Introduction
Esophageal cancer is recognized as one of the most
aggressive types of malignancy, with a poor prognosis and low
overall survival rate, making it a significant global health
concern. The main histological type of this cancer, particularly
common in East Asian populations, is esophageal squamous cell
carcinoma (ESCC) (1–3). Patients with ESCC still have high
risks of lymph node (LN) metastasis and poor long-term overall
survival (OS) outcomes (4–6). Depending on the clinical stage,
patients often undergo preoperative neoadjuvant chemotherapy,
chemoradiotherapy or chemotherapy immunization, followed by
postoperative immunotherapy maintenance. Despite advancements in
treatment strategies, esophagectomy remains the primary treatment
approach for ESCC within multimodal therapy (7–10).
Surgical outcomes in esophagectomy are influenced by
various patient-specific factors, underscoring the necessity for
personalized treatment approaches tailored to individual clinical
scenarios (11–13). While manual suturing (MS) has
traditionally been the method of choice (14–16),
the emergence of mechanical suturing (MeS) as a viable alternative
has garnered interest (17,18). MS, also known as hand-sewn
anastomosis, is a traditional surgical technique used to join the
two ends of the esophagus following resection. This method provides
greater flexibility in tailoring the anastomosis to the specific
anatomical and physiological conditions of the patient; however, it
is typically more time-consuming. By contrast, MeS, or stapled
anastomosis, is a more modern technique that employs a mechanical
stapler to connect the esophageal tissues. This approach creates a
secure and uniform anastomosis, ensuring consistency in tissue
approximation while reducing operative time. Additionally, the use
of a stapler requires specific equipment and may incur higher costs
compared to manual suturing. MeS was first reported by Sugimura
et al (15), and later
improved by Orringer et al (19). Both MS and MeS techniques have their
specific advantages and disadvantages. Studies by Kondra et
al (20), Singhal et al
(21) and Singh et al
(22) suggest that MeS
significantly reduces the incidence of postoperative anastomotic
leaks and strictures in esophageal cancer surgeries, considering it
a safe and effective technique. When MeS is difficult or fails, MS
becomes an important alternative (23). Initial comparisons between MS and
MeS have indicated similar immediate postoperative outcomes and
complication rates, hinting at a potential shift in surgical
techniques (24). However,
controversy remains over which suturing method is better and
long-term survival data comparing these techniques in esophagectomy
are lacking. Such research is essential for refining surgical
practices and enhancing the long-term prognosis of patients
undergoing esophagectomy for ESCC.
The present study aimed to account for various
potential confounders in order to ensure an unbiased and precise
comparison of the long-term outcomes linked to each technique. This
will be achieved through a comprehensive causal inference analysis
to compare the long-term outcomes of MS and MeS following
esophagectomy.
Materials and methods
Patients and methods
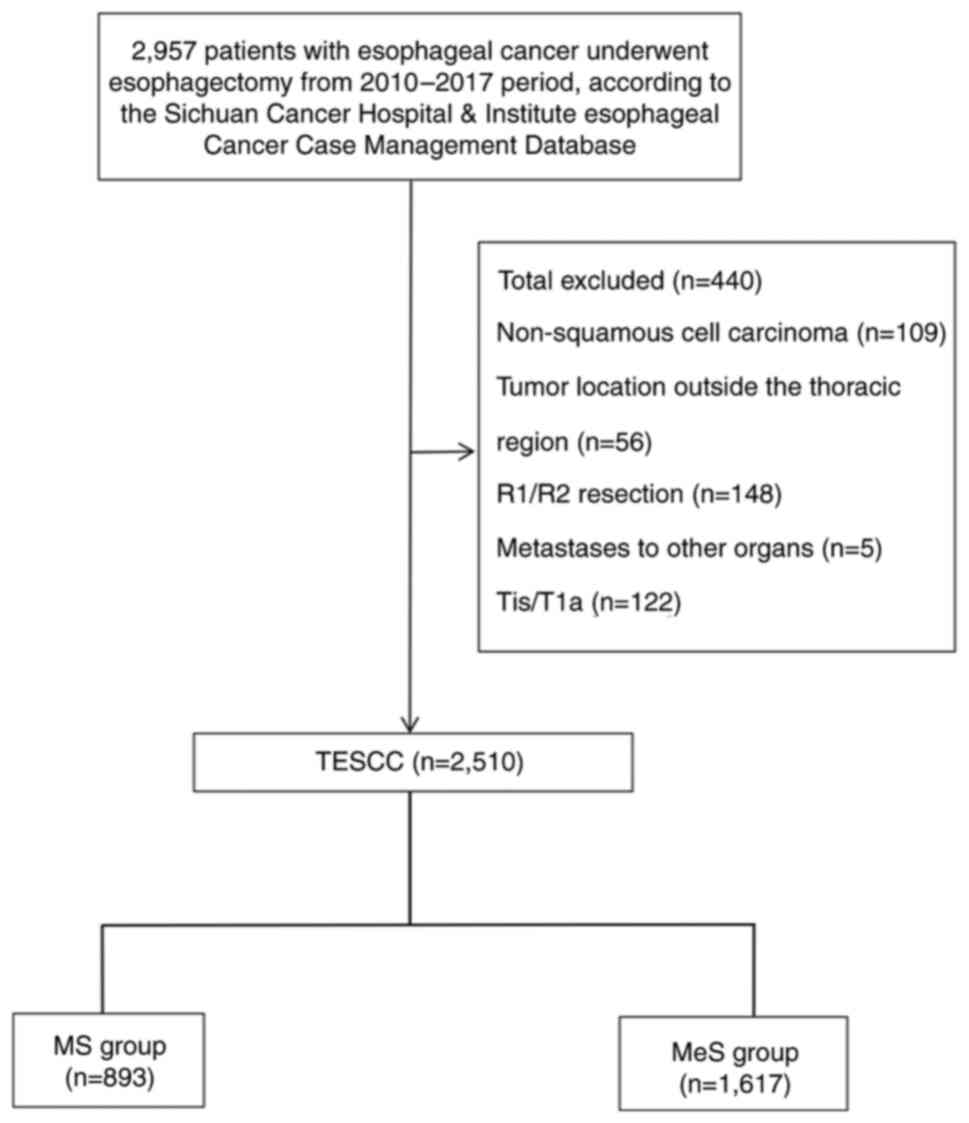
This retrospective study examined data from 2,510
patients diagnosed with ESCC who underwent esophagectomy at Sichuan
Cancer Hospital (Sichuan, China) between January 2010 and December
2017. The data were obtained from the Esophageal Cancer Case
Management database of Sichuan Cancer Hospital and Institute. The
study aimed to compare the long-term OS outcomes between MS and MeS
techniques used in esophagectomy. Patients included in the study
