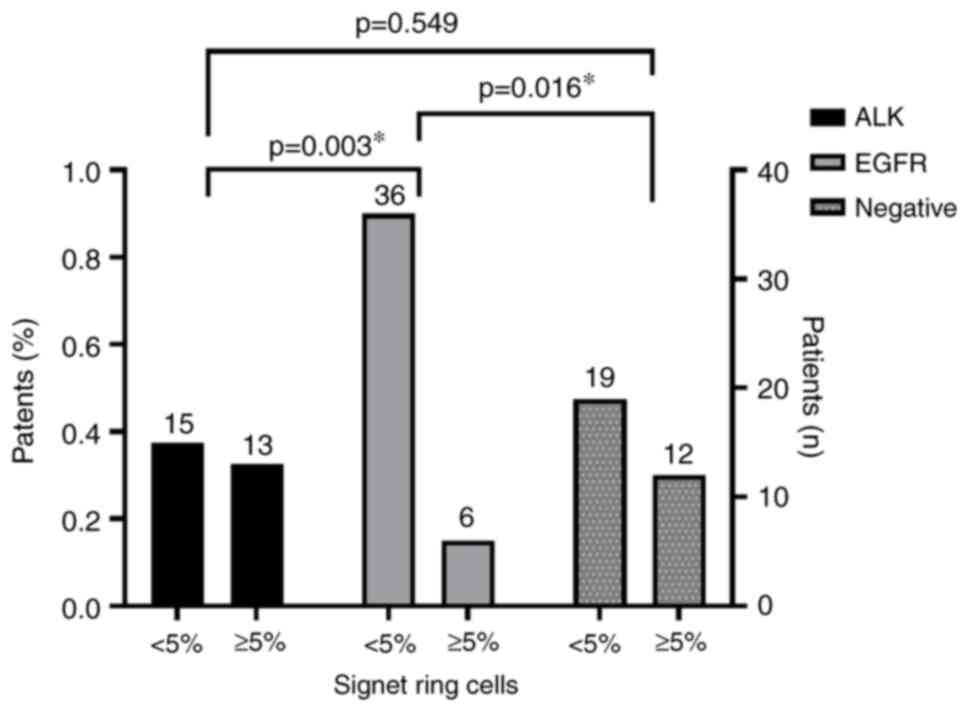
vacuoles, and the presence of signet ring cells (Fig. 2) were statistically significantly
more frequently observed features in the ALK group (P<0.01;
Fig. 3, Fig. 4, Fig.
5).
No statistically significant differences were found
regarding the amount of inflammatory infiltrate, stroma and
cribriform cell arrangement between the groups. Additionally, the
groups exhibited no statistically significant differences in
nuclear size and a degree of nuclear atypia.
A binary logistic regression analysis was conducted
to evaluate the association relationship and predictive accuracy of
specific microscopic features (cell arrangement, nucleoli
visibility, intracytoplasmic vacuoles, signet ring cells and
necrosis in smears) in determining the mutational status of lung
adenocarcinoma (Tables VI and
VII). When compared with the
negative group, the collective influence of these variables as
predictors of EGFR positivity was statistically significant
(P=0.029), with pseudo-R2 values ranging from 0.171 to
0.280. Notably, focal necrosis in smears was associated with a
5.2-fold reduction in the likelihood of EGFR positivity. The
presence of signet ring cells led to a 96.5% decrease in the
probability of EGFR positivity (P<0.05). Incorporating sex and
smoking status into the model enhanced its predictive accuracy by
14%, increasing it from 72.60 to 86.30%. The corresponding
confusion matrix is presented in Tables VIIIA and IXA. Cross-validation of the model is
provided in Table SI, Table SII, Table SIII and Figs. S2 and S3.
 | Table VI.Binary logistic regression model for
mutation status of lung adenocarcinoma according to
micromorphological features (cell arrangement, nucleoli visibility,
intra-cytoplasmic vacuoles, signet ring cells, and necrosis in
smears). |
Table VI.
