Introduction
Intravascular large B-cell lymphoma (IVLBCL) is a
rare extranodal lymphoma characterized by the proliferation of
lymphoma cells almost within the lumina of large and small vessels
(1,2). Its pathogenesis remains obscure but is
likely related to changes in cell migration properties and
endothelium adhesion molecules inhibiting the extravasation of
lymphocytes (3). IVLBCL has an
estimated incidence rate of ~0.5 cases per million individuals
worldwide (4). Due to its unique
presentation, the diagnosis of IVLBCL is often delayed, as its
clinical symptoms can vary widely and affect multiple organ
systems, including the skin, central nervous system and bone marrow
(5). Consequently, patients may
present with a range of nonspecific symptoms such as fever,
respiratory distress and ground-glass opacities (GGOs) (1). The R-CHOP regimen, which includes
rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisone, is commonly used for treatment (1). Early recognition and diagnosis are
crucial for improving prognosis. Previous data show that the median
survival time of patients with IVLBCL is ~1 year, and the prognosis
is poor (5). Staging of IVLBCL is
difficult and still not satisfactory (1). Therefore, differentiating IVLBCL from
pneumonia and interstitial lung disease presents a diagnostic
challenge (6).
In the present case, a CT-guided biopsy of the
lesion was performed. While the pathological morphology has been
previously reported (1,7), the cytological morphological
characteristics of IVLBCL cells under a microscope with an
oil-immersion objective lens were described in the present report.
The present case report offers valuable insights that may serve as
a crucial reference for improving early clinical identification and
diagnosis of IVLBCL.
Case report
A 65-year-old female patient presented to The Second
Hospital of Dalian Medical University (Dalian, China) in October
2023, and experienced persistent cough and expectoration lasting
for 4 months, accompanied by intermittent fever with fluctuating
temperatures of ~37.3°C. Chest CT scans performed at Wafangdian
Central Hospital (Dalian, China) and Dalian Central Hospital
(Dalian, China) revealed scattered subpleural GGO in both lungs,
prompting suspicion of bacterial, viral or other pathogenic
infections. Treatment with levofloxacin, penicillin (dosage
unspecified) and oral prednisone (reducing dose from 50 to 15 mg)
yielded no significant improvement. At 5 days before admission to
the respiratory department (The Second Hospital of Dalian Medical
University, Dalian, China) in October 2023, the temperature of the
patient reached 38.2°C. A subsequent chest CT scan revealed
multiple round GGOs in the subpleural regions of both lungs,
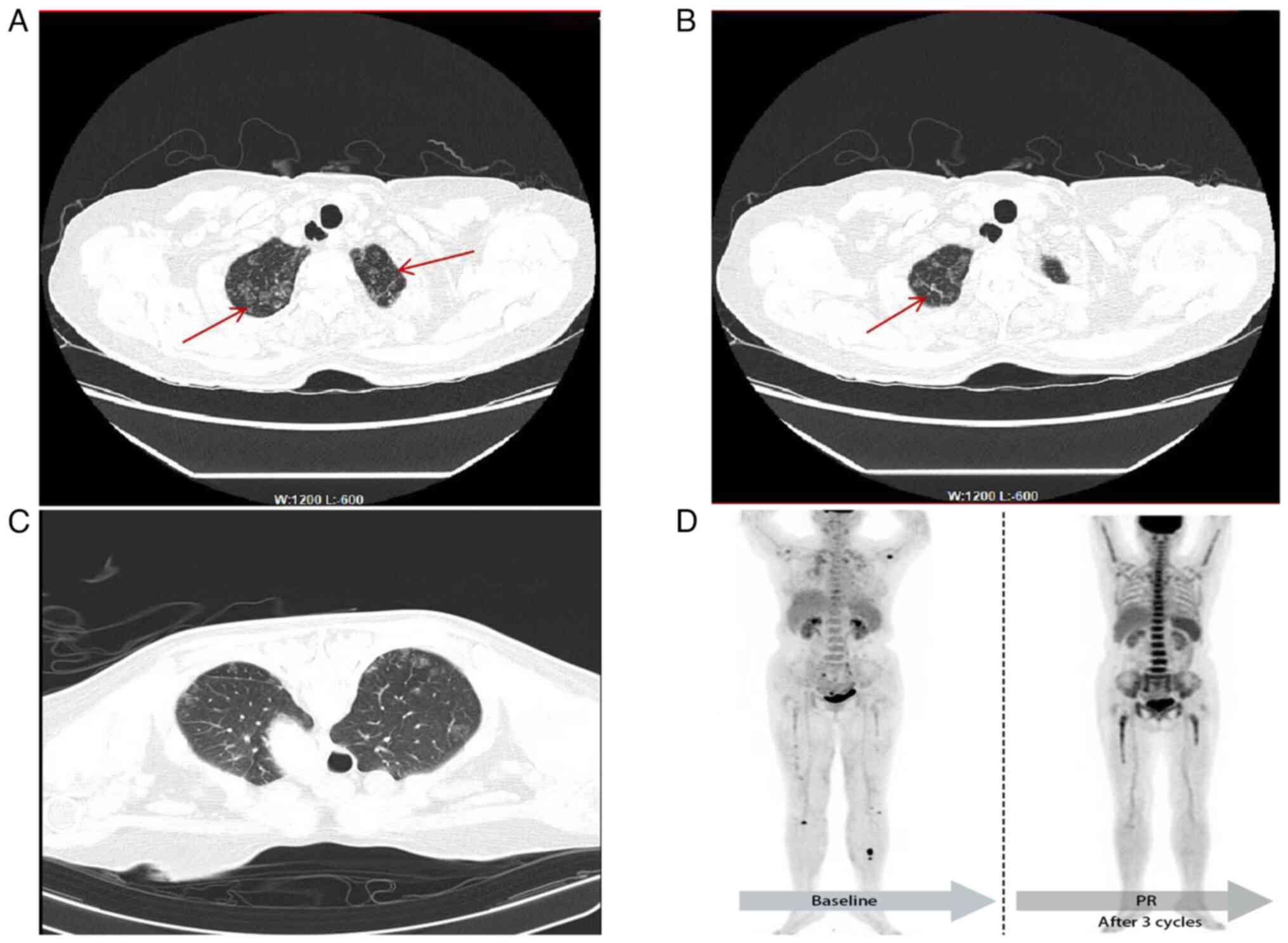
suggesting viral pneumonia (Fig. 1A and
B).
