Introduction
According to the 2021 central nervous system (CNS)
tumor classification by the World Health Organization (WHO),
primary mesenchymal CNS tumors, including solitary fibrous tumors
(SFTs), are rare and typically originate from the meninges
(1). CNS SFTs show an age-adjusted
incidence rate of 3.77 per 10,000,000 individuals (2), constituting 0.22% of all CNS tumors
(3). A systematic review of 563
patients (average age, 41 years) revealed a slight predominance of
CNS SFTs in males (55%, 246/450) and recurrence in 57% of cases
(158/277) (4). Another systematic
review found extracranial metastasis in 28% of cases (251/904),
with a predilection for lung, liver, bone and pleural metastases
(5). Additionally, WHO grade III
was associated with a 1.88-fold increased risk of extracranial
metastasis (5). The standard CNS
SFT treatment involves surgery and adjunctive radiation therapy
(6); however, CNS SFTs that
metastasize to multiple extracranial lesions are not amenable to
surgical resection, and radiation therapy for localized
extra-meningeal SFTs does not extend the overall survival time
(6). Furthermore, the efficacy of
conventional chemotherapy is limited (6). No other standard treatment strategies
have been established.
According to the 2021 WHO classification of CNS
tumors, CNS SFTs are defined as ‘fibroblastic neoplasms’,
categorized as ‘mesenchymal and non-meningothelial tumors’
(1). These tumors are graded as
follows: Grade 1 [<5 mitoses/10 high-power fields (HPFs)], grade
2 (≥5 mitoses/10 HPF, without necrosis) and grade 3 (≥5 mitoses/10
HPF, with necrosis). Patients with CNS SFTs harbor the nerve growth
factor I-A [also known as early growth response (EGR) 1] binding
protein (NAB) 2::signal transducer and activator of transcription
(STAT) 6 fusion gene, resulting from chromosomal inversion at the
12q13 locus (1). The classification
suggests that CNS SFTs could be placed in the same group as
pleural-origin SFTs; however, the precise cellular origin of CNS
SFTs remains unclear (1).
NAB2 and STAT6 are localized to the nucleus and
cytoplasm, respectively. However, immunostaining of CNS SFTs has
revealed nuclear localization of STAT6 owing to the presence of the
NAB2::STAT6 fusion gene, enabling their differentiation from
meningiomas (1,7,8). The
NAB2::STAT6 fusion protein, mediated by EGR1, activates target
genes, including fibroblast growth factor (FGF) 2, platelet-derived
growth factor (PDGF) D and receptor tyrosine kinases, including FGF
receptor (FGFR) 1 and neurotrophic tyrosine receptor kinase 1, all
involved in cell proliferation (9).
The EGR1 target genes include vascular endothelial growth factor
(VEGF) A and basic FGF, indicating their involvement in tumor
angiogenesis (6).
Pazopanib, a tyrosine kinase inhibitor, has multiple
targets, including VEGF receptor (VEGFR)1/2/3, PDGF receptor α/β
and FGFR1/3 (10,11). In particular, pazopanib inhibits
VEGFR2, further suppressing angiogenesis (11–13).
Pazopanib demonstrated prolonged median progression-free survival
in a phase 3 trial involving 369 patients with metastatic
soft-tissue sarcoma (14); however,
no patients with CNS SFT were included in the study. Therefore,
while pazopanib exhibits significant efficacy against extracranial
soft-tissue sarcoma, its effectiveness in CNS SFT remains unproven.
Pazopanib has been approved for the treatment of advanced renal
cell carcinoma and soft-tissue sarcoma in the United States and the
European Union (11). Additionally,
pazopanib may be considered in cases where extracranial SFTs
originate from the pleura and are classified as malignant soft
tissue tumors, despite the conventional restriction on pazopanib
application for CNS SFTs; however, its efficacy in the treatment of
CNS SFTs remains unclear. In the present study, 3 cases of
high-grade CNS SFTs with multiple extracranial metastases that were
treated with on-label pazopanib are described to examine the
efficacy of pazopanib in treating CNS SFTs.
Case report
Cases
In total, 3 consecutive cases of CNS SFTs with
multiple extracranial metastases were treated with pazopanib at
Kochi Health Sciences Center between January 2018 and April 2024.
The standard oral dose of pazopanib was set at 800 mg daily based
on a previous phase 3 trial (14).
A reduced dose of 600 mg daily was administered based on patient
conditions or adverse events related to pazopanib, as reported
previously in a phase 2 trial (15). Figs.
S1 and S2 show the baseline
whole-body images and representative pathological findings,
respectively.
Case 1
A 51-year-old male patient who developed weakness in
the right lower limb was diagnosed with a parasagittal sinus mass
in December 2011. The patient underwent an initial tumor resection
that same month and was diagnosed with atypical meningioma.
Intracranial multifocal tumor regrowth necessitated multiple tumor
resections and γ-knife treatments. The third tumor resection in
September 2022 revealed a WHO grade 3 CNS SFT. After the sixth
γ-knife therapy in October 2022, the patient was referred to Kochi
Health Sciences Center with a Karnofsky Performance Status (KPS) of
60%. The patient exhibited right-hand dexterity impairment, right
lower limb paresis and right lower limb sensory impairment. A
whole-body computed tomography (CT) revealed multiple masses in the
liver, pelvic cavity bones and a right cervical lymph node. A
biopsy of the right sacral lesion confirmed SFT and pazopanib (800
mg daily) was initiated in January 2023. The pelvic cavity tumor
was enlarged 2 months later, prompting a ~3-week interruption of
pazopanib treatment and the total resection of the enlarged tumor.