Binary logistic regression model for
mutation status of lung adenocarcinoma according to
micromorphological features (cell arrangement, nucleoli visibility,
intra-cytoplasmic vacuoles, signet ring cells, and necrosis in
smears).
| Model (H1) | df | χ2 | P-value | McFaden | Negelkerke | Tjur | Cox and Snell |
|---|
| EGFR vs.
negative | 64 | 17.0223 | 0.029 | 0.171 | 0.280 | 0.227 | 0.227 |
| ALK vs.
negative | 49 | 19.163 | 0.024 | 0.235 | 0.370 | 0.250 | 0.277 |
| EGFR vs. ALK | 60 | 44.361 | <0.001 | 0.471 | 0.635 | 0.515 | 0.469 |
 | Table VII.Binary logistic regression model for
mutation status of lung adenocarcinoma according to
micromorphological features (cell arrangement, nucleoli visibility,
intra-cytoplasmic vacuole, signet ring cells, and necrosis in
smears), sex and smoking habits. |
Table VII.
Binary logistic regression model for
mutation status of lung adenocarcinoma according to
micromorphological features (cell arrangement, nucleoli visibility,
intra-cytoplasmic vacuole, signet ring cells, and necrosis in
smears), sex and smoking habits.
| Model (H1) | df | χ2 | P-value | McFaden | Negelkerke | Tjur | Cox and Snell |
|---|
| EGFR vs.
negative | 24 | 52.295 | <0.001 | 0.525 | 0.687 | 0.580 | 0.512 |
| ALK vs.
negative | 46 | 29.849 | 0.003 | 0.366 | 0.523 | 0.416 | 0.397 |
| EGFR vs. ALK | 57 | 49.719 | <0.001 | 0.528 | 0.687 | 0.564 | 0.509 |
 | Table VIII.Confusion matrix of classification
model for mutation status in lung adenocarcinoma according to
micromorphological features. |
Table VIII.
Confusion matrix of classification
model for mutation status in lung adenocarcinoma according to
micromorphological features.
| A, Predicted EGFR
and Negative. |
|---|
|
|---|
| Observed | EGFR | Negative | Accuracy (%) |
|---|
| EGFR | 31 | 11 | 73.81 |
| Negative | 9 | 22 | 70.97 |
| Accuracy |
|
| 72.60 |
|
| B, Predicted ALK
and Negative. |
|
|
Observed | ALK |
Negative | Accuracy
(%) |
|
| ALK | 18 | 10 | 64.29 |
| Negative | 7 | 24 | 77.42 |
| Accuracy |
|
| 71.19 |
|
| C, Predicted
EGFR and ALK. |
|
|
Observed | EGFR | ALK | Accuracy
(%) |
|
| EGFR | 35 | 7 | 83.33 |
| ALK | 4 | 24 | 85.71 |
| Accuracy |
|
| 84.29 |
 | Table IX.Confusion matrix of classification
model for mutation status in lung adenocarcinoma according to
micromorphological features with sex and smoking habits of the
patients. |
Table IX.
Confusion matrix of classification
model for mutation status in lung adenocarcinoma according to
micromorphological features with sex and smoking habits of the
patients.
| A, Predicted EGFR
and Negative. |
|---|
|
|---|
| Observed | EGFR | Negative | Accuracy (%) |
|---|
| EGFR | 38 | 4 | 90.47 |
| Negative | 6 | 25 | 80.65 |
| Accuracy |
|
| 86.30 |
|
| B, Predicted ALK
and Negative. |
|
|
Observed | ALK |
Negative | Accuracy
(%) |
|
| ALK | 20 | 8 | 71.43 |
| Negative | 3 | 28 | 90.32 |
| Accuracy |
|
| 81.36 |
|
| C, Predicted
EGFR and ALK |
|
|
Observed | EGFR | ALK | Accuracy
(%) |
|
| EGFR | 37 | 5 | 88.10 |
| ALK | 6 | 22 | 78.57 |
| Accuracy |
|
| 84.29 |
Similarly, the overall impact of the studied
variables as predictors of ALK positivity, relative to the negative
group, was statistically significant (P=0.024), with
pseudo-R2 values ranging from 0.235 to 0.370. However,
the individual predictors did not reach statistical significance.
