Introduction
Human microbiota
The human microbiota comprises diverse microbial
communities that inhabit various regions inside and outside the
human body. These communities include bacteria, fungi and viruses
(1). These microbiota colonize
distinct anatomical sites such as the oral cavity (2), skin (3), intestinal tract (4), reproductive tract (5) and even the brain (6). Each anatomical site provides a unique
physiological environment, fostering the growth and proliferation
of specific microorganisms tailored to those conditions (7,8).
Consequently, every part of the body harbors its own distinct
microbial community. Disruptions in the regulatory mechanisms that
govern the microbiota of the host, whether caused by infections,
dietary changes or lifestyle factors, can disturb the delicate
balance between the microbiota and the host (9). These disturbances often result in
dysbiosis, marked by an imbalance in the microbial composition
within the body. Dysbiosis can manifest in various ways, including
alterations in the abundance of specific microbial taxa, changes in
microbial diversity or shifts in the metabolic activities of the
microbiota (10). This dysbiotic
state can have significant implications for human health, as it is
increasingly recognized to contribute to the development or
progression of various diseases, such as colorectal cancer,
inflammatory bowel disease, and non-alcoholic fatty liver disease
(11,12).
Tumor microenvironment (TME)
The concept of the TME was organically proposed by
Stephen Paget, an assistant surgeon at the Royal London Hospital,
over a century ago (13). Paget
introduced the ‘seed and soil’ hypothesis of cancer, suggesting
that the microenvironment surrounding tumors play a critical role
in their growth and spread. TME refers to the intricate milieu in
which tumor cells reside (14). It
includes surrounding blood vessels, immune cells, fibroblasts,
myeloid-derived inflammatory cells, various signaling molecules and
extracellular matrix (ECM) (15).
It serves as a dynamic ecosystem that influences the survival and
progression of tumor cells. The interplay between tumor cells and
immune cells, along with the complex signaling networks and
molecular interactions, profoundly shape the fate of the tumor
(16).
The present review focuses on the interaction
between fungi and inflammatory response within the TME.
Specifically, this study explores the potential role of fungi in
tumor development through their interactions with immune cells.
Understanding these interactions may offer novel insights into
innovative therapeutic strategies that target TEM.
Relationship among human microbiota, TME and
tumors
Recent advancements in molecular biology, genomics
and high-throughput sequencing technology have shed light on the
critical role of the microbiota in maintaining organismal
homeostasis and influencing cancer development (17). The contribution of microorganisms
and microbiota to carcinogenesis can be broadly categorized into
altering the balance of host cell proliferation and death,
modulating immune system function, and influencing host metabolism
(11). Notably, certain microbial
species, such as pks+ Escherichia coli and Clostridium
nucleatum, have been implicated in colorectal carcinogenesis
through modulating the TME and directly inducing gene mutations in
epithelial cells (18).
Although bacteria and viruses have been extensively
studied in relation to cancer (17), fungi have received less attention.
Emerging evidence highlights the importance of fungi in cancer
development by influencing the TME (19,20). A
comprehensive study analyzing the mycobiomes of 17,401 tissue and
blood samples across 35 cancer types has revealed a noteworthy
positive correlation between fungal communities and cancer
incidence (21). This finding
emphasizes the potential role of fungi in tumorigenesis across
diverse cancer types. Fungi exhibit heterogeneous distributions
within different cancers, suggesting a nuanced relationship with
the progression of specific tumor types. This variability in fungal
presence and abundance highlights the complexity of their
interactions within the TME and their potential impact on tumor
behavior (22).
While the precise mechanisms by which fungi
contribute to cancer development are still being elucidated,
several fungi have been implicated in this process. Candida
albicans, Malassezia spp., and Aspergillus spp.
have garnered attention for their potential roles in oral squamous
cell carcinoma, pancreatic ductal adenocarcinoma and lung
adenocarcinoma. These fungi, along with others that yet to be fully
characterized, represent intriguing targets for further
investigation into their mechanisms of action and their
implications for cancer development.
Candida albicans and cancer
The association between Candida spp. and
gastrointestinal cancers, particularly gastric (23) and colon cancer (24), have been documented. Candida
albicans, a common fungus in the human body, is typically found
as a commensal organism, mainly in the colon and vagina. However,
when the disturbance in the host-fungus balance can lead to
excessive growth of C. albicans, leading to various health
issues such as serious candidiasis (25) and current vulvovaginal candidiasis.
The potential link between C. albicans and oral tumors was
first observed in the 1960s (26).
Subsequent reports have strengthened the evidence, suggesting a
correlation between the presence of C. albicans in the oral
mucosa and the development of oral squamous cell carcinoma (OSCC)
(27–29). The association between C.
albicans and OSCC is an example of explored fungus-cancer
connections, the evidence for such associations (30–32).
Abnormal colonization of the intestine by C. albicans and
Malassezia furfur promotes the development and progression
of HCC. In the integrated experiment, compared with the control
group, the weight and volume of HCC tumors in the Candida
albicans and Malassezia furfur groups significantly
increased (33).
Malassezia and cancer
The connection between Malassezia and cancer,
particularly pancreatic ductal adenocarcinoma (PDA), has been
highlighted in a case report from 2022 (34) The report observed a significant
increase of ~3,000-fold in the number of fungi in PDA compared with
normal pancreatic tissue in both humans and mice subjects (34). To further investigate this
relationship, researchers established models of slowly progressive
and invasive PDA. The authors revealed that removing the mycobiome
(the fungal community) protects against tumor growth, whereas
re-introducing Malassezia accelerates oncogenesis,
potentially through the glycans of the fungal wall binding to
mannose-binding lectin, activating the complement cascade (34) and accelerating pancreatic cancer
progression (35). Other
experiments also confirmed that fungal pancreatic carcinogenesis
occurred due to the activation of the complement cascade and the
secretion of IL-33 and IL-6 (36,37).
In addition to PDA, elevated levels of
Malassezia are also found in intestinal mucosa samples from
patients with Crohn's disease compared with healthy individuals.
Crohn's disease is closely associated with the development of
colorectal cancer (CRC). This finding suggests the potential for
early CRC diagnosis by assessing the presence of Malassezia
in intestinal mucosa samples (38).
Aspergillus and cancer
The aflatoxin produced by Aspergillus flavus
has confirmed to be one of the most potent natural carcinogens,
known to cause liver cancer. Since its association with live cancer
was first reported in 1990, researchers have extensively explored
the potential mechanisms by which this fungus acts as a carcinogen
(39). Through the use of
Multicohort Fecal Metagenomic Analytics, scientists have also
identified an association between Aspergillus lambellii and
CRC (40).
However, current research primarily focuses on
investigating the differences in Aspergillus abundance in
the feces of patients with CRC compared with healthy individuals.
While it has been established that fungi, including Aspergillus
lambellii, can serve as biomarkers for CRC diagnosis, the
specific mechanisms underlying this relationship are not yet fully
understood (40). In the
exploration of the correlation between Aspergillus and lung
adenocarcinoma (LUAD), researchers have observed an enrichment of
tumor resident Aspergillus not only in patients with LUAD,
but also in three distinct homologous lung cancer mouse models.
