Introduction
As is well documented, neuroendocrine neoplasms
(NENs) are rare malignancies that originate from the diffuse
neuroendocrine cell system, and are more prevalent in the
gastroenteropancreatic (GEP) tract and lung (1). Despite accounting for 1.2% of all
pancreatic malignancies (2), the
incidence of NEN of the pancreas (pNEN) has notably increased in
both the USA and Europe in recent years. In 2018, the
age-standardized incidence rate of pNEN reached one case per
100,000 residents, with an average annual growth rate of 110.6% in
Europe (3). For pNEN, the estimated
5-year overall survival (OS) has been reported to decrease from 87%
at stage I to 71% at stage III and 26% at stage IV (3).
Neuroendocrine neoplasms (NENs) exhibit high
heterogeneity and can be classified based on the embryonic origin
of the corresponding tissues of the primary tumor into three
groups: Foregut (bronchopulmonary, stomach, duodenum, biliary tract
and pancreas), midgut (jejunum, ileum, appendix and proximal
colon), and hindgut (distal colon and rectum) NENs (4). The rectum and pancreas are the most
common sites of occurrence in Asian populations, while in Caucasian
populations in Europe and America, the midgut and pancreas are the
most common sites (4). In addition,
these NENs can be classified into functioning and non-functioning
types, with functioning tumors often secreting hormones such as
insulin or glucagon, leading to distinct clinical syndromes
(5). In the present case, the tumor
originated from the pancreas and was non-functional.
The management of pNEN relies on the stage and grade
of the tumor at the time of diagnosis. Surgical intervention is the
gold-standard treatment for patients diagnosed at an early stage,
whereas systemic therapy, such as chemotherapy, and interventional
treatment, such as transarterial chemoembolization (TACE), remain
the primary treatments for locally advanced disease or for patients
with metastatic pNEN who are ineligible for surgery (4). However, the majority of pNENs are
diagnosed at locally advanced or metastatic stages (6). Patients with advanced pancreatic NENs
may present with symptoms related to hormone excess, abdominal
pain, weight loss and gastrointestinal disturbances.
Histologically, these tumors are characterized by a proliferation
of uniform cells with granular cytoplasm and may exhibit varying
degrees of differentiation. For this population, the role of
surgery is therefore often palliative, whereas chemotherapy has
limited activity (7–9).
The present case report outlines the case of a
patient with multiple metastatic pNEN who underwent numerous
sessions of radiofrequency ablation (RFA) and treatment with
systemic somatostatin analogs (SSAs). After receiving the targeted
therapy surufatinib and TACE, the patient achieved a favorable
response.
Case report
Patient case
A 54-year-old woman hospitalized at The First
Affiliated Hospital of Xinxiang Medical University (Xinxiang,
China) without prior medical history was originally diagnosed with
moderately differentiated pNEN during a routine physical
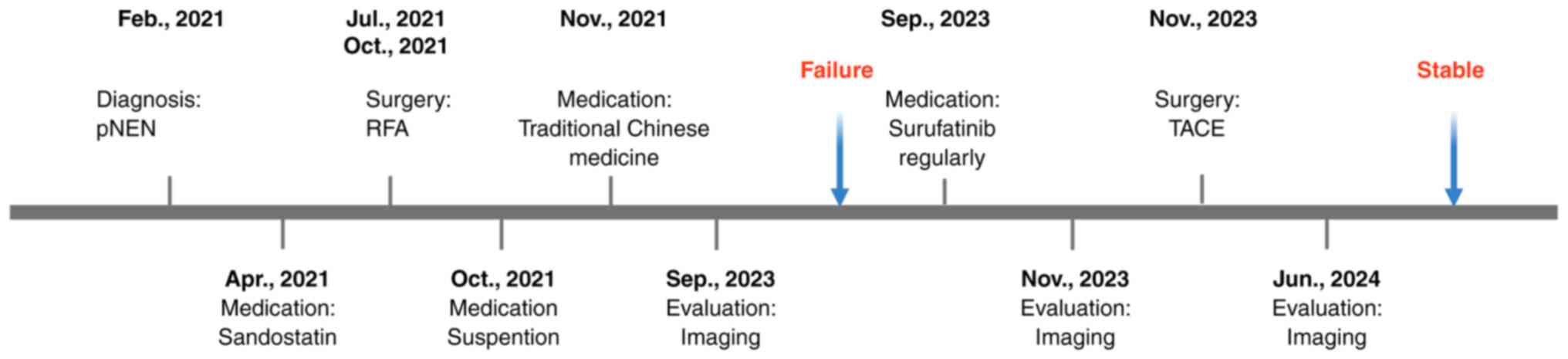
examination in February 2021. Fig.
