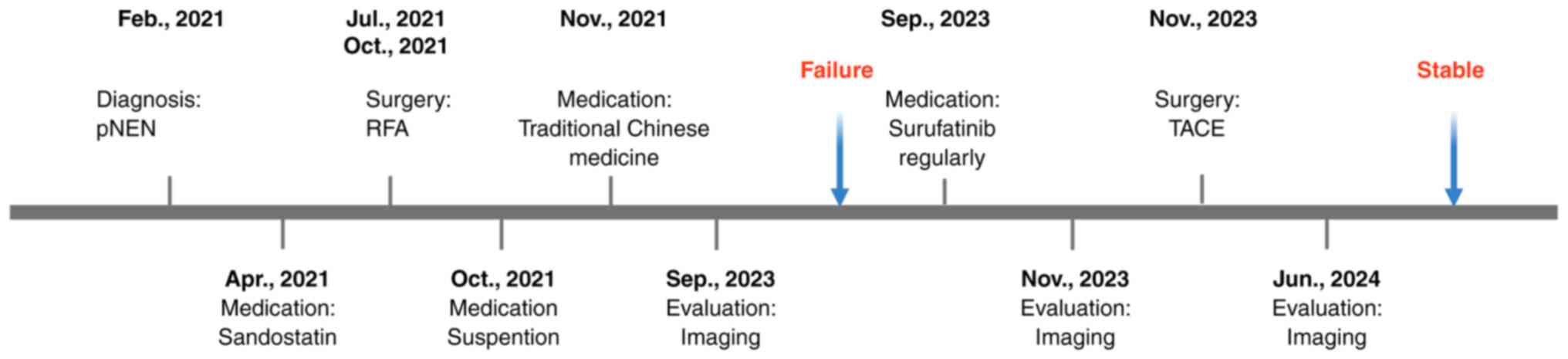
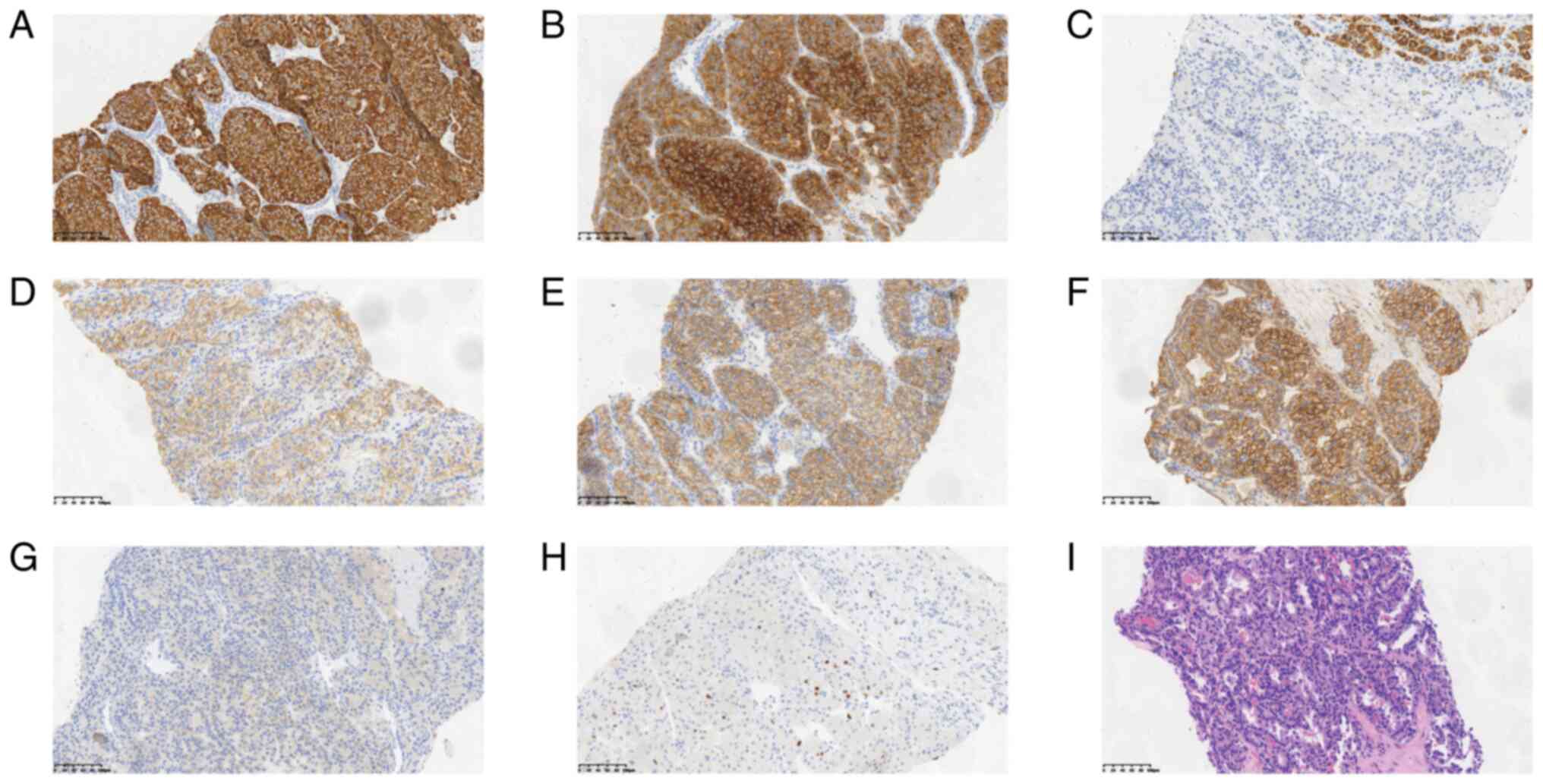
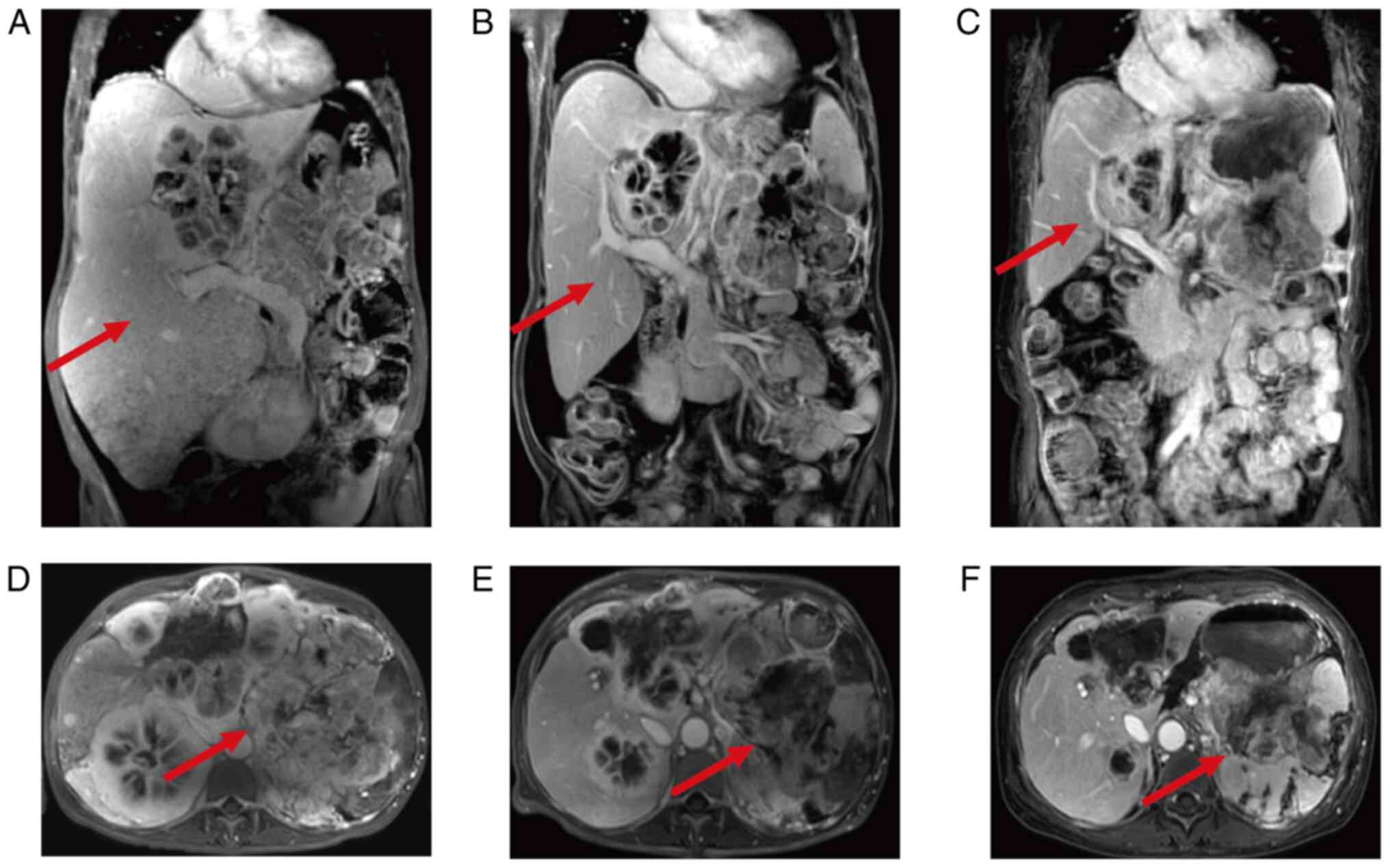
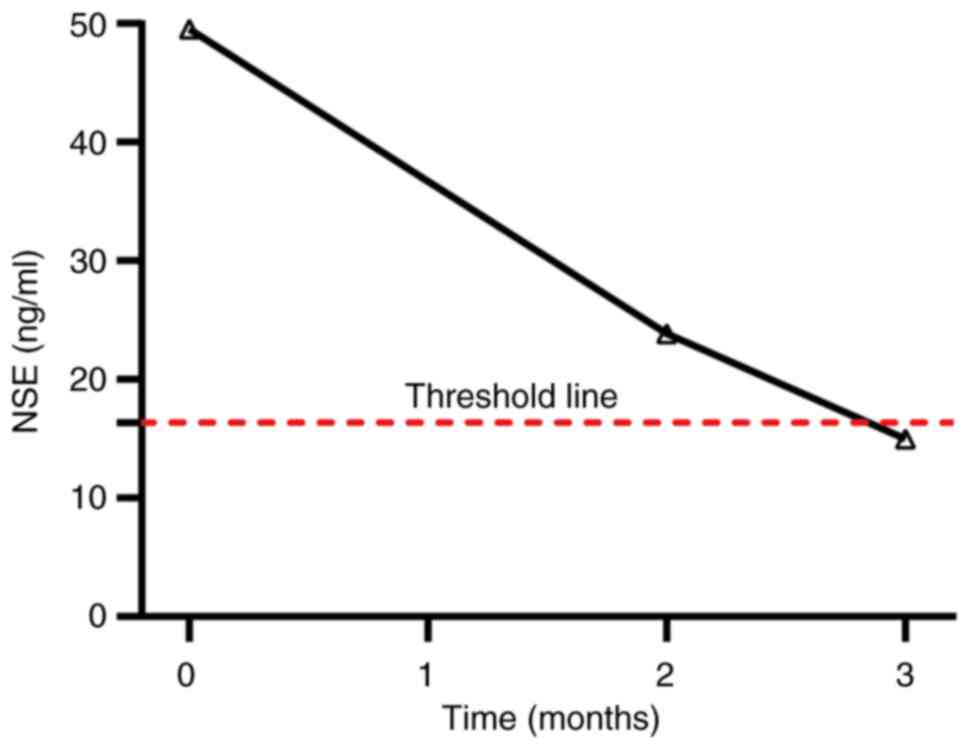
|
1
|
Pavel M, Öberg K, Falconi M, Krenning EP,
Sundin A, Perren A and Berruti A; ESMO Guidelines Committee.
Electronic address, : simpleclinicalguidelines@esmo.org:
Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 31:844–860. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White BE, Rous B, Chandrakumaran K, Wong
K, Bouvier C, Van Hemelrijck M, George G, Russell B,
Srirajaskanthan R and Ramage JK: Incidence and survival of
neuroendocrine neoplasia in England 1995–2018: A retrospective,
population-based study. Lancet Reg Health Eur. 23:1005102022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinese Anti-Cancer Association
Neuroendocrine Tumor Professional Committee: Neuroendocrine tumor
management guidelines of the Chinese anti-cancer association (2022
Edition). China Oncology. 32:545–580. 2022.(In Chinese).
|
|
5
|
Sultana Q, Kar J, Verma A, Sanghvi S, Kaka
N, Patel N, Sethi Y, Chopra H, Kamal MA and Greig NH: A
comprehensive review on neuroendocrine neoplasms: Presentation,
pathophysiology and management. J Clin Med. 12:51382023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Metz DC and Jensen RT: Gastrointestinal
neuroendocrine tumors: Pancreatic endocrine tumors.
