Introduction
In total, 10–20% of patients with early-stage
non-small cell lung cancer (NSCLC) treated with stereotactic body
radiation therapy (SBRT) experience local failure (1–3). Some
locoregional failures, particularly local-only failures, can be
salvaged with curative-intent treatment. Hence, the early detection
and treatment of local failure may lead to improved clinical
outcomes. The American Society of Clinical Oncology (ASCO) and
European Society for Medical Oncology (ESMO) guidelines for lung
cancer recommend a 6-monthly computed tomography (CT) examination
during the first 2 years after curative-intent treatment for
detecting treatable tumor recurrence (4,5). Then,
the main target of surveillance imaging shifts from detecting tumor
recurrence to a new second lung cancer after the first 2 years.
However, local failure in early-stage NSCLC treated with SBRT can
occur even after 2 years (6).
Previous studies of locally advanced NSCLC and NSCLC at various
stages treated with chemoradiotherapy (7) and radiotherapy (8), respectively, have revealed that
prognosis is poor for tumors that reoccur within a short period. In
patients with NSCLC treated with complete resection, a short
interval between initial resection and tumor recurrence remains a
significant factor for poor prognosis (9,10).
Therefore, curative-intent treatment for patients with NSCLC may
improve prognosis. If this is also applicable to early-stage NSCLC
treated with SBRT, detecting delayed local failure may be more
beneficial for patient survival than detecting early recurrence
after SBRT.
In the present study, features of late local failure
(LLF; local failure >2 years after SBRT) for NSCLC treated with
SBRT were investigated and compared with early local failure (ELF;
local failure ≤2 years after SBRT).
Materials and methods
Patient selection
Medically inoperable patients with stage IA1-IIA
(Union for International Cancer Control 8th Edition) (11) NSCLC treated with SBRT at the
National Hospital Organization Shikoku Cancer Center (Matsuyama,
Japan) between July 2006 and March 2014 were retrospectively
evaluated by reviewing the medical records. Synchronous or
metachronous multiple NSCLC cases in which it was difficult to
identify the primary lesion that caused distant and/or regional
failure were excluded from the present study. The present study was
approved by the Ethics Committee of National Hospital Organization
Shikoku Cancer Center (approval. no. 2021-67) and, owing to the
retrospective nature of the present study, the opt-out method was
applied regarding patient consent. SBRT was only performed in
patients with an Eastern Cooperative Oncology Group (ECOG)
performance status of ≥2 (12).
Thoracic surgeons, thoracic oncologists and radiation oncologists
discussed the indications for SBRT. Bronchoscopy and/or CT-guided
needle aspiration biopsy (CT-NAB) were conducted for pathological
diagnosis. For patients whose lung tumors were not pathologically
proven, SBRT was performed only when a continuous increase in the
overall tumor size, solid component size or density of ground glass
were observed over time via serial CT. In all patients, the age,
sex, clinical stage, pathology of tumors and SBRT dose were
available from the records. However, certain basic data, including
blood type, height, body weight, obesity, smoking, drinking or
dietary habits, cancer-causing occupational exposure, concomitant
diseases such as diabetes or hypertension and long-term medication,
were unavailable.
Procedures for SBRT
For SBRT, the internal target volume (ITV) was
defined as lesions that could be visualized on slow-scan CT images
(4 sec, 2 mm thickness). For the planning target volume, a 5 mm
margin was added to the ITV contours. For SBRT, 8–11 non-coplanar
static 4 MV photon beams were used. Typical SBRT doses were 48 Gy
in four fractions [biological effective dose
(BED)10=106.6] for T1 tumors and 60 Gy in five fractions
(BED10=132) for T2 tumors, with an isocenter
prescription.
Follow-up studies
Local, regional and distant failures were diagnosed
using serial follow-up CT images. Follow-up CT was conducted every
2–6 months after SBRT for the first 2–3 years. Thereafter,
follow-up CT scans were performed once to thrice yearly. Follow-up
CT was continued when patients were able or willing to visit the
hospital. Whole-body 18F-fluorodeoxyglucose-positron
emission tomography/CT (FDG-PET/CT) was performed when tumor
recurrence was suspected.