1 depicts the timeline of the present case report. In February
2021, the patient underwent ultrasonography-guided liver biopsy,
liver lesion RFA and pancreatic lesion RFA. The results of the
morphological analysis on the pNEN revealed a Ki-67 proliferation
index of 10%, with 5 mitotic figures/2 mm2 and the
absence of necrosis. Postoperative pathology delineated that the
liver lesions were NENs (grade G2, stage IV, T4N0M1), according to
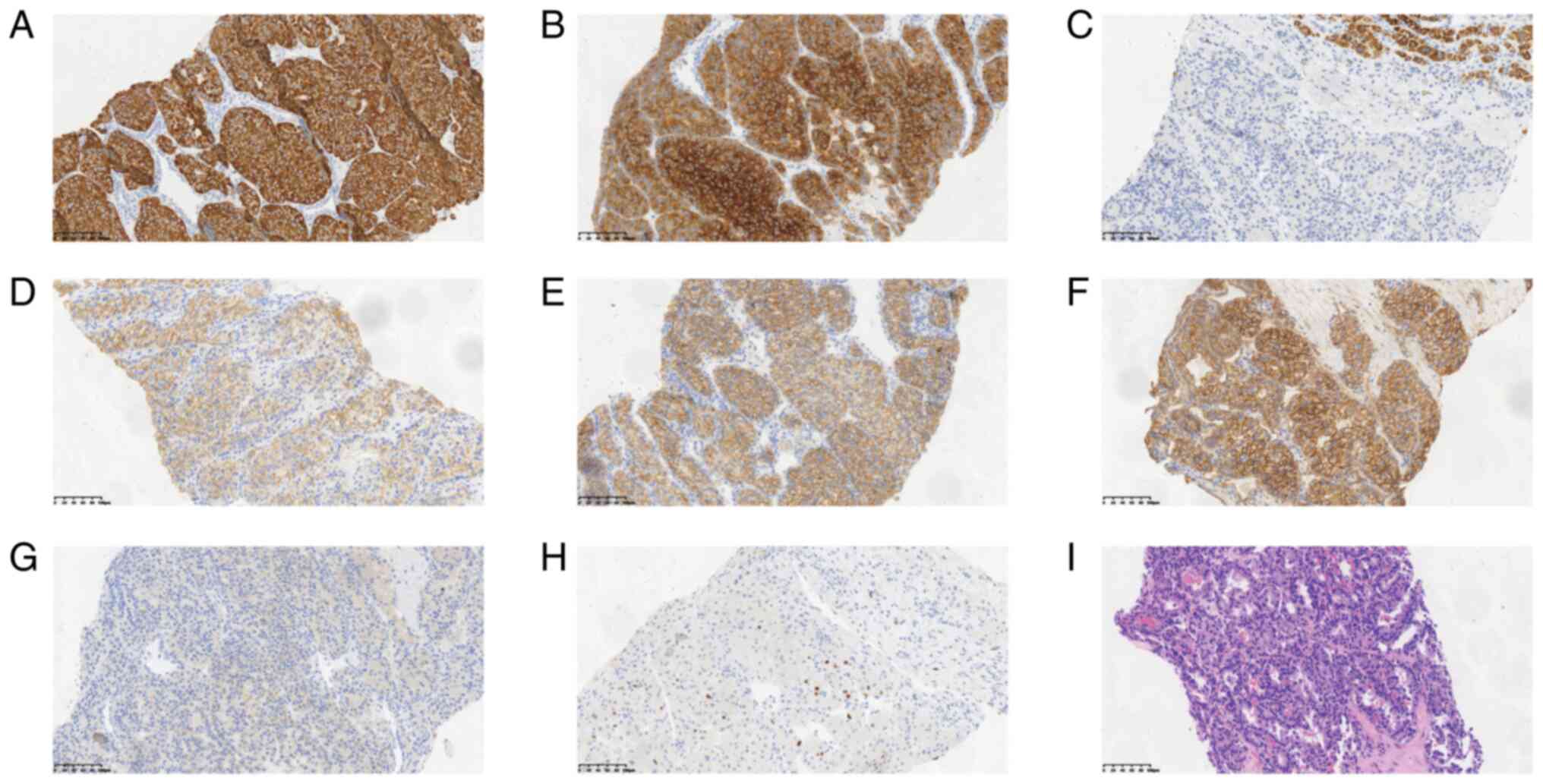
the American Joint Committee on Cancer 8th edition (10). Immunohistochemistry results of the
pNEN (Fig. 2A-H) showed that the
tumor had the following characteristics: CK19(+), SYN(+), HEP(−),
somatostatin receptor (SSTR)2(+), E-cadherin(+), β-catenin(membrane
+), P504S(−) and Ki67(+ 10%). Furthermore, histopathological
examination of the tumor (routine hematoxylin and eosin staining)
depicted that tumor cells were arranged in a cribriform pattern
(Fig. 2I). Additionally, the cells
exhibited atypia, with marginally abundant cytoplasm, oval nuclei
of different sizes, deeply stained nuclei and few mitoses. The
nuclear membranes and nucleoli were difficult to visualize.
Notably, metastases were not detected outside of the liver.
According to the National Comprehensive Cancer Network guidelines
(11), the patient was administered
Sandostatin LAR (20 mg intravenously; q4w) to suppress tumor growth
in April 2021. Following the seventh cycle in October 2021, the
patient self-discontinued Sandostatin LAR due to side effects, such
as dizziness, outside the hospital setting.
After an imaging evaluation (including assessment of
the target, non-target and new lesions) according to the Response
Evaluation Criteria in Solid Tumors version 1.1 (12), the condition of the patient was
considered stable. To further reduce the tumor burden, the patient
underwent two sessions of percutaneous RFA under ultrasound
guidance for liver lesions in July and October 2021. Between
November 2021 and September 2023, the patient declared that they
self-administered traditional Chinese herbal medicine, and they did
not attend the hospital for a follow-up appointment. Notably, no
other information was provided regarding the traditional Chinese
medicine taken, including the composition, dosage and
administration.
In September 2023, the patient presented with
abdominal distension and generalized itching, prompting her to be
re-hospitalized to undergo abdominal paracentesis and drainage.