The classification model demonstrated an overall accuracy of 70%.
When sex and smoking status were included, the precision of the
model increased by 10%. The confusion matrix is displayed in
Tables VIIIB and IXB. Cross-validation of the model is
provided in Table SIV, Table SV, Table SVI and Figs. S4 and S5.
Furthermore, the present study assessed the
predictive accuracy of the model between the ALK and EGFR groups.
The combined effect of the variables as predictors was highly
significant (P<0.001), with pseudo-R2 values ranging
from 0.471 to 0.635. Focal necrosis in smears emerged as a
significant predictor, reducing the likelihood of EGFR positivity
by 87.77%, while the presence of signet ring cells was associated
with a 13.75-fold increase in the probability of ALK positivity.
The overall accuracy of the model was 84.29%. After incorporating
sex and smoking status into the regression model, the accuracy for
predicting ALK positivity decreased, whereas the accuracy for EGFR
positivity increased, with the overall accuracy remaining
unchanged. The classification performance metrics are detailed in
Tables VIIIC and IXC. Cross-validation of the model is
provided in Table SVII, Table SVIII, Table SIX and Figs. S6 and S7.
Discussion
World cancer statistics indicate a male predominance
in the incidence and prevalence of lung cancer (1). In the present study, there was an
equal representation of sex in the ALK group, while females
predominated in the EGFR group. Studies on patients with ALK-EML4
gene rearrangement have shown varying results regarding sex
distribution (21–24). However, consistent findings across
multiple studies confirm that EGFR-positive lung cancer is more
common in female patients (23–25).
Nonetheless, exceptions, such as a study from Egypt, reported a
higher representation of male patients (25). As regards the smoking habits, there
is no consensus in patients with ALK-EML4 gene rearrangement
regarding the association with smoking (17–20).
The results of the present study align with some of the literature
findings indicating that non-smokers are more commonly associated
with EGFR driver mutations (23–26).
The results of the present study confirm that
certain cytological and histological features of lung
adenocarcinoma are associated with the mutational status of the
tumor. This association can aid in selecting appropriate samples
for testing and may serve as a valuable supplement to the numerous
predictive models currently under development.
Signet ring cells are histologically and
cytologically characteristic cells found in tumors of the
gastrointestinal system, particularly in the stomach and colon, as
well as in ovarian tumors. According to certain studies, tumors
containing signet ring cells comprising 10% of the sample represent
7% of adenocarcinomas, while in all lung cancers, they account for
~1.5% (27,28).
A previous study by Japanese authors revealed that
specific cytomorphological characteristics, including a pink
cytoplasm, vesicular cytoplasm, and smears with predominantly
individually distributed cells, suggest ALK testing positivity.
However, these features cannot replace testing. Nevertheless, in
the absence of histological samples for immunohistochemical
analysis, these parameters could aid in predicting ALK positivity
(8). Nishino et al (10) demonstrated a statistically
significant presence of signet ring cells, micropapillary
arrangement, and hepatoid cell appearance in ALK-positive
adenocarcinomas. They proposed a scoring system with a high
sensitivity (88%) and negative predictive value (87%), but low
specificity (45%) and positive predictive value (49%) (10). Incorporating useful cyto- and
histomorphological parameters into predictive systems could develop
a score with higher predictive values than those mentioned. In the
present study, intracytoplasmic vacuoles and signet ring cells were
statistically significantly more frequent in the ALK group compared
with the EGFR and negative groups. Although no studies comparing
EGFR and ALK groups were found, studies examining ALK vs.
ALK-negative groups identified signet ring cells as a statistically
significant parameter of ALK positivity (12). In the present study, the ALK group
samples had a higher percentage of signet ring cells, although this
difference was not statistically significant. An important
consideration in identifying signet ring cells is their mimics,
such as vacuolar or fatty degeneration. However, the present study
did not address this consideration, representing a limitation
(28).