Salvage treatment for failure
Salvage treatment was administered whenever
feasible. Typical curative-intent salvage treatments for local
failure included salvage surgery or re-irradiation (SBRT or
conventional three-dimensional conformal radiotherapy of ≥60 Gy for
the entire lesion). Patients with distant and/or widespread
regional failure were treated with supportive care to alleviate
distressing symptoms.
Statistical analysis
Overall survival after local failure was calculated
from the diagnosis of local failure. The Kaplan-Meier method was
used to estimate the overall survival rates and local failure-free
rates, and the statistical differences were evaluated using the
log-rank test. Differences in the incidence of local-only, regional
and distant failures were assessed using Fisher's exact test. All
statistical analyses were performed using the StatView software
(version 5.0; SAS Institute, Inc.).
Results
Study Population
Between July, 2006 and March, 2014, 244 NSCLC tumors
from 206 patients were treated with SBRT at the National Hospital
Organization Shikoku Cancer Center. Of these patients, 33 received
SBRT for synchronous or metachronous multiple NSCLC and were
subsequently excluded from the present study since it was difficult
to identify the primary lesion that caused distant and/or regional
failure. In the present study, multiple NSCLC tumors were defined
as independent lung tumors that were identified metachronously or
simultaneously regardless of the location and were diagnosed as
primary NSCLC by two radiologists based on the imaging findings,
disease course and medical history. The remaining 173 patients (173
tumors) were included in the present study (Table I).
 | Table I.Characteristics of the included
patients (n=173). |
Table I.
Characteristics of the included
patients (n=173).
| Characteristics | Value |
|---|
| Median age (range),
years | 79 (58–92) |
| Sex, n |
|
| Male | 113 |
|
Female | 60 |
| Stage, n |
|
| Stage
I | 137 |
| Stage
IIA | 36 |
| Histology of tumors,
n |
|
|
Adenocarcinoma | 69 |
| Squamous
cell carcinoma | 24 |
|
Other/unspecified non-small
cell cancer | 4 |
|
Unproven | 76 |
| SBRT dose (the
isocenter dose), Gy |
|
|
Range | 48.0–62.5 |
|
Median | 48.0 |
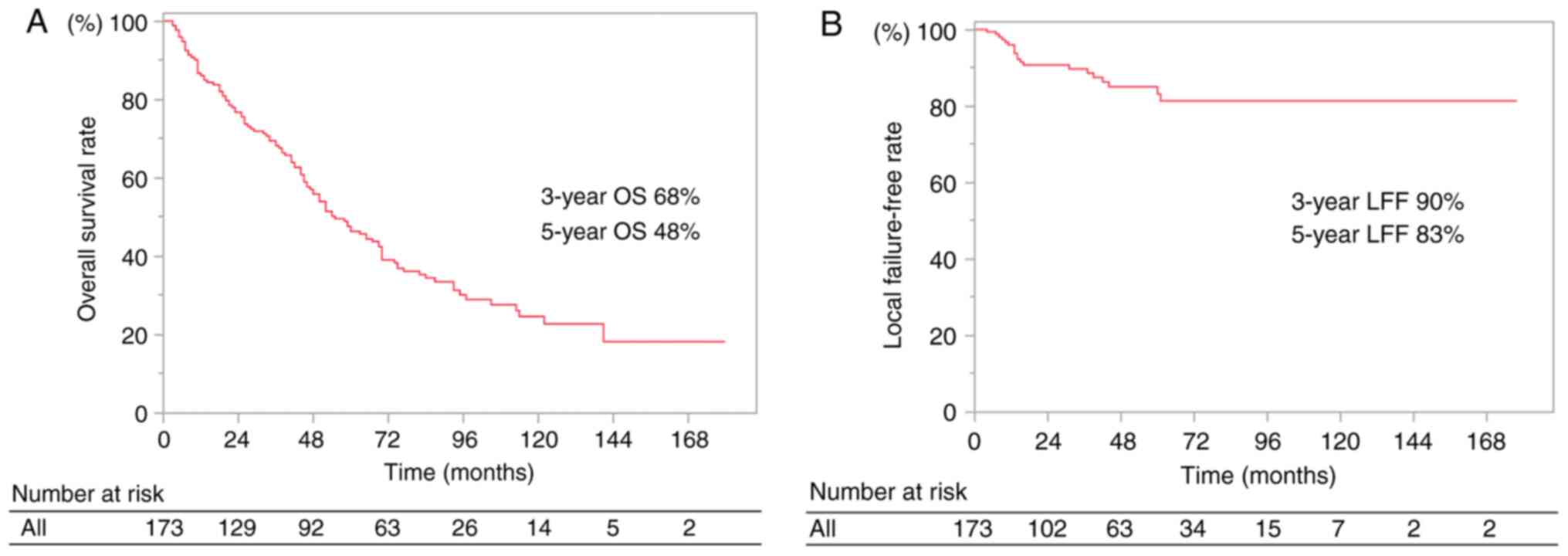
The median follow-up time from SBRT was 50 months
(range, 3–180 months) for survival and 31 months (1–178 months) for
CT follow-up. The median and mean doses of SBRT were 48.0 and 50.1
Gy, respectively (range, 48.0–62.5 Gy). The 3- and 5-year overall