Upon admission, the detection of extractable nuclear
antigen (ENA) was performed using a Diagnostic Kit for ENA (cat.
no. ENA-17; Guangzhou Kangrun Biotech Co., Ltd.) on a fully
automated immunoblot analyzer (HELIA BLOT; Guangzhou Kangrun
Biotech Co., Ltd.) using the immunoblotting method, and this was
negative, indicating that the patient did not have an autoimmune
disease. The results of tumor markers, including α-fetoprotein,
carbohydrate antigen (CA)-199, CA-242, CA-125, CA-153,
carcinoembryonic antigen (CEA), prostate-specific antigen and
neuron-specific enolase (NSE), detected using an
electrochemiluminescence method (Shenzhen New Industries Biomedical
Engineering Co., Ltd.), were all within the normal range.
Bronchoscopy and bronchoalveolar lavage detected no abnormal cells.
However, hemoglobin and albumin levels were decreased, while serum
ferritin, lactate dehydrogenase and inflammatory marker levels were
elevated compared with the normal range (Table I), indicating a possible tumor. The
clinicians performed a CT-guided puncture biopsy of the lesion in
the upper lobe of the right lung. The procedure was conducted
smoothly. At the conclusion of the lung puncture, upon removal of
the puncture needle, a synchronous CT scan revealed a small amount
of needle-induced bleeding at the puncture site. No gas shadows
were observed in the heart or major vessels, and no other
complications were noted (Fig. 1C).
The smear was then sent to the laboratory for cytological
examination. After 20 min of Wright-Giemsa staining [Wright's
stain: 1 g of dried Wright's stain was placed in a mortar and 500
ml methanol (concentration, ≥99.8%) was added, followed by grinding
at room temperature until the stain was dissolved. Giemsa Stain:
7.6 g Giemsa stain powder was dissolved in 500 ml methanol and 500
ml glycerol wad added, followed by grinding at room temperature
until the stain was dissolved. Solution A: Wright's stain and
Giemsa's stain were mixed at a ratio of 10:1 for use. Solution B:
Buffer solution (pH 6.4–6.8; weakly acidic): 30 ml 1%
KH2PO4, 20 ml 1%
Na2HPO4 and H2O (fresh) up to
1,000 ml. Staining was performed at room temperature. First, at
room temperature, 0.5 ml of Solution A (Solution A contains a
fixative with a concentration of methanol ≥99.8%) was added for
fixation for 30 sec, and then 1 ml Solution B was added for
staining for 18 min, followed by rinsing with tap water and drying
for 2 min], observation under an Olympus BX43 (Olympus Corporation)
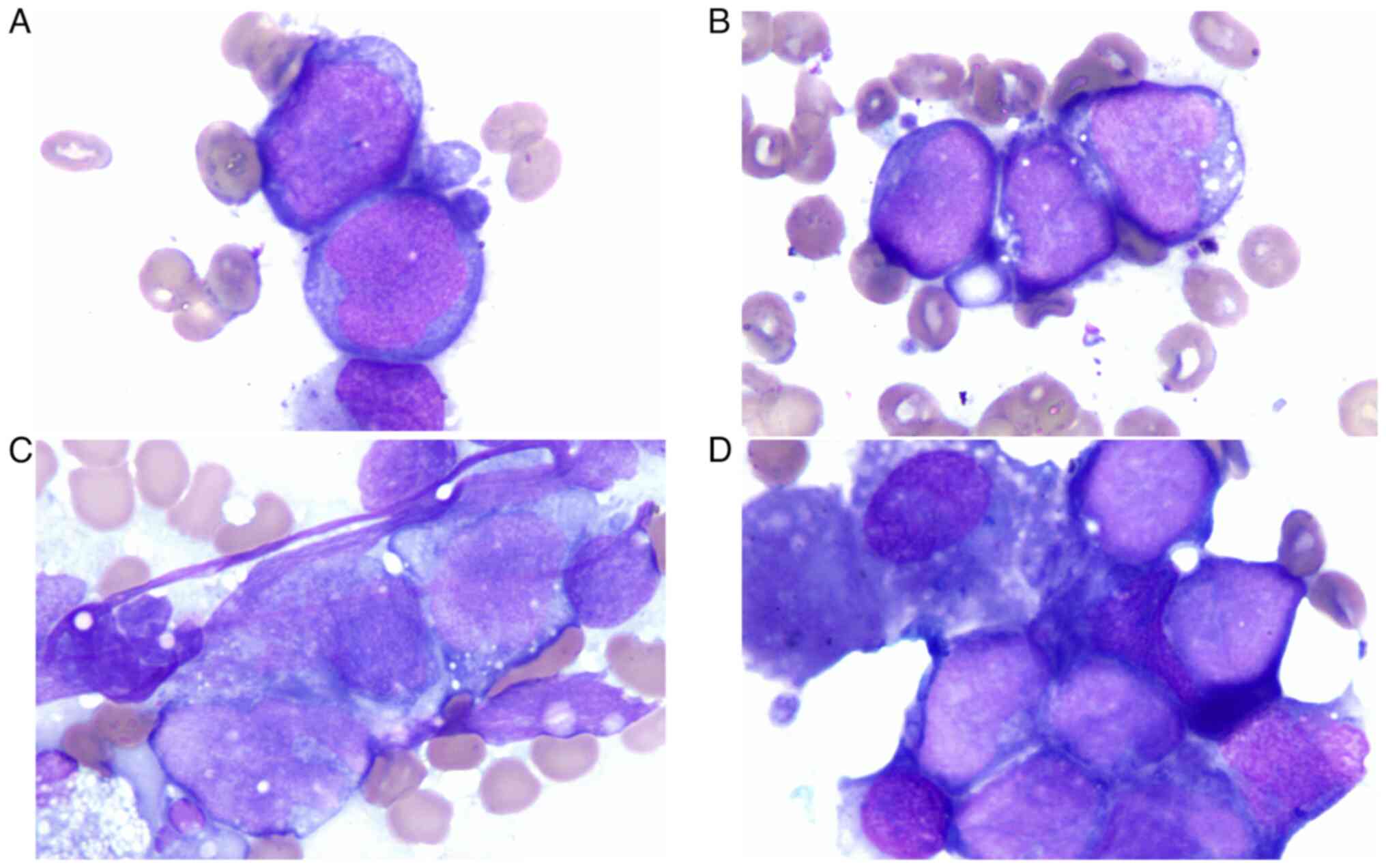
light microscope using an oil-immersion objective lens revealed
clusters of abnormal cells. These cells were slightly larger than
mature lymphocytes, round or oval in shape; with large nuclei,
smooth in outline, oval to irregular, uniform and delicate
chromatin; with indistinct or occasional nucleoli; with scant
cytoplasm, some with vacuoles; and exhibited an embedded and
adhesion-like pattern (Fig. 2).