had undergone esophagectomy for ESCC within the specified period
and met the criteria of thoracic ESCC staging according to the 8th
edition TNM staging system by the Union for International Cancer
Control/American Joint Committee on Cancer (25). Exclusion criteria encompassed
non-squamous cell carcinoma histology in a surgical specimen,
tumors located outside the thoracic esophagus, evidence of distant
metastasis, R1/R2 resection (indicative of incomplete tumor
removal), stage pTis/T1a, receipt of preoperative neoadjuvant
therapy and missing data (Fig.
1).
Outcome measures
Demographic data, smoking and alcohol consumption
history, clinical stage and tumor characteristics were collected
for each patient. Clinical staging was reviewed by multiple experts
and pathological findings were confirmed by two pathologists, with
a third pathologist's review for accuracy. Patients were followed
up every 3 months for the first 2 years post-surgery and then every
6 months for the next 3–5 years. OS, defined as the time from
surgery to death from any cause or last known follow-up, served as
the primary endpoint.
Statistical analysis
Categorical variables were presented as percentages
and analyzed using chi-square or Fisher's exact test, as
appropriate. The Kaplan-Meier method was used to generate survival
curves and differences were assessed using the log-rank test.
Univariate and multivariate logistic regression using the Cox
proportional hazards model identified independent predictors of OS,
providing hazard ratios (HRs) and 95% confidence intervals (CIs).
To minimize selection bias and balance baseline characteristics
between the MS and MeS Groups, four causal inference methods was
performed. Propensity scores were calculated using logistic
regression based on age, sex and other relevant baseline
characteristics [propensity score matching (PSM)]. A 1:1
nearest-neighbor matching algorithm without replacement and a
caliper width of 0.25 times the standard deviation of the logit of
the propensity score were used. Sensitivity analyses with inverse
probability of treatment weighting (IPTW), overlap weighting (OW)
and standardized mortality ratio weighting (SMRW) validated the
findings. Statistical significance was set at a two-sided P-value
of <0.05. Analyses were conducted using SPSS version 23.0 (IBM
Corp.) and RStudio software with R 4.3 (The R Foundation).
Results
Demographic data
The initial dataset included 2,957 patients
diagnosed with ESCC who underwent esophagectomy at Sichuan Cancer
Hospital between January 2010 and December 2017. After applying the
exclusion criteria, 440 patients were excluded from the analysis.
Specifically, 109 patients were excluded due to non-squamous cell
carcinoma histology, 56 for tumor locations outside the thoracic
region, 148 for R1/R2 resections indicating incomplete tumor
removal, 5 for metastases to other organs, 122 for early-stage
(pTis/T1a) disease and 7 for missing data. Consequently, a total of
2,510 patients were eligible for the study, as shown in the study
flowchart in Fig. 1. A total of
82.1% of the patients (n=2,060) were male and 17.9% (n=450) were
female. Approximately 57.5% of the patients (n=1,443) were above
stage III, with 5.8% of the patients (n=212) aged >75 years
(Table I). Before PSM, there were
statistically significant differences between the MS and MeS groups
in terms of sex, KPS, smoking history, tumor location, thoracic
surgery, abdominal surgery, surgical approach and clinical
treatment modality. However, after PSM, these baseline
characteristics were well-balanced between the two groups, ensuring
comparability for subsequent analyses.
 | Table I.Demographic characteristics of the
MeS group and the MS group. |
Table I.