survival rates were 68 and 48%, respectively and the 3- and 5-year
local failure-free rates were 90 and 83%, respectively (Fig. 1).
Features of local failure according to
the failure time
Of the 173 included patients, 20 experienced local
failure. LLF and ELF occurred in 7 and 13 patients, respectively
(Table II). The differences in
baseline factors between the ELF and LLF groups were not
statistically significant (Table
II). The proportions of squamous cell carcinoma and
adenocarcinoma were 38 and 23%, respectively, in the ELF cases and
14 and 29%, respectively, in the LLF cases (Table SI). In addition, pathologically
unproven tumors were 31% in the ELF and 57% in the LLF (Table SI). The median time to local
failure was 14 months (range, 4–61 months), 42 months (range, 31–61
months) and 13 months (range, 4–16 months) for all local failure,
LLF and ELF cases, respectively.
 | Table II.Characteristics of the 20 patients
with local failure. |
Table II.
Characteristics of the 20 patients
with local failure.
| Characteristics | Early local failure,
n=13 | Late local failure,
n=7 | P-valuea |
|---|
| Median age (range),
years | 78 (68–85) | 77 (61–88) |
|
| Age, n (%) |
|
|
|
| ≤80
years | 8 (61.5) | 6 (85.7) | 0.35 |
| >80
years | 5 (38.5) | 1 (14.3) |
|
| Sex, n (%) |
|
|
|
| Male | 10 (76.9) | 3 (42.9) | 0.17 |
|
Female | 3 (23.1) | 4 (57.1) |
|
| TNMb, n (%) |
|
|
|
| I | 9 (69.2) | 6 (85.7) | 0.61 |
| IIA | 4 (30.8) | 1 (14.3) |
|
| Appearance, n
(%) |
|
|
|
|
Solid | 13 (100.0) | 6 (85.7) | 0.35 |
| GGN | 0 (0.0) | 1 (14.3) |
|
| History of multiple
primary cancer, n (%) |
|
|
|
| Yes | 7 (53.8) | 2 (28.6) | 0.64 |
| No | 6 (46.2) | 5 (71.4) |
|
| Mean SBRT dose
(range), Gy | 52.6 (48.0–60.0) | 50.1 (48.0–62.5) |
|
| SBRT, n (%) |
|
|
|
| 48
Gy | 9 (69.2) | 4 (57.1) | 0.65 |
| >48
Gy | 4 (30.8) | 3 (42.9) |
|
| Sq/Ad/other/UP
pathology, n | 6/2/1/4 | 1/0/0/6 |
|
| Median time to
local failure (range), months | 13 (4–16) | 43 (31–61) |
|
| Median interval
between CT-detected local failure and the previous CTc (range), months | 3 (1–6) | 6 (4–12) |
|
| Curative intent
salvage treatment, n (%) |
|
|
|
|
Yes | 9 (69.2) | 0 (0.0) | <0.01 |
| No | 4 (30.8) | 7 (100.0) |
|
The incidence of local-only failure was lower in the
LLF than in the ELF cases (Table
III). Among the 20 local failures, local-only failure was
observed in 14% (1/7) of LLF cases and 77% (10/13) of ELF cases,
which was statistically significant (Fisher's exact test, P=0.02).