In addition to mucinous components, other markers
were examined as predictors for ALK positivity. Psammoma bodies and
a ‘Club cell-like’ cytological pattern are statistically
significant markers (11). ‘Club
cell-like’ cells exhibit projections of eosinophilic cytoplasm at
the apical compartment lining the surface of papillary cell
arrangements (29). In a previous
study from Japan (8), the presence
of this cytological pattern and papillary tumor growth were
predictors of ALK-positive tumors. The results of the present study
revealed papillary growth in 21% of ALK-positive tumors, while no
such cases were found in the EGFR and negative groups.
Despite the significance of cytomorphological
parameters in predicting mutations, data on precise parameters
remain insufficient (14–16). Blons et al (30) observed an association between the
lepidic growth pattern of adenocarcinoma and EGFR status. Sharma
et al (14) demonstrated a
statistically significant presence of acini and single-layer cell
bands in EGFR-positive vs. EGFR-negative lung cancer samples. The
present study analyzed histological samples dominated by acini
formations; however, no statistically significant differences were
found compared with the ALK and negative groups. The aforementioned
study also suggested an association of EGFR mutations with nuclear
atypia and chromatin distribution, noting that mild nuclear atypia
was more common in EGFR-positive tumors (14). However, in the present study, there
were no statistically significant differences in nuclear atypia
between groups, and severe nuclear atypia dominated within each
group, contrary to the previous findings (14). The results from an American study
revealed that an acinar growth arrangement was significantly more
common in EGFR-positive lung adenocarcinomas, with the absence of
solid growth serving as a predictor for EGFR negativity, as all
solid adenocarcinomas in their sample were EGFR-negative (15). Similarly, in the present study,
solid growth arrangement was more common in the ALK and negative
groups, although the interpretation of this result should consider
the patient-to-group ratio.
The prediction of molecular analysis positivity has
been explored through various diagnostic modalities. Song et
al (31) introduced a deep
learning model based on computed tomography and clinicopathological
data, successfully predicting ALK positivity with accuracy,
sensitivity and specificity of 76.65, 77.44 and 76.32%,
respectively. By comparison, the regression model in the present
study had an accuracy of 71.19% with only microscopic features. The
addition of sex and smoking habits increased the accuracy to
81.36%.
The present study has limitations which should be
mentioned. The use of samples obtained by different bronchoscopy
sampling techniques in one such limitation. This limitation was
unavoidable due to the rarity of the ALK mutation in the
population. However, potential differences in the frequency of
cyto- and histomorphological characteristics was investigated among
samples obtained through different sampling methods. No
statistically significant differences were observed, indicating no
connection between morphological characteristics and the sampling
method. It is worth noting that other studies employing similar
methodologies also encountered challenges with different sampling
methods (8,10–12).
Another limitation of the present study is the
inability to validate the model using external data. Due to the
rarity of the mutation, all ALK-mutated adenocarcinomas at the
authors' institution with adequate cytological and histological
samples were included in the analysis, leaving no additional cases
for external verification. However, this limitation is partially
mitigated by performing cross-validation using the k-nearest
neighbors test, the results of which are provided in Table SI, Table SII, Table SIII, Table SIV, Table SV, Table SVI, Table SVII, Table SVIII, Table SIX.
In conclusion, the present study revealed
differences in sex distribution and smoking habits between the
groups, alongside statistically significant differences in specific
morphological parameters. These findings suggest the potential
inclusion of these parameters in future models for predicting the
mutational status of NSCLC. Recognizing characteristic patterns in
adenocarcinoma samples associated with specific mutational statuses
can facilitate the triage of samples for appropriate molecular
analyses. However, it is noteworthy that while morphological
analyses provide valuable insights, they are not a substitute for
molecular testing.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NG, AL and SG performed a cytological and
histological assessment on the samples, and final review of the
manuscript. VS and BZ participated in research design and
critically reviewed the intellectual content of the manuscript. AI
and TL participated in the analysis and interpretation of the
results, while MB and SKL analyzed the research findings, focusing
on clinical characteristics and reviewed a discussion based on
these insights. All authors read and approved the final version of
the manuscript. NG, AL and SG confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved (approval nos.