Through these models, it has been found that Aspergillus
promotes lung tumor progression by mediating the expansion and
activation of myeloid-derived suppressor cells via IL-1β signaling
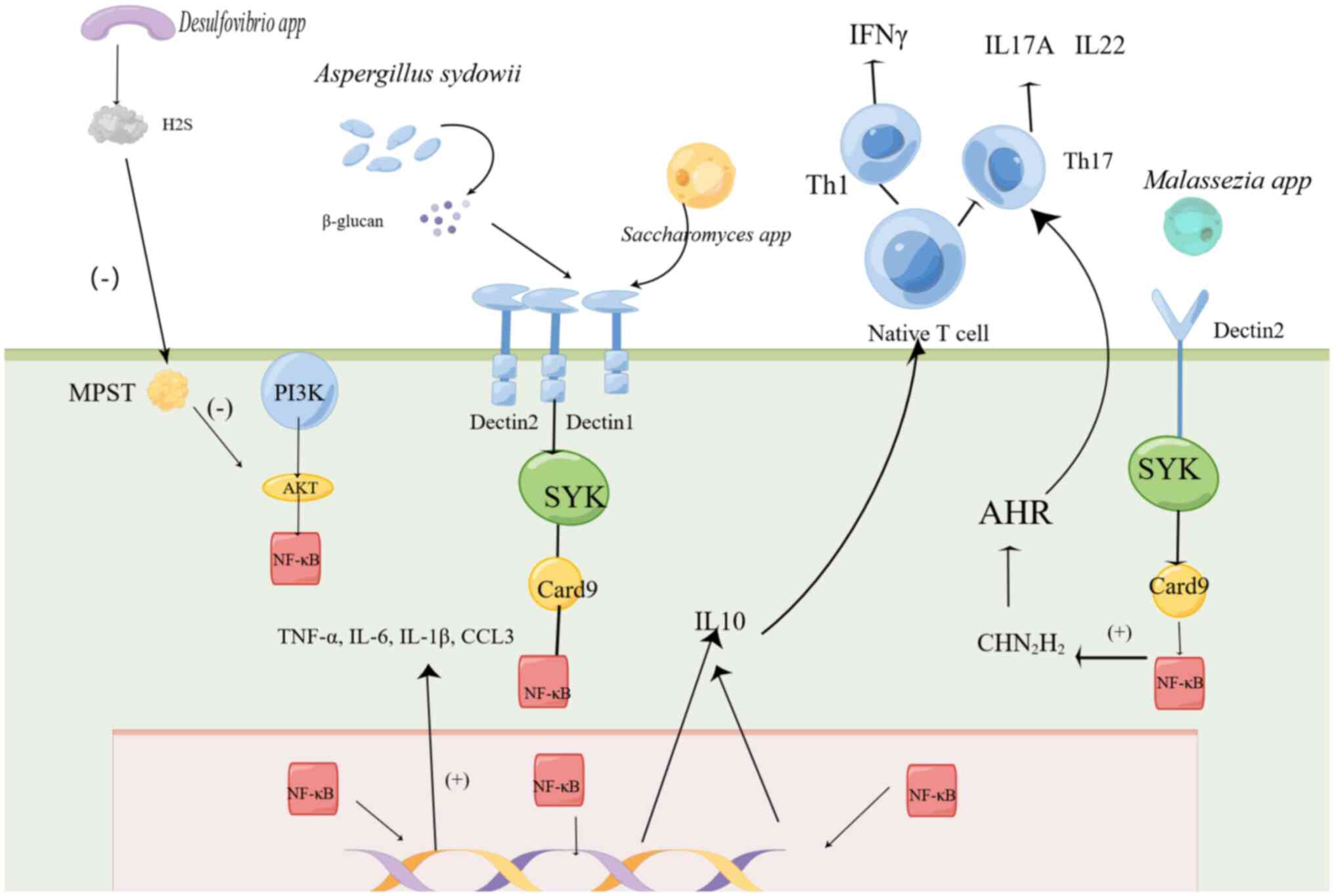
(41). How does this fungus
activate the signaling pathway, as shown in Fig. 1. This process inhibits cytotoxic T
lymphocytes activity and impedes the accumulation of
PD-1+CD8+T cells (41), thereby facilitating tumor
growth.
Other fungi and cancer
In addition to well-known fungi such as
Malassezia, C. albicans and Aspergillus, other less
common fungi species have also been contributed to tumor occurrence
and development through dysbiosis of the microbial community.
Advanced sequencing technologies such as whole-genome shotgun
sequencing and high-throughput sequencing have been used by
research to identify changes in these fungi in tumor patients
compared with healthy individuals. For instance, there is an
observed increase in the abundance of Trichophoron and
Malassezia in the intestinal mucosa of patients with CRC
(42), as well as Nakaseomyces
and Skeletocutis in patients with pancreatic ductal
adenocarcinoma (43).
Aspergillus lambellii is closely related to the CRC-enriched
bacterium Fusobacterium nucleatum, and this type of fungi
can promote CRC cell proliferation and tumor growth in xenograft
mice (40). Through pan-pathogen
array (PathoChip), the 18S ribosomal RNA signal of dendritic spores
has been detected in almost all ovarian cancer samples, in addition
to the distinctive features of Pneumocystis, Acremonium,
Cladophialophora, Malassezia and Pleistophora
microsporidia (44). It has
been revealed that >95% of ovarian cancer samples correlate with
the features of Rhizomucor, Rhodotorula, Alternaria and
Geotrichum, but the features of Geotrichum are also
detected in all control samples (44). Thus, all the aforementioned
mentioned fungi except Geotrichum deserve to be noticed in
ovarian cancer.
Although these findings warrant further
investigation, they underscore the importance of analyzing fungal
dysbiosis in comprehending the mechanisms underlying cancer
occurrence. Recent studies have also suggested an association
between certain fungal species and tumor transcriptional profiles
(21,22) or CRC stage (22,45),
but a specific causal relationship is unknown.
While the link between these less common fungal
species, as shown in Table I, and
carcinogenesis may require further confirmation through additional
clinical studies, it is evident that the relationship between
tumors and fungi is intricate. Dysbiosis of fungi can significantly
impact the immune response within the tumor microenvironment,
thereby influencing tumor occurrence and progression.
 | Table I.Fungi related to different
tumors. |
Table I.
Fungi related to different
tumors.
| Cancer type | Number of clinical
patients | Associated
fungi | (Refs.) |
|---|
| Oral cancer | 2 OSCC samples | Candida
albicans | (26) |
| Oral cancer | 103 OED or OSCC
samples vs. 120 healthy samples | Candida
albicans | (32) |
| Oral cancer | 52 OSCC samples vs.
104 healthy samples | Candida
albicans | (31) |
| Pancreatic
cancer | 134 control
individuals with normal pancreases, 98 patients with pancreatic
cysts and 74 patients with PDAC |
Skeletocutis;
Nakaseomyces | (114) |
| Pancreatic
cancer | 22 patients with
LTS PDAC and 21 patients with STS PDAC |
Saccharopolyspor | (115) |
| Pancreatic
cancer | Mouse models of
pancreatic cancer | Alternaria;
Malassezia | (84) |
| GC | Cancer lesions and
adjacent non-cancerous tissue in 45 GC cases | Candida
albicans | (23) |
| Pancreatic
cancer | - |
Malassezia | (34) |
| Lung cancer | 32 lung cancer
samples | Candida | (116) |
| Lung cancer | 52 LUAD vs. 10
non-malignant nodule tissues |
Aspergillus | (41) |
| Prostate
cancer | 50 PCa samples and
15 control samples | Fungi | (117) |
| Liver cancer | - | Aspergillus
flavus | (39) |
| Liver cancer | 2 fecal samples
from 34 patients with HCC, 20 cirrhotic patients and 18 healthy
controls | Candida
albicans; Malassezia | (33) |
| CRC | 454 healthy samples
vs. 350 adenoma samples vs. 525 CRC samples | Aspergillus
lambellii; Cordyceps sp.; RAO-2017; Erysiphe
pulchra; Moniliophthora perniciosa; Sphaerulina
musiva Phytophthora capsica; Aspergillus kawachii | (40) |
| CRC | - | Candida
dubliniensis; Candida guilliermondii; Candida
tropicalis; Saccharomyces eubayanus; Cyberlindnera
jadinii; Candida glabrata | (22) |
| CRC | 184 CRC samples vs.