Additional γ-knife therapy was administered for intracranial tumor
growth in September 2023. Pazopanib was discontinued in October
2023 owing to an infection necessitating a sequestrectomy. In March
2024, both intracranial and extracranial residual tumors showed
progressive disease (PD), necessitating additional γ-knife therapy.
The enlarged sacral tumor reduced the KPS to 50% by April 2024, and
heavy-ion radiotherapy was planned. The patient presented with
worsening right lower limb paresis and pain from the right buttock
to the right lower limb. A slowly enlarging liver lesion was also
observed on a whole-body CT. Accordingly, heavy-ion radiotherapy
(70.4 Gy, relative biological effectiveness, in 16 fractions) was
administered for the right sacral lesion in June-July 2024,
followed by stereotactic body radiotherapy (48 Gy in 4 fractions)
for the liver lesion in August 2024. However, in September 2024,
the patient presented with left facial paresis, dysphagia and left
hemiparesis, and a whole-body CT revealed a rapid increase in
multiple intracranial lesions. The patient refused all possible
treatments, including the resumption of pazopanib, and palliative
care was initiated. The patient experienced pazopanib-related
adverse events of grade 3 diarrhea and grade 1 increase in serum
bilirubin levels (1.3 mg/dl; normal range 0.2–1.2 mg/dl). The
patient was treated with probiotics for diarrhea. Subsequently, the
diarrhea and hyperbilirubinemia improved with the discontinuation
of pazopanib.
Case 2
A 60-year-old female patient with dizziness and
headache was initially diagnosed with a left infratentorial mass.
The patient promptly underwent an initial tumor resection in
September 2006 and was diagnosed with hemangiopericytoma. However,
tumor regrowth, including a continuous extension to the left
supratentorial region, necessitated a second resection with three
γ-knife treatments by November 2022. Rapid tumor growth after the
third γ-knife treatment led to the transfer of the patient to Kochi
Health Sciences Center, with a KPS of 40%. The patient presented
with impaired consciousness, aphasia and right hemiparesis. The
patient promptly underwent a partial tumor resection of the rapidly
enlarged left supratentorial tumor, confirmed as WHO grade 3 CNS
SFT, in February 2023. A whole-body CT revealed multiple bone and
lung metastases, and a biopsy of the thoracic vertebra 1 lesion
confirmed SFT. Consequently, pazopanib (600 mg daily) was initiated
on day 0. However, bleeding from the postoperative supratentorial
lesion on day 6 resulted in a KPS of 30% and a 4-day interruption
of pazopanib treatment. Eventually, the patient achieved stable
disease on day 42 according to the Choi criteria (16). However, postoperative hydrocephalus
necessitated further surgery, leading to a preoperative
interruption of pazopanib on day 57. The patient showed improvement
in right upper limb paresis. After removing the residual tumor in
the left supratentorial region on day 65, the KPS score improved to
40%. However, rapid growth of the residual tumor in the left
infratentorial region occurred on day 66, and the patient died on
day 70. The pazopanib-related adverse event was a grade 3
intracranial hemorrhage.
Case 3
A 39-year-old female patient with diplopia was
diagnosed with a large left frontal convexity tumor in September
2014 necessitating semi-emergency resection, revealing a WHO grade
2 hemangiopericytoma. Magnetic resonance imaging (MRI) conducted in
September 2017 and 2018 revealed two skull lesions, prompting
referral to Kochi Health Sciences Center, with a KPS of 100%. The
patient did not exhibit any neurological deficits. An
18F-fluorodeoxyglucose positron emission tomography-CT
scan performed in October 2018 revealed multiple liver, kidney and
bone lesions. Consequently, the patient underwent posterior
fixation for cervical vertebra 6 (C6) mass compression and a left
sacral lesion biopsy, which confirmed a WHO grade 2
SFT/hemangiopericytoma. Denosumab (120 mg, subcutaneous injection
every 4 weeks) was initiated that month for the treatment of
multiple lytic bone lesions and proton therapy (65 Gy equivalent in
26 fractions) was administered to the enlarged soft tissue mass
derived from C6. The patient presented with numbness in the right
fingers. Pazopanib (800 mg daily) was initiated in January 2019 but
was reduced to 600 mg after 1 month owing to grade 2 hypertension.
The patient had been on calcium channel blockers and angiotensin II
inhibitors for blood pressure control prior to the initiation of
pazopanib; however, due to an increase in blood pressure after
pazopanib initiation, adjustments to these antihypertensive
medications and a reduction in the pazopanib dosage were required
to maintain blood pressure control. Partial response was achieved
(according to the Choi criteria) in April 2019. The patient showed
right-hand dexterity impairment in November 2019. Although
pazopanib was administered for 3.5 years, PD necessitated treatment
changes, including the administration of two chemotherapeutic
agents: Trabectedin (1.2 mg/m2) in July 2022 and
eribulin (1.4 mg/m2) in October 2023, followed by plans
to resume pazopanib in April 2024, despite a KPS of 80%. The
patient then exhibited right upper limb paresis. Throughout the 9
years, the patient did not experience a recurrence of the initial
intracranial tumor. However, one of the two skull lesions protruded
slightly outward and the extracranial soft tissue tumors
progressed, whereas the lytic bone lesions transformed into
sclerotic lesions, indicating disease stabilization. Pazopanib
treatment was resumed in May 2024. A whole-body CT in June 2024
showed a decrease in the density of multiple systemic lesions;
however, a whole-body CT in October 2024 indicated a subsequent
increase in density. The tumor size exhibited a gradual tendency to
increase.
