Introduction
Adenoid cystic carcinoma (ACC) is a rare malignant
tumour predominantly originating in the salivary glands, accounting
for ~10% of salivary gland tumours and 1% of head and neck cancers
(1). However, it can also occur in
other anatomical locations, including the lungs, trachea, breast,
uterus, prostate and skin. Of note, studies have indicated that
nonsalivary gland primary ACC accounts for approximately half of
all ACC cases (2), whereas
intraosseous ACC (IACC) comprises <0.4% of all ACC cases
(3). ACC can present at any age,
but it is most frequently diagnosed in individuals aged 40 to 60
years, with a slight female predominance, and it lacks specific
clinical signs or symptoms (4).
Characterized as a slow-growing, invasive tumour, ACC often
infiltrates peripheral nerves, leading to local recurrence or
distant metastasis, most commonly affecting the lung, followed by
the bones, liver, brain and other organs. The overall survival rate
for patients with ACC ranges from 68 to 90% (5), but this rate decreases to 52 and 28%
at 10 and 15 years, respectively. Diagnostic procedures for ACC
include imaging tests such as ultrasound, computed tomography (CT)
and magnetic resonance imaging (MRI). Fine-needle aspiration
histopathological examination offers superior diagnostic accuracy.
The primary treatment strategy involves surgical excision of the
tumour, supplemented by postoperative radiotherapy and chemotherapy
(6). As ACC is frequently detected
at an advanced stage due to its slow course, preoperative diagnosis
plays a crucial role. This case report aims to increase awareness
of this uncommon disease.
Case report
A 47-year-old man presented with recurrent