204 healthy samples | Aspergillus
flavus; Kwoniella mangrovensis; Pseudogymnoascus
sp; VKM F-4518; Debaryomyces fabryi; A.
sydowii; Moniliophthora perniciosa; K.
heavenensis; A. ochraceoroseus; Talaromyces
islandicus; Malassezia globosa; Pseudogymnoascus
sp; VKM F-4520; A. rambellii; Pneumocystis
murina; Nosemia apis | (118) |
| CRC | 74 CRC samples vs.
28 healthy samples |
Trichosporon;
Malassezia | (42) |
| Ovarian cancer | 99 ovarian cancer
samples and 20 matched and 20 unmatched control samples |
Pneumocystis; Acremonium;
Cladophialophora; Malassezia; Pleistophora;
microsporidia; Rhizomucor; Rhodotorula;
Alternaria | (45) |
Effect of immune cells induced by fungi in
the TME
Fungi can elicit various immune responses within the
TME through interactions with immune cells. The presence of fungi
in the TME can activate immune cells, leading to both
pro-inflammatory and anti-inflammatory responses.
Mast cells (MCs)
MCs are important resident sentinel in TEM,
recognizing fungal cells and playing a crucial role in the immune
response. The response of MCs to fungal cells depends on their
morphology and MC subtype (46).
Connective tissue mast cells predominantly respond to the mycelial
state of C. albicans by promoting the production of
anti-inflammatory cytokines (47).
By contrast, mucosal mast cells (MMC) tend to react differently,
promoting death and damage in response to yeast state of C.
albicans (48).
During C. albicans infection in vivo,
mast cells exhibit a dual role. They contribute to the initial
inflammatory pathology (48,49)
while also playing a crucial role in controlling fungal growth and
spreading, as well as activating the Th1 immune response for memory
protection during reinfection (49). In leaky gut models,
Candida-driven IL-9 and MMC contribute to the loss of
barrier function, dissemination of the fungus (50) and inflammation (51,52).
This highlights the ability of C. albicans to exploit the
versatility of the IL-9/MC axis for both symbiosis and
pathogenesis. The IL-9/MC axis, by integrating signals from
disturbed host/microbiota homeostasis, may serve as a marker for
distinguishing between pathogenic and protective roles of fungi in
the gut (49).
Candida-infected MCs have been shown to
enhance the crawling ability of macrophages and to promote their
chemotaxis towards the site of infection, suggesting an important
role in modulating macrophage responses during C. albicans
infections (53). As MCs maintain
equilibrium between the host and commensal fungi such as C.
albicans, their modulation of macrophage activity would likely
helps limit fungal growth during infections (54). However, quiescent MCs can notably
inhibit phagocytosis of C. albicans by macrophages in a
contact-dependent manner (47).
This property may indeed be implicated in suppressing macrophage
activity, potentially aiding in immune evasion within the
tumors.
Neutrophils
Neutrophils are an essential component of the innate
immune system, equipped with both neutrophilic and specialized
granules that aid in the digestion of bacteria and foreign bodies.
They possess strong chemotaxis and phagocytosis capabilities,
making them quick response at fungal infection sites (55). Studies have also found that
neutrophils have a close association with tumor development in the
TME (56–58). Depleting neutrophils leads to
increased tumor growth, proliferation and invasiveness in mouse
model of inflammation-induced and sporadic colon tumors (59).
Candidalysin, a peptide toxin secreted by C.
albicans, play a crucial role in the interaction between the
fungus and host cells. Candidalysin activates the EGFR-ERK pathway
in a ligand-dependent manner (60),
leading to the release of transcription factor c-Fos, granulocyte
colony-stimulating factor and granulocyte-macrophage
colony-stimulating factor. These factors are essential for
recruiting neutrophils to clear infections and can also promote
cancer development.
While neutrophils are generally associated with
anti-tumor activity due to their ability to eliminate pathogens and
produce cytotoxic molecules, the potential influence of C.
albicans on the TEM through its interaction with neutrophils
via candidalysin secretion raises intriguing questions (61). It is plausible that C.
albicans could modulate the TME to promote tumor progression by
exploiting its interaction with neutrophils.
Macrophages
Macrophages are known as the first line of defense
against pathogens. They are responsible for recognizing,
phagocytosing and degrading cellular debris and pathogens.
Additionally, they play a crucial role in presenting antigens to T
cells and inducing the expression of co-stimulatory molecules by
other antigen-presenting cells (62). Recent research has revealed a
dichotomy in macrophage polarization, identified as two distinct
forms: M1 and M2. M1 macrophages produce type I pro-inflammatory
cytokines, participate in antigen presentation and exert an
antitumor role (63). Conversely,
M2 macrophages produce type II cytokines that promote
anti-inflammatory responses and possess pro-tumor functions
(64). In fact, macrophages play a
pivotal role in supporting various aspects of tumor progression and
tumor cell invasion (65).
Mechanistically, it has been discovered that
phenylpyruvic acid derived from C. albicans directly binds
to sirtuin 2, leading to an increase in reactive oxygen species
(ROS) production (66). ROS, in
turn, modulate various cellular signaling pathways primarily
involving transcription factors NF-κB and STAT3 (67), hypoxia-inducible factor-1α (68), kinases, growth factors, cytokines
and other proteins and enzymes. These pathways have been implicated
in various aspects of cancer biology, including cellular
transformation, inflammation, tumor survival, proliferation,
invasion, angiogenesis and metastasis (69,70).
In a mouse model lacking the c-type lectin Dectin-3 (Dectin-3-/-),
increased C. albicans load triggers glycolysis in
macrophages and secretion of IL-7, which induces IL-22 production
in type 3 innate lymphoid cells (ILC3) via the aryl hydrocarbon
receptor and STAT3 signaling pathway (71). Notably, IL-22 has been shown to
promote tumor growth in several models (72,73).
This aligns with the fact that IL-22 is a potent activator of STAT3
in epithelial cells (74), which is
required for the development of colitis-associated cancer (75). Based on these findings, it is
reasonable to speculate that C. albicans promotes
macrophages activation via the IL-22/STAT3 axis, contributing to
the complex interplay between fungal infection and cancer
development.
Similar effects on macrophages have been reported in
another yeast species, including Malassezia. The enrichment
of Malassezia in fungal communities found in mouse and human
pancreatic tumors suggests a potential carcinogenic role for this
organism (31), possibly through
its influence on the immune system of the host, such as suppressing
immunity via the inhibition of M1 macrophage differentiation.
Therefore, targeting fungal populations such as Malassezia
or modulating inflammatory responses associated with these fungi
may hold significant promise in reshaping the TEM and enhancing
cancer immunotherapy. By disrupting the immunosuppressive effects
of these fungi, it may be possible to enhance the antitumor immune
response and improve the effectiveness of immunotherapy for cancer
treatment.