Imaging assessments
In the present study, it was investigated how
pazopanib affected the 3 cases. First, to assess the tumor
backgrounds in the 3 cases, the baseline CT tumor characteristics
measured at Kochi Health Sciences Center were compared (Table I). The methods of the statistical
analysis are detailed in Appendix
S1. No significant difference was observed in the intracranial
tumor densities between Cases 1 and 2 (P=0.290). However, the
extracranial tumor density was higher in Case 3 than Case 2
(P=0.005), likely due to the inclusion of a lung lesion in Case 2
and the high-density range in Case 3. The intracranial tumor size
was larger in Case 2 than Case 1 (P=0.049). Case 1 had only
supratentorial tumors, whereas in Case 2, partial resection of the
supratentorial tumor led to a predominance of infratentorial tumors
(P=0.003). No significant differences were observed in the
extracranial tumor sizes among the three cases (P=0.226).
 | Table I.Baseline tumor characteristics via
computed tomography. |
Table I.
Baseline tumor characteristics via
computed tomography.
| Tumor
characteristics | Case 1 | Case 2 | Case 3 | P-value |
|---|
| Tumor density (HU),
[n (%)] |
|
|
|
|
|
Intracranial | 85.75
(63.80–104.00), | 77.45
(66.55–80.00), | NA | 0.290a |
|
| [16 (32.0)] | [4 (8.0)] |
|
|
|
Extracranial | 97.85
(77.50–118.00), | 82.90
(69.10–95.05), | NA | 0.315b |
|
| [4 (8.0)] | [7 (14.0)] |
|
|
|
Extracranial | 97.85
(77.50–118.00), | NA | 138.00
(126.00–143.00), | 0.063b |
|
| [4 (8.0)] |
| [19 (38.0)] |
|
|
Extracranial | NA | 82.90
(69.10–95.05), | 138.00
(126.00–143.00), | 0.005b |
|
|
| [7 (14.0)] | [19 (38.0)] |
|
| Tumor size (mm), [n
(%)] |
|
|
|
|
|
Intracranial | 7.38
(5.39–13.93), | 24.36
(12.29–38.51), | NA |
0.049a |
|
| [16 (32.0)] | [4 (8.0)] |
|
|
|
Extracranial | 11.52
(7.69–16.64), | 20.79
(18.91–22.80), | 16.24
(10.44–20.03), | 0.226c |
|
| [4 (8.0)] | [7 (14.0)] | [19 (38.0)] |
|
| Intracranial tumor
locations, n (%) |
|
|
|
|
|
Supratentorial | 16 (80) | 1 (5) | NA |
0.003d |
|
Infratentorial | 0 (0.0) | 3 (15) | NA |
|
Subsequently, to evaluate the efficacy of pazopanib
on intracranial and extracranial lesions in CNS SFT, imaging
assessments pre- and post-treatment in the 3 cases were conducted.
The details of the methods are provided in Appendix S1. Fig. 1 depicts the representative CT and
MRI findings for each case. In all cases, by observational
assessment, except for the right sacral tumor in Case 1, the CT
density or MRI intensity decreased after pazopanib initiation or
resumption and increased after interruption. Case 3 showed clear
decreases in tumor CT densities after pazopanib initiation. In Case
1, by observational assessment, the size of the intracranial tumor
decreased with pazopanib treatment and increased without it. In
Case 2, the infratentorial tumors grew rapidly upon interruption of
treatment, occupying the infratentorial region and compressing the
brainstem.