Demographic characteristics of the
MeS group and the MS group.
|
|
| Before PSM |
| After PSM |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Total | MeS group
(n=1,617) | MS group
(n=893) | P-value | MeS group
(n=643) | MS group
(n=643) | P-value |
|---|
| Sex |
|
|
| 0.017 |
|
| 0.783 |
|
Male | 2,060 (82.1) | 1,349 (83.4) | 711 (79.6) |
| 508 (79.0) | 512 (79.6) |
|
|
Female | 450 (17.9) | 268 (16.6) | 182 (20.4) |
| 135 (21.0) | 131 (20.4) |
|
| Age, years |
|
|
| 0.916 |
|
| 0.900 |
|
<75 | 2,365 (94.2) | 1,523 (94.2) | 842 (94.3) |
| 610 (94.9) | 609 (94.7) |
|
|
≥75 | 145 (5.8) | 94 (5.8) | 51 (5.7) |
| 33 (5.1) | 34 (5.3) |
|
| KPS |
|
|
| <0.001 |
|
| 0.908 |
|
≥90 | 1,419 (56.5) | 798 (49.4) | 621 (69.5) |
| 410 (63.8) | 408 (63.5) |
|
|
≤80 | 1,091 (43.5) | 819 (50.6) | 272 (30.5) |
| 233 (36.2) | 235 (36.5) |
|
| Smoker |
|
|
| <0.001 |
|
| 0.954 |
| No | 1,340 (53.4) | 724 (44.8) | 616 (69.0) |
| 411 (63.9) | 412 (64.1) |
|
|
Yes | 1,170 (46.6) | 893 (55.2) | 277 (31.0) |
| 232 (36.1) | 231 (35.9) |
|
| Drinker |
|
|
| 0.157 |
|
| 0.852 |
| No | 1,841 (73.3) | 1,171 (72.4) | 670 (75.0) |
| 467 (72.6) | 464 (72.2) |
|
|
Yes | 669 (26.7) | 446 (27.6) | 223 (25.0) |
| 176 (27.4) | 179 (27.8) |
|
| Pathologic
differentiation grade |
|
|
| 0.204 |
|
| 0.755 |
| Well
G1 | 455 (18.1) | 279 (17.3) | 176 (19.7) |
| 120 (18.7) | 128 (19.9) |
|
|
Moderate G2 | 1,046 (41.7) | 691 (42.7) | 355 (39.8) |
| 270 (42.0) | 258 (40.1) |
|
| Poor or
undifferentiated G3 | 1,009 (40.2) | 647 (40.0) | 362 (40.5) |
| 253 (39.3) | 257 (40.0) |
|
| Lymphovascular
invasion |
|
|
| 0.147 |
|
| 1.000 |
| No | 442 (17.6) | 1,319 (81.6) | 749 (83.9) |
| 529 (82.3) | 529 (82.3) |
|
|
Yes | 2,068 (82.4) | 298 (18.4) | 144 (16.1) |
| 114 (17.7) | 114 (17.7) |
|
| Nerve invasion |
|
|
| 0.133 |