Dendritic cells (DCs)
DCs play a crucial role in both initiating the
primary host immune response and enhancing the secondary host
immune response. As the most potent antigen presenting cell, DCs
excel at stimulating the activation and proliferation of naive T
cells, making them primary initiators of specific immunity.
Recent findings have revealed that the yeast form of
C. albicans induces Th17 cell responses through a mechanism
that requires Dectin-1-mediated expression of IL-6 by DCs (76). This underscores the potential of DCs
to not only mediate immune responses to fungal infections but also
to exert influences on the TEM. However, despite the critical role
of DCs in promoting T cell responses (77), the interaction between fungi and DCs
within the TME remains relatively unstudied. Understanding the
dynamics of this interaction, along with the broader interplay
between tumors, immune cells and fungi in the TME, will continue to
be a prominent focus in future studies.
Natural Killer (NK) cells
In recent years, the importance of NK cells in
antifungal immunity (43,78) has been recognized, in addition to
their well-known role in combating viruses and tumors. While NK
cells are capable of recognizing and eliminating C.
albicans, fungal cells can also inhibit NK cells by
manipulating the immune checkpoint receptor T cell immunoreceptor
with Ig and ITIM domains in both humans and mice (79). Despite this emerging understanding,
there is a lack of studies investigating in the interaction between
NK cells and C. albicans within TME. More in-depth research
is needed to further explore this interaction.
T cells
Regulatory T cells (Treg cells)
Treg cells, a subpopulation of CD4+ T
cells, play a crucial role in immune homeostasis by preventing
autoimmunity, controlling excessive inflammation and maintaining
immune tolerance. In normal physiological conditions, Treg cells
regulate the proliferation and activation of T and B cells, as well
as the homeostasis of innate cytotoxic lymphocytes (80). However, the immunosuppressive effect
of Treg cells can lead to immune escape of tumor cells, indirectly
promoting tumor cell proliferation and enhancing tumor cells
infiltration capacity (81).
In a study by Ahmadi et al (82), mice were divided into four groups:
Normal, tumor, Candida infected and tumor/Candida
groups. The results demonstrated that Candida infection led
to an increase in the Treg population in the TME in the
experimental group compared with the uninfected control group.
Similarly, in a mouse model of lung cancer constructed by Liu et
al, a higher proportion of Tregs was observed in the TME of
mice exposed to Aspergillus sydowii (41). These experiments collectively
suggest that fungi may contribute to the occurrence and development
of tumors by inhibiting antitumor immune responses through
mechanisms involving Treg cells. However, the specific signaling
pathways and mechanisms underlying this response remain unclear and
warrant further investigation. Understanding these mechanisms could
provide insights into potential therapeutic strategies for
targeting fungal-induced immunosuppression in cancer.
T helper (Th)2 cells
Th2 cells and ILC2 have been shown to stimulate
tumor growth by secreting pro-tumorigenic cytokines such as IL-4,
IL-5 and IL-13 (83). Notably, the
cancer-cell-specific deletion of IL-33 has been found to reduce the
recruitment Th2 and ILC2, thereby promoting tumor regression
(84,85). Notably, the secretion of IL-33 has
been found to be dependent on the intra-tumoral fungal mycobiome
(86). Specifically,
Malassezia in the pancreas has been implicated in promoting
the occurrence of pancreatic tumors by promoting the secretion of
IL-33. Genetic deletion of IL-33 or anti-fungal treatment can
decrease TH2 and ILC2 infiltration and increases survival (84).
According to a 2016 report, all cases of atrophying
pityriasis versicolor had Malassezia infiltration within the
stratum corneum and were surrounded by focal interfacial dermatitis
mediated by Th2 cell (87). These
findings may reveal the generation of Malassezia-primed Th2
cells.
Th17 cells
C. albicans regulates Th17 cells
proliferation, leading to increased secretion of IL-17A, IL-17F,
and IL-22. IL-17 activates NF- κB through the MAP kinase pathway,
enhancing its biological activity (88). IL-17A is a pro-inflammatory cytokine
that recruits neutrophils and promotes their proliferation,
maturation and chemotaxis. It also synergistically activates T
cells and promote cell maturation (89).
A recent study revealed that C. albicans
infection induces the expression of Gasdermin E (GSDME) in a
specific subset of human Th17 cells (90). GSDME is a membrane pore-forming
molecule that induces rapid cell death, suggesting its potential
role as a cancer checkpoint (91).
Investigating GSDME expression on the surface of Th17 cells
following C. albicans infection could provide valuable novel
insights for tumor diagnosis. Further research is required to
explore the implications of C. albicans-mediated GSDME
response on Th17 cells within TEM. Understanding the impact of ton
tumor development, progression and therapy response could lead to
innovative approaches to cancer diagnosis and treatment (90).
CD8+ T cells
CD8+ T cells play a key role in
eliminating intracellular infections and malignant cells and
provide long-term protective immunity (92). There are multiple subpopulations of
CD8+ T cells, each with distinct effector functions and
cytotoxic potential; for example, Tc1s has special cytotoxic
activity and can effectively kill tumor cells and cells containing
intracellular pathogens (93). Tc17
can produce IL17, which acts on tumor cells or other cells within
the TME (94). These subpopulations
have also been detected in the TME (95)
On the other hand, CD8+ T cells
themselves can target the fungus to produce and secrete large
amounts of IFN-γ and protease granzyme B to control the infection
(96). For example, in a mouse
model of lung adenocarcinoma, the proportion of CD8+ T
cells in the TME increased after Aspergillus sydowii
infection, revealing that CD8+ T cells and fungi reach a
delicate balance in the TME to regulate tumor development (41).
Future perspectives
The presence of specific fungi within the TME holds
promise as novel diagnostic or prognostic marker for specific types
of cancer. Recent research by Narunsky Haziza et al in 2022
investigated the relationship between clinical data of various
cancer patients and fungal species, revealing positive associations
between fungi and various clinical outcomes. For instance, fungi
were found to be positively associated with total survival time of
patients with breast cancer, progression free survival time in
patients with ovarian cancer, immunotherapeutic response in
patients with melanoma and detection of early cancer (21). These findings suggest that fungi
could serve as valuable biomarkers and potential therapeutic
targets in cancer management. The analyses of gut microbiota as a
screening, prognostic or predictive biomarkers have already begun
to meet clinical applications, offering a promising avenue for
preventing cancer, enhancing treatment efficacy and reducing
adverse treatment effects through microbiota regulation (97).
Recent studies have also highlighted the significant
role of fungi in cancer treatment and drug resistance. In addition
to the direct approach of reducing Th2 and ILC2 infiltration
through antifungal therapy, which can improve the survival rate of
patients with cancer (86), the use
of some fungal metabolites, such as polysaccharides and
triterpenes, in conjunction with immune therapy, has garnered
attention for their antitumor effects through various mechanisms
(98). Polysaccharides can serve as
key antitumor metabolites in numerous fungi infections through
their impacts on host immunity. They exhibit diverse effects on
host immunity by enhancing immunity, inhibiting tumor cell growth
and inducing tumor cell apoptosis (99). Polysaccharides derived from
Ganoderma atrum have been reported to activate macrophages,
improve immunity and inhibit colorectal tumor growth through a TLR4
dependent signaling pathway (100). In addition to inhibiting tumor
growth, Ganoderma polysaccharides have shown promise in
antihepatocellular cancer treatment by modulating macrophage
polarization (86) or increasing
the ratio of the effector T cells to Tregs (101). Another example is Poria
cocos polysaccharides, which have been implicated in exerting
antitumor effects. According to experiments conducted by Jing et
al, Poria cocos polysaccharides have the ability to induce
apoptosis in ovarian cancer cells (102).