When local failure was detected by CT, regional failure was
observed in 71% (5/7) of LLF cases and 8% (1/13) of ELF cases
(Fisher's exact test, P=0.01), whereas distant failure was observed
in 57% (4/7) and 15% (2/13) of LLF and ELF cases respectively
(Fisher's exact test, P=0.12).
 | Table III.Patterns of failure in patients who
experienced local failure. |
Table III.
Patterns of failure in patients who
experienced local failure.
| Failure
patterns | ELF, n=13 | LLF, n=7 |
P-valuea |
|---|
| Local only, n
(%) | 10 (77) | 1 (14) | 0.02 |
| Local + regional, n
(%) | 1 (8) | 2 (29) | 0.27 |
| Local + distant, n
(%) | 2 (15) | 1 (14) | >0.99 |
| Local + regional +
distant, n (%) | 0 (0) | 3 (43) | 0.03 |
| Distant, n (%) | 2 (15) | 4 (57) | 0.12 |
| Regional, n
(%) | 1 (8) | 5 (71) | 0.01 |
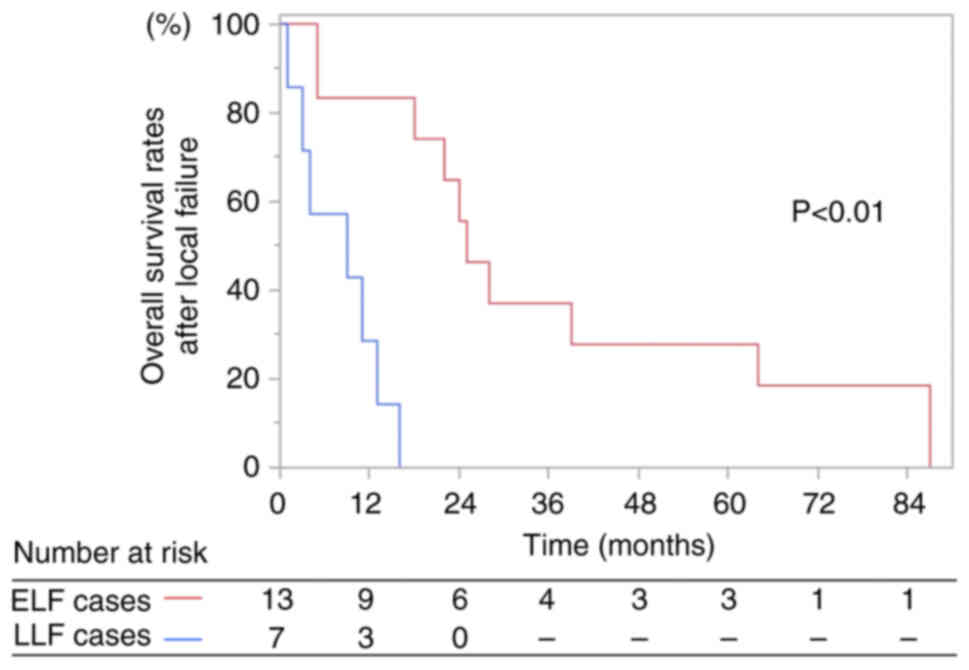
The median survival times after local failure were 9
months (range, 1–16 months) and 25 months (range, 2–87 months) for
patients with LLF and ELF, respectively (Fig. 2). The 1- and 2-year overall survival
rates after local failure were 29 and 0%, respectively, in LLF
cases, and 83 and 56%, respectively, in ELF cases (log-rank test,
P<0.01 at 1-year; Fig. 2).