|
| 0.945 |
| No | 487 (19.4) | 1289 (79.7) | 734 (82.2) |
| 513 (79.8) | 514 (79.9) |
|
|
Yes | 2,023 (80.6) | 328 (20.3) | 159 (17.8) |
| 130 (20.2) | 129 (20.1) |
|
| Tumor location |
|
|
| <0.001 |
|
| 0.951 |
|
Upper | 587 (23.4) | 330 (20.4) | 257 (28.8) |
| 169 (26.3) | 166 (25.8) |
|
|
Middle | 1,367 (54.5) | 893 (55.2) | 474 (53.1) |
| 364 (56.6) | 363 (56.5) |
|
|
Lower | 556 (22.2) | 394 (24.4) | 162 (18.1) |
| 110 (17.1) | 114 (17.7) |
|
| Pathological T
category |
|
|
| 0.260 |
|
| 0.987 |
| T1 | 202 (8.0) | 120 (7.4) | 82 (9.2) |
| 62 (9.6) | 66 (10.3) |
|
| T2 | 509 (20.3) | 333 (20.6) | 176 (19.7) |
| 124 (19.3) | 123 (19.1) |
|
| T3 | 1,584 (63.1) | 1,033 (63.9) | 551 (61.7) |
| 392 (61.0) | 389 (60.5) |
|
| T4 | 215 (8.6) | 131 (8.1) | 84 (9.4) |
| 65 (10.1) | 65 (10.1) |
|
| Pathological N
category |
|
|
| 0.272 |
|
| 0.903 |
| N0 | 1,084 (43.2) | 702 (43.4) | 382 (42.8) |
| 280 (43.5) | 282 (43.9) |
|
| N1 | 761 (30.3) | 505 (31.2) | 256 (28.7) |
| 190 (29.5) | 197 (30.6) |
|
| N2 | 450 (17.9) | 281 (17.4) | 169 (18.9) |
| 111 (17.3) | 109 (17.0) |
|
| N3 | 215 (8.6) | 129 (8.0) | 86 (9.6) |
| 62 (9.6) | 55 (8.6) |
|
| 8th TNM stage |
|
|
| 0.182 |
|
| 0.927 |
| I | 202 (8.0) | 120 (7.4) | 82 (9.2) |
| 61 (9.5) | 62 (9.6) |
|
| II | 865 (34.5) | 573 (35.4) | 292 (32.7) |
| 215 (33.4) | 218 (33.9) |
|
|
III | 1,152 (45.9) | 746 (46.1) | 406 (45.5) |
| 284 (44.2) | 288 (44.8) |
|
| IV | 291 (11.6) | 178 (11.0) | 113 (12.7) |
| 83 (12.9) | 75 (11.7) |
|
| Thoracic
surgery |
|
|
| 0.022 |
|
| 0.695 |
|
MIE | 1,199 (47.8) | 745 (46.1) | 454 (50.8) |
| 359 (55.8) | 352 (54.7) |
|
| OE | 1,311 (52.2) | 872 (53.9) | 439 (49.2) |
| 284 (44.2) | 291 (45.3) |
|
| Abdominal
surgery |
|
|
| <0.001 |
|
| 0.822 |
|
MIE | 964 (38.4) | 674 (41.7) | 290 (32.5) |