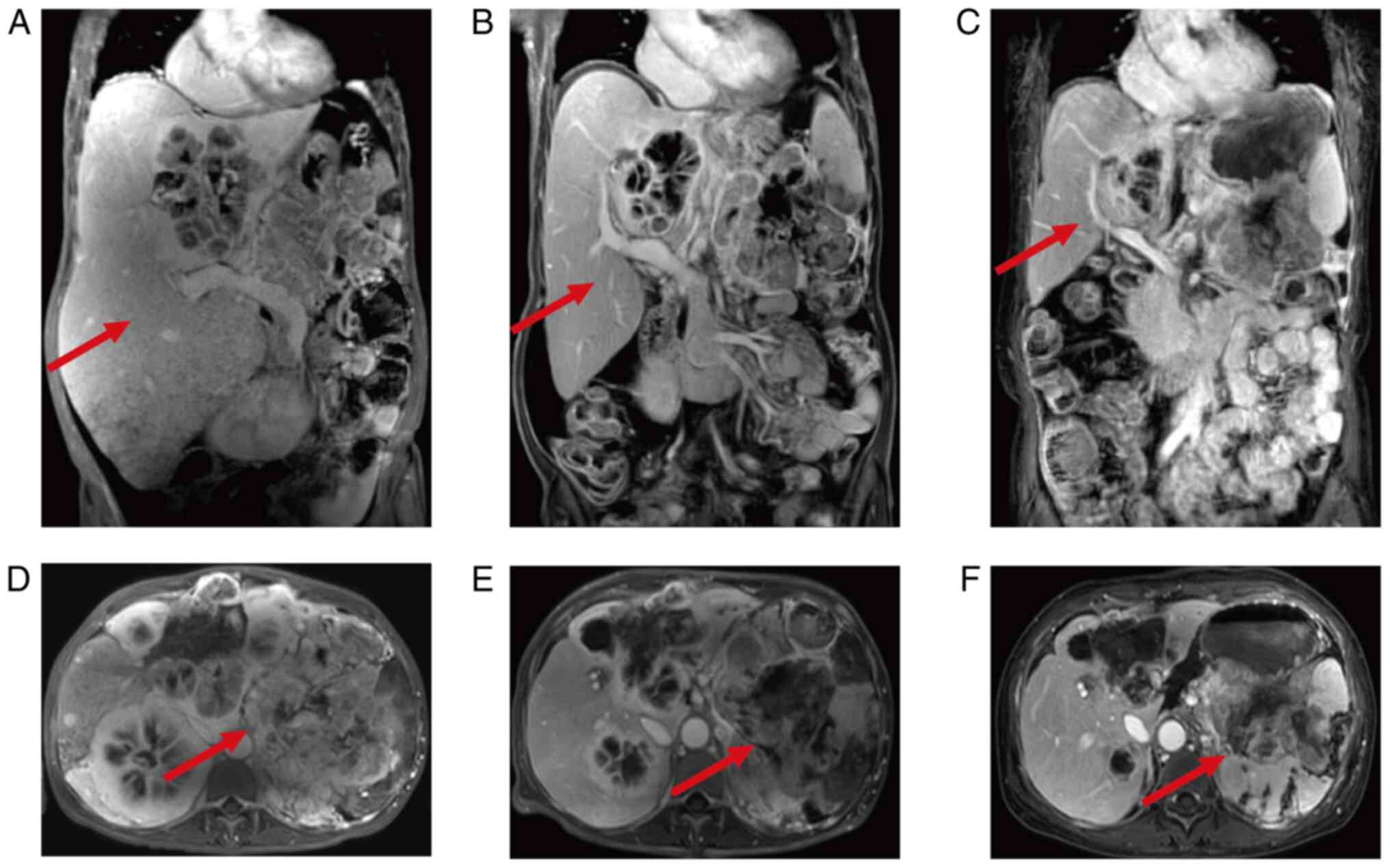
Subsequently, magnetic resonance cholangiopancreatography (MRCP)
dynamic enhancement imaging (Fig. 3A
and D) indicated that the range and number of pancreatic tail
and liver lesions had increased compared with in June 2022,
reflecting disease progression. Notably, the right kidney was
displaced and rotated due to compression. Subsequently, the patient
underwent three sessions of temporary hemodialysis to mitigate
renal dysfunction induced by right kidney compression, and closed
peritoneal drainage to relieve abdominal discomfort due to ascites.
After a multi-disciplinary treatment (MDT) consultation and
considering the associated high risk, no further
ultrasonography-guided biopsy was performed to reassess the
pathological staging of the lesion progression. Meanwhile, the
patient was treated with surufatinib (300 mg orally, qd) and
received two doses of the short-acting SSA octreotide (0.05 mg
subcutaneous injection, q12 h) to enhance treatment
effectiveness.