Considering the size, arrangement and morphological features of the
abnormal cells, along with the prevalence of various lung
malignancies, small cell lung cancer (SCLC) was initially suspected
(8). However, SCLC typically
presents as a hilar mass and bulky mediastinal lymphadenopathy that
cause persistent cough and dyspnea. Furthermore, SCLC is
characterized by a rapid doubling time, high growth fraction and
early development of widespread metastases (9). In the present case, the presentation
of the patient diverged markedly from these hallmarks of SCLC, and
tumor markers such as NSE, CEA and CA-125 were negative. Given
these discrepancies, it was possible to provisionally exclude the
diagnosis of SCLC. For the malignant tumor cells with this
morphological appearance, the initial suspicion was lymphoma cells.
The morphology and arrangement of the cells were not consistent
with diffuse large B-cell lymphoma (DLBCL) (10), and the mediastinum and other lymph
nodes were not enlarged, and thus, DLBCL cells were also excluded.
The cellular morphology observed by oil immersion microscopy
differed from that reported for small cell carcinoma and DLBCL
(8,10). Combined with the aforementioned
clinical presentation and examination results, this raised the
suspicion of primary pulmonary intravascular lymphoma (8–10).
Therefore, it was strongly recommended that the patient should be
transferred to the hematology department, and that pathology and
immunohistochemical staining should be performed. H&E staining
revealed capillary or sinusoidal structures. The tissues were fixed
in 10% neutral formalin overnight at room temperature for 12 h. The
slices (thickness, 4 µm) were placed in xylene I for 10 min, xylene
II for 10 min, anhydrous ethanol I for 1 min, anhydrous ethanol II
for 1 min, 95% ethanol I for 1 min, 95% ethanol II for 1 min, 90%
ethanol for 1 min and 80% ethanol for 1 min, and then washed with
tap water for 1 min. The sections were then stained with Harris
hematoxylin for 1 min at room temperature, washed with tap water
for 1 min, differentiated with 1% hydrochloric acid ethanol for 30
sec at room temperature, and rinsed with tap water for 5 min. The
sections were then stained in eosin staining solution at 37°C for
30 sec, and washed with tap water for 30 sec. Thereafter, the
slices were placed into 85% ethanol for 20 sec, 90% ethanol for 30
sec, 95% ethanol I for 1 min, 95% ethanol II for 1 min, absolute
ethanol I for 2 min, absolute ethanol II for 2 min, and xylene I,
xylene II and xylene III for 2 min, respectively. After the last
step, the slices were removed from xylene and dried before being
sealed with neutral gum. Finally, the slides were examined under a
light microscope (Olympus Corporation) and images were acquired.
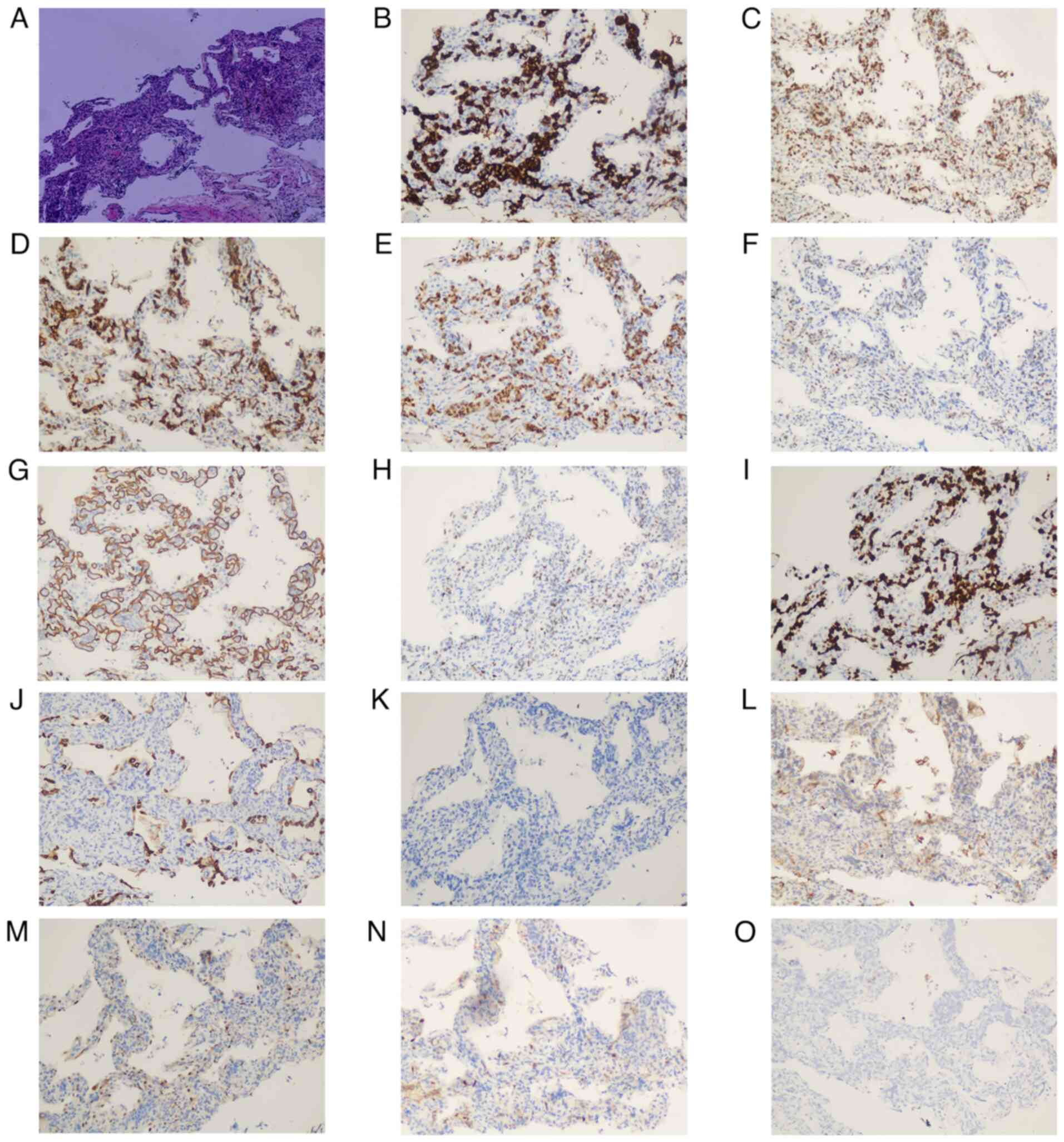
Within these small blood vessels, individual or small clusters of
tumor cells were observed. The cells exhibited consistent
morphology with marked atypia, large deeply stained nuclei, and no
transitional zone between the tumor cells and the surrounding lung
tissue (Fig. 3A). Based on the
morphological characteristics, the pathologist initially suspected
lymphoma (1). Subsequently,
immunohistochemistry (IHC) was performed. ICH detection kits, which
included ready-to-use blocking reagents, primary and secondary
antibodies, and other necessary reagents that required no dilution,
were used according to the instructions provided with each kit. The