right-sided chest pain persisting for more than two weeks presented
to Affiliated Hospital of Zunyi Medical University (Zunyi, China)
in May 2023, without any cough, fever or dyspnoea. The patient had
a past medical history of coronary artery disease and coronary
angiography revealed 60–70% stenosis of the right coronary artery.
Over the past year, the patient had experienced recurrent chest
pain, with notable exacerbation of right-sided chest pain in the
preceding two weeks. The patient had no history of surgery, trauma
or familial predisposition. Physical examination did not reveal any
significant cardiopulmonary abnormalities.
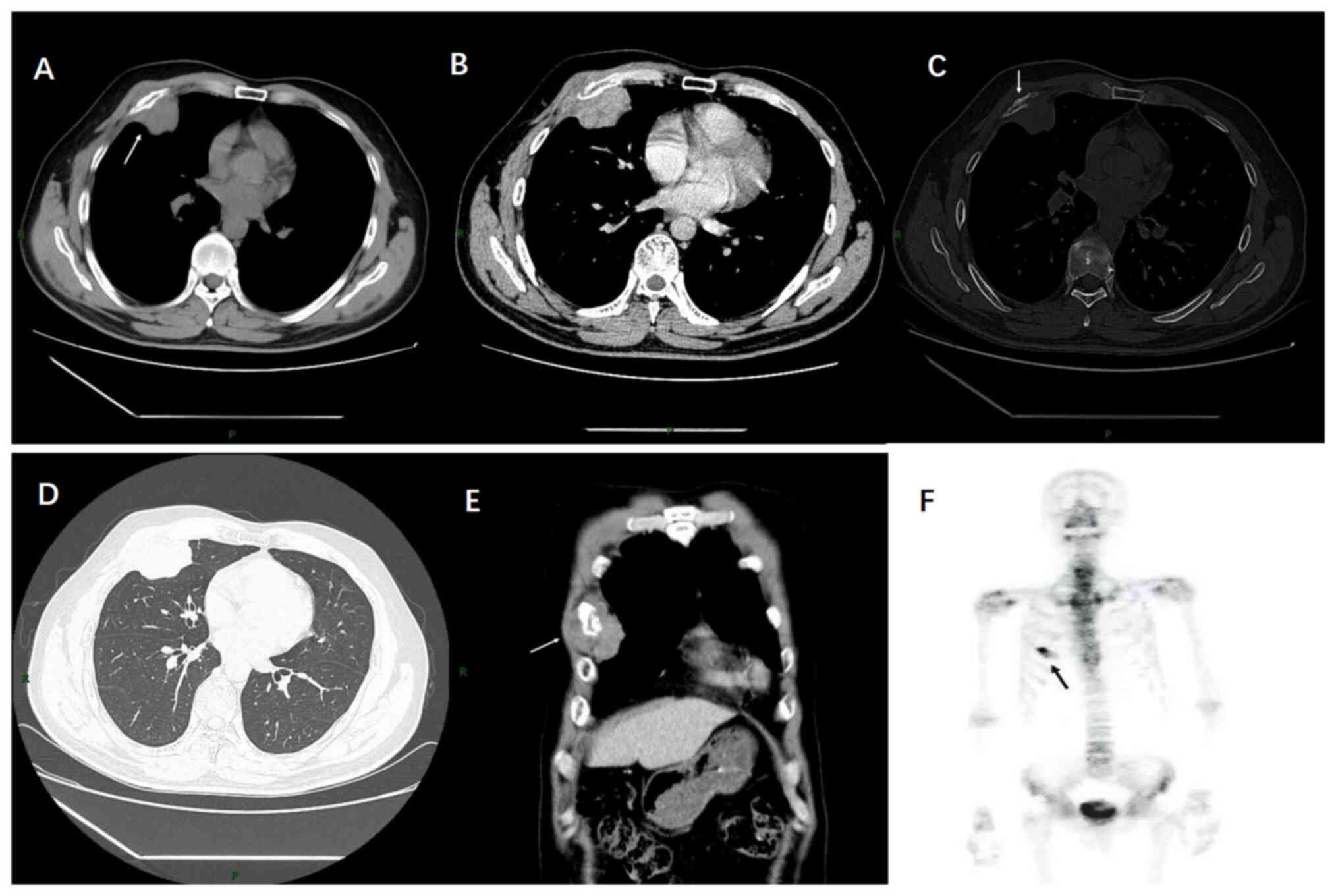
Thoracic CT imaging revealed a soft-tissue mass in
the right anterior chest wall, ~5.7×4.2×5.4 cm in size, which
bulged out towards the thoracic cavity (Fig. 1A). The tumour exhibited marked
uneven enhancement (Fig. 1B) and
encased the anterior segment of the fourth rib on the right side,
with areas of osteolytic bone destruction, but no calcification was
observed (Fig. 1C). There was no
evidence of intrapulmonary infiltrates or enlarged lymph nodes in
the mediastinum or axilla (Fig. 1D and
E). Based on these findings, the CT suggested a diagnosis of a
malignant tumour. Common malignant tumours arising in the ribs
include chondrosarcoma, osteosarcoma, Ewing sarcomas and
plasmacytoma (7). All of these
tumours can manifest as osteolytic bone destruction with soft
tissue mass formation, which is difficult to distinguish on
imaging. Certainly, the possible presence of metastasis needs to be
identified. Whole-body bone scintigraphy revealed an increased
uptake in the right 4th rib (Fig.
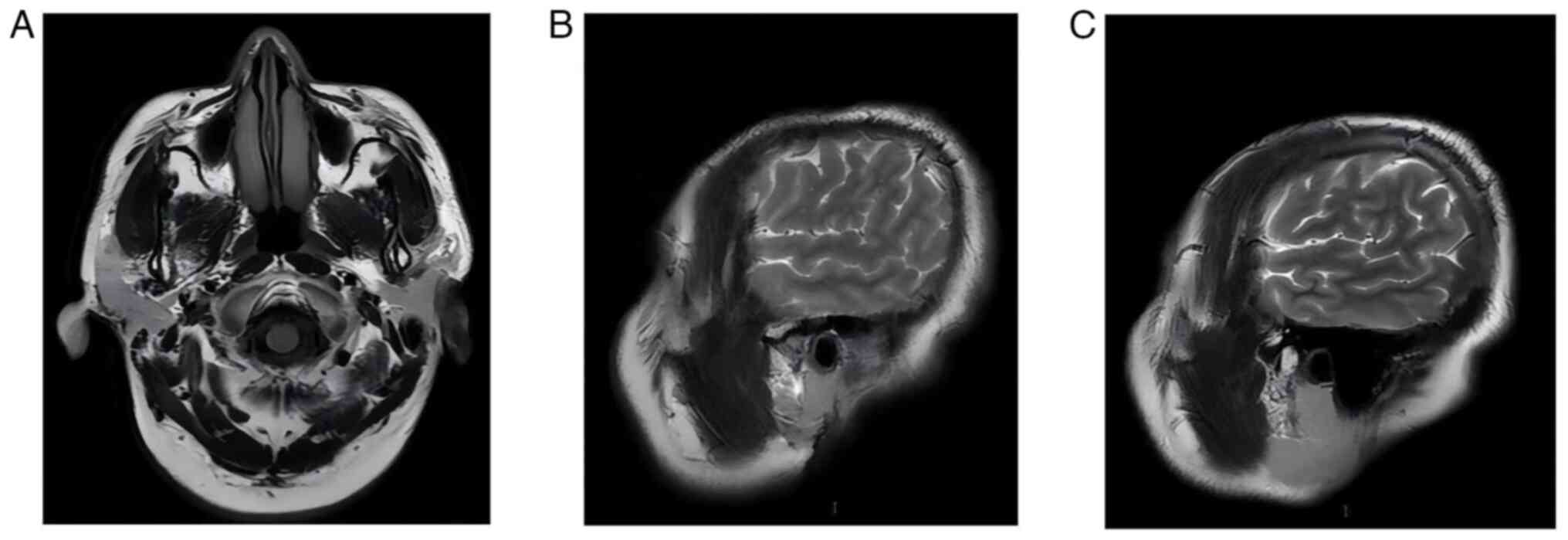