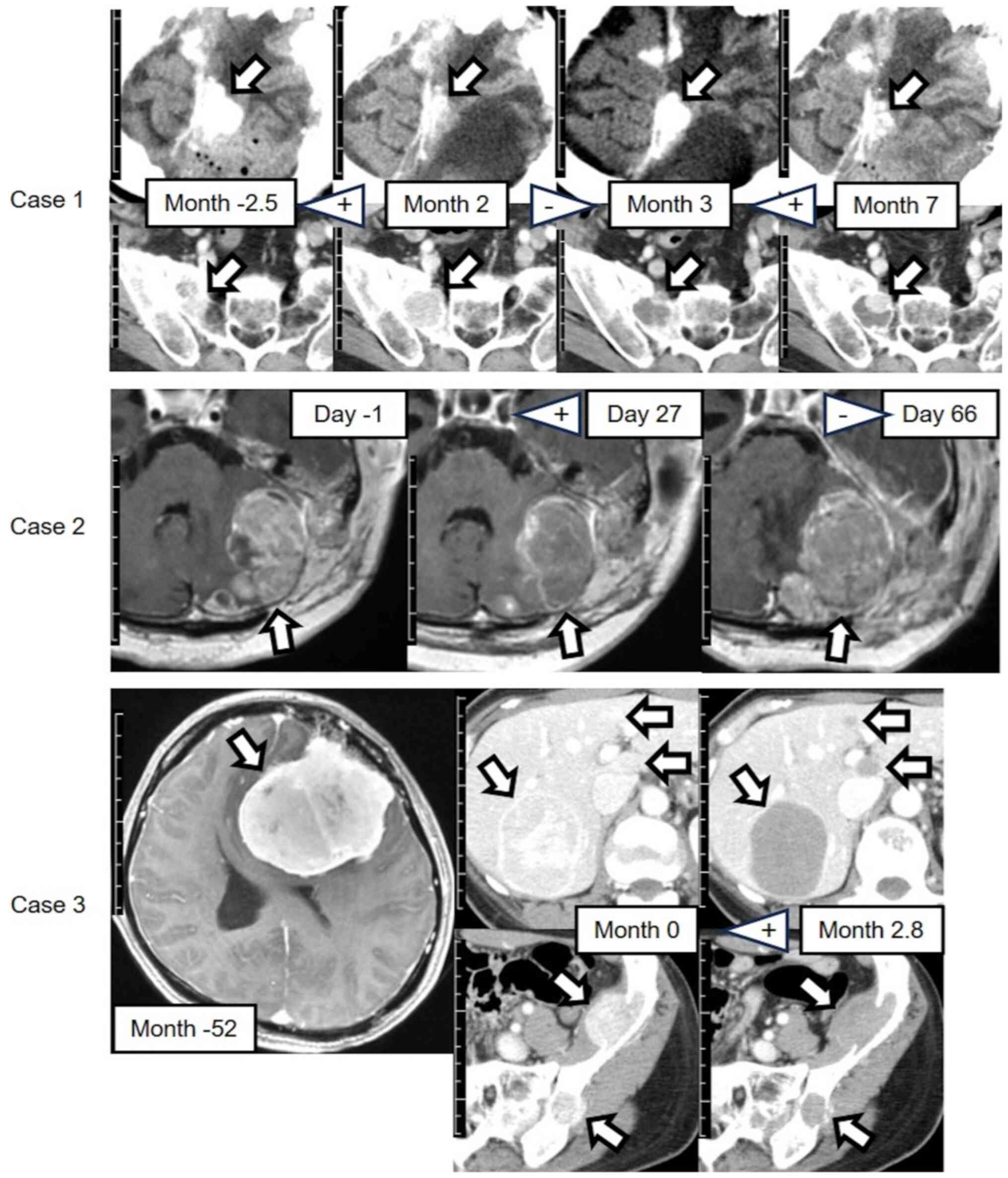 | Figure 1.Contrast-enhanced CT or head
T1-weighted images of the 3 cases. The arrows indicate the
representative tumors. The months or days indicate the time since
initiation of pazopanib treatment. + indicates initiation or
resumption of pazopanib treatment; - indicates interruption of
pazopanib treatment. Scale bar, 1 cm per division. Case 1: Upper
row, left supratentorial falx tumors, head CT; lower row, right
sacral tumor, pelvic CT. Case 2: T1-weighted images showing left
infratentorial tumors. Case 3: Upper row, liver tumors, abdominal
CT; lower row, left iliac tumors, pelvic CT; contrast-enhanced head
T1-weighted image shows the initial primary tumor with no
recurrence after resection. The image was provided by a previous
institute, whose imaging equipment and conditions differed from
those described in Appendix S1.
CT, computed tomography. |
To further evaluate the efficacy of pazopanib in the
3 cases, waterfall plots presenting the change ratios from pre- to
post-pazopanib initiation, interruption or resumption were
constructed (Fig. 2). The imaging
evaluation methods are detailed in Appendix S1. In Case 1, after pazopanib
initiation, most intracranial and extracranial tumor CT densities
generally decreased (Fig. 2A). By
contrast, the size of approximately half of the intracranial tumors
and most of the extracranial tumors increased (Fig. 2B). Indeed, pazopanib was
subsequently interrupted in Case 1 and surgical removal of an
enlarged extracranial tumor was performed. Following interruption,
the CT densities increased in approximately half of the
intracranial tumors but decreased in all extracranial tumors
(Fig. 2C). The size of most
intracranial and extracranial tumors increased (Fig. 2D) but without rapid enlargement. In
Case 1 after pazopanib resumption, the CT densities decreased in
all intracranial and extracranial tumors (Fig. 2E). However, the size of more than
half of the intracranial and extracranial tumors increased
(Fig. 2F). In Case 2,
contrast-enhanced head MRIs were used for the evaluation as
contrast-enhanced whole-body CTs were not performed after pazopanib
interruption. After pazopanib initiation in Case 2, the size of
more than half of the intracranial tumors decreased, but an
extracranial tumor (a left skull base bone lytic tumor) increased
in size (Fig. 2G). However, after
pazopanib interruption, all tumor sizes increased (Fig. 2H). The enlargement was rapid,
leading to death. In Case 3, since there was no recurrence of the
primary intracranial tumor, the evaluation focused solely on
extracranial tumors based on a contrast-enhanced whole-body CT
conducted 2.8 months after pazopanib initiation, as subsequent
follow-up imaging was limited to plain whole-body CTs. After
pazopanib initiation in Case 3, the CT densities decreased in all
extracranial tumors (Fig. 2I), and
the tumor size decreased in most extracranial tumors (Fig. 2J).