Treatment efficacy was assessed based on subsequent
MRCP reevaluations compared with the initial MRCP result (Fig. 3A and D; September 2023). After 2
months, MRCP dynamic enhancement imaging demonstrated a decrease in
the size of the pNEN and multiple metastatic lesions (Fig. 3B and E; November 2023), indicative
of stable disease progression. After that, the patient underwent
TACE, a procedure involving chemotherapy (ethiodized poppyseed oil,
4.8-g injection) and embolization through the hepatic artery, in
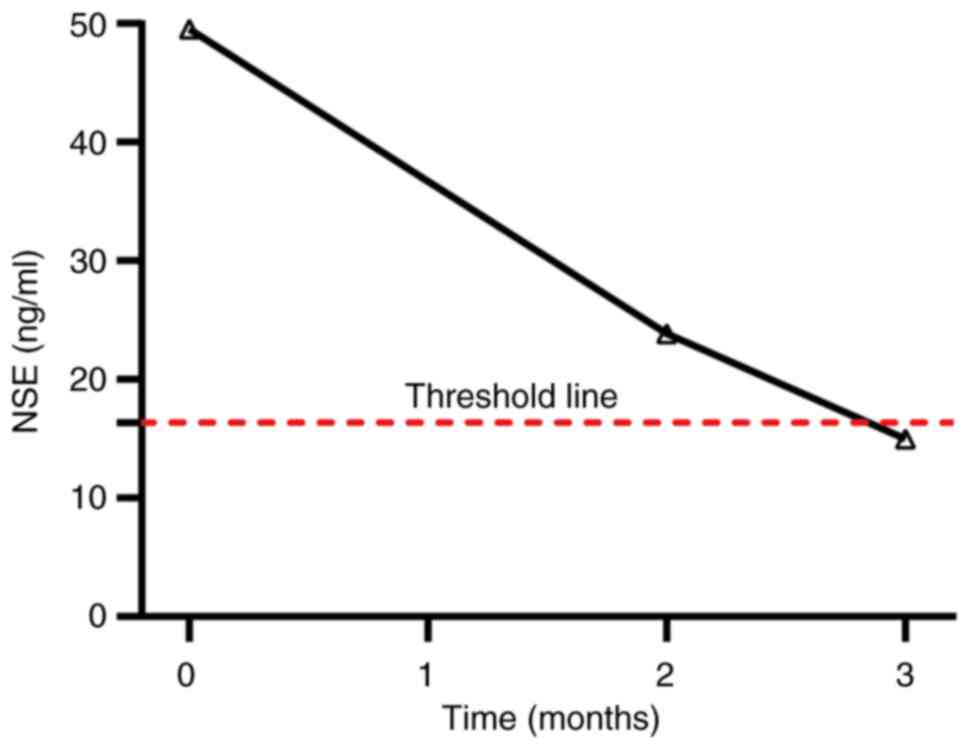
November 2023. After 3 months of targeted therapy, neuron-specific
enolase (NSE) levels had decreased to within the reference range
(0–17.5 ng/ml) (Fig. 4). The MRCP
reevaluation in June 2024 revealed a stable condition (Fig. 3C and F). The latest follow-up visit
was in mid-June 2024.
By June 2024, the progression-free survival (PFS)
time of the patient was 12 months, and a total of 41 months had
passed since the initial diagnosis of pNEN. The patient had
achieved stable disease following evaluation.
Pathology
Hematoxylin and eosing staining
Tissues were fixed in 10% neutral buffered formalin
at room temperature for 24 h before being sectioned into 4-µm thick
slices. The sections were then deparaffinized at 45°C for ~5 min.
Next, the slides were immersed in hematoxylin at 25°C for 5–10 min,
differentiated in hydrochloric acid alcohol at 25°C and then
stained with eosin at 25°C for 1–3 min, followed by rinsing with
water. Finally, the sections were mounted with neutral gum sealant.
The quality of staining was evaluated using a LEICA DM1000 LED
microscope (Leica Microsystems GmbH) at a magnification of
×200.
Immunohistochemical staining
The tissue sections were deparaffinized at 45°C for
5 min, followed by immersion in an immunohistochemical antigen
retrieval solution (neutral pH) at 95°C for 20 min. The sections
were then respectively immersed in distinct primary antibody
solutions (diluted 1:100), including those for CK19 (cat. no.
ZM-0074), SYN (cat. no. ZA-0506), HEP (cat. no. ZM-0131),
somatostatin receptor 2 (cat. no. ZA-0587), E-cadherin (cat. no.
ZA-0565), β-catenin (cat. no. ZA-0646), P504S (cat. no. ZA-0227)
and Ki67 (cat. no. TA500265) (all Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.), and incubated at room temperature for 1–2
h. After washing, the sections were incubated with a suitable
secondary antibody (horseradish-conjugated goat anti-rabbit/mouse
IgG; diluted 1:200; cat. no. PV-8000; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.). Finally, the substrate solution
DAB (cat. no. ZLI-9017; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) was added, and the color development
reaction was observed.
NSE detection
Fasting venous blood was collected and centrifuged
at ×1,170 g (25°C for 10 min) to separate the serum. The level of
NSE was then detected using the Chemiluminescent Immunoassay with
the Roche Cobas e602 (both Roche Diagnostics GmbH), following the
manufacturer's instructions.
Discussion
The complexity and heterogeneity of NENs pose
numerous challenges in their treatment. Surgical management is the
preferred option for patients with early-stage tumors and achieves
satisfactory outcomes (13,14); however, the prognosis of patients
with advanced and metastatic pNEN remains suboptimal (15,16),
with comprehensive treatment being the mainstay approach (1). The present case report provides
valuable insights into the challenges and implications for the
diagnosis and treatment of this specific subgroup of patients.
In the present study the patient was diagnosed with
advanced pNEN with liver metastases. Notably, hepatic metastatic
lesions pose a greater risk due to compression of abdominal organs
compared with the primary tumor, and removing the primary tumor
provides relatively minimal improvement in treatment effectiveness
or survival time for the patient (11). The classification of neuroendocrine
neoplasms (NENs) into functional and non-functional types is based
on hormone secretion. In the present case, it was classified as
non-functional. In addition, for G1/G2 non-functional pNENs,
radical surgery should be pursued. If the tumor involves adjacent
organs or tissues, radical resection of both the primary tumor and
the affected organs or tissues is recommended. If the tumor is
associated with liver metastases, the surgical plan should be based
on the resectability of the primary tumor and the classification of
the liver metastases (4).