1F). Therefore, the patient underwent a CT of the chest and
whole abdomen and an MRI of the head, which did not reveal any
significant metastases (Fig. 2).
Considering the high likelihood of the tumour being malignant and
the absence of obvious metastases, direct surgical excision of the
mass was recommended for a definitive diagnosis without a prior
biopsy.
After surgical contraindications were excluded, the
patient underwent surgery to remove the mass. An incision ~15 cm in
length was made in the 5th intercostal space of the right anterior
chest wall, with layer-by-layer dissection through the skin and
muscle to expose the fourth rib. A cauliflower-like tumour, ~2×4 cm
in size, was observed on the surface of the right 4th rib, growing
outwards with an intact envelope, with the inferior edge of the
tumour affecting the 5th rib. The side of the tumour adjacent to
the chest cavity was smooth with no lung invasion. The intercostal
muscle and thickened soft tissue from the lower edge of the 4th rib
and the upper edge of the 6th rib were taken as the upper and lower
incision margins, respectively. The left incision margin followed
the fourth rib proximal to the right side of the sternum and the
right incision margin extended 3 cm distal to the fourth rib. The
tumour was completely excised and the right chest wall was
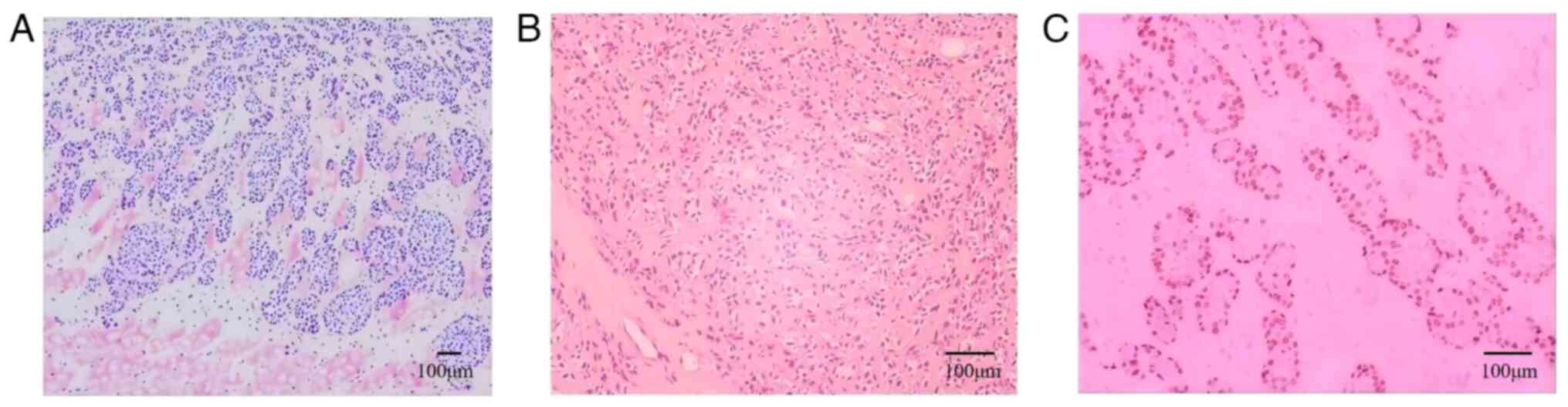
reconstructed. Postoperative histopathological examination
(8) of different parts of the
tumour revealed tumour cells arranged in sheets and nests
surrounded by ductal and myoepithelial cells, and skeletal muscle
infiltration was observed (Fig. 3A and
B). All surgical margins were pathologically negative.