Curative-intent salvage treatment, including salvage
surgery and salvage radiotherapy with ≥60 Gy for the entire lesion,
was not performed for any patients with LLF (0/7), but was
performed in 69% (9/13) of patients with ELF (Fisher's exact test,
P<0.01; Table II). Of the 9
patients with ELF in which curative-intent salvage therapy was
performed, surgery was performed for 3 patients and ≥60 Gy
radiotherapy of the entire lesion was administered for 6
patients.
Discussion
To the best of our knowledge, the present study is
the first to show the poor prognosis of patients after LLF
following the treatment of NSCLC with SBRT. The median survival
time after local failure was significantly shorter in the LLF
compared with the ELF cases (6.5 vs. 25 months). The incidence of
local-only failure was also significantly lower in the LLF compared
with the ELF cases (14 vs. 77%). Reportedly, in outcomes of NSCLC
treated with conventionally fractionated radiotherapy or surgery,
delayed failure (>6–12 months from treatment) may be associated
with an improved prognosis compared with early failure (5–8).
However, LLF after SBRT was shown to be associated with poor
prognosis in the present study.
In the present study, most LLF cases had distant
and/or regional metastases when local failure was detected.
Curative-intent salvage treatment is often difficult among LLF
cases due to these metastases. After SBRT, radiation fibrosis of
the lungs often masks signs of local failure (13). We hypothesize that LLF tumors grow
latently in the radiation fibrosis of the lung and continue to be a
seed for metastasis for a relatively long period before local
recurrence becomes apparent. The benefit of surveillance imaging
for detection compared with ELF may be relatively small since the
potential for curative-intent salvage treatment remains relatively
small for LLF. The frequency of follow-up CT scans after the first
2 years may also be decreased for patients with NSCLC treated with
SBRT, as recommended by the ASCO and ESMO guidelines (4,5).
Previous reports have shown an association between
the pathological subtypes of NSCLC and the incidence of LLF after
SBRT, in which there is a trend towards a higher incidence of LLF
in adenocarcinoma than in squamous cell carcinoma. Specifically,
Shintani et al (6) reported
that the median time to local failure was 1.3 and 2.1 years for
squamous cell carcinoma and adenocarcinoma, respectively. The study
mentioned that frequent follow-up in the first 2 years is
necessitated for squamous cell carcinoma, whereas careful follow-up
beyond the first 2 years is warranted for adenocarcinoma. Woody
et al (14) reported that
the time to local failure was 14.9 and 18.9 months for squamous
cell carcinoma and adenocarcinoma, respectively. In the present
study, the proportions of squamous cell carcinoma and
adenocarcinoma were 38 and 23%, respectively, in the ELF cases and
14 and 29%, respectively, in the LLF cases. Despite the relatively
high and low proportion of adenocarcinomas in the LLF and ELF
cases, respectively, 57% of LLF and 31% of ELF cases had
pathologically unproven tumors. Hence, the pathological features of
LLF and ELF could not be adequately analyzed in the present study
due to the low number of available cases.