Triterpenes represent another common antitumor
component found in fungi. Feng et al (103) studied the inhibitory effects of
Ganoderma triterpenoids on cell proliferation and tumor
growth, revealing their ability to induce cell death in lung cancer
cells in vitro. Additionally, in a Lewis tumor-bearing mouse
model, Ganoderma triterpenoids significantly delayed its
tumor growth (104). Although
research on this ingredient primarily focuses on in vitro
and mouse experiments, lacking clinical trial data, its potential
therapeutic effects warrant further exploration. Investigation
whether Ganoderma triterpenoids has a specific effect on the
treatment of patients with lung cancer is an important step for
future exploration. Furthermore, assessment of their safety and
toxicity are also worth further research. However, regardless, the
combined use of these products in clinical settings is also
essential.
Anticancer drug resistance is present in almost all
types of cancers (105). Research
on the relationship between the immune microenvironment and tumor
resistance is advancing rapidly. T-cell depletion is a
well-documented factor in promoting resistance to anticancer drugs,
and strategies to enhance T-cell infiltration show promise in
overcoming this anticancer drug resistance. Notably, fungi have
been increasingly recognized for their influence on immune function
and their potential role in cancer treatment. A study utilizing a
fecal microbiota transplantation to boost the effectiveness of PD-1
therapy in drug-resistant melanoma patients marks a significant
breakthrough (106). It
underscores the potential for manipulating the microbiome,
including fungi, to enhance responses to immunotherapy (107). As research continues to uncover
fungal-bacterial communities capable of significantly improving
tumor treatment outcomes, there is hope for extending these
benefits beyond melanoma to other cancer types treated with
antitumor therapies.
Both animal experiments and clinical studies have
found the significant impact of gut microbiota (108) on enhancing the anticancer effects
of checkpoint inhibitors such as CTLA-4, PD-1, and PD-L1 in
treating lung cancer (109), renal
cell carcinoma (110) and melanoma
(106,107,111). Changes in gut microbiota appear to
enhance the effectiveness of PD-1 inhibitors by possibly activating
the IL-12 signaling pathway, which boosts the response of Th1 cells
and activates T lymphocytes (108). Modulating the proportion of
probiotics and fungi within the gut microbiota, potentially through
antifungal drugs, could further optimize the efficacy of PD-1
inhibitors.
Numerous studies have demonstrated the capacity of
gut microbiota to influence the body's immune function and bolster
the efficacy of immunotherapy, indicating that manipulating gut
microbiota could emerge as a crucial element in tumor prevention
and immunotherapy (81,112,113). The highlighted therapies
underscore the significant interplay between fungi and the immune
system, presenting considerable promise in tumor prevention and
treatment. However, it is essential to conduct additional clinical
trials to confirm the efficacy and safety of these approaches.
Conclusions
In conclusion, the relationship between fungi,
immunity and the TEM is intricate and significant. Fungi possess
the ability to modulate immunity through diverse mechanisms,
consequently influencing the TME. The cytokines produced by
different immune cell phenotypes play a crucial mediator in
determining tumor outcomes.
Studying the interaction between the tumor immune
system and microbiota holds promise for uncovering novel
therapeutic targets. It is essential to gain a deeper understanding
of molecular mechanisms underlying fungal actions in tumors. This
knowledge will contribute to the discovery of novel therapeutic
targets and such endeavors will not only facilitate the discovery
of new therapeutic targets but also aid in identifying valuable
biomarkers for improved cancer treatment strategies.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Nature
Science Foundation of China (grant no. NM 82272358), the Key
Research and Development Plan of Jining (grant nos. NM 2023YXNS001
and NM 2022YXNS127) and the Medicine health science and Technology
Development Plan of Shandong Province (grant no. 202202070556).
Availability of data and materials
Not applicable.
Authors' contributions
JZ wrote the original draft. YF participated in the
revision of the draft manuscript. DM supervised the study,
visualized the data, and reviewed and edited the manuscript. DS
provided resources, visualized the data, and reviewed and edited
the paper. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Sayed A, Aleya L and Kamel M:
Microbiota's role in health and diseases. Environ Sci Pollut Res
Int. 28:36967–36983. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao L, Xu T, Huang G, Jiang S, Gu Y and
Chen F: Oral microbiomes: More and more importance in oral cavity
and whole body. Protein Cell. 9:488–500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Byrd AL, Belkaid Y and Segre JA: The human
skin microbiome. Nat Rev Microbiol. 16:143–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sommer F, Anderson JM, Bharti R, Raes J
and Rosenstiel P: The resilience of the intestinal microbiota
influences health and disease. Nat Rev Microbiol. 15:630–638. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen C, Song X, Wei W, Zhong H, Dai J, Lan
Z, Li F, Yu X, Feng Q, Wang Z, et al: The microbiota continuum
along the female reproductive tract and its relation to
uterine-related diseases. Nat Commun. 8:8752017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ottman N, Smidt H, de Vos WH and Belzer C:
The function of our microbiota: Who is out there and what do they
do? Front Cell Infect Microbiol. 2:1042012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zoetendal EG, Rajilic-Stojanovic M and de
Vos WM: High-throughput diversity and functionality analysis of the
gastrointestinal tract microbiota. Gut. 57:1605–1615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davenport ER, Sanders JG, Song SJ, Amato
KR, Clark AG and Knight R: The human microbiome in evolution. BMC
Biol. 15:1272017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garrett WS: Cancer and the microbiota.
Science. 348:80–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D,
Xiao C, Zhu D, Koya JB, Wei L, Li J and Chen ZS: Microbiota in
health and diseases. Signal Transduct Target Ther. 7:1352022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Zhou J and Wang L: Role and
mechanism of gut microbiota in human disease. Front Cell Infect
Microbiol. 11:6259132021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Belvoncikova P, Maronek M and Gardlik R:
Gut dysbiosis and fecal microbiota transplantation in autoimmune
diseases. Int J Mol Sci. 23:107292022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
14
|
Preisler HD, Bjornsson S, Mori M and
Barcos M: Granulocyte differentiation by friend leukemia cells.
Cell Differ. 4:273–283. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Izraely S and Witz IP: Site-specific
metastasis: A cooperation between cancer cells and the metastatic
microenvironment. Int J Cancer. 148:1308–1322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bicher HI, Hetzel FW, Sandhu TS, Frinak S,
Vaupel P, O'Hara MD and O'Brien T: Effects of hyperthermia on
normal and tumor microenvironment. Radiology. 137:523–530. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie Y, Xie F, Zhou X, Zhang L, Yang B,
Huang J, Wang F, Yan H, Zeng L, Zhang L and Zhou F: Microbiota in
tumors: From understanding to application. Adv Sci (Weinh).
9:e22004702022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang C, Liu H, Gong X, Wen B, Chen D, Liu
J and Hu F: Analysis of different components in the peritumoral
tissue microenvironment of colorectal cancer: A potential prospect
in tumorigenesis. Mol Med Rep. 14:2555–2565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vallianou N, Kounatidis D, Christodoulatos
GS, Panagopoulos F, Karampela I and Dalamaga M: Mycobiome and
cancer: What is the evidence? Cancers (Basel). 13:31492021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narunsky-Haziza L, Sepich-Poore GD,
Livyatan I, Asraf O, Martino C, Nejman D, Gavert N, Stajich JE,
Amit G, González A, et al: Pan-cancer analyses reveal
cancer-type-specific fungal ecologies and bacteriome interactions.