| 285 (44.3) | 281 (43.7) |
|
| OE | 1,542 (61.4) | 939 (58.1) | 603 (67.5) |
| 358 (55.7) | 362 (56.3) |
|
|
None |
| 4 (0.2) | 0 (0.0) |
|
|
|
|
| Surgical
approach |
|
|
| <0.001 |
|
| 0.631 |
|
McKeown | 1,779 (70.9) | 1,009 (62.4) | 770 (86.2) |
| 528 (82.1) | 534 (83.0) |
|
|
Iovr-Lewis | 691 (27.5) | 583 (36.1) | 108 (12.1) |
| 109 (17.0) | 106 (16.5) |
|
|
Sweet | 4 (0.2) | 4 (0.2) | 0 (0.0) |
|
|
|
|
| Left thoracotomy
and laparotomy | 36 (1.4) | 21 (1.3) | 15 (1.7) |
| 6 (0.9) | 3 (0.5) |
|
| Clinical treatment
modality |
|
|
| <0.001 |
|
| 0.939 |
|
Preoperative CT or | 46 (1.8) | 24 (1.5) | 22 (2.5) |
| 16 (2.5) | 18 (2.8) |
|
| RT/CRT
plus surgery |
|
|
|
|
|
|
|
| Surgery
alone | 1,269 (50.6) | 687 (42.5) | 582 (65.2) |
| 395 (61.4) | 395 (61.4) |
|
| Surgery
plus postoperative | 1,195 (47.6) | 906 (56.0) | 289 (32.4) |
| 232 (36.1) | 230 (35.8) |
|
| CT or
RT/CRT |
|
|
|
|
|
|
|
OS
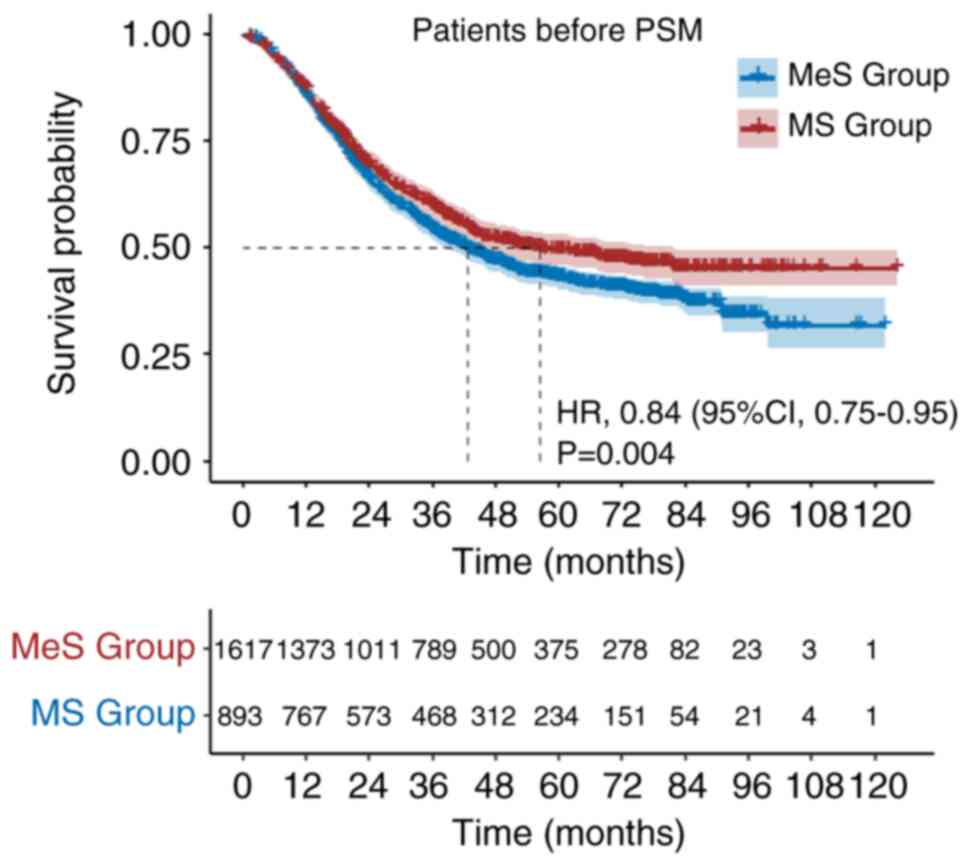
The study included 1,617 (64.4%) patients in the MeS
group and 893 (35.6%) patients in the MS group. The median
follow-up duration was 63.97 months. Patients in the MeS group had
a median OS of 42.67 months (95% CI: 38.55–46.78), while those in
the MS group had a median OS of 56.47 months. The 1-, 3- and 5-year
OS rates were 86, 54 and 43% for patients in the MeS group, and 87,
60 and 50% for patients in the MS group, respectively [Mes vs. MS
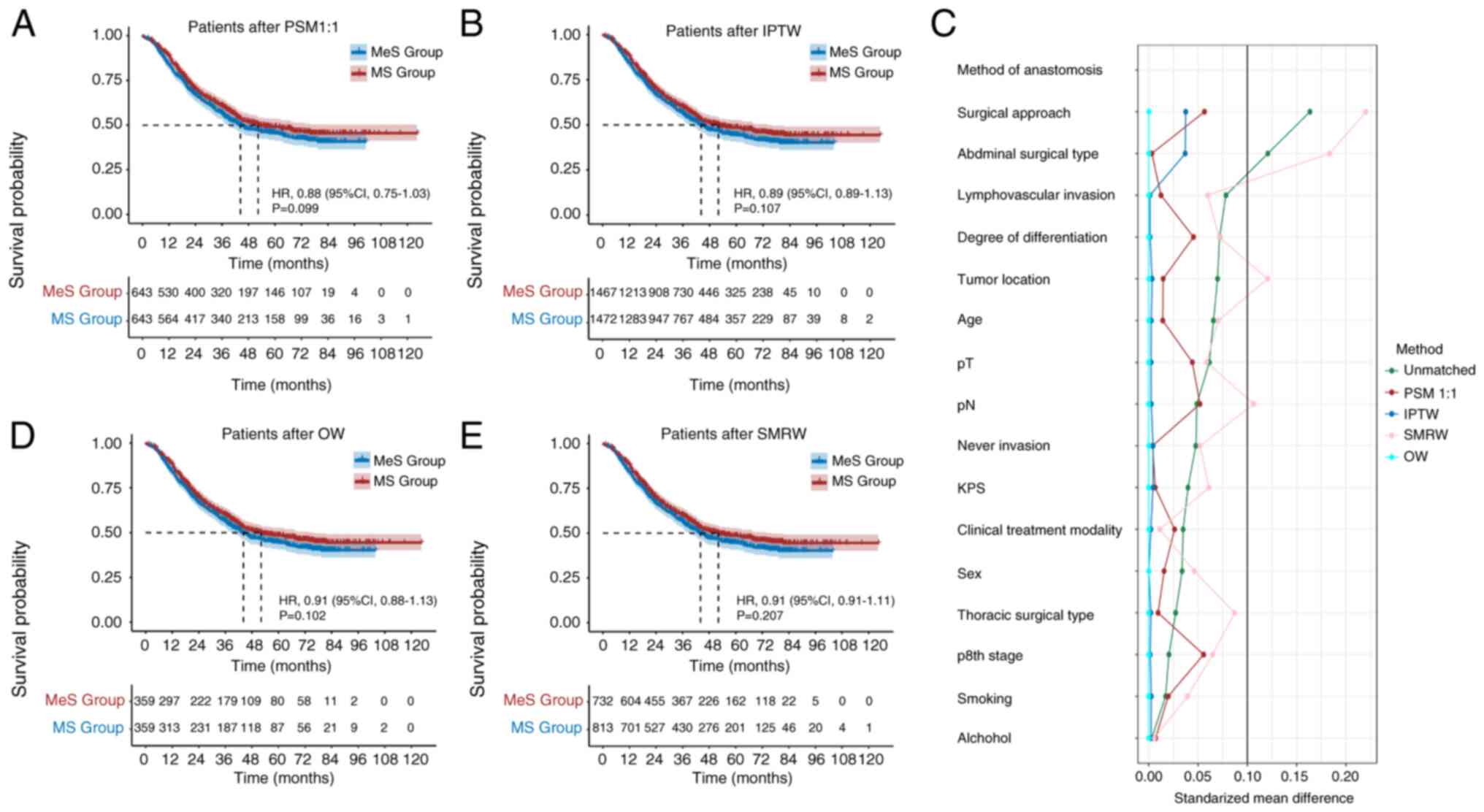
hazard ratio (HR): 0.84; 95% CI: 0.75–0.95; P=0.004; Fig. 2]. Following 1:1 PSM, the analysis
showed no significant differences between the MeS and MS groups
(HR: 0.88; 95% CI: 0.75–1.03; P=0.099; Fig. 3A). Similar results were observed
after applying the IPTW, OW and SMRW methods (HR: 0.89; 95% CI:
0.89–1.13; P=0.107; Fig. 3B/HR:
0.91; 95% CI: 0.88–1.13; P=0.102; Fig.