Immunohistochemical testing (9)
revealed the following findings: Cytokeratin (CK) (++++), vimentin
(+++), CK7 (++), CK5/6 (positive), tumor protein 63 (P63) (++),
S-100 proteins (S-100) (++), smooth muscle actin (SMA) (++),
epithelial membrane antigen (++) and Ki-67 ~50% + (Fig. 3C) (cat. nos. RAB-0050, MAB-0735,
MAB-0820, MAB-0744, MAB-0694, Kit-0007, MAB-0890, Kit-0011 and
MAB-0672, respectively; Fuzhou Maixin Biotech). The final
pathological diagnosis was solid-type adenoid cystic carcinoma,
grade III.
The patient was advised to undergo fluorescence
in situ hybridization (FISH) or next-generation sequencing
tests to identify potential genetic mutations, as well as
postoperative chemotherapy or radiotherapy. However, the patient
declined these and opted for regular follow-up. Half a month after
discharge, the patient suddenly experienced dyspnoea and noticed
blood oozing from the surgical incision. Emergency investigations
revealed a heart rate of 149 beats/min (reference range, 60–90
beats/min), a blood pressure of 90/60 mmHg (reference range,
90–140/60–90 mmHg) and a haemoglobin level of 78 g/l (reference
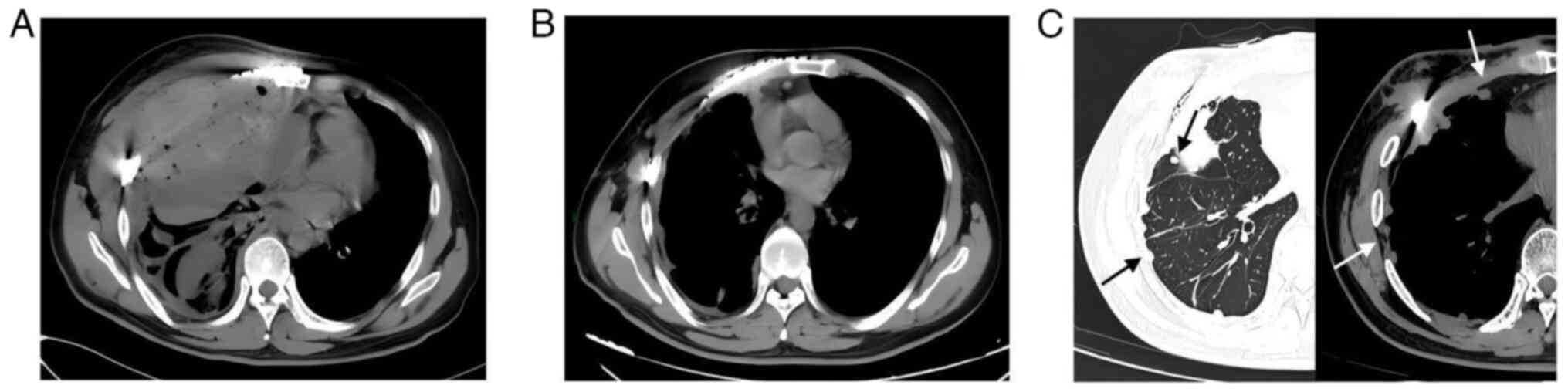
range, 120–160 g/l). A chest CT revealed right-sided
haemopneumothorax with pulmonary atelectasis (Fig. 4A). An immediate emergency
thoracotomy was performed to explore and control the bleeding. The
patient's vital signs were stable 5 days after the operation and a
repeat chest CT revealed a significant reduction in pneumothorax
(Fig. 4B); the patient was
subsequently discharged. During this period, the patient was again
advised to undergo chemotherapy or radiotherapy, but the patient
continued to refuse. At 16 months post-surgery, a chest CT revealed
pleural and intrapulmonary metastasis (Fig. 4C). The patient was transferred to a
separate hospital for chemotherapy consisting of paclitaxel at 330
mg and carboplatin at 600 mg every three weeks. The patient remains
under monthly follow-up.