Cell. 185:3789–3806.e17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dohlman AB, Klug J, Mesko M, Gao IH,
Lipkin SM, Shen X and Iliev ID: A pan-cancer mycobiome analysis
reveals fungal involvement in gastrointestinal and lung tumors.
Cell. 185:3807–3822. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong M, Xiong Y, Zhao J, Gao Z, Ma J, Wu
Z, Song Y and Hong X: Candida albicans disorder is associated with
gastric carcinogenesis. Theranostics. 11:4945–4956. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramirez-Garcia A, Rementeria A,
Aguirre-Urizar JM, Moragues MD, Antoran A, Pellon A,
Abad-Diaz-de-Cerio A and Hernando FL: Candida albicans and cancer:
Can this yeast induce cancer development or progression? Crit Rev
Microbiol. 42:181–193. 2016.PubMed/NCBI
|
|
25
|
Conti HR and Gaffen SL: Host responses to
Candida albicans: Th17 cells and mucosal candidiasis. Microbes
Infect. 12:518–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williamson DM: Chronic hyperplastic
candidiasis and squamous carcinoma. Br J Dermatol. 81:125–127.
1969. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gupta SR, Gupta N, Sharma A, Xess I, Singh
G and Mani K: The association of Candida and antifungal therapy
with pro-inflammatory cytokines in oral leukoplakia. Clin Oral
Investig. 25:6287–6296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bastiaan RJ and Reade PC: The prevalence
of Candida albicans in the mouths of tobacco smokers with and
without oral mucous membrane keratoses. Oral Surg Oral Med Oral
Pathol. 53:148–151. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daftary DK, Mehta FS, Gupta PC and
Pindborg JJ: The presence of Candida in 723 oral leukoplakias among
Indian villagers. Scand J Dent Res. 80:75–79. 1972.PubMed/NCBI
|
|
30
|
Healy CM and Moran GP: The microbiome and
oral cancer: More questions than answers. Oral Oncol. 89:30–33.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alnuaimi AD, Wiesenfeld D, O'Brien-Simpson
NM, Reynolds EC and McCullough MJ: Oral Candida colonization in
oral cancer patients and its relationship with traditional risk
factors of oral cancer: A matched case-control study. Oral Oncol.
51:139–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCullough M, Jaber M, Barrett AW, Bain L,
Speight PM and Porter SR: Oral yeast carriage correlates with
presence of oral epithelial dysplasia. Oral Oncol. 38:391–393.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Chen C, Chai D, Li C, Qiu Z,
Kuang T, Liu L, Deng W and Wang W: Characterization of the
intestinal fungal microbiome in patients with hepatocellular
carcinoma. J Transl Med. 21:1262023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aykut B, Pushalkar S, Chen R, Li Q,
Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, et al:
The fungal mycobiome promotes pancreatic oncogenesis via activation
of MBL. Nature. 574:264–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishikawa T, Itoh F, Yoshida S, Saijo S,
Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T and
Yamasaki S: Identification of distinct ligands for the C-type
lectin receptors mincle and dectin-2 in the pathogenic Fungus
Malassezia. Cell Host Microbe. 13:477–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zambirinis CP, Pushalkar S, Saxena D and
Miller G: Pancreatic cancer, inflammation, and microbiome. Cancer
J. 20:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sparmann A and Bar-Sagi D: Ras-induced
interleukin-8 expression plays a critical role in tumor growth and
angiogenesis. Cancer Cell. 6:447–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Limon JJ, Tang J, Li D, Wolf AJ, Michelsen
KS, Funari V, Gargus M, Nguyen C, Sharma P, Maymi VI, et al:
Malassezia Is associated with Crohn's disease and exacerbates
colitis in mouse models. Cell Host Microbe. 25:377–388.e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nixon MW: Aflatoxin and liver cancer.
Lancet. 335:11651990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin Y, Lau HC, Liu Y, Kang X, Wang Y, Ting
NL, Kwong TN, Han J, Liu W, Liu C, et al: Altered mycobiota
signatures and enriched pathogenic Aspergillus rambellii are
associated with colorectal cancer based on multicohort fecal
metagenomic analyses. Gastroenterology. 163:908–921. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu NN, Yi CX, Wei LQ, Zhou JA, Jiang T,
Hu CC, Wang L, Wang YY, Zou Y, Zhao YK, et al: The intratumor
mycobiome promotes lung cancer progression via myeloid-derived
suppressor cells. Cancer Cell. 41:1927–1944. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao R, Kong C, Li H, Huang L, Qu X, Qin N
and Qin H: Dysbiosis signature of mycobiota in colon polyp and
colorectal cancer. Eur J Clin Microbiol Infect Dis. 36:2457–2468.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Shi ZW, Strickland AB and Shi M:
Cryptococcus neoformans infection in the central nervous system:
The battle between host and pathogen. J Fungi (Basel). 8:10692022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Banerjee S, Tian T, Wei Z, Shih N, Feldman
MD, Alwine JC, Coukos G and Robertson ES: The ovarian cancer
oncobiome. Oncotarget. 8:36225–36245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu NN, Jiao N, Tan JC, Wang Z, Wu D, Wang
AJ, Chen J, Tao L, Zhou C, Fang W, et al: Multi-kingdom microbiota
analyses identify bacterial-fungal interactions and biomarkers of
colorectal cancer across cohorts. Nat Microbiol. 7:238–250. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dudeck A, Köberle M, Goldmann O, Meyer N,
Dudeck J, Lemmens S, Rohde M, Roldán NG, Dietze-Schwonberg K,
Orinska Z, et al: Mast cells as protectors of health. J Allergy
Clin Immunol. 144:S4–S18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Zuani M, Paolicelli G, Zelante T, Renga
G, Romani L, Arzese A, Pucillo CEM and Frossi B: Mast cells respond
to candida albicans infections and modulate macrophages
phagocytosis of the Fungus. Front Immunol. 9:28292018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abraham SN and St John AL: Mast
cell-orchestrated immunity to pathogens. Nat Rev Immunol.
10:440–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Renga G, Moretti S, Oikonomou V, Borghi M,
Zelante T, Paolicelli G, Costantini C, De Zuani M, Villella VR,
Raia V, et al: IL-9 and mast cells are key players of candida
albicans commensalism and pathogenesis in the gut. Cell Rep.
23:1767–1778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gerard R, Sendid B, Colombel JF, Poulain D
and Jouault T: An immunological link between Candida
albicanscolonization and Crohn's disease. Crit Rev Microbiol.
41:135–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu B, Yang MQ, Yu TY, Yin YY, Liu Y, Wang
XD, He ZG, Yin L, Chen CQ and Li JY: Mast cell tryptase promotes
inflammatory bowel disease-induced intestinal fibrosis. Inflamm
Bowel Dis. 27:242–255. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nalleweg N, Chiriac MT, Podstawa E,
Lehmann C, Rau TT, Atreya R, Krauss E, Hundorfean G, Fichtner-Feigl
S, Hartmann A, et al: IL-9 and its receptor are predominantly
involved in the pathogenesis of UC. Gut. 64:743–755. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Richardson JP, Moyes DL, Ho J and Naglik
JR: Candida innate immunity at the mucosa. Semin Cell Dev Biol.