3D and HR: 0.91; 95% CI: 0.91–1.11; P=0.207; Fig. 3E). Fig.
3C illustrates that for the 1:1 PSM, IPTW and OW methods, the
standardized mean difference for all variables is <0.1.
Risk factors
Factors significantly affecting OS after
esophagectomy were identified through univariate analyses. These
factors included drinking (P<0.01), smoking (P<0.01), age
(P<0.01), Karnofsky performance status (KPS) scores (P<0.01),
sex (P<0.01), thoracic surgical type (P<0.01), abdominal
surgical type (P=0.02), surgical approach (P<0.01), tumor grade
(P<0.01), lymphovascular invasion (P<0.01), nerve invasion
(P<0.01), method of anastomosis (P<0.01), pathological T
category (P<0.01), pathological N category (P<0.01) and TNM
stage according to the eighth edition (P<0.01; Fig. 4). Multivariate analyses indicated
that drinking (P<0.01), age (P<0.01), sex (P=0.03), method of
anatomosis (P=0.03), lymphovascular invasion (P=0.04), pathological
T3 category (P=0.06), pathological N2 category (P<0.01) and
pathological N3 category (P=0.03) were independent influencing
factors for OS after esophagectomy compared with other factors
(Fig. 4).
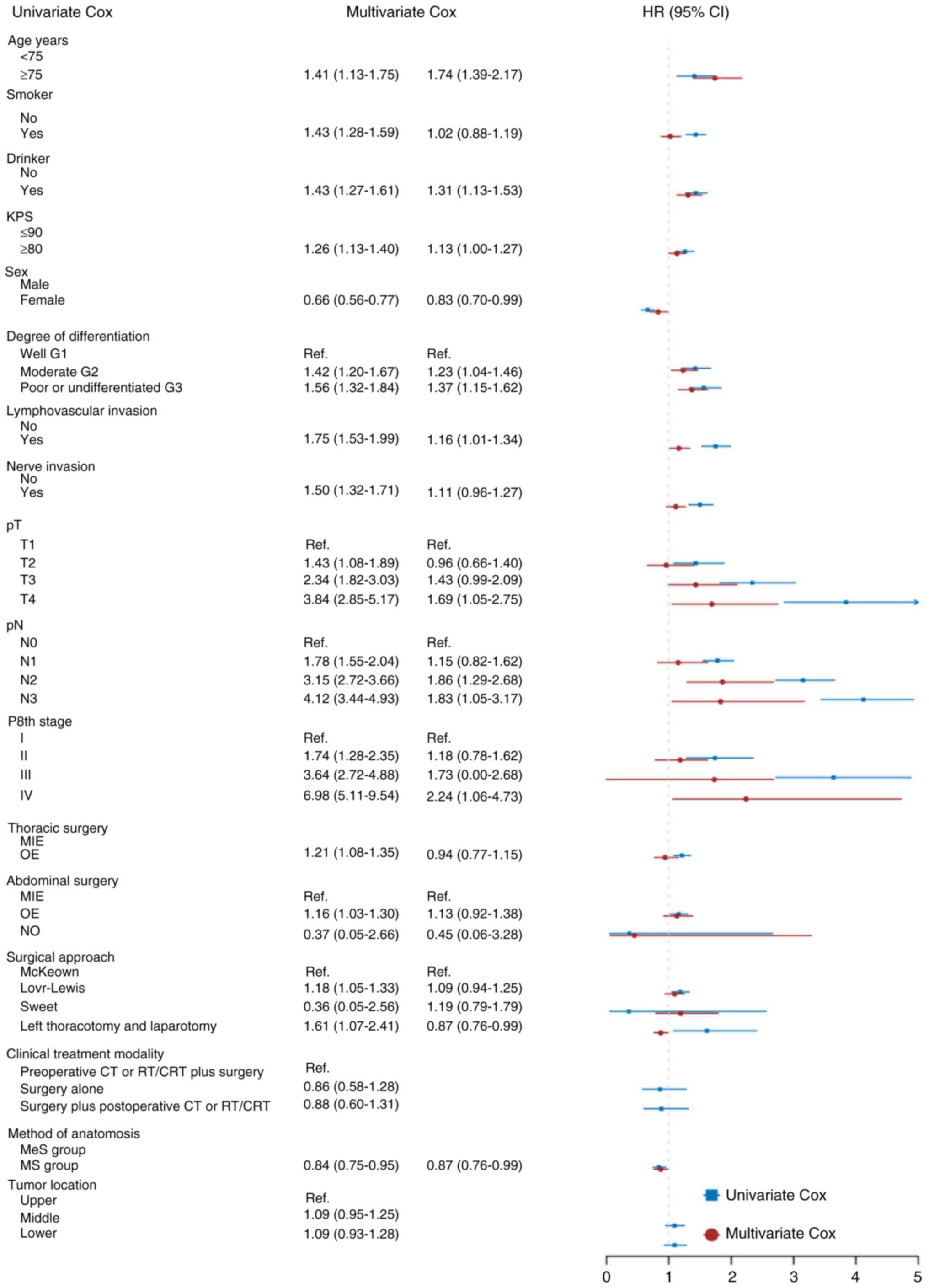 | Figure 4.Univariate and multivariate Cox
regression analyses regarding factors affecting patient survival.