Gastroenterology. 135:1469–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunz PL, Reidy-Lagunes D, Anthony LB,
Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK,
Klimstra DS, et al: Consensus guidelines for the management and
treatment of neuroendocrine tumors. Pancreas. 42:557–577. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Öberg K, Knigge U, Kwekkeboom D and Perren
A; ESMO Guidelines Working Group, : Neuroendocrine
gastro-entero-pancreatic tumors: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23 (Suppl
7):vii124–vii130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kos-Kudła B, Hubalewska-Dydejczyk A,
Kuśnierz K, Lampe P, Marek B, Nasierowska-Guttmejer A,
Nowakowska-Duława E, Pilch-Kowalczyk J, Sowa-Staszczak A, Rosiek V,
et al: Pancreatic neuroendocrine neoplasms-management guidelines
(recommended by the Polish Network of Neuroendocrine Tumours).
Endokrynol Pol. 64:459–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition ajcc cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah MH, Goldner WS, Benson AB, Bergsland
E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, et
al: Neuroendocrine and adrenal tumors, version 2.2021, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
19:839–868. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falconi M, Eriksson B, Kaltsas G, Bartsch
DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G,
Klöppel G, et al: ENETS consensus guidelines update for the
management of patients with functional pancreatic neuroendocrine
tumors and non-functional pancreatic neuroendocrine tumors.
Neuroendocrinology. 103:153–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jilesen APJ, van Eijck CHJ, Hof KH, van
Dieren S, Gouma DJ and van Dijkum EJMN: Postoperative
complications, in-hospital mortality and 5-year survival after
surgical resection for patients with a pancreatic neuroendocrine
tumor: A systematic review. World J Surg. 40:729–748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mou Y, Wang ZY, Tan CL, Chen YH, Liu XB
and Ke NW: The role of primary tumor resection in patients with
pancreatic neuroendocrine tumors with liver metastases. Front
Oncol. 12:8381032022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang A, Sherman SK, Howe JR and Sahai V:
Progress in the management of pancreatic neuroendocrine tumors. Ann
Rev Med. 73:213–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang Y, Wu W, Nie Y and Chen J:
Interpretation on the Chinese Guideline for Diagnosis and Treatment
of Neuroendocrine Neoplasms from The China Anti-Cancer Association
(2022). Medical Journal of Peking Union Medical College Hospital.
14:94–100. 2023.(In Chinese).
|
|
18
|
Yu EY, Petrylak DP, O'Donnell PH, Lee JL,
van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T,
Harrison MR, et al: Enfortumab vedotin after PD-1 or PD-L1
inhibitors in cisplatin-ineligible patients with advanced
urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2
trial. Lancet Oncol. 22:872–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
VanderWalde A, Bellasea SL, Kendra KL,
Khushalani NI, Campbell KM, Scumpia PO, Kuklinski LF, Collichio F,
Sosman JA, Ikeguchi A, et al: Ipilimumab with or without nivolumab
in PD-1 or PD-L1 blockade refractory metastatic melanoma: A
randomized phase 2 trial. Nat Med. 29:2278–2285. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polak P, Fu L and Foulkes WD: PD-1 and
PD-L1 blockade plus chemotherapy in endometrial cancer. N Engl J
Med. 389:8662023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bongiovanni A, Maiorano BA, Azzali I,
Liverani C, Bocchini M, Fausti V, Di Menna G, Grassi I, Sansovini
M, Riva N and Ibrahim T: Activity and safety of immune checkpoint
inhibitors in neuroendocrine neoplasms: A systematic review and
meta-analysis. Pharmaceuticals (Basel). 14:4762021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris PE and Zhernosekov K: The evolution
of PRRT for the treatment of neuroendocrine tumors; What comes
next? Front Endocrinol (Lausanne). 13:9418322022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strosberg J, El-Haddad G, Wolin E,
Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H,
et al: Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine
tumors. N Engl J Med. 376:125–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambrosini V, Kunikowska J, Baudin E, Bodei
L, Bouvier C, Capdevila J, Cremonesi M, de Herder WW, Dromain C,
Falconi M, et al: Consensus on molecular imaging and theranostics
in neuroendocrine neoplasms. Eur J Cancer. 146:56–73. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmona-Bayonas A, Jiménez-Fonseca P,
Lamarca Á, Barriuso J, Castaño Á, Benavent M, Alonso V,
Riesco-Martínez MDC, Alonso-Gordoa T, Custodio A, et al: Prediction
of progression-free survival in patients with advanced,
well-differentiated, neuroendocrine tumors being treated with a
somatostatin analog: The GETNE-TRASGU study. J Clin Oncol.