89:58–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiao Q, Luo Y, Scheffel J, Zhao Z and
Maurer M: The complex role of mast cells in fungal infections. Exp
Dermatol. 28:749–755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu SC, Yu HS, Yen FL, Lin CL, Chen GS and
Lan CC: Neutrophil extracellular trap formation is increased in
psoriasis and induces human beta-defensin-2 production in epidermal
keratinocytes. Sci Rep. 6:311192016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miksch RC, Schoenberg MB, Weniger M, Bösch
F, Ormanns S, Mayer B, Werner J, Bazhin AV and D'Haese JG:
Prognostic impact of tumor-infiltrating lymphocytes and neutrophils
on survival of patients with upfront resection of pancreatic
cancer. Cancers (Basel). 11:392019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Raftopoulou S, Valadez-Cosmes P, Mihalic
ZN, Schicho R and Kargl J: Tumor-mediated neutrophil polarization
and therapeutic implications. Int J Mol Sci. 23:32182022.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Que H, Fu Q, Lan T, Tian X and Wei X:
Tumor-associated neutrophils and neutrophil-targeted cancer
therapies. Biochim Biophys Acta Rev Cancer. 1877:1887622022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Triner D, Devenport SN, Ramakrishnan SK,
Ma X, Frieler RA, Greenson JK, Inohara N, Nunez G, Colacino JA,
Mortensen RM and Shah YM: Neutrophils restrict tumor-associated
microbiota to reduce growth and invasion of colon tumors in mice.
Gastroenterology. 156:1467–1482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nikou SA, Zhou C, Griffiths JS, Kotowicz
NK, Coleman BM, Green MJ, Moyes DL, Gaffen SL, Naglik JR and Parker
PJ: The Candida albicans toxin candidalysin mediates distinct
epithelial inflammatory responses through p38 and EGFR-ERK
pathways. Sci Signal. 15:eabj69152022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Swamydas M, Gao JL, Break TJ, Johnson MD,
Jaeger M, Rodriguez CA, Lim JK, Green NM, Collar AL, Fischer BG, et
al: CXCR1-mediated neutrophil degranulation and fungal killing
promote Candida clearance and host survival. Sci Transl Med.
8:322ra102016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Strizova Z, Benesova I, Bartolini R,
Novysedlak R, Cecrdlova E, Foley LK and Striz I: M1/M2 macrophages
and their overlaps-myth or reality? Clin Sci (Lond). 137:1067–1093.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gu P, Liu R, Yang Q, Xie L, Wei R, Li J,
Mei F, Chen T, Zeng Z, He Y, et al: A metabolite from commensal
Candida albicans enhances the bactericidal activity of macrophages
and protects against sepsis. Cell Mol Immunol. 20:1156–1170. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li J, Lan T, Zhang C, Zeng C, Hou J, Yang
Z, Zhang M, Liu J and Liu B: Reciprocal activation between
IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and
survival of non-small cell lung cancer cells. Oncotarget.
6:1031–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cheng CW, Kuo CY, Fan CC, Fang WC, Jiang
SS, Lo YK, Wang TY, Kao MC and Lee AY: Overexpression of Lon
contributes to survival and aggressive phenotype of cancer cells
through mitochondrial complex I-mediated generation of reactive
oxygen species. Cell Death Dis. 4:e6812013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang C, Cao S, Toole BP and Xu Y: Cancer
may be a pathway to cell survival under persistent hypoxia and
elevated ROS: A model for solid-cancer initiation and early
development. Int J Cancer. 136:2001–2011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhu Y, Shi T, Lu X, Xu Z, Qu J, Zhang Z,
Shi G, Shen S, Hou Y, Chen Y and Wang T: Fungal-induced glycolysis
in macrophages promotes colon cancer by enhancing innate lymphoid
cell secretion of IL-22. EMBO J. 40:e1053202021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dmitrieva-Posocco O, Dzutsev A, Posocco
DF, Hou V, Yuan W, Thovarai V, Mufazalov IA, Gunzer M, Shilovskiy
IP, Khaitov MR, et al: Cell-type-specific responses to
interleukin-1 control microbial invasion and tumor-elicited
inflammation in colorectal cancer. Immunity. 50:166–180.e7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kirchberger S, Royston DJ, Boulard O,
Thornton E, Franchini F, Szabady RL, Harrison O and Powrie F:
Innate lymphoid cells sustain colon cancer through production of
interleukin-22 in a mouse model. J Exp Med. 210:917–931. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bollrath J, Phesse TJ, von Burstin VA,
Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T,
Canli O, Schwitalla S, et al: gp130-mediated stat3 activation in
enterocytes regulates cell survival and cell-cycle progression
during colitis-associated tumorigenesis. Cancer Cell. 15:91–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Keir ME, Yi T, Lu TT and Ghilardi N: The
role of IL-22 in intestinal health and disease. J Exp Med.
217:e201921952020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kashem SW, Igyarto BZ, Gerami-Nejad M,
Kumamoto Y, Mohammed JA, Jarrett E, Drummond RA, Zurawski SM,
Zurawski G, Berman J, et al: Candida albicans morphology and
dendritic cell subsets determine T helper cell differentiation.
Immunity. 42:356–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pittet MJ, Di Pilato M, Garris C and
Mempel TR: Dendritic cells as shepherds of T cell immunity in
cancer. Immunity. 56:2218–2230. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schmidt S, Condorelli A, Koltze A and
Lehrnbecher T: NK cells and their role in invasive mold infection.
J Fungi (Basel). 3:252017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Charpak-Amikam Y, Lapidus T, Isaacson B,
Duev-Cohen A, Levinson T, Elbaz A, Levi-Schaffer F, Osherov N,
Bachrach G, Hoyer LL, et al: Candida albicans evades NK cell
elimination via binding of Agglutinin-Like Sequence proteins to the
checkpoint receptor TIGIT. Nat Commun. 13:24632022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Horii M and Matsushita T: Regulatory B
cells and T cell regulation in cancer. J Mol Biol. 433:1666852021.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wu J, Wang S, Zheng B, Qiu X, Wang H and
Chen L: Modulation of gut microbiota to enhance effect of
checkpoint inhibitor immunotherapy. Front Immunol. 12:6691502021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ahmadi N, Ahmadi A, Kheirali E, Yadegari
MH, Bayat M, Shajiei A, Amini AA, Ashrafi S, Abolhassani M, Faezi
S, et al: Systemic infection with Candida albicans in breast tumor
bearing mice: Cytokines dysregulation and induction of regulatory T
cells. J Mycol Med. 29:49–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Protti MP and De Monte L: Thymic stromal
lymphopoietin and cancer: Th2-dependent and -independent
mechanisms. Front Immunol. 11:20882020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Alam A, Levanduski E, Denz P,
Villavicencio HS, Bhatta M, Alhorebi L, Zhang Y, Gomez EC, Morreale
B, Senchanthisai S, et al: Fungal mycobiome drives IL-33 secretion
and type 2 immunity in pancreatic cancer. Cancer Cell.