OS, overall survival; MS, manual suturing; MeS, mechanical
suturing; HR, hazard ratio; KPS, Karnofsky performance status; CRT,
chemoradiotherapy; CT, chemotherapy; MIE, minimally invasive
esophagectomy; OE, open esophagectomy; RT, radiotherapy. |
Discussion
The impact of mechanical vs. manual suturing
techniques on OS in patients undergoing esophagectomy is a critical
consideration. In the present study, initially, patients in the MS
group showed a superior median OS compared to the MeS group,
suggesting a significant influence of the suturing technique on
postoperative outcomes. However, subsequent analysis following PSM
revealed that the differences between groups were not statistically
significant. This implies that when matched for similar baseline
characteristics, the choice of suturing technique may not be as
crucial for OS as previously thought. The similarity in survival
outcomes between MS and MeS provides surgeons with the flexibility
to select a technique based on other factors, such as their
proficiency, patient-specific anatomical or physiological
considerations, and resource availability. Further investigation
through univariate and multivariate analyses highlights other
factors that have a more substantial impact on survival outcomes.
Lifestyle factors like the drinking and smoking status, as well as
clinical indicators, such as age, KPS scores, sex, tumor grade and
the presence of lymphovascular and nerve invasion, emerge as
significant predictors of OS. These results were also confirmed in
corresponding studies and emphasized the multifaceted nature of
survival following esophagectomy (26), where the surgical technique is just
one of several variables influencing patient outcomes.
The ongoing discussion and evolution of treatment
strategies for esophageal cancer emphasize a comprehensive approach
that combines surgery with various adjuvant therapies to improve
patient outcomes (26–29). Surgical resection remains crucial in
esophageal cancer treatment, but the complexity of the disease
necessitates a comprehensive strategy involving neoadjuvant
chemoradiotherapy, chemotherapy, combined chemotherapy and
immunotherapy, as well as postoperative immunotherapy maintenance
(7,29–32).
The debate among surgical experts revolves around
the extent of LN dissection and the decision on thoracic duct
resection, both crucial in treating esophageal cancer (33–35).
LN involvement is a key factor in predicting adverse outcomes
(4,5). The present study showed that there is
no statistically significant difference in long-term survival
between traditional manual anastomosis and mechanical anastomosis,
suggesting both are viable options. The choice should be
personalized based on the patient's condition and the surgeon's
skill. As we delve deeper into treatment combinations, surgical
techniques like LN dissection and thoracic duct management will
continue to play a pivotal role. Future research should focus on
not just the technical aspects of esophageal cancer surgery but
also on integrating these techniques with other treatments to
enhance survival, quality of life and patient-centered
outcomes.
There are several limitations that should be
acknowledged in the present study. The retrospective design of the
study, despite using propensity score matching to minimize bias,
inherently limits the ability to establish causality. Prospective
randomized controlled trials are necessary to definitively compare
the efficacy and safety of MS and MeS techniques. The data were
collected from a single institution, which may limit the
generalizability of the findings. Multi-center studies could
provide a more representative overview of outcomes and potentially
validate the applicability of these results across different
surgical settings and populations. The study focused on OS as the
primary endpoint, neglecting other important outcomes such as
post-operative recovery, quality of life and complication rates.