antibody kits used included AE1/AE3 (cat. no. ZM-0069), CD20 (cat.
no. ZM-0039), CD5 (cat. no. ZA-0510), CD10 (cat. no. ZM-0283),
Bcl-6 (cat. no. ZM-0011), Bcl-2 (cat. no. ZA-0536), multiple
myeloma oncogene 1 (MUM1; cat. no. ZA-0583), C-Myc (cat. no.
ZA-0555), CyclinD1 (cat. no. ZM-0039), p53 (cat. no. ZM-0408), CD34
(cat. no. ZM-0046), CD30 (cat. no. ZM-0043) and Ki-67 (cat. no.
ZM-0166), all of which were purchased from Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd. The tissues were fixed in 10%
neutral formalin overnight at room temperature for 12 h. After
sectioning the samples to a thickness of 4 µm, deparaffinization
and hydration (at room temperature by soaking in anhydrous ethanol,
95% ethanol and 85% ethanol for 3 min each, followed by a 1-min
rinse with tap water) pretreatments were conducted. The EDTA
antigen retrieval solution was heated to boiling in a stainless
steel pot on an induction cooker at high power, and then the
sections were placed on a heat-resistant slide rack and immersed in
the retrieval solution to heat for 20 min. Subsequently, the
solution was allowed to cool naturally for 10 min, and once the
liquid in the pot had cooled to room temperature, the sections were
removed and rinsed with distilled water for 3 min twice. A drop of
endogenous peroxidase blocking agent was added and sections were
incubated at room temperature for 10 min. Subsequently, 100 µl
primary antibody was added and sections were incubated at room
temperature for 60 min, followed by the addition of 100 µl
enzyme-labeled secondary antibody and incubation for an additional
15 min at room temperature. For chromogenic staining,
3,3′-diaminobenzidine (DAB) was used, and sections were incubated
at room temperature for 3–5 min, rinsed with tap water and
counterstained with hematoxylin for 5 min at room temperature.
Finally, the location and intensity of the markers were observed
under a light microscope. Additionally, in situ
hybridization Epstein-Barr encoding region (EBER) detection was
performed. The EBER detection kit (cat. no. ISH-7001; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.), which included blocking
reagent, pepsin and HRP-conjugated anti-digoxigenin antibody (all
reagents were ready to use without dilution), was used according to
the manufacturer's instructions. The tissues were fixed in 10%
neutral formalin overnight at room temperature for 12 h. After
fixation, the tissue was paraffin-embedded. After sectioning the
samples to a thickness of 4 µm, the sections were placed in
deparaffinization solution and soaked for 10 min, which was
repeated three times. After removing the excess liquid, the
sections were placed in anhydrous ethanol for soaking for 3 min,
which was repeated three times. The sections were then air-dried
for 10 min. Subsequently, 100 µl blocking solution was added, and
the sections were incubated at room temperature in the dark for 10
min. After washing off the blocking solution with pure water,
gradient ethanol dehydration was performed at room temperature (75,
95 and 100% for 2 min each), followed by air-drying. Subsequently,
100 µl pepsin solution was added, and the sections were incubated
at 37°C for 20 min. After discarding the pepsin solution, gradient
ethanol dehydration was carried out at room temperature (75, 95 and
100% for 2 min each), followed by air-drying. Next, 10 µl
digoxigenin-labeled EBER probe was added, and the sections were
covered with a silanized cover slip, and sealed with rubber cement.