Discussion
ACC is a tumour predominantly arising in the
salivary glands, with a relatively high incidence in the
submandibular glands, minor salivary glands and mucous glands of
the sinuses and oropharynx (1).
Although ACC can manifest in other locations, its occurrence in
bones is exceedingly rare, with few documented cases in the maxilla
and mandible (10,11). The following diagnostic criteria
have been established for primary IACCs: i) Definite radiographic
evidence of osteolysis, ii) intact cortical plates, iii) an intact
mucous membrane overlying the lesion, iv) no definite primary
tumour in the salivary glands, and v) histological confirmation of
ACC (12). In the patient of the
present study, the tumour was located in the right 4th rib and a CT
revealed significant osteolytic bone destruction. Whole-body bone
scintigraphy also revealed a rib tumour. Surgery and postoperative
histology confirmed that the tumour arose from the ribs and
immunohistochemistry verified the diagnosis of ACC. These findings
satisfied the diagnostic criteria for primary IACCs. The
pathogenesis of IACC is not clear, though certain scholars suggest
that primary ACC in the mandible may originate from the ectopic
entry of adjacent salivary gland tissue into the lingual cortex
during embryonic development. Another theory posits that the
tumours originate from the metaplasia and malignant transformation
of epithelial cells (13). In the
present case, the tumour was located far from any salivary gland
and it was hypothesized that it originated from the malignant
transformation of epithelial cells.
ACC occurring in bone typically presents with
worm-like bone destruction, with an intact, thin or perforated bone
cortex and the formation of soft-tissue-density masses on CT, which
can be mildly enhanced. Osteosclerosis is less common (3,14,15).
Differentiating IACCs from other malignant bone tumours on imaging
is challenging; thus, pathology is the gold standard for diagnosis.
Histologically, ACC exhibits biphasic differentiation of ductal and
myoepithelial cells, with three primary growth patterns:
Cribriform, tubular and solid, with cribriform being the most
prevalent. The ACC grade correlates with its growth pattern: Grade
I ACC has predominantly tubular growth, Grade II ACC is cribriform
and Grade III ACC has a solid component exceeding 30% (16). Owing to their biphasic
differentiation, ductal epithelial cells frequently express CK7 and
CD117, while myoepithelial cells express S-100, P63, SMA and
calponin (3). In terms of molecular
pathology, ACC frequently exhibits a t(6;9) translocation,
producing a v-myb avian myelobastosis viral oncogene homolog
(MYB):nuclear factor I/B gene fusion and leading to the
overexpression of the MYB oncoprotein (17). MYB oncoprotein detection is also
used to confirm the diagnosis of ACC. NOTCH1 mutations have been
detected in recurrent/metastatic ACC and are associated with a poor
prognosis (18). These findings
also indicate that the NOTCH1 and MYB genes are promising
therapeutic targets.
The differential diagnosis for the patient of the
present study included the following: i) Chondrosarcoma, the most
common malignant rib tumour characterized by worm-like bone
destruction with soft tissue mass formation and stromal
calcification. Pathologically, chondrosarcomas exhibit neoplastic
chondrocytes and cartilage stroma, which are often calcified.
Immunohistochemistry has shown no specificity, S-100 is positive
and IDH1 and IDH2 mutations are present in certain tumours
(19). ii) Solitary plasmacytoma:
On CT, there is commonly well-circumscribed chisel-like bone
destruction, soft tissue masses and obvious destruction and
interruption of the bone cortex; at times, a ‘soap bubble’
appearance can be observed. Histologically, it shows clonal plasma
cell proliferation without bone marrow spread and immunoglobulin
levels are generally normal (20).
iii) Ewing's sarcoma: Primarily affecting patients <20 years of
age. The typical imaging findings are osteolytic bone destruction
with a soft tissue mass and a characteristic ‘onion skin’
periosteal reaction. Histologically, Ewing's sarcoma typically
features closely arranged small round cell tumours, and
immunohistochemical characteristics include strong and diffuse
positive expression of CD99 (21).