37:2571–2580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rinke A, Müller HH, Schade-Brittinger C,
Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker
M, et al: Placebo-controlled, double-blind, prospective, randomized
study on the effect of octreotide LAR in the control of tumor
growth in patients with metastatic neuroendocrine midgut tumors: A
report from the PROMID study group. J Clin Oncol. 27:4656–4663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caplin ME, Pavel M, Ćwikła JB, Phan AT,
Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L,
et al: Lanreotide in metastatic enteropancreatic neuroendocrine
tumors. New Engl J Med. 371:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui J, Jiao F, Li Q, Wang Z, Fu D, Liang
J, Liang H, Xia T, Zhang T, Zhang Y, et al: Chinese society of
clinical oncology (CSCO): Clinical guidelines for the diagnosis and
treatment of pancreatic cancer. J Natl Cancer Cent. 2:205–215.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larghi A, Rizzatti G, Rimbaş M, Crino SF,
Gasbarrini A and Costamagna G: EUS-guided radiofrequency ablation
as an alternative to surgery for pancreatic neuroendocrine
neoplasms: Who should we treat? Endosc Ultrasound. 8:220–226. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khoury T, Sbeit W, Fusaroli P, Campana D,
Brighi N, Napoleon B and Lisotti A: Safety and efficacy of
endoscopic ultrasound-guided radiofrequency ablation for pancreatic
neuroendocrine neoplasms: Systematic review and meta-analysis. Dig
Endosc. 36:395–405. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen R, Chen Q, Cheng Z, Yu F, Chen X and
Tan H: A retrospective cohort study of Qizhen YiLiu prescription
combined with somatostatin analogues in the treatment of advanced
pancreatic neuroendocrine tumors. Journal of China-Japan Friendship
Hospital. 38:139–143. 2024.(In Chinese).
|
|
32
|
Li M, Dou D, Jie L, Zuo G, Liu Q and Tan
H: Efficacy analysis of Traditional Chinese Medicine Combined with
Somatostatin Analogues in the Treatment of Advanced
Gastroenteropancreatic Neuroendocrine Tumors. Journal of Clinical
Oncology. 22:238–242. 2017.(In Chinese).
|
|
33
|
Salvia AL, Espinosa-Olarte P,
Riesco-Martinez MDC, Anton-Pascual B and Garcia-Carbonero R:
Targeted cancer therapy: What's new in the field of neuroendocrine
neoplasms? Cancers (Basel). 13:17012021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu J, Shen L, Bai C, Wang W, Li J, Yu X,
Li Z, Li E, Yuan X, Chi Y, et al: Surufatinib in advanced
pancreatic neuroendocrine tumours (SANET-p): A randomised,
double-blind, placebo-controlled, phase 3 study. Lancet Oncol.
21:1489–1499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cazzato RL, Hubelé F, Marini PD, Ouvrard
E, Salvadori J, Addeo P, Garnon J, Kurtz JE, Greget M, Mertz L, et
al: Liver-directed therapy for neuroendocrine metastases: From
interventional radiology to nuclear medicine procedures. Cancers
(Basel). 13:63682021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanabar R, Barriuso J, McNamara MG,
Mansoor W, Hubner RA, Valle JW and Lamarca A: Liver embolisation
for patients with neuroendocrine neoplasms: Systematic review.
Neuroendocrinology. 111:354–369. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia Z and Wang W: Yttrium-90
radioembolization for unresectable metastatic neuroendocrine liver
tumor: A systematic review. Eur J Radiol. 100:23–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minh DD, Chapiro J, Gorodetski B, Huang Q,
Liu C, Smolka S, Savic LJ, Wainstejn D, Lin M, Schlachte T, et al:
Intra-arterial therapy of neuroendocrine tumour liver metastases:
Comparing conventional TACE, drug-eluting beads TACE and yttrium-90
radioembolisation as treatment options using a propensity score
analysis model. Eur Radiol. 27:4995–5005. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guiu B, Deschamps F, Aho S, Munck F,
Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger
M, et al: Liver/biliary injuries following chemoembolisation of
endocrine tumours and hepatocellular carcinoma: Lipiodol vs.
drug-eluting beads. J Hepatol. 56:609–617. 2012. View Article : Google Scholar : PubMed/NCBI
|