The sections were hybridized and incubated at 37°C for 4 h (in
situ hybridization was performed in a moist chamber). The
rubber cement was carefully removed, and the slides were immersed
in PBS buffer for 10 min, allowing the cover slips to fall off
naturally. A hydrophobic pen was used to draw a circle around the
tissue. The slides were then rinsed with PBS buffer for 2 min,
which was repeated three times. All aforementioned operations were
carried out at room temperature. Subsequently, 50 µl HRP-conjugated
anti-digoxigenin antibody was added, and the sections were
incubated at 37°C for 30 min, followed by rinsing with PBS buffer
for 2 min, which was repeated three times. Freshly prepared DAB
chromogenic solution was added at room temperature, and the
sections were incubated for 10 min. The sections were then rinsed
with tap water, counterstained with hematoxylin at room temperature
for 10 sec, differentiated and rinsed to blue. Finally, the
sections were dehydrated and cleared, and the result was observed
under a light microscope. The IHC and EBER results were issued by
the pathology department 2 days later. The results showed AE1/AE3
(−) (Fig. 3J), which primarily
ruled out an epithelial origin of the tumor (11), and CD20 (+) (Fig. 3B), CD5 (−) (Fig. 3K), CD10 (−) (Fig. 3L), Bcl-6 (+) (Fig. 3C), Bcl-2 (diffuse strong+) (Fig. 3D), MUM1 (+) (Fig. 3E), C-Myc (30%+) (Fig. 3H), CyclinD1 (−) (Fig. 3M), p53 (30%+) (Fig. 3F), CD34 (vessels+) (Fig. 3G), CD30 (−) (Fig. 3N), Ki-67 (+; >90%) (Fig. 3I) and EBER (−) (Fig. 3O). The patient was ultimately
diagnosed with IVLBCL (2). In the
present case, the patient declined molecular biological and genetic
testing. Subsequent positron emission tomography-CT (PET-CT)
demonstrated metabolic enhancement in multiple sites throughout the
body (Fig. 1D), involving the lung,
bone, bone marrow, pituitary gland, right temporal region and
various subcutaneous soft tissues, and the clinical stage was IV
(1,12). Treatment with rituximab (600 mg),
cyclophosphamide (1.3 g), doxorubicin (80 mg), vincristine (2 mg)
and prednisone (15 mg) (R-CHOP) combined with zanubrutinib (160 mg)
was administered for 7 cycles over a total of 150 days, causing the
hemoglobin levels to gradually increase, while the erythrocyte
sedimentation rate, CRP, ferritin, lactate dehydrogenase, and
inflammatory cytokine levels gradually decreased to normal levels
and a stable clinical condition (Table
I). Currently, the interim PET-CT revealed a reduction in the
extent of GGO and a decrease or complete resolution of abnormal
glucose metabolism throughout the body (Fig. 1D). The patient was advised to have
follow-up appointments every 3 months, which would likely include
blood tests, imaging studies and physical examinations to monitor
for any signs of recurrence or complications. The patient was in
good condition in July 2024.
 | Table I.Laboratory findings of the
patient. |
Table I.
Laboratory findings of the
patient.
| Variable, unit
(RI) | Pre-ADM | Pre-ADM | ADM | Post-ADM | Post-ADM | Post-ADM |
|---|
| Hospitalization
day | −98 | −17 | 0 | 23 | 44 | 65 |
| Treatment
phase | - | - | Baseline | Post-C1 | Post-C2 | Post-C3 |
|
|
|
| R-CHOP | R-CHOP | R-CHOP | R-CHOP |
| HB, g/l (115–150
g/l) | 91 | 88 | 77 | 94 | 102 | 111 |
| Alb, g/l (40–55
g/l) | 24.00 | 22.74 | 23.90 | 32.58 | 34.54 | 37.21 |
| ESR, mm/h (0–20
mm/h) | 80 | 106 | 105 | 67 | 19 | 20 |
| CRP, mg/l (0–10
mg/l) | 36.28 | 44.93 | 100.07 | 11.00 | 3.33 | 3.64 |
| FER, ng/ml (10–291
ng/ml) | 399.41 | 726.76 | 721.64 | 76.85 | – | - |
| PCT, ng/ml (0–0.06
ng/ml) | 0.02 | 0.16 | 0.09 | - | - | - |
| LDH, U/l (120–250
U/l) | - | 281.25 | 329.81 | 286.72 | 189.58 | 253.60 |
| IL-2R, U/ml
(223–710 U/ml) | 236 | - | 4382 | 671 | 562 | 669 |
| IL-6, pg/ml (0–3.4
pg/ml) | 17.9 | - | 49.0 | 5.9 | 7.1 | 4.5 |
| IL-10, pg/ml (0–9.1
pg/ml) | 126.8 | - | >1,000.0 | <5.0 | <5.0 | 5.8 |
| TNF-α, pg/ml (0–8.1
pg/ml) | 1.4 | - | 13.9 | 10.6 | 16.1 | 20.5 |
Discussion
Clinically, IVLBCL predominantly affects the
elderly, with no significant sex disparity in prevalence (5). Currently, IVLBCL is clinically
categorized into three variants based on presentation: Cutaneous,
classic and hemophagocytic. These variants exhibit varying
prognoses, with the cutaneous variant generally being associated
with an improved outcome in young women in Western countries
(1). The hemophagocytic variant is
more prevalent in Asian countries, accounting for 79% of IVLBCL
cases in Asia (13), and is
characterized by multiorgan failure, hepatosplenomegaly and
pancytopenia. The present case, exhibiting primary lung involvement
and persistent anemia without leukopenia or thrombocytopenia, was a
classic variant with pulmonary primary IVLBCL and a rare
manifestation in Asian patients.
Similar to the present case, a number of patients
with primary pulmonary IVLBCL have been easily misdiagnosed with
pneumonia or even overlooked, treated with steroids and experienced
worsening symptoms (14,15). The diagnosis frequently took over a
month or longer from atypical symptom onset (16), and some patients were diagnosed
incidentally (17). Yamamoto et
al (18) reported a case of
IVLBCL in which the patient showed no overt symptoms but was
incidentally found to have multiple GGOs during examination for a
pancreatic cyst. A subsequent surgical lung biopsy led to the
diagnosis of IVLBCL. In this instance, pulmonary IVLBCL may have
progressed slowly without noticeable symptoms (18). IVLBCL is likely to be overlooked
because it does not form visible extravascular masses, and only
autopsy can confirm it (19). An
autopsy review revealed pulmonary involvement in ~60% of cases
(20), while a single-center study
found diffuse GGO on chest CT in 23.8% of patients (21). Therefore, early diagnosis of primary
pulmonary IVLBCL remains a clinical challenge.