40:153–167.e11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jou E, Rodriguez-Rodriguez N, Ferreira AF,
Jolin HE, Clark PA, Sawmynaden K, Ko M, Murphy JE, Mannion J, Ward
C, et al: An innate IL-25-ILC2-MDSC axis creates a
cancer-permissive microenvironment for Apc mutation-driven
intestinal tumorigenesis. Sci Immunol. 7:eabn01752022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li GL, Tang JF, Tan WL, Zhang T, Zeng D,
Zhao S, Ran JH, Li J, Wang YP and Chen DL: The anti-hepatocellular
carcinoma effects of polysaccharides from Ganoderma lucidum by
regulating macrophage polarization via the MAPK/NF-κB signaling
pathway. Food Funct. 14:3155–3168. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Levy JMS and Magro C: Atrophying
pityriasis versicolor as an idiosyncratic T cell-mediated response
to Malassezia: A case series. J Am Acad Dermatol. 76:730–735. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Poggi A, Catellani S, Musso A and Zocchi
MR: Gammadelta T lymphocytes producing IFNgamma and IL-17 in
response to Candida albicans or mycobacterial antigens possible
implications for acute and chronic inflammation. Curr Med Chem.
16:4743–4749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Coffelt SB, Kersten K, Doornebal CW,
Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M,
Hawinkels LJAC, Jonkers J and de Visser KE: IL-17-producing γδ T
cells and neutrophils conspire to promote breast cancer metastasis.
Nature. 522:345–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chao YY, Puhach A, Frieser D, Arunkumar M,
Lehner L, Seeholzer T, Garcia-Lopez A, van der Wal M, Fibi-Smetana
S, Dietschmann A, et al: Human TH17 cells engage gasdermin E pores
to release IL-1α on NLRP3 inflammasome activation. Nat Immunol.
24:295–308. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liang WF, Gong YX, Li HF, Sun FL, Li WL,
Chen DQ, Xie DP, Ren CX, Guo XY, Wang ZY, et al: Curcumin activates
ros signaling to promote pyroptosis in hepatocellular carcinoma
HepG2 cells. In Vivo. 35:249–257. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Reina-Campos M, Scharping NE and Goldrath
AW: CD8+ T cell metabolism in infection and cancer. Nat
Rev Immunol. 21:718–738. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Farhood B, Najafi M and Mortezaee K:
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A
review. J Cell Physiol. 234:8509–8521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Picard FSR, Lutz V, Brichkina A, Neuhaus
F, Ruckenbrod T, Hupfer A, Raifer H, Klein M, Bopp T, Pfefferle PI,
et al: IL-17A-producing CD8+ T cells promote PDAC via
induction of inflammatory cancer-associated fibroblasts. Gut.
72:1510–1522. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Han J, Khatwani N, Searles TG, Turk MJ and
Angeles CV: Memory CD8+ T cell responses to cancer.
Semin Immunol. 49:1014352020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pearce EL and Shen H: Making sense of
inflammation, epigenetics, and memory CD8+ T-cell differentiation
in the context of infection. Immunol Rev. 211:197–202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fernandes MR, Aggarwal P, Costa RG, Cole
AM and Trinchieri G: Targeting the gut microbiota for cancer
therapy. Nat Rev Cancer. 22:703–722. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xu J, Chen F, Wang G, Liu B, Song H and Ma
T: The versatile functions of G. Lucidum Polysaccharides and G.
Lucidum Triterpenes in cancer radiotherapy and chemotherapy. Cancer
Manag Res. 13:6507–6516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tian W, Huang J, Zhang W, Wang Y, Jin R,
Guo H, Tang Y, Wang Y, Lai H and Leung EL: Harnessing natural
product polysaccharides against lung cancer and revisit its novel
mechanism. Pharmacol Res. 199:1070342024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang S, Nie S, Huang D, Li W and Xie M:
Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26
tumor-bearing mice. Food Chem. 136:1213–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li A, Shuai X, Jia Z, Li H, Liang X, Su D
and Guo W: Ganoderma lucidum polysaccharide extract inhibits
hepatocellular carcinoma growth by downregulating regulatory T
cells accumulation and function by inducing microRNA-125b. J Transl
Med. 13:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jing T, Guo Y and Wei Y: Carboxymethylated
pachyman induces ferroptosis in ovarian cancer by suppressing
NRF1/HO-1 signaling. Oncol Lett. 23:1612022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Feng L, Yuan L, Du M, Chen Y, Zhang MH, Gu
JF, He JJ, Wang Y and Cao W: Anti-lung cancer activity through
enhancement of immunomodulation and induction of cell apoptosis of
total triterpenes extracted from Ganoderma luncidum (Leyss. ex Fr.)
Karst. Molecules. 18:9966–9981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bin G and Gui-Zhen Y: Effects of Ganoderma
applanatum polysaccharide on cellular and humoral immunity in
normal and sarcoma-180 transplanted mice. Phytother. 5:134–138.
1991. View Article : Google Scholar
|
|
105
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Davar D, Dzutsev AK, McCulloch JA,
Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding
Q, Pagliano O, et al: Fecal microbiota transplant overcomes
resistance to anti-PD-1 therapy in melanoma patients. Science.
371:596–602. 2021. View Article : Google Scholar
|
|
107
|
Routy B, Lenehan JG, Miller WH Jr, Jamal
R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L,
Punčochář M, et al: Fecal microbiota transplantation plus anti-PD-1
immunotherapy in advanced melanoma: A phase I trial. Nat Med.
29:2121–2132. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Qian X, Zhang HY, Li QL, Ma GJ, Chen Z, Ji
XM, Li CY and Zhang AQ: Integrated microbiome, metabolome, and
proteome analysis identifies a novel interplay among commensal
bacteria, metabolites and candidate targets in non-small cell lung
cancer. Clin Transl Med. 12:e9472022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Derosa L, Routy B, Fidelle M, Iebba V,
Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G,
et al: Gut bacteria composition drives primary resistance to cancer
immunotherapy in renal cell carcinoma patients. Eur Urol.
78:195–206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lu Y, Yuan X, Wang M, He Z, Li H, Wang J
and Li Q: Gut microbiota influence immunotherapy responses:
Mechanisms and therapeutic strategies. J Hematol Oncol. 15:472022.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang H, Hong Y, Wu T, Ben E, Li S, Hu L
and Xie T: Role of gut microbiota in regulating immune checkpoint
inhibitor therapy for glioblastoma. Front Immunol. 15:14019672024.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kohi S, Macgregor-Das A, Dbouk M, Yoshida
T, Chuidian M, Abe T, Borges M, Lennon AM, Shin EJ, Canto MI and
Goggins M: Alterations in the duodenal fluid microbiome of patients
with pancreatic cancer. Clin Gastroenterol Hepatol. 20:e196–e227.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Riquelme E, Zhang Y, Zhang L, Montiel M,
Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, Lucas AS, et al:
Tumor microbiome diversity and composition influence pancreatic
cancer outcomes. Cell. 178:795–806.e12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Apostolou P, Tsantsaridou A, Papasotiriou
I, Toloudi M, Chatziioannou M and Giamouzis G: Bacterial and fungal
microflora in surgically removed lung cancer samples. J
Cardiothorac Surg. 6:1372011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Banerjee S, Alwine JC, Wei Z, Tian T, Shih
N, Sperling C, Guzzo T, Feldman MD and Robertson ES: Microbiome
signatures in prostate cancer. Carcinogenesis. 40:749–764. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong
SH, Ng SC, Chan FKL, Sung JJY and Yu J: Enteric fungal microbiota
dysbiosis and ecological alterations in colorectal cancer. Gut.
68:654–662. 2019. View Article : Google Scholar : PubMed/NCBI
|