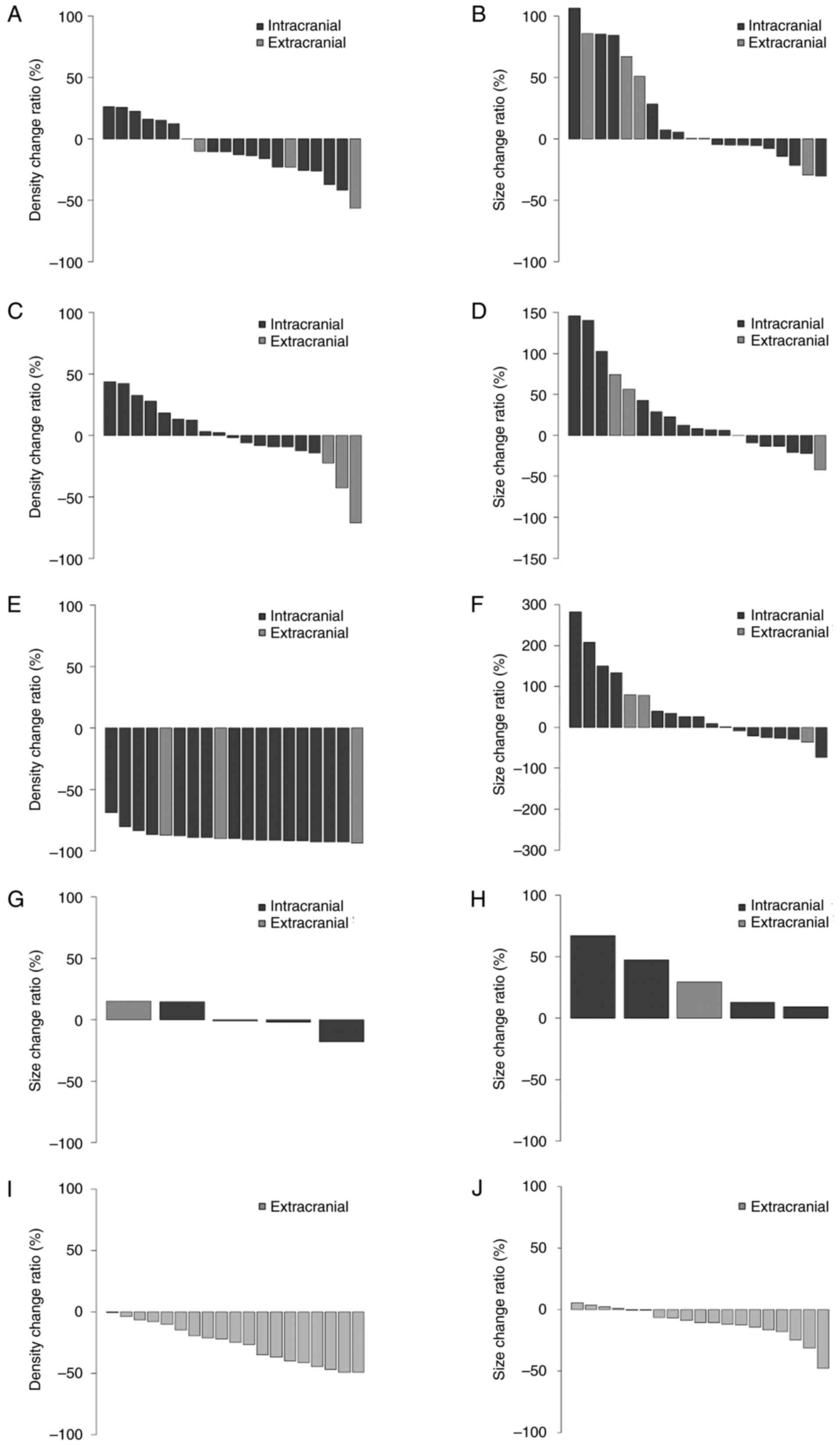 | Figure 2.Waterfall plots display change ratios
from pre- to post-pazopanib initiation, interruption or resumption.
Distributions of (A) tumor density and (B) size change ratios in 20
CT lesions (intracranial, 16; extracranial, 4) in Case 1 after
pazopanib initiation. Distributions of (C) tumor density and (D)
size change ratios in 19 CT lesions (intracranial, 16;
extracranial, 3) in Case 1 after pazopanib interruption.
Distributions of (E) tumor density and (F) size change ratios in 19
CT lesions (intracranial, 16; extracranial, 3) in Case 1 after
pazopanib resumption. Distributions of tumor size change ratios in
5 magnetic resonance imaging lesions (intracranial, 4;
extracranial, 1) in Case 2 after pazopanib (G) initiation and (H)
interruption. Distributions of (I) tumor density and (J) size
change ratios in 19 extracranial CT lesions in Case 3 after
pazopanib initiation. CT, computed tomography. |
Discussion
CNS SFTs show high rates of extracranial metastasis
(5), leading to unresectable
systemically enlarging lesions that pose challenges in patient
management. The results of the present study suggest a deviation
from the typical surgical treatment approach, indicating a
potential alternative strategy or response to the unique
characteristics of CNS SFTs. The present study also clarified the
changes in tumor density and/or size from pre- to post-pazopanib
initiation, emphasizing the significant concerns regarding
pazopanib interruption. This is clinically important and
noteworthy, as it has not been previously reported in studies on
patients with CNS SFTs.
Pazopanib efficacy has been assessed in a phase 3
trial of 369 patients with metastatic soft-tissue sarcoma without
CNS involvement (14). The median
progression-free survival was 4.6 months in patients who underwent
pazopanib therapy, significantly surpassing that observed with the
placebo (1.5 months), whereas the median overall survival did not
increase after pazopanib therapy. Pazopanib was also tested in a
phase 2 trial for systemic SFTs in 36 patients, 5 of whom had
meningeal involvement (15).
However, the efficacy of pazopanib in CNS SFTs has not been
individually assessed. Pazopanib is preferred for the treatment of
SFTs but not for CNS SFTs, according to the National Comprehensive
Cancer Network Guidelines version 1.2024 (https://www.nccn.org/). Pazopanib was shown to reduce
the intracranial SFT volumes in 2 cases (WHO grades 2 and 3) after
4–6 months (17). Another study
reported that extracranial metastatic lesions shrank after 3–4
months of pazopanib therapy (18).