Generally, resection of the primary tumor helps improve symptoms,
and liver metastases can also be managed through surgery combined
with interventional treatments. Specifically, when both the primary
and metastatic lesions are resectable, radical surgery should be
attempted; if the primary tumor is resectable but resection of the
metastases is difficult, effective debulking surgery (with ≥70%
debulking) combined with interventional treatment for liver
metastases is recommended. When metastases cannot be resected,
resection of the primary tumor may offer some benefit, which
requires a comprehensive evaluation of the primary tumor size,
overall tumor burden and any complications from local compression
(4). When the primary tumor cannot
be resected but metastases can be, resection of the metastases
alone is typically not recommended (17). Imaging evaluation revealed that the
patient in the present study did not meet the criteria for curative
resection of the primary tumor and metastatic lesions. Therefore,
the initial treatment plan included multiple sessions of RFA
combined with medical therapy to reduce tumor burden including the
liver metastases and the primary tumor and alleviate symptoms,
followed by a liver biopsy to confirm the pathological grade of the
tumor.
The objectives of medical treatment for functional
NENs (the pNEN in this case was non-functional) are to attenuate
symptoms caused by hormone release and to delay tumor growth.
Antitumor therapies for NENs include biological agents, targeted
drugs, cytotoxic chemotherapies and immunotherapy. Systemic
chemotherapy is typically not recommended as the first-line
treatment for G1/G2 grade gastrointestinal neuroendocrine tumors
(GI-NETs), and is only reserved for cases where other therapeutic
approaches, including biologics, tyrosine kinase inhibitors, and
peptide receptor radionuclide therapy (PRRT), have failed (1).
In recent years, immune checkpoint inhibitors
(ICIs), particularly those targeting PD-1/PD-L1, have shown
clinical efficacy across various types of cancer (18–20).
However, their use in NENs remains at an exploratory stage
(21). A systematic review
evaluating the role of ICIs both as single agents and in
combination in NENs reported a pooled overall response rate (ORR)
and a disease control rate of 10 and 42%, respectively. In
addition, the median PFS was 4.1 months, while the median OS was 11
months (21). Notably, the ORRs
from existing clinical trials of ICIs (21) are generally low.
PRRT involves labeling radioactive isotopes that
emit α or β particles onto tumor-targeting peptides (22). The results of a phase III (NETTER-1)
trial indicated that the estimated PFS at 20 months was 65.2% in
patients with advanced midgut neuroendocrine tumors treated with
the PRRT 177 Lu-DOTATATE and 10.8% in the same patients treated
with octreotide (23). However, due
to the heterogeneity and complexity of pNENs, the effectiveness of
PRRT warrants further prospective clinical studies to validate its
efficacy (24).
Extensively used SSAs, such as long-acting
octreotide and lanreotide autogels, exert anti-proliferative and
pro-apoptotic effects by binding to SSTR (25). Notably, the PROMID phase III
clinical trial corroborated the efficacy of SSAs in delaying tumor
progression (26). Clinical
research on metastatic patients demonstrated that, in pNEN,
lanreotide significantly prolonged PFS compared with the placebo,
with a median PFS of 18.0 months versus not reached, and the
estimated rates of PFS at 24 months were 65.1 and 33.0% in the
lanreotide group and placebo group, respectively (27). The European Society for Medical
Oncology consensus recommends SSAs as the first-line treatment
option for advanced GEP-NETs and NENs with unknown primary sites
that are SSTR-positive, have slow growth rates and a Ki-67
proliferation index of 10% (1).
Therefore, SSAs were administered in the present case to further
prevent tumor growth, given the stable postoperative condition of
the patient. The patient received seven cycles of an SSA
(Sandostatin LAR 20 mg, q4w) from April 2021 to October 2021, and
self-discontinued Sandostatin LAR due to dizziness, a known adverse
reaction associated with SSAs, outside the hospital setting.
According to the Chinese Society of Clinical Oncology (CSCO)
guidelines, long-acting SSAs include long-acting octreotide
(Sandostatin LAR) and lanreotide (28). However, lanreotide was only
officially approved for the treatment of GI-NETs and pNENs in China
on March 29, 2024. Prior to this, due to the drug availability, it
could not be considered a first-line option.
The present patient was medicated with Sandostatin.
After 3 months of recovery and follow-up, imaging assessments
revealed that the disease remained stable, and the patient
underwent two sessions of percutaneous RFA targeting the hepatic
lesions in July and October 2021. RFA is a local ablative technique
used to treat liver metastases from NENs in patients who are not
eligible for curative surgical resection (29) by delivering high-frequency
electrical currents to generate heat, which destroys tumor tissue.
This procedure can assist in managing the size and number of liver
metastases, alleviate symptoms and potentially extend survival
(30). For patients with a limited
number of liver metastases, hepatic resection or ablative
therapies, such as RFA or microwave ablation, may be promptly
performed in conjunction with systemic treatments (1). Notably, a systematic review and
meta-analysis involving 292 patients with pNENs documented a pooled
complete radiological response of 87.1% and a pooled partial
response of 11.4% (30).
In the present study, the patient developed ascites
and generalized itching 2 years after the last RFA, attributed to
portal hypertension from liver metastasis and malignant effusion.