The present study had certain limitations. First,
this was a retrospective study using obsolete medical records from
a single institution with a limited sample size; the available
data, including blood type, height, body weight, obesity, smoking,
drinking or dietary habits, cancer-causing occupational exposure,
concomitant diseases such as diabetes or hypertension and long-term
medication, were also limited; hence, further studies are warranted
to confirm the differences in the features of LLF and ELF. Second,
it is often difficult to assess local recurrence after SBRT. To
address this issue, in the present study, an initial diagnosis was
based on a single CT image. However, CT imaging findings, such as
increased consolidation or the loss of an air-bronchogram in the
treated area over time, were also considered. Additionally, these
findings were combined with clinical symptoms, elevated tumor
markers and increased FDG accumulation via FDG/PET-CT to make a
more comprehensive decision. Although local recurrence was
diagnosed with as much care as possible, careful interpretation is
still required. However, in the present study, local recurrence was
not proven in the majority of cases as tumors with inflammation
also showed increased FDG uptake even in the absence of local
recurrence (15,16). Therefore, careful interpretation
will be required regarding the results of FDG uptake as an
indicator of local recurrence after SBRT. Third, pathological
confirmation could not be obtained for most tumors reviewed in the
present study as some patients did not undergo CT-NAB for
pathological confirmation. For these patients, the diagnosis of
lung cancer was based on careful CT observations over time
(FDG-PET/CT was performed when necessary). The treatment outcomes
of clinically diagnosed lung cancer are comparable to those of
pathologically proven NSCLC when various clinical findings are
integrated and carefully diagnosed (17). Since careful CT follow-up was
performed before SBRT, we consider that the diagnosis of lung
cancer was reliable. Furthermore, since follow-up CT was performed
more frequently in the first 2 years after SBRT compared with later
years, an effect similar to ‘lead-time bias’ may potentially
contribute to generating differences in survival time after local
failure between the LLF and ELF cases. However, the median interval
between CT-detected local failure and previous CT was 6 and 3
months for the LLF and ELF cases, respectively, which was not a
significant difference. Finally, information regarding the cause of
death was unavailable since information on the date of death was
obtained from the cancer registry database for a number of
patients.
In conclusion, for patients with stage I–IIA NSCLC
treated with SBRT, the prognosis after local failure was
significantly improved in the ELF cases compared with the LLF
cases. Curative-intent treatment is often not performed in patients
with LLF due to the frequent distant and/or regional failure.
Considering the features of LLF cases, it seems reasonable to
decrease the frequency of follow-up CT for detecting tumor
recurrence after the first 2 years post-SBRT, as recommended by the
ASCO and ESMO guidelines.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available to preserve individuals' privacy under ‘the
Personal Information Protection Law’ but may be requested from the
corresponding author.
Authors' contributions
KM and YH designed the study. KM and YH confirm the
authenticity of all the raw data. YH analyzed the data. KM, YH, HK,
KN, TU, HS, DH, TN, YK, YS, TK and MY collected the patient data
and drafted the article. All authors collaborated in writing the
discussion section and in discussing the interpretation of the
results. KM prepared the manuscript and YH edited the manuscript.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures involving human participants were
performed in accordance with the ethical standards of the
institutional research committee and with the 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
This retrospective study was approved by the Ethics Committee of
National Hospital Organization Shikoku Cancer Center (Matsuyama,
Japan; approval. no. 2021-67). The opt-out method was applied
regarding patient consent due to the retrospective nature of the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASCO
|
American Society of Clinical
Oncology
|
|
BED
|
biological effective dose
|
|
CT
|
computed tomography
|
|
CT-NAB
|
CT-guided needle aspiration biopsy
|
|
ELF
|
early local failure
|
|
ESMO
|
European Society for Medical
Oncology
|
|
FDG-PET
|
18F-fluorodeoxyglucose-positron emission tomography
|
|
ITV
|
internal target volume
|
|
LLF
|
late local failure