Screening methods for primary pulmonary IVLBCL
mainly include random skin biopsy, PET-CT, bronchoscopy brushings,
bronchoalveolar lavage, transbronchial lung biopsy (TBLB) and
surgical tissue biopsy (15,16,21–28).
Studies have indicated that most IVLBCL lesions exist within the
subcutaneous adipose tissue vessels, and random skin biopsy can be
used for early diagnosis (21–23).
However, this method has limitations, with lower sensitivity and
potential false-negative results due to the variations in skin
biopsy puncture depth and location (24). Due to the high incidence of
cutaneous melanoma in Western countries, the diagnostic efficiency
of random skin biopsy is relatively high in Western countries
(25). PET-CT is used in the early
diagnosis of isolated pulmonary IVLBCL (26,27).
However, Zhu et al (15)
found that only 1 case in their study presented pulmonary mild
18F-fluorodeoxyglucose (FDG) uptake on PET, which was
atypical. Furthermore, Nguyen et al (16) reported that 3 patients (including 1
of the autopsied patients) failed to demonstrate any parenchymal
FDG uptake in the lungs. GGO imaging findings in the lungs are
nonspecific for this disease, and bronchoscopy brushings or
bronchoalveolar lavage have limited sampling sites and specimen
volumes (28). TBLB may
occasionally yield the evidence of lung involvement with IVLBCL;
however, the small size of the bronchoscopic biopsy specimens
limits its sensitivity in diagnosing microvascular disease
processes such as IVLBCL (16).
Compared with surgical tissue biopsy, CT-guided percutaneous biopsy
is a precise and minimally invasive method. Furthermore, additional
insights can be provided by quickly staining the punctured cells
under a microscope.
To the best of our knowledge, the present report was
the first to provide a morphological description of primary
pulmonary IVLBCL cells under an oil-immersion objective. The size
and arrangement of IVLBCL cells resembled those of SCLC. Based on
the observations in the present case, IVLBCL cells exhibited
chromatin (uniform and delicate, with visible nucleoli and less
cytoplasm) that is more similar to that of original immature
lymphocytes. However, SCLC cells (8) are more regular in shape, round, ovate
or angular, with finely granular or deeply stained chromatin, and
no or inconspicuous nucleoli, and have sparse cytoplasm compared
with IVLBCL cells. Furthermore, IVLBCL cells need to be
distinguished from lung-infiltrating DLBCL cells (10), which are larger, have more
pronounced nucleoli, more cytoplasm and a diffusely distributed
pattern; however, IVLBCL cells exhibit an embedded and
adhesion-like pattern. Therefore, the aforementioned three types of
cells can be identified based on morphology and distribution
pattern. Additionally, both Hodgkin's lymphoma (HL) and infectious
mononucleosis (IM) initially present with fever in the early
clinical stage (29,30). However, the morphological
characteristics of HL include typical Reed-Sternberg cells
(29), while IM is characterized by
the presence of typical atypical lymphocytes (30), both of which can be distinguished
from IVLBCL. Combined with clinical manifestations (such as
enlarged lymph nodes and laboratory tests), morphology can provide
important clues for the diagnosis of IVLBCL.
Studies on the molecular biology and cytogenetics of
IVLBCL are scarce. Only a limited number of investigations have
reported on the clonal rearrangement, gene mutations and
chromosomal rearrangements of the immunoglobulin heavy chain in
this rare disease (15,31–34).
Tanaka et al (31) found
that lymphoplasmacytic lymphoma clones utilize the J4 segment of
the immunoglobulin heavy chain gene, while IVLBCL clones utilize
the J6 segment. Next-generation sequencing analysis has indicated
that IVLBCL exhibits a non-germinal center B-cell gene expression
profile, characterized by hyperactivation of the NF-κB pathway
(32). Furthermore, high mutation
frequencies have been observed in several genes, including MYD88
(57%), CD79B (67%), SETD1B (57%) and major histocompatibility
complex, class I, B (57%) (33).
Fujikura et al (34) found
that most cases of their archive had recurrent abnormalities in
chromosomes 6, 8 and 19, with the most common being 6q13, 8p11 and
19q13, and chromosome 4/8 losses and marker chromosomes were also
detected in the study. In a case report of primary pulmonary
IVLBCL, Zhu et al (15)
detected gene recombination of Igκ-VJ and Igκ-V/in by PCR. In the
present case, the patient declined molecular biological and genetic
testing. Due to the rarity of primary pulmonary IVLBCL, genetic
research specific to this subtype is still lacking.
At present, there is no specific clinical staging
standard for IVLBCL. In clinical practice, the Ann Arbor staging
system for lymphoma is typically used for assessment (12). The cutaneous variant, involving only
a single extranodal organ (skin), is usually classified as stage I
(1,12). Notably, patient survival is strongly
associated with the number of cutaneous lesions, and although
classified as stage I, most patients with multiple skin
manifestations suffered a relapse within 1 year of treatment and
showed a worse outcome (35). The
classic variant and the hemophagocytic variant are characterized by
high aggressiveness and rapid disease progression, and they often
present with diffuse or widespread extranodal organ involvement at
diagnosis, such as the nervous system, bone marrow, spleen and
liver, and are therefore typically classified as stage IV (1). IVLBCL is characterized by insidious
onset and easy metastasis, and the Ann Arbor staging system
(12) is inadequate for accurate
assessment, highlighting the need for more precise staging criteria
(1).
Current data involve only individual case reports
because of the rarity of IVLBCL and lack of prospective multicenter
trials providing definitive treatment strategies (4,5,7,14,15,21,24,31).