Grade 3–4 adverse events of pazopanib treatment include
hypertension (3–29%), lymphopenia (4–14%), diarrhea (4–8%),
elevated alanine aminotransferase (0–19%), aspartate
aminotransferase (2–8%) and bilirubin (0–6%) levels, bleeding (2%),
hypoglycemia (0–5%), hyperglycemia (0–3%) (12), pneumothorax (2–3%), and
thrombocytopenia (2–3%) (19). In
the present study, the adverse events associated with pazopanib
were assessed according to the Common Terminology Criteria for
Adverse Events, version 5.0 (20).
However, the criteria do not specify clear discontinuation
guidelines for the medication, and we considered the following:
Case 1 had grade 3 diarrhea and grade 1 hyperbilirubinemia, which
resolved after pazopanib discontinuation. Antidiarrheal agents were
also required for the management of diarrhea. Case 2 had
postoperative grade 3 intracranial hemorrhage, necessitating
temporary interruption, whereas Case 3 required a pazopanib dose
reduction and increased antihypertensive treatment due grade 2
hypertension. Thus, managing the adverse events required
interruption, discontinuation, dose reduction and symptomatic
treatments. Interruption and discontinuation may require prompt
resumption and medication changes, as demonstrated in Cases 2 and
3, respectively; however, the changes have limitations regarding
medication selection. The mechanisms by which pazopanib causes
diarrhea, hemorrhage and hypertension have been proposed as
submucosal fat accumulation in the gastrointestinal tract (21), targeting kinase events downstream of
glycoprotein VI and other platelet receptors (22), imbalance in vasoconstrictors and
vasodilators, capillary depletion and direct renal impairment
(23).
A PubMed (https://pubmed.ncbi.nlm.nih.gov/) search for
English-language literature published between January 2000 and
April 2024 was conducted using the terms ‘meninges’, ‘solitary
fibrous tumor’, ‘hemangiopericytoma’ and ‘pazopanib’. The search
yielded only two previous studies (17,24); a
comparison with the cases of the present study is summarized in
Table II. Maeda et al
(24) reported the off-label use of
temozolomide and bevacizumab in 4 SFT cases, including only 1 CNS
SFT case additionally treated with pazopanib. The treatment
response was evaluated based on the Response Evaluation Criteria in
Solid Tumors, version 1.1 (RECIST 1.1) (6,25) and
Choi criteria. However, several results from these studies were
unavailable. Shorter progression-free survival was observed in the
present study compared with that observed in the Apra et al
study (17), although the efficacy
of pazopanib was comparable between the two studies.
 | Table II.Literature review and comparison with
cases in the present study. |
Table II.
Literature review and comparison with
cases in the present study.
|
|
|
|
|
|
| Best overall
response | PFS, months | Rapid tumor growth
after pazopanib interruption |
|
|
|
|---|
| Authors, year | Age, years | Sex | WHO grade | Primary tumor sites
in the CNS | Locations of
metastases |
|
|
| OS, months | Prognosis | (Refs.) |
|---|
| RECIST | Choi | RECIST | Choi | Intracranial | Extracranial |
|---|
| Apra et al,
2018 | 31 | M | 3 | Bil.
Infratentorial | None | PR | NA | 4 | ND | ND | ND | ND | ND | (17) |
|
| 52 | F | 2 | Rt. temporal | ND | PR | NA | 6 | ND | ND | ND | ND | ND |
|
| Maeda et al,
2020a | 52 | F | NA |
Supra/infratentorial | Liver, lung | NDb | NDb | ND | ND | ND | ND | NDc | Dead | (24) |
| Present study | 51 | M | 3 | Lt. parietal | Pelvic cavity,
liver, bone, lymph noded | NAe | NAe | 2 | 2 | No | No | 16+ | Alive | - |
|
| 60 | F | 3 | Lt.
infratentorial | Lung, bone | PD | SD | 1.4 | 1.4 | Yes | NA | 2.3 | Dead |
|
|
| 39 | F | 2 | Lt. frontal | Liver, kidney,
bone | SD | PR | 2.8 | 2.8 | No | No | 64+ | Alive |
|
A case report of uterine carcinosarcoma with right
lung metastasis described rapid tumor growth following pazopanib
interruption; tumor reduction was observed following pazopanib
resumption, suggesting the benefit of early pazopanib resumption
after interruption (26). In the
present study, Case 1 experienced some pazopanib interruptions but
no rapid growth, whereas Case 2 exhibited rapid growth upon
pazopanib interruption. By contrast, trabectedin was promptly
initiated in Case 3 upon discontinuation of pazopanib, resulting in
no rapid growth. Thus, pazopanib interruption does not necessarily
induce rapid growth. The critical factors for rapid growth
following interruption remain unclear. However, dose reduction or
prompt medication changes may help prevent rapid growth. Regarding
tumor growth and mortality after interruption, the supratentorial
lesions in Case 1 were enlarged without mortality. By contrast, the
infratentorial lesions in Case 2 were enlarged with perifocal
edema, causing fatal brainstem compression. Case 2 had larger
baseline tumors than those of Case 1, and growth in a confined
infratentorial region could have contributed to the mortality.