|
|
NSCLC
|
non-small cell lung cancer
|
|
SBRT
|
stereotactic body radiation
therapy
|
References
|
1
|
Bradley JD, El Naqa I, Drzymala RE, Trovo
M, Jones G and Denning MD: Stereotactic body radiation therapy for
Early-stage Non-small-cell lung cancer: The pattern of failure is
distant. Int J Radiat Oncol Biol Phys. 77:1146–1150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Onishi H, Araki T, Shirato H, Nagata Y,
Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, et
al: Stereotactic hypofractionated high-dose irradiation for stage I
nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in
a Japanese multiinstitutional study. Cancer. 101:1623–1631. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senthi S, Lagerwaard FJ, Haasbeek CJ,
Slotman BJ and Senan S: Patterns of disease recurrence after
stereotactic ablative radiotherapy for early stage Non-small-cell
lung cancer: A retrospective analysis. Lancet Oncol. 13:802–809.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider BJ, Ismaila N, Aerts J, Chiles
C, Daly ME, Detterbeck FC, Hearn JWD, Katz SI, Leighl NB, Levy B,
et al: Lung cancer surveillance after definitive curative-intent
therapy: ASCO guideline. J Clin Oncol. 38:753–766. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Postmus PE, Kerr KM, Oudkerk M, Senan S,
Waller DA, Vansteenkiste J, Escriu C and Peters S; ESMO Guidelines
Committee, : Early and locally advanced non-small-cell lung cancer
(NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 28 (Suppl_4):iv1–iv21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shintani T, Matsuo Y, Iizuka Y, Mitsuyoshi
T and Mizowaki T: A retrospective Long-term follow-up study of
stereotactic body radiation therapy for Non-small cell lung cancer
from a single institution: Incidence of late local recurrence. Int
J Radiat Oncol Biol Phys. 100:1228–1236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamamoto Y, Kataoka M, Nogami N, Kozuki T,
Kato Y, Shinohara S and Shinkai T: Factors affecting survival time
after recurrence of non-small-cell lung cancer treated with
concurrent chemoradiotherapy. Jpn J Radiol. 30:249–254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McAvoy S, Ciura K, Wei C, Rineer J, Liao
Z, Chang JY, Palmer MB, Cox JD, Komaki R and Gomez DR: Definitive
reirradiation for locoregionally recurrent non-small cell lung
cancer with proton beam therapy or intensity modulated radiation
therapy: Predictors of high-grade toxicity and survival outcomes.
Int J Radiat Oncol Biol Phys. 90:819–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki H, Suzuki A, Tatematsu T, Shitara
M, Hikosaka Y, Okuda K, Moriyama S, Yano M and Fujii Y: Prognosis
of recurrent non-small cell lung cancer following complete
resection. Oncol Lett. 7:1300–1304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takenaka T, Yano T, Yamazaki K, Okamoto T,
Hamatake M, Shimokawa M and Mori M; Kyushu University Lung Surgery
Study Group Japan, : Survival after recurrence following surgical
resected non-small cell lung cancer: A multicenter, prospective
cohort study. JTCVS Open. 10:370–381. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lababede O and Meziane MA: The Eighth
edition of TNM staging of lung cancer: Reference chart and
diagrams. Oncologist. 23:844–848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang K, Senthi S, Palma DA, Spoelstra FO,
Warner A, Slotman BJ and Senan S: High-risk CT features for
detection of local recurrence after stereotactic ablative
radiotherapy for lung cancer. Radiother Oncol. 109:51–57. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woody NM, Stephans KL, Andrews M, Zhuang
T, Gopal P, Xia P, Farver CF, Raymond DP, Peacock CD, Cicenia J, et
al: A histologic basis for the efficacy of SBRT to the lung. J
Thorac Oncol. 12:510–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugawara Y, Braun DK, Kison PV, Russo JE,
Zasadny KR and Wahl RL: Rapid detection of human infections with
fluorine-18 fluorodeoxyglucose and positron emission tomography:
Preliminary results. Eur J Nucl Med. 25:1238–1243. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kubota R, Yamada S, Kubota K, Ishiwata K,
Tamahashi N and Ido T: Intratumoral distribution of
fluorine-18-fluorodeoxyglucose in vivo: High accumulation in
macrophages and granulation tissues studied by
microautoradiography. J Nucl Med. 33:1972–1980. 1992.PubMed/NCBI
|
|
17
|
Takeda A, Kunieda E, Sanuki N, Aoki Y, Oku
Y and Handa H: Stereotactic body radiotherapy (SBRT) for solitary
pulmonary nodules clinically diagnosed as lung cancer with no
pathological confirmation: Comparison with non-small-cell lung
cancer. Lung Cancer. 77:77–82. 2012. View Article : Google Scholar : PubMed/NCBI
|