The main points of differentiation are summarized in Table I (19–21).
 | Table I.Differential diagnoses of primary rib
intraosseous adenoid cystic carcinoma. |
Table I.
Differential diagnoses of primary rib
intraosseous adenoid cystic carcinoma.
| Pathology | Age group/clinical
feature | Imaging findings | Pathological
feature |
|---|
| Chondrosarcoma | 40–60 years old;
distant metastasis is rare; mostly occurring at the costochondral
junction | Stromal
calcification | Neoplastic
chondrocytes and cartilage stroma; S-100 positive |
| Solitary
plasmacytoma | 40–50 years old;
plasma cells proliferate abnormally | ‘Soap bubble’
appearance | Clonal plasma
cell |
| Ewing sarcomas | Individuals under 30
years of age; early metastasis and easy to relapse | ‘Onion skin’
periosteal reaction | Small round cells;
CD99 strongly positive |
Although ACC is a slow-growing tumour, it remains
invasive and is characterized by frequent perineural invasion.
Current evidence-based guidelines recommend radical surgery with
postoperative radiotherapy (PORT). The principle of surgery is to
excise the lesion to a negative margin (5). The extent of neural infiltration can
be assessed preoperatively via MRI to plan a surgical procedure.
The degree of lymph node clearance can be determined by the surgeon
based on the patient's condition. ACC occurring in the head and
neck region poses surgical challenges due to complex anatomy; at
this point, ensuring that the surgical margins are negative is
difficult. One study suggested that neural infiltration and
positive cut margins do not affect survival in patients with neural
infiltration who are receiving PORT (22). The typical PORT prescription dose is
54–71 Gy, with a median of 64 Gy (23). Indications for PORT include a large
primary tumour, nerve invasion, positive margins, or if the surgeon
considers the primary tumour unresectable (24). Multiple studies have demonstrated
that surgery combined with radiotherapy improves the 5-year local
control rate compared with surgery alone. However, certain studies
indicate that PORT can only play a role in local control and has no
effect on disease-free or overall survival. This effect was more
pronounced in intermediate- and high-risk patients and was not
significant in low-risk patients (24,25).
The toxicity and cost of treatment also need to be considered.
Systemic therapy is primarily used for ACC where
metastasis and recurrence have occurred and can be considered when
surgery and radiotherapy are not possible (5). There is no standard medication
regimen, though common regimens include platinum-based single-agent
or combination therapies, and objective response rates are higher
for combination programs. These drugs include
cisplatin/vinorelbine, cisplatin/doxorubicin/cyclophosphamide,
carboplatin/paclitaxel and gemcitabine/carboplatin (26). However, combination regimens may
limit the use of multiple drugs because of their toxicity and side
effects, such as nephrotoxicity, peripheral neuropathy, vomiting,
myelosuppression and hearing loss (27).
The prognosis of patients with ACC, an indolent
malignant tumour, is influenced by various factors. Positive
surgical margins, nerve infiltration and solid growth patterns are
associated with poor outcomes (28). Studies have also identified receptor
tyrosine kinase c-KITT (CD117), VEGF and lymphovascular invasion as
poor prognostic markers (23,29).
In addition, the site of tumour origin and metastasis also has an
impact on prognosis. ACC in major salivary glands has a better
prognosis, whereas ACC in the nasal sinus area has a worse
prognosis (22). Patients with
lung-only metastases have a better prognosis than those with
bone-only metastases (30). The
patient of the present study developed pleural and intrapulmonary
metastases, but the patient did not receive PORT and the specific
prognosis needs to be followed up continuously.
The present case is the first case of primary ACC of
the rib reported to date, and there are several limitations: The
patient was not tested for MYB and was not subjected to FISH
analysis, and despite a negative surgical margin, the patient did
not receive any postoperative radiotherapy, and lung and pleural
metastases were discovered 16 months after surgery.