24-VII/10 and 25-VIII/10) by the Professional and Ethical Board of
Institute for Pulmonary Diseases of Vojvodina (Sremska Kamenica,
Serbia). The Institutional Review Board waived the requirement for
informed consent due to the retrospective design of the study with
no risk of identity exposure for patients. The present study did
not include any minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Šutić M, Vukić A, Baranašić J, Försti A,
Džubur F, Samaržija M, Jakopović M, Brčić L and Knežević J:
Diagnostic, predictive, and prognostic biomarkers in non-small cell
lung cancer (NSCLC) management. J Pers Med. 11:11022021. View Article : Google Scholar
|
|
4
|
Nicholson AG, Tsao MS, Beasley MB, Borczuk
AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S,
et al: The 2021 WHO classification of lung tumors: Impact of
advances since 2015. J Thorac Oncol. 17:362–387. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina H,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang F, Wang C, Yang P, Sun P and Liu J:
Pathological cytomorphologic features and the percentage of ALK
FISH-positive cells predict pulmonary adenocarcinoma prognosis: A
prospective cohort study. World J Surg Oncol. 19:2782021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofman P: ALK in non-small cell lung
cancer (NSCLC) pathobiology, epidemiology, detection from tumor
tissue and algorithm diagnosis in a daily practice. Cancers.
9:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyata K, Morita S, Dejima H, Seki N,
Matsutani N, Mieno M, Kondo F, Soejima Y, Tanaka F and Sawabe M:
Cytological markers for predicting ALK-positive pulmonary
adenocarcinoma. Diagn Cytopathol. 45:963–970. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshida A, Tsuta K, Nakamura H, Kohno T,
Takahashi F, Asamura H, Sekine I, Fukayama M, Shibata T, Furuta K
and Tsuda H: Comprehensive histologic analysis of ALK-rearranged
lung carcinomas. Am J Surg Pathol. 35:1126–1134. 2011. View Article : Google Scholar
|
|
10
|
Nishino M, Klepeis VE, Yeap BY, Bergethon
K, Morales-Oyarvide V, Dias-Santagata D, Yagi Y, Mark EJ, Iafrate
AJ and Mino–Kenudson M: Histologic and cytomorphologic features of
ALK-rearranged lung adenocarcinomas. Mod Pathol. 25:1462–1472.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pareja F, Crapanzano JP, Mansukhani MM,
Bulman WA and Saqi A: Cytomorphological features of ALK-positive
lung adenocarcinomas: Psammoma bodies and signet ring cells. Cancer
Cytopathol. 123:162–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ha SY, Ahn J, Roh MS, Han J, Lee JJ, Lee B
and Yim J: Cytologic features of ALK-positive pulmonary
adenocarcinoma. Korean J Pathol. 47:252–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russo A, Franchina T, Riccardi GRR, Picone
A, Ferraro G, Zanghi M, Toscano G, Giordano A and Adamo V: A decade
of EGFR inhibition in EGFR-mutated non-small cell lung cancer
(NSCLC): Old successes and future perspectives. Oncotarget.
6:26814–26825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma S, Gupta N, Singh N, Chaturvedi R,
Behera D and Rajwanshi A: Cytomorphological features as predictors
of epidermal growth factor receptor mutation status in lung
adenocarcinoma. Cytojournal. 15:112018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dacic S, Shuai Y, Yousem S, Ohori P and
Nikiforova M: Clinicopathological predictors of EGFR/KRAS
mutational status in primary lung adenocarcinomas. Mod Pathol.
23:159–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu H, Pan Y, Li Y, Wang L, Wang R, Zhang
Y, Li H, Ye T, Zhang Y, Luo X, et al: Oncogenic mutations are
associated with histological subtypes but do not have an
independent prognostic value in lung adenocarcinoma. Onco Targets
Ther. 7:1423–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi Y, Yokose T, Kawamura K, Iwasaki
S, Murata Y, Onuma S, Hasebe T, Nagai K, Sasaki S and Ochiai A:
Cytologic factors associated with prognosis in patients with
peripheral adenocarcinoma of the lung measuring 3 cm or less in
greatest dimension. Cancer. 105:44–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravaioli S, Bravaccini S, Tumedei MM,
Pironi F, Candoli P and Puccetti M: Easily detectable
cytomorphological features to evaluate during ROSE for rapid lung
cancer diagnosis: From cytology to histology. Oncotarget.