In September 2023, the patient underwent MRCP dynamic enhancement
imaging, which revealed disease progression, with an increase in
the range and number of pancreatic tail and liver lesions compared
with in June 2022. The patient used traditional Chinese herbal
medicine treatment between November 2021 to September 2023, which
may not have effectively inhibited tumor progression. Despite
earlier studies (31,32) reporting that Chinese herbal medicine
can delay the progression of NENs, these were largely single-center
studies with limited sample sizes. Therefore, the efficacy of
traditional Chinese herbal medicine in the treatment of NENs
requires further exploration.
To determine progression in the pathological grading
of the tumor, a second biopsy is necessary; however, after
thoroughly discussing the risks (including infection, bleeding,
pain, tissue damage and the possibility of more serious
complications) associated with a second biopsy with the MDT, the
patient declined to undergo the procedure. Additionally, the
patient experienced abdominal distension due to tumor progression,
with compression-induced right kidney displacement leading to renal
function abnormalities, rendering them unable to tolerate RFA.
Surufatinib is a small-molecule inhibitor targeting
vascular endothelial growth factor receptor and fibroblast growth
factor receptor 1 (33). The
SANET-p phase III clinical trial revealed that the median PFS was
10.9 months in the surufatinib group and 3.7 months in a placebo
group (34). In June 2021, the
Chinese National Medical Products Administration approved
surufatinib for the treatment of individuals with locally advanced
or metastatic, progressive, non-functional, well-differentiated
(G1, G2) pNENs, offering a novel treatment option for patients with
NENs. Accordingly, the patient described in the present study was
treated with surufatinib to diminish the tumor burden. According to
the CSCO guidelines, everolimus and surufatinib are both
recommended as optional medications (28). In the RADIANT-3 study, for the
treatment of advanced pNENs, the median PFS for the everolimus
group versus the placebo group was 11.0 versus 4.6 months
(P<0.01) (35); for the
surufatinib group versus the placebo group, it was 10.9 versus 3.7
months (P=0.0011). The ORR was 5% for the everolimus group and 19%
for the surufatinib group (34).
Due to the higher ORR of sunitinib, this medication was chosen for
treatment in the present case.
Recently, a phase II clinical trial (TALENT)
investigating the efficacy of lenvatinib for the treatment of
advanced NENs highlighted an ORR of 29.9%, with 44.2% in pancreatic
neuroendocrine tumors and 16.4% in gastrointestinal neuroendocrine
tumors at 23 months. Furthermore, the median (range) duration of
response was 19.9 (8.4–30.8) and 33.9 (10.6–38.3) months in the
panNET and GI-NET groups, respectively. The median PFS was 15.7
months (27). Lenvatinib has
demonstrated the highest ORR among targeted therapies for GEP-NETs
to date.
A total of 1 month after initiating treatment with
surufatinib, MRCP imaging indicated that the tumor was stable. In
November 2023, the patient underwent TACE, taking into account the
high tumor burden. Local treatment of unresectable liver metastases
is crucial, and various methods can be adopted, encompassing
transcatheter arterial embolization (TAE), TACE and transarterial
radioembolization (TARE) through the hepatic artery (36). Considering that the majority of the
blood supply of NEN liver metastases (NENLMs) originates from the
hepatic artery, the hepatic artery approach is regarded as an
effective treatment for whole-liver involvement. In a previous
study, the median PFS and OS were 18.4 and 40.7 months,
respectively, for patients with NENs who underwent liver
embolization (37). According to
another study, the median survival time of patients with liver
metastases secondary to NENs who underwent TARE was 28 months, with
1-, 2- and 3-year survival rates of 72.5, 57 and 45%, respectively
(38). Overall, multiple sessions
of interventional therapy are recommended for patients with a tumor
burden exceeding 50% to minimize the risk of complications
(1).
To date, studies have established that the efficacy
between TAE and TACE is comparable (39). Drug-eluting beads (used in TAE and
TACE) used in patients with NENLM can significantly increase the
risk of hepatic and biliary damage, and increase the risk of
hepatic abscess by 6.6 times (40);
therefore, they are not recommended for use in patients with
NENLM.
There are limitations to the present study. The
first limitation is regarding the methods used to monitor the
conditions of the patient. We encourage patients to undergo any
nuclear imaging examinations, since they provide detailed
functional, metabolic and molecular information about the lesions,
including very small lesions. However, due to the high cost of
18F-PET-CT and its exclusion from medical insurance coverage in
China, the patient did not undergo this examination. Other
radiotracer PET-CT scans are not widely available in most hospitals
in China; therefore, the patient did not receive any nuclear
imaging examinations. Second, the level of SSTR expression may be
of guiding significance for the applicability and efficacy of PRRT.
Currently, since PRRT is not offered at our institution or nearby
medical facilities, SSTR testing was not performed for the present
patient. Third, NENs exhibit high spatiotemporal heterogeneity,
with potential variability in metastases at different sites and
times. Therefore, the pathological characteristics of lesions in
the advanced stage may change, thus affecting the choice of
treatment. It is therefore advisable to perform a second biopsy;
however, the patient in the present case report did not undergo a
secondary biopsy due to the risk of puncture. Additionally, the
potential interference of the traditional Chinese herbal medicine
with the patient outcomes cannot be overlooked.