In conclusion, the current study presented a rare
case of ACC of the rib, combined with its CT, bone imaging and
histopathological findings, and it was indicated to be a solid
grade III ACC with a primary origin in the rib. This previously
unreported site for ACC enhances the current understanding of the
disease.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and Technology Program of
Guizhou, China (grant Guizhou Science and Technology Cooperation
Support [2021] general no. 432).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SLi: Literature search and manuscript writing. BL:
Examined the patient. SLu: Pathological review. RF and SLi:
Obtained and analyzed patient data and performed the patient
follow-up. KJ: Conception and critical review. SLi and BL confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was provided by the Biomedical
Research Ethics Committee of the Affiliated Hospital of Zunyi
Medical University (Zunyi, China; approval no. KLLY-2023-042).
Patient consent for publication
The patient provided written informed consent for
the case study to be published, including case information and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cantu G: Adenoid cystic carcinoma. An
indolent but aggressive tumour. Part A: from aetiopathogenesis to
diagnosis. Acta Otorhinolaryngol Ital. 41:206–214. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chowsilpa S, An D and Maleki Z: Adenoid
cystic carcinoma cytology: Salivary gland and nonsalivary gland.
Diagn Cytopathol. 48:1282–1289. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu C, Shen W, Cheng Y, Yu D and Zhu H:
Primary and recurrent intraosseous adenoid cystic
carcinoma-analysis of two cases and literature review. Medicina
(Kaunas). 60:1002024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nightingale J, Lum B, Ladwa R, Simpson F
and Panizza B: Adenoid cystic carcinoma: a review of clinical
features, treatment targets and advances in improving the immune
response to monoclonal antibody therapy. Biochim Biophys Acta Rev
Cancer. 1875:1885232021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Y, Peng Z, Wang Y, Gao K, Liu Y, Fan
R, Zhang H, Xie Z and Jiang W: Current opinions on diagnosis and
treatment of adenoid cystic carcinoma. Oral Oncol. 130:1059452022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng Y, Xu L, Chen Z, Wu H, Zou H, Zhang
T, Liu G, Liu Z, Yin C, Ma L, et al: Prognosis of adenoid cystic
carcinoma in head and neck region treated with different regimens-A
single-centre study. Cancer Med. 12:2368–2377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas M and Shen KR: Primary tumors of
the osseous chest wall and their management. Thorac Surg Clin.
27:181–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinese Society of Pathology; Pathology
Committee of China Anti-Cancer Association; Sarcoma Committee of
China Anti-Cancer Association; Pathology Group of Sarcoma Committee
of Chinese Society of Clinical Oncology; Pathology Committee of
Chinese Research Hospital Association; Expert Committee of Clinical
Practice Guideline for Pathological Sampling and Standardized
Report of Bone Tumor (2023 version), . Clinical practice guideline
for pathological sampling and standardized report of bone tumor
(2023 version). Zhonghua Bing Li Xue Za Zhi. 52:1098–1106. 2023.(In
Chinese). PubMed/NCBI
|
|
9
|
Hussar P, Popovska-Percinic F, Blagoevska
K, Järveots T and Dūrītis I: Immunohistochemical Study of Glucose
Transporter GLUT-5 in Duodenal Epithelium in Norm and in T-2
Mycotoxicosis. Foods. 9:8492020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki E, Yamagata K, Hagiwara T, Takasaki
R, Fukuzawa S, Uchida F, Ishibashi-Kanno N and Bukawa H: A case of
primary intraosseous adenoid cystic carcinoma of the mandible. Case
Rep Dent. 2023:24220862023.PubMed/NCBI
|
|
11
|
Nascimento de Aquino S, Silvestre Verner
F, Alvares Cabral R, Najar Rios CH, Paes de Almeida O and
Sanchez-Romero C: Adenoid cystic carcinoma with myoepithelial
predominance affecting maxilla and maxillary sinus. J Stomatol Oral
Maxillofac Surg. 120:55–60. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carlos-Bregni R, Vidaurre EC, Carolina
Netto A, Leon JE and Almeida OP: Primary intraosseous adenoid
cystic carcinoma of the mandible: Histopathological and
immunohistochemical analysis. Pathol Oncol Res. 15:659–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shome S, Mallick A, Kundu S and Gayen S:
Gnathic variant of primary adenoid cystic carcinoma: A unique case
report. J Cancer Res Ther. 18:286–290. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Guo X, Yu K, Shen X, Liu J, Zhao T
and Gu H: Adenoid cystic carcinoma of head and neck: Summary and
review of imaging findings. Heliyon. 9:e219012023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savithri V, Suresh R, Janardhanan M,
Aravind T and Mohan M: Primary intraosseous adenoid cystic
carcinoma with widespread skeletal metastases showing features of
high-grade transformation. Head Neck Pathol. 15:715–722. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Morais EF, de Farias Morais HG, de
Almeida Freitas R and Coletta RD: Prognostic significance of
histopathological parameters for salivary gland adenoid cystic
carcinoma. Dent J (Basel). 11:2622023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Persson M, Andersson MK, Sahlin PE, Mitani
Y, Brandwein-Weber MS, Frierson HF Jr, Moskaluk C, Fonseca I,
Ferrarotto R, Boecker W, et al: Comprehensive molecular
characterization of adenoid cystic carcinoma reveals tumor
suppressors as novel drivers and prognostic biomarkers. J Pathol.