8:11199–11205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radić J, Nikolić I, Kolarov-Bjelobrk I,
Vasiljević T, Djurić A, Vidović V and Kožik B: Prognostic and
predictive significance of primary tumor localization and HER2
expression in the treatment of patients with KRAS wild-type
metastatic colorectal cancer: Single-centre experience from serbia.
J Pers Med. 14:8792024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marotti JD, Schwab MC, McNulty NJ, Rigas
JR, DeLong PA, Memoli VA, Tsongalis GJ and Padmanabhan V:
Cytomorphologic features of advanced lung adenocarcinomas tested
for EGFR and KRAS mutations: A retrospective review of 50 cases.
Diagn Cytopathol. 41:15–21. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noronha V, Ramaswamy A, Patil VM, Joshi A,
Chougule A, Kane S, Kumar R, Sahu A, Doshi V, Nayak L, et al: ALK
positive lung cancer: clinical profile, practice and outcomes in a
developing country. PLoS One. 11:e01607522016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou X, Chen H, Liu Y, Gong S, Zhudai M and
Shen L: Clinicopathological and computed tomography features of
patients with early-stage non-small-cell lung cancer harboring ALK
rearrangement. Cancer Imaging. 23:202023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang HJ, Lim HJ, Park JS, Cho YJ, Yoon HI,
Chung JH, Lee JH and Lee CT: Comparison of clinical characteristics
between patients with ALK-positive and EGFR-positive lung
adenocarcinoma. Respir Med. 108:388–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Q, Huang Q, Yu Z and Wu H: Clinical
characteristics of non-small cell lung cancer patients with EGFR
mutations and ALK&ROS1 fusions. Clin Respir J. 16:216–225.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou JY, Zheng J, Yu ZF, Xiao WB, Zhao J,
Sun K, Wang B, Chen X, Jiang LN, Ding W and Zhou JY: Comparative
analysis of clinicoradiologic characteristics of lung
adenocarcinomas with ALK rearrangements or EGFR mutations. Eur
Radiol. 25:1257–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mourabiti AY, Sqalli Houssaini M, Benfares
A, Bouardi NE, Lamrani MYA, Fatemi HE, Serraj M, Amara B, Qjidaa H,
Smahi M, et al: Clinical and radiological features associated with
EGFR mutation in non-small-cell lung cancer: A study of 149 cases.
Egypt J Radiol Nucl Med. 54:1712023. View Article : Google Scholar
|
|
27
|
Tsuta K, Ishii G, Yoh K, Nitadori J-I,
Hasebe T, Nishiwaki Y, Endoh Y, Kodama T, Nagai K and Ochiai A:
Primary lung carcinoma with signetring cell carcinoma components:
Clinicopathological analysis of 39 cases. Am J Surg Pathol.
28:868–874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boland JM, Wampfler JA, Jang JS, Wang X,
Erickson–Johnson MR, Oliveira AM, Yang P, Jen J and Yi ES:
Pulmonary adenocarcinoma with signet ring cell features: A
comprehensive study from 3 distinct patient cohorts. Am J Surg
Pathol. 38:1681–1688. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyata-Morita K, Morita S, Matsutani N,
Kondo F, Soejima Y and Sawabe M: Frequent appearance of club cell
(Clara cell)-like cells as a histological marker for ALK-positive
lung adenocarcinoma. Pathol Int. 69:688–696. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blons H, Côté JF, Le Corre D, Riquet M,
Fabre-Guilevin E, Laurent-Puig P and Danel C: Epidermal growth
factor receptor mutation in lung cancer are linked to
bronchioloalveolar differentiation. Am J Surg Pathol. 30:1309–1315.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song Z, Liu T, Shi L, Yu Z, Shen Q, Xu M,
Huang Z, Cai Z, Wang W, Xu C and Sun J: The deep learning model
combining CT image and clinicopathological information for
predicting ALK fusion status and response to ALK-TKI therapy in
non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging.
48:361–371. 2021. View Article : Google Scholar : PubMed/NCBI
|