Despite challenges in selecting treatments for
patients with advanced pNEN, it is vital to conduct a comprehensive
analysis of factors, such as tumor location, functional status,
differentiation level, proliferation index, SSTR expression, tumor
burden and disease progression, to formulate the most appropriate
treatment schedule for each patient. In the present case report, a
patient with advanced multiple metastatic pNEN was successfully
managed via multiple sessions of RFA, TAE and targeted therapy. The
present case emphasizes the role of tailored treatment strategies
considering patient comorbidities and tumor biology, and the
significance of secondary puncture biopsy despite the high risk
involved, which may provide survival benefits for patients with
advanced or metastatic pNEN.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ contributed to writing the original draft,
investigation and methodology. XC was responsible for
conceptualization, investigation, methodology, generated figures,
and writing, reviewing and editing the manuscript. HoZ contributed
to investigation, obtaining resources and data validation. HaZ was
involved in investigation, obtaining resources and data validation.
WD also participated in investigation, obtaining resources and data
validation. ZM handled conceptualization, investigation,
methodology, funding acquisition, supervision, and writing,
reviewing and editing the manuscript. ZM and XC confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent to
participate.
Patient consent for publication
The patient provided written informed consent for
publication of this report and the associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pavel M, Öberg K, Falconi M, Krenning EP,
Sundin A, Perren A and Berruti A; ESMO Guidelines Committee.
Electronic address, : simpleclinicalguidelines@esmo.org:
Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 31:844–860. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White BE, Rous B, Chandrakumaran K, Wong
K, Bouvier C, Van Hemelrijck M, George G, Russell B,
Srirajaskanthan R and Ramage JK: Incidence and survival of
neuroendocrine neoplasia in England 1995–2018: A retrospective,
population-based study. Lancet Reg Health Eur. 23:1005102022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinese Anti-Cancer Association
Neuroendocrine Tumor Professional Committee: Neuroendocrine tumor
management guidelines of the Chinese anti-cancer association (2022
Edition). China Oncology. 32:545–580. 2022.(In Chinese).
|
|
5
|
Sultana Q, Kar J, Verma A, Sanghvi S, Kaka
N, Patel N, Sethi Y, Chopra H, Kamal MA and Greig NH: A
comprehensive review on neuroendocrine neoplasms: Presentation,
pathophysiology and management. J Clin Med. 12:51382023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Metz DC and Jensen RT: Gastrointestinal
neuroendocrine tumors: Pancreatic endocrine tumors.
Gastroenterology. 135:1469–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunz PL, Reidy-Lagunes D, Anthony LB,
Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK,
Klimstra DS, et al: Consensus guidelines for the management and
treatment of neuroendocrine tumors. Pancreas. 42:557–577. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Öberg K, Knigge U, Kwekkeboom D and Perren
A; ESMO Guidelines Working Group, : Neuroendocrine
gastro-entero-pancreatic tumors: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23 (Suppl
7):vii124–vii130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kos-Kudła B, Hubalewska-Dydejczyk A,
Kuśnierz K, Lampe P, Marek B, Nasierowska-Guttmejer A,
Nowakowska-Duława E, Pilch-Kowalczyk J, Sowa-Staszczak A, Rosiek V,
et al: Pancreatic neuroendocrine neoplasms-management guidelines
(recommended by the Polish Network of Neuroendocrine Tumours).
Endokrynol Pol. 64:459–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition ajcc cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah MH, Goldner WS, Benson AB, Bergsland
E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, et
al: Neuroendocrine and adrenal tumors, version 2.2021, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
19:839–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falconi M, Eriksson B, Kaltsas G, Bartsch
DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G,
Klöppel G, et al: ENETS consensus guidelines update for the
management of patients with functional pancreatic neuroendocrine
tumors and non-functional pancreatic neuroendocrine tumors.
Neuroendocrinology. 103:153–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jilesen APJ, van Eijck CHJ, Hof KH, van
Dieren S, Gouma DJ and van Dijkum EJMN: Postoperative
complications, in-hospital mortality and 5-year survival after
surgical resection for patients with a pancreatic neuroendocrine
tumor: A systematic review. World J Surg. 40:729–748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mou Y, Wang ZY, Tan CL, Chen YH, Liu XB
and Ke NW: The role of primary tumor resection in patients with
pancreatic neuroendocrine tumors with liver metastases. Front
Oncol. 12:8381032022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang A, Sherman SK, Howe JR and Sahai V:
Progress in the management of pancreatic neuroendocrine tumors. Ann
Rev Med. 73:213–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang Y, Wu W, Nie Y and Chen J:
Interpretation on the Chinese Guideline for Diagnosis and Treatment
of Neuroendocrine Neoplasms from The China Anti-Cancer Association
(2022). Medical Journal of Peking Union Medical College Hospital.
14:94–100. 2023.(In Chinese).