The chemotherapy regimen remains uncertain, and the R-CHOP
chemoimmunotherapy remains a first-line option (1,2). The
cutaneous variant of IVLBCL, albeit being less aggressive, should
be treated in the same way as the other variants (1). Notably, in patients without central
nervous system (CNS) involvement at initial diagnosis, the risk of
CNS recurrence is as high as 18% in R-CHOP-treated patients
(36). Consequently, the addition
of drugs with an improved CNS bioavailability, such as high-dose
methotrexate, represents a possible strategy (36). Furthermore, capitalizing on the high
frequency of MYD88 and CD79 mutations observed in patients with
IVLBCL, a clinical trial demonstrated that zanubrutinib plus R-CHOP
had promising efficacy and safety as a front-line regimen in
IVLBCL, even in patients with CNS involvement (37). Additionally, Kato et al
(38) suggested that consolidation
therapy with autologous stem cell transplantation following
remission can serve as an effective treatment strategy.
In the present case, the patient declined genetic
testing; however, the clinical symptoms were relieved after R-CHOP
treatment. Interim PET-CT revealed a reduction in the extent of GGO
and a decrease or complete resolution of abnormal glucose
metabolism throughout the body. In the future, close follow-up will
be maintained to monitor the long-term prognosis of the
patient.
In conclusion, the diagnosis of primary pulmonary
IVLBCL is often challenging because of nonspecific signs and
symptoms, causing potential misdiagnosis and overlook. Primary
pulmonary IVLBCL should be considered in patients presenting with
recurrent fever, GGO in the lungs, and ineffective responses to
anti-inflammatory and anti-infection treatments. Timely puncture
cytology of the lesion site should be performed to identify the
cause of the disease, ensuring that the patients receive accurate
treatment effectively. This approach can help avoid misdiagnosis
due to nonspecific clinical symptoms.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WJ and XX were responsible for data analysis and
manuscript drafting. ML and CZ were responsible for collecting the
data and drafting the work. WJ and XX contributed to design and
revised the manuscript critically for important intellectual
content. The authors have accepted responsibility for the entire
content of this manuscript and approved its submission. WJ and XX
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ponzoni M, Campo E and Nakamura S:
Intravascular large B-cell lymphoma: A chameleon with multiple
faces and many masks. Blood. 132:1561–1567. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lymphoid Disease Group, Chinese Society of
Hematology, Chinese Medical Association, . Lymphoma Expert
Committee of Chinese Society of Clinical Oncology (CSCO): Chinese
expert consensus on the diagnosis and management of intravascular
large B cell lymphoma (2023). Zhonghua Xue Ye Xue Za Zhi.
44:177–181. 2023.(In Chinese). PubMed/NCBI
|
|
3
|
Baptista P, Aguiar E, Fonseca E, Pinto R
and Trigo F: Intravascular large B-cell lymphoma presenting with
haemophagocytic syndrome. Br J Haematol. 204:2151–2152. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferreri AJM, Campo E, Seymour JF, Willemze
R, Ilariucci F, Ambrosetti A, Zucca E, Rossi G, López-Guillermo A,
Pavlovsky MA, et al: Intravascular lymphoma: Clinical presentation,
natural history, management and prognostic factors in a series of
38 cases, with special emphasis on the ‘cutaneous variant’. Br J
Haematol. 127:173–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Zhang Y, Zhu Y and Zhang W:
Prognosis of intravascular large B cell lymphoma (IVLBCL): Analysis
of 182 patients from global case series. Cancer Manag Res.
12:10531–10540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsue K, Abe Y, Narita K, Kobayashi H,
Kitadate A, Takeuchi M, Miura D and Takeuchi K: Diagnosis of
intravascular large B cell lymphoma: Novel insights into
clinicopathological features from 42 patients at a single
institution over 20 years. Br J Haematol. 187:328–336. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cakir E, Demirag F and Aydin M:
Cytopathologic differential diagnosis of small cell carcinoma and
poorly differentiated non-small cell carcinoma in bronchial lavage
specimens using a regression analysis. APMIS. 118:150–155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ganti AKP, Loo BW, Bassetti M, Blakely C,
Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M,
et al: Small cell lung cancer, version 2.2022, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
19:1441–1464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Debliquis A, Voirin J, Harzallah I, Maurer
M, Lerintiu F, Drénou B and Ahle G: Cytomorphology and flow
cytometry of brain biopsy rinse fluid enables faster and
multidisciplinary diagnosis of large B-cell lymphoma of the central
nervous system. Cytometry B Clin Cytom. 94:182–188. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinkus GS, Etheridge CL and O'Connor EM:
Are keratin proteins a better tumor marker than epithelial membrane
antigen? A comparative immunohistochemical study of various
paraffin-embedded neoplasms using monoclonal and polyclonal
antibodies. Am J Clin Pathol. 85:269–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Armitage JO: Staging non-Hodgkin lymphoma.