261:256–268. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thierauf J, Ramamurthy N, Jo VY, Robinson
H, Frazier RP, Gonzalez J, Pacula M, Dominguez Meneses E, Nose V,
Nardi V, et al: Clinically integrated molecular diagnostics in
adenoid cystic carcinoma. Oncologist. 24:1356–1367. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdulfatah E, Rottmann D, Morag Y,
Pantanowitz L, Udager AM, Hao W and Lucas DR: Conventional
chondrosarcoma of the rib cage and sternum: clinicopathological and
molecular analysis of 27 patients treated at a single institution.
Hum Pathol. 136:63–74. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laasri K, El Hamzi A, Halfi MI, Allaoui M,
El Fenni J, En Nafaa I and Lahkim M: Solitary plasmacytoma of the
rib: A rare tumor to keep in mind: Case report. Radiol Case Rep.
18:214–217. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soeroso NN, Ramadhani N and Tarigan SP:
Ewing sarcoma with intra thoracic and multiple extra thoracic
metastases in a young adult male: A case report. Int J Surg Case
Rep. 118:1096422024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zupancic M, Näsman A, Berglund A, Dalianis
T and Friesland S: Adenoid Cystic Carcinoma (AdCC): A clinical
survey of a large patient cohort. Cancers (Basel). 15:14992023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zupancic M, Nasman A, Friesland S and
Dalianis T: Adenoid cystic carcinoma, clinical presentation,
current treatment and approaches towards novel therapies.
Anticancer Res. 44:1325–1334. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Zheng ZQ, Chen FP, Yan JY, Huang
XD, Li F, Sun Y and Zhou GQ: Role of postoperative radiotherapy in
nonmetastatic head and neck adenoid cystic carcinoma. J Natl Compr
Canc Netw. 18:1476–1484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tasoulas J, Divaris K, Theocharis S,
Farquhar D, Shen C, Hackman T and Amelio AL: Impact of tumor site
and adjuvant radiotherapy on survival of patients with adenoid
cystic carcinoma: A SEER database analysis. Cancers (Basel).
13:5892021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kacew AJ and Hanna GJ: Systemic and
targeted therapies in adenoid cystic carcinoma. Curr Treat Options
Oncol. 24:45–60. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dewenter I, Otto S, Kakoschke TK, Smolka W
and Obermeier KT: Recent advances, systemic therapy, and molecular
targets in adenoid cystic carcinoma of the head and neck. J Clin
Med. 12:14632023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimoda H, Teshima M, Murase T, Nagao T,
Kusafuka K, Nakaguro M, Urano M, Taguchi KI, Yamamoto H, Kano S, et
al: Prognostic scores for patients with salivary adenoid cystic
carcinoma without lymph node metastasis. Oral Oncol.
145:1064912023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lombardi D, Tomasoni M, Lorini L, Gurizzan
C, Tomasini D, Ardighieri L, Battocchio S, Bozzola A, Mattavelli D,
Paderno A, et al: Baseline prognostic factors affecting survival in
recurrent and/or metastatic salivary gland adenoid cystic
carcinoma. Oral Oncol. 126:1057642022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turchan WT, Korpics MC, Rooney M, Koshy M
and Spiotto MT: Impact of anatomic site of distant metastasis on
survival in salivary gland cancers. Head Neck. 43:2589–2601. 2021.
View Article : Google Scholar : PubMed/NCBI
|