|
|
18
|
Yu EY, Petrylak DP, O'Donnell PH, Lee JL,
van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T,
Harrison MR, et al: Enfortumab vedotin after PD-1 or PD-L1
inhibitors in cisplatin-ineligible patients with advanced
urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2
trial. Lancet Oncol. 22:872–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
VanderWalde A, Bellasea SL, Kendra KL,
Khushalani NI, Campbell KM, Scumpia PO, Kuklinski LF, Collichio F,
Sosman JA, Ikeguchi A, et al: Ipilimumab with or without nivolumab
in PD-1 or PD-L1 blockade refractory metastatic melanoma: A
randomized phase 2 trial. Nat Med. 29:2278–2285. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polak P, Fu L and Foulkes WD: PD-1 and
PD-L1 blockade plus chemotherapy in endometrial cancer. N Engl J
Med. 389:8662023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bongiovanni A, Maiorano BA, Azzali I,
Liverani C, Bocchini M, Fausti V, Di Menna G, Grassi I, Sansovini
M, Riva N and Ibrahim T: Activity and safety of immune checkpoint
inhibitors in neuroendocrine neoplasms: A systematic review and
meta-analysis. Pharmaceuticals (Basel). 14:4762021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris PE and Zhernosekov K: The evolution
of PRRT for the treatment of neuroendocrine tumors; What comes
next? Front Endocrinol (Lausanne). 13:9418322022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strosberg J, El-Haddad G, Wolin E,
Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H,
et al: Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine
tumors. N Engl J Med. 376:125–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambrosini V, Kunikowska J, Baudin E, Bodei
L, Bouvier C, Capdevila J, Cremonesi M, de Herder WW, Dromain C,
Falconi M, et al: Consensus on molecular imaging and theranostics
in neuroendocrine neoplasms. Eur J Cancer. 146:56–73. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmona-Bayonas A, Jiménez-Fonseca P,
Lamarca Á, Barriuso J, Castaño Á, Benavent M, Alonso V,
Riesco-Martínez MDC, Alonso-Gordoa T, Custodio A, et al: Prediction
of progression-free survival in patients with advanced,
well-differentiated, neuroendocrine tumors being treated with a
somatostatin analog: The GETNE-TRASGU study. J Clin Oncol.
37:2571–2580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rinke A, Müller HH, Schade-Brittinger C,
Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker
M, et al: Placebo-controlled, double-blind, prospective, randomized
study on the effect of octreotide LAR in the control of tumor
growth in patients with metastatic neuroendocrine midgut tumors: A
report from the PROMID study group. J Clin Oncol. 27:4656–4663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caplin ME, Pavel M, Ćwikła JB, Phan AT,
Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L,
et al: Lanreotide in metastatic enteropancreatic neuroendocrine
tumors. New Engl J Med. 371:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui J, Jiao F, Li Q, Wang Z, Fu D, Liang
J, Liang H, Xia T, Zhang T, Zhang Y, et al: Chinese society of
clinical oncology (CSCO): Clinical guidelines for the diagnosis and
treatment of pancreatic cancer. J Natl Cancer Cent. 2:205–215.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larghi A, Rizzatti G, Rimbaş M, Crino SF,
Gasbarrini A and Costamagna G: EUS-guided radiofrequency ablation
as an alternative to surgery for pancreatic neuroendocrine
neoplasms: Who should we treat? Endosc Ultrasound. 8:220–226. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khoury T, Sbeit W, Fusaroli P, Campana D,
Brighi N, Napoleon B and Lisotti A: Safety and efficacy of
endoscopic ultrasound-guided radiofrequency ablation for pancreatic
neuroendocrine neoplasms: Systematic review and meta-analysis. Dig
Endosc. 36:395–405. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen R, Chen Q, Cheng Z, Yu F, Chen X and
Tan H: A retrospective cohort study of Qizhen YiLiu prescription
combined with somatostatin analogues in the treatment of advanced
pancreatic neuroendocrine tumors. Journal of China-Japan Friendship
Hospital. 38:139–143. 2024.(In Chinese).
|
|
32
|
Li M, Dou D, Jie L, Zuo G, Liu Q and Tan
H: Efficacy analysis of Traditional Chinese Medicine Combined with
Somatostatin Analogues in the Treatment of Advanced
Gastroenteropancreatic Neuroendocrine Tumors. Journal of Clinical
Oncology. 22:238–242. 2017.(In Chinese).
|
|
33
|
Salvia AL, Espinosa-Olarte P,
Riesco-Martinez MDC, Anton-Pascual B and Garcia-Carbonero R:
Targeted cancer therapy: What's new in the field of neuroendocrine
neoplasms? Cancers (Basel). 13:17012021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu J, Shen L, Bai C, Wang W, Li J, Yu X,
Li Z, Li E, Yuan X, Chi Y, et al: Surufatinib in advanced
pancreatic neuroendocrine tumours (SANET-p): A randomised,
double-blind, placebo-controlled, phase 3 study. Lancet Oncol.
21:1489–1499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cazzato RL, Hubelé F, Marini PD, Ouvrard
E, Salvadori J, Addeo P, Garnon J, Kurtz JE, Greget M, Mertz L, et
al: Liver-directed therapy for neuroendocrine metastases: From
interventional radiology to nuclear medicine procedures. Cancers
(Basel). 13:63682021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanabar R, Barriuso J, McNamara MG,
Mansoor W, Hubner RA, Valle JW and Lamarca A: Liver embolisation
for patients with neuroendocrine neoplasms: Systematic review.
Neuroendocrinology. 111:354–369. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia Z and Wang W: Yttrium-90
radioembolization for unresectable metastatic neuroendocrine liver
tumor: A systematic review. Eur J Radiol. 100:23–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minh DD, Chapiro J, Gorodetski B, Huang Q,
Liu C, Smolka S, Savic LJ, Wainstejn D, Lin M, Schlachte T, et al:
Intra-arterial therapy of neuroendocrine tumour liver metastases:
Comparing conventional TACE, drug-eluting beads TACE and yttrium-90
radioembolisation as treatment options using a propensity score
analysis model. Eur Radiol. 27:4995–5005. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guiu B, Deschamps F, Aho S, Munck F,
Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger
M, et al: Liver/biliary injuries following chemoembolisation of
endocrine tumours and hepatocellular carcinoma: Lipiodol vs.
drug-eluting beads. J Hepatol. 56:609–617. 2012. View Article : Google Scholar : PubMed/NCBI
|