CA Cancer J Clin. 55:368–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murase T, Nakamura S, Kawauchi K,
Matsuzaki H, Sakai C, Inaba T, Nasu K, Tashiro K, Suchi T and Saito
H: An Asian variant of intravascular large B-cell lymphoma:
Clinical, pathological and cytogenetic approaches to diffuse large
B-cell lymphoma associated with haemophagocytic syndrome. Br J
Haematol. 111:826–834. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bae HJ, Chon GR, Kim DJ, Lee SH and Ahn
JY: A case of intravascular large B-cell lymphoma of lung
presenting with progressive multiple nodules on chest computed
tomography. Respir Med Case Rep. 21:108–112. 2017.PubMed/NCBI
|
|
15
|
Zhu M, Chang Y, Fan H, Shi J, Zhu B and
Mai X: Primary pulmonary intravascular large B-cell lymphoma
misdiagnosed as pneumonia: Four case reports and a literature
review. Oncol Lett. 25:2342023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen TT, Sekiguchi H, Yi ES and Ryu JH:
Occult diffuse neoplasm in the lungs: Intravascular large B-cell
lymphoma. Am J Med. 134:926–929. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davis JW, Auerbach A, Crothers BA, Lewin
E, Lynch DT, Teschan NJ and Schmieg JJ: Intravascular large B-cell
lymphoma. Arch Pathol Lab Med. 146:1160–1167. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto R, Okagaki N, Sakamoto H, Tanaka
Y, Takeda A, Maruguchi N, Nakamura S, Matsumura K, Ueyama M,
Ikegami N, et al: Intravascular large B-cell lymphoma presenting as
pulmonary ground-glass nodules that progressed slowly over several
months with no overt symptoms. Intern Med. 63:559–563. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng JW and Li JH: Intravascular large
B-cell lymphoma. N Engl J Med. 389:21882023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu H, Chen G, Zhang R and Jin X: Primary
intravascular large B-cell lymphoma of lung: A report of one case
and review. Diagn Pathol. 7:702012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enzan N, Kitadate A, Tanaka A and Matsue
K: Incisional random skin biopsy, not punch biopsy, is an
appropriate method for diagnosis of intravascular large B-cell
lymphoma: A clinicopathological study of 25 patients. Br J
Dermatol. 181:200–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsue K, Abe Y, Kitadate A, Miura D,
Narita K, Kobayashi H, Takeuchi M, Enzan N, Tanaka A and Takeuchi
K: Sensitivity and specificity of incisional random skin biopsy for
diagnosis of intravascular large B-cell lymphoma. Blood.
133:1257–1259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
MacGillivary ML and Purdy KS:
Recommendations for an approach to random skin biopsy in the
diagnosis of intravascular B-cell lymphoma. J Cutan Med Surg.
27:44–50. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SR, Ko CJ, Nelson CA, Ramachandran S
and Gehlhausen JR: Random skin biopsies for diagnosis of
intravascular large B-cell lymphoma: Retrospective analysis of 31
biopsies from a US dermatology inpatient consultative service with
literature review. J Am Acad Dermatol. 88:714–716. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rozenbaum D, Tung J, Xue Y, Hoang MP and
Kroshinsky D: Skin biopsy in the diagnosis of intravascular
lymphoma: A retrospective diagnostic accuracy study. J Am Acad
Dermatol. 85:665–670. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu F, Wang Z, Xing X, Yu M and Shi B: The
Value of 18F-FDG PET/CT in diagnostic procedure of intravascular
large B-cell lymphoma presenting fever of unknown origin and
pulmonary hypertension as an initial manifestation. Clin Nucl Med.
41:506–507. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spencer J, Dusing R, Yap W, Hill J and
Walter C: Intravascular large B-cell lymphoma presenting with
diffusely increased pulmonary fluorodeoxyglucose uptake without
corresponding CT abnormality. Radiol Case Rep. 14:260–264. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mondoni M, Rinaldo RF, Carlucci P,
Terraneo S, Saderi L, Centanni S and Sotgiu G: Bronchoscopic
sampling techniques in the era of technological bronchoscopy.
Pulmonology. 28:461–471. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas RK, Re D, Wolf J and Diehl V: Part
I: Hodgkin's lymphoma-molecular biology of Hodgkin and
Reed-Sternberg cells. Lancet Oncol. 5:11–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luzuriaga K and Sullivan JL: Infectious
mononucleosis. N Engl J Med. 362:1993–2000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka Y, Kobayashi Y, Maeshima AM, Oh SY,
Nomoto J, Fukuhara S, Kitahara H, Munakata W, Suzuki T, Maruyama D
and Tobinai K: Intravascular large B-cell lymphoma secondary to
lymphoplasmacytic lymphoma: A case report and review of literature
with clonality analysis. Int J Clin Exp Pathol. 8:3339–3343.
2015.PubMed/NCBI
|
|
32
|
Gonzalez-Farre B, Ramis-Zaldivar JE,
Castrejón de Anta N, Rivas-Delgado A, Nadeu F, Salmeron-Villalobos
J, Enjuanes A, Karube K, Balagué O, Cobo F, et al: Intravascular
large B-cell lymphoma genomic profile is characterized by
alterations in genes regulating NF-κB and immune checkpoints. Am J
Surg Pathol. 47:202–211. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimada K, Yoshida K, Suzuki Y, Iriyama C,
Inoue Y, Sanada M, Kataoka K, Yuge M, Takagi Y, Kusumoto S, et al:
Frequent genetic alterations in immune checkpoint-related genes in
intravascular large B-cell lymphoma. Blood. 137:1491–1502. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujikura K, Yamashita D, Yoshida M,
Ishikawa T, Itoh T and Imai Y: Cytogenetic complexity and
heterogeneity in intravascular lymphoma. J Clin Pathol. 74:244–250.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Breakell T, Waibel H, Schliep S, Ferstl B,
Erdmann M, Berking C and Heppt MV: Intravascular large B-cell
lymphoma: A review with a focus on the prognostic value of skin
involvement. Curr Oncol. 29:2909–2919. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimada K, Murase T, Matsue K, Okamoto M,
Ichikawa N, Tsukamoto N, Niitsu N, Miwa H, Asaoku H, Kosugi H, et
al: Central nervous system involvement in intravascular large
B-cell lymphoma: A retrospective analysis of 109 patients. Cancer
Sci. 101:1480–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Jia C, Wang W, Zhang L, Cao X, Li
J, Zhang W and Zhou D: The interim analysis from a prospective
single-center phase 2 study of Zanubrutinib plus R-CHOP in
treat-naïve intravascular large B cell lymphoma. Blood. 138 (Suppl
1):S35632021. View Article : Google Scholar
|
|
38
|
Kato K, Mori T, Kim SW, Sawa M, Sakai T,
Hashimoto H, Taguchi J, Oyake T, Kurahashi S, Imada K, et al:
Outcome of patients receiving consolidative autologous peripheral
blood stem cell transplantation in the frontline treatment of
intravascular large B-cell lymphoma: Adult lymphoma working group
of the Japan society for hematopoietic cell transplantation. Bone
Marrow Transplant. 54:1515–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|