Nevertheless, the present study has some
limitations. First, the baseline contrast-enhanced CT for Case 1
was obtained 2.5 months before pazopanib initiation (Table SI) and the tumor sizes increased
during this time. This explains the large tumor size changes from
pre- to post-pazopanib initiation. This also diverged from strict
adherence to the RECIST 1.1 and Choi criteria. Follow-up imaging
periods varied across the cases (Table
SI). Case 3 underwent only one follow-up contrast-enhanced
whole-body CT. Therefore, consistent follow-up imaging is required
in further studies. Second, normalization was not feasible for
evaluating quantitative MRI intensity changes owing to variations
in the machines and imaging conditions for MRI. Thus, further
evaluation with MRI may be required. Third, in Case 3, denosumab
caused sclerotic lytic bone lesions, possibly affecting the
efficacy of pazopanib. However, denosumab is ineffective for soft
tissue lesions. Fourth, the present study describes the experience
of only 3 cases, and it does not assess the robustness or
generalizability of the results. Fifth, prognostic tumor biomarkers
for CNS SFTs have not been established. Interferon-stimulated gene
15 (27) and p53 (28) have been reported as potential
prognostic factors for SFTs; however, there has been no specific
mention for CNS SFTs. The present study lacked data on these
biomarkers, necessitating further investigation.
In conclusion, pazopanib may inhibit both
intracranial and extracranial tumor growth in CNS SFTs with
multiple extracranial metastases. In the present study, 1 patient
received pazopanib for >3 years. Further research and case
studies are required to determine the efficacy of pazopanib.
However, caution is warranted regarding rapid tumor growth
following pazopanib interruption. Thus, abrupt interruption should
be avoided and gradual tapering or prompt transition to alternative
agents is recommended.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Jun Iwata and Dr
Manabu Matsumoto (Department of Pathology, Kochi Health Sciences
Center, Kochi, Japan) for providing the pathological images, Mr.
Yuichi Taniguchi (Department of Medical Technology, Kochi Health
Sciences Center, Kochi, Japan) for supplying the pathology
protocols, and Mr. Masaki Oka and Mr. Yoshiaki Wada (Department of
Medical Technology, Kochi Health Sciences Center, Kochi, Japan) for
their assistance in preparing the CT and MRI protocols.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TT, NM, KN and HI conceptualized and designed the
study, developed the data collection instruments, collected data,
conducted the initial analyses, analyzed, interpreted and validated
the data as well as reviewed and revised the manuscript. NK, DY,
YK, TM and HN coordinated and supervised the data collection and
validation and reviewed the manuscript. TT drafted the manuscript.
All authors have read and approved the final version of the
manuscript. TT and HI confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study was performed in-line with the principles
of the Declaration of Helsinki. Approval was granted by the Ethics
Committee of Kochi Health Sciences Center (Kochi, Japan; date:
February 13, 2024; approval no. 231080). We declare that this study
complies with the CARE reporting guidelines. Written informed
consent was obtained from the patients or from family members if
the patient had passed away.
Patient consent for publication
Research participants provided informed consent for
the publication of the manuscript and images.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Takaya Tsuno ORCID ID: 0000-0002-9446-2374
Glossary
Abbreviations
Abbreviations:
|
CNS
|
central nervous system
|
|
SFT
|
solitary fibrous tumor
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
WHO
|
World Health Organization
|
|
HPF
|
high-power field
|
|
EGR
|
early growth response
|
|
NAB
|
nerve growth factor I-A binding
protein
|
|
STAT
|
signal transducer and activator of
transcription
|
|
FGF
|
fibroblast growth factor
|
|
PDGF
|
platelet-derived growth factor
|
|
FGFR
|
FGF receptor
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
VEGF receptor
|
|
KPS
|
Karnofsky Performance Status
|
|
PD
|
progressive disease
|
|
RECIST 1.1
|
Response Evaluation Criteria In Solid
Tumors, version 1.1
|
References
|
1
|
WHO Classification of Tumours Editorial
Board, . Mesenchymal, non-meningothelial tumours involving the CNS.
Central Nervous System Tumours. WHO Classification of Tumours (5th
edition). International Agency for Research on Cancer; Lyon: pp.
299–305. 2021
|
|
2
|
Kinslow CJ, Bruce SS, Rae AI, Sheth SA,
McKhann GM, Sisti MB, Bruce JN, Sonabend AM and Wang TJC:
Solitary-fibrous tumor/hemangiopericytoma of the central nervous
system: A population-based study. J Neurooncol. 138:173–182. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trifiletti DM, Mehta GU, Grover S and
Sheehan JP: Clinical management and survival of patients with
central nervous system hemangiopericytoma in the national cancer
database. J Clin Neurosci. 44:169–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rutkowski MJ, Sughrue ME, Kane AJ, Aranda
D, Mills SA, Barani IJ and Parsa AT: Predictors of mortality
following treatment of intracranial hemangiopericytoma. J
Neurosurg. 113:333–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ratneswaren T, Hogg FRA, Gallagher MJ and
Ashkan K: Surveillance for metastatic hemangiopericytoma-solitary
fibrous tumors-systematic literature review on incidence,
predictors and diagnosis of extra-cranial disease. J Neurooncol.
138:447–467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Bernardi A, Dufresne A, Mishellany F,
Blay JY, Ray-Coquard I and Brahmi M: Novel therapeutic options for
solitary fibrous tumor: Antiangiogenic therapy and beyond. Cancers
(Basel). 14:10642022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schweizer L, Koelsche C, Sahm F, Piro RM,
Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, et al:
Meningeal hemangiopericytoma and solitary fibrous tumors carry the
NAB2-STAT6 fusion and can be diagnosed by nuclear expression of
STAT6 protein. Acta Neuropathol. 125:651–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao F, Ling C, Shi L, Commins D, Zada G,
Mack WJ and Wang K: Inversion-mediated gene fusions involving
NAB2-STAT6 in an unusual malignant meningioma. Br J Cancer.
109:1051–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robinson DR, Wu YM, Kalyana-Sundaram S,
Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, et al:
Identification of recurrent NAB2-STAT6 gene fusions in solitary
fibrous tumor by integrative sequencing. Nat Genet. 45:180–185.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pottier C, Fresnais M, Gilon M, Jérusalem
G, Longuespée R and Sounni NE: Tyrosine Kinase inhibitors in
cancer: Breakthrough and challenges of targeted therapy. Cancers
(Basel). 12:7312020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y and Adjei AA: Targeting
angiogenesis in cancer therapy: Moving beyond vascular endothelial
growth factor. Oncologist. 20:660–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamberg P, Verweij J and Sleijfer S:
(Pre-)clinical pharmacology and activity of pazopanib, a novel
multikinase angiogenesis inhibitor. Oncologist. 15:539–547. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar R, Knick VB, Rudolph SK, Johnson JH,
Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE,
Onori JA, et al: Pharmacokinetic-pharmacodynamic correlation from
mouse to human with pazopanib, a multikinase angiogenesis inhibitor
with potent antitumor and antiangiogenic activity. Mol Cancer Ther.
6:2012–2021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martin-Broto J, Stacchiotti S, Lopez-Pousa
A, Redondo A, Bernabeu D, de Alava E, Casali PG, Italiano A,
Gutierrez A, Moura DS, et al: Pazopanib for treatment of advanced
malignant and dedifferentiated solitary fibrous tumour: A
multicentre, single-arm, phase 2 trial. Lancet Oncol. 20:134–144.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi H, Charnsangavej C, Faria SC,
Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA and
Benjamin RS: Correlation of computed tomography and positron
emission tomography in patients with metastatic gastrointestinal
stromal tumor treated at a single institution with imatinib
mesylate: Proposal of new computed tomography response criteria. J
Clin Oncol. 25:1753–1759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Apra C, Alentorn A, Mokhtari K,
Kalamarides M and Sanson M: Pazopanib efficacy in recurrent central
nervous system hemangiopericytomas. J Neurooncol. 139:369–372.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SJ, Kim ST, Park SH, Choi YL, Park JB,
Kim SJ and Lee J: Successful use of pazopanib for treatment of
refractory metastatic hemangiopericytoma. Clin Sarcoma Res.
4:132014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura T, Matsumine A, Kawai A, Araki N,
Goto T, Yonemoto T, Sugiura H, Nishida Y, Hiraga H, Honoki K, et
al: The clinical outcome of pazopanib treatment in Japanese
patients with relapsed soft tissue sarcoma: A Japanese
musculoskeletal oncology group (JMOG) study. Cancer. 122:1408–1416.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Common Terminology Criteria for Adverse
Events (CTCAE), . V5.0, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf
|
|
21
|
Liu J, Yan S, Du J, Teng L, Yang R, Xu P
and Tao W: Mechanism and treatment of diarrhea associated with
tyrosine kinase inhibitors. Heliyon. 10:e275312024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tullemans BME, Nagy M, Sabrkhany S,
Griffioen AW, Oude Egbrink MGA, Aarts M, Heemskerk JWM and Kuijpers
MJE: Tyrosine kinase inhibitor pazopanib inhibits platelet
procoagulant activity in renal cell carcinoma patients. Front
Cardiovasc Med. 5:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Justice CN, Derbala MH, Baich TM, Kempton
AN, Guo AS, Ho TH and Smith SA: The impact of pazopanib on the
cardiovascular system. J Cardiovasc Pharmacol Ther. 23:387–398.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda O, Ohka F, Maesawa S, Matsuoka A,
Shimokata T, Mitsuma A, Urakawa H, Nakamura S, Shimoyama Y,
Nakaguro M, et al: Solitary fibrous tumor/hemangiopericytoma
treated with temozolomide plus bevacizumab: A report of four cases
and literature review. Nagoya J Med Sci. 82:631–644.
2020.PubMed/NCBI
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sawayama S, Murakami R, Aki M, Kawaguchi
Y, Takao Y, Nonogaki H, Goto T and Yamauchi C: Efficacy of
pazopanib in FGFR1-amplified uterine carcinosarcoma: A case report.
Gynecol Oncol Rep. 41:1009932022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mondaza-Hernandez JL, Moura DS,
Lopez-Alvarez M, Sanchez-Bustos P, Blanco-Alcaina E,
Castilla-Ramirez C, Collini P, Merino-Garcia J, Zamora J,
Carrillo-Garcia J, et al: ISG15 as a prognostic biomarker in
solitary fibrous tumour. Cell Mol Life Sci. 79:4342022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Napolitano A, Moura DS, Hindi N,
Mondaza-Hernandez JL, Merino-Garcia JA, Ramos R, Dagrada GP,
Stacchiotti S, Graziano F, Vincenzi B and Martin-Broto J:
Expression of p53 as a biomarker of pazopanib efficacy in solitary
fibrous tumours: Translational analysis of a phase II trial. Ther
Adv Med Oncol. 14:175883592211161552022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giannini C, Rushing EJ, Hainfellner JA,
Bouvier C, Figarella-Branger D, von Deimling A, Wesseling P and
Antonescu CR: Solitary fibrous tumour/haemangioparicytoma. WHO
Classification of Tumours of the Central Nervous System. (Revised
4th edition). International Agency for Research on Cancer; Lyon:
pp. 248–254. 2016
|
