Furthermore, while the significance of preoperative neoadjuvant
therapy, particularly for patients with T2/T3N0-1 ESCC, has been
highlighted in studies like CROSS (8) and NEOCRTEC5010As the founder of the
Department of Thoracic Surgery, he gave guidance and suggestions
for the design and data collection of this study, and also gave
guidance on each clinical technical issue at the discussion
meeting
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural
Science Foundation of China (grant no. 82472663), the National Key
Research and Development Program (grant no. 2022YFC2403400),
International Cooperation Projects of the Science and Technology
Department of Sichuan Province (grant no. 2024YFHZ0322), the
Sichuan Key Research and Development Project from the Science and
Technology Department of Sichuan Province (grant nos. 2023YFS0044,
2023YFQ0056 and 2022YFQ0008), the Chengdu Science and Technology
Bureau Key Research Project (grant no. 2024-YF05-00797-SN), the Wu
Jieping Clinical Research Projects (grant no. 320.6750.2023-05-141)
and the Sichuan Province Clinical Key Specialty Construction
Project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Study conception and design: SLu, KL, LJ, JX, SLi,
ZW, HC, WH, CW, KW, HL, QZ, HZ, QF, QW, YH, LP and XL. Acquisition,
analysis or interpretation of data: SLu, KL, LJ, JX, SLi, ZW, HC,
WH, CW, KW, HL, QZ, HZ, QF, QW, YH, LP and XL. Drafting of the
article: KL. Revising the article critically for important
intellectual content: SLu, KL, LJ, JX, SLi, ZW, HC, WH, CW, KW, HL,
QZ, HZ, QF, QW, YH, LP and XL. Statistical analysis: SLu. Obtained
funding: XL. Administrative, technical or material support: YH and
LP. Study supervision: XL, LP. SLu and XL confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in this study involving
human participants were in accordance with the Declaration of
Helsinki (as revised in 2013). The study was approved by the Ethics
Committee for Medical Research and New Medical Technology of
Sichuan Cancer Hospital (Chengdu, China; approval no.
SCCHEC-02-2022-050).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
LN
|
lymph node
|
|
OS
|
overall survival
|
|
TNM
|
tumor-node-metastasis
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
PSM
|
performing propensity score
matching
|
|
KPS
|
Karnofsky performance status
|
|
IPTW
|
inverse probability of treatment
weighting
|
|
OW
|
overlap weighting
|
References
|
1
|
Frick C, Rumgay H, Vignat J, Ginsburg O,
Nolte E, Bray F and Soerjomataram I: Quantitative estimates of
preventable and treatable deaths from 36 cancers worldwide: A
population-based study. Lancet Glob Health. 11:e1700–e1712. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li K, Nie X, Li C, He W, Wang C, Du K, Li
K, Liu K, Li Z, Lu S, et al: Mapping of lymph node metastasis and
efficacy index in thoracic esophageal squamous cell carcinoma: A
large-scale retrospective analysis. Ann Surg Oncol. 30:5856–5865.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tachimori Y, Ozawa S, Numasaki H,
Matsubara H, Shinoda M, Toh Y, Udagawa H, Fujishiro M, Oyama T and
Uno T; Registration Committee for Esophageal Cancer of the Japan
Esophageal Society, : Efficacy of lymph node dissection by node
zones according to tumor location for esophageal squamous cell
carcinoma. Esophagus. 13:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li K, Leng X, He W, Du K, Li C, Liu K,
Wang C, Liu G, Li Z, Jiang L, et al: Resected lymph nodes and
survival of patients with esophageal squamous cell carcinoma: an
observational study. Int J Surg. 109:2001–2009. 2023.PubMed/NCBI
|
|
7
|
Kelly RJ, Ajani JA, Kuzdzal J, Zander T,
Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre
A, et al: Adjuvant nivolumab in resected esophageal or
gastroesophageal junction cancer. N Engl J Med. 384:1191–1203.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shapiro J, van Lanschot JJB, Hulshof MCCM,
van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven
HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al:
Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for
oesophageal or junctional cancer (CROSS): Long-term results of a
randomised controlled trial. Lancet Oncol. 16:1090–1098. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H,
Gong L, Liu H, Tian F, Lu Q, et al: Tislelizumab combined with
chemotherapy as neoadjuvant therapy for surgically resectable
esophageal cancer: A prospective, single-arm, phase II study
(TD-NICE). Int J Surg. 103:1066802022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Neoadjuvant
chemoradiotherapy followed by surgery versus surgery alone for
locally advanced squamous cell carcinoma of the esophagus
(NEOCRTEC5010): A phase III multicenter, randomized, open-label
clinical trial. J Clin Oncol. 36:2796–2803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alwatari Y, Freudenberger DC, Khoraki J,
Bless L, Payne R, Julliard WA, Shah RD and Puig CA: Emergent
esophagectomy in patients with esophageal malignancy is associated
with higher rates of perioperative complications but no independent
impact on short-term mortality. J Chest Surg. 57:160–168. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugiyama F, Kanda M, Shimizu D, Umeda S,
Inokawa Y, Hattori N, Hayashi M, Tanaka C, Nakayama G and Kodera Y:
Absence of hypercoagulation status after neoadjuvant treatment is
associated with favorable prognosis in patients undergoing subtotal
esophagectomy for esophageal squamous cell carcinoma. Ann Surg
Oncol. 31:3417–3425. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Zijden CJ, van der Sluis PC,
Mostert B, Nuyttens JJME, Spaander MCW, Toxopeus ELA, Valkema R,
Beerepoot LV, van Halteren HK, Lagarde SM and Wijnhoven BPL:
Induction chemotherapy followed by response evaluation and
esophagectomy for advanced esophageal cancer. Eur J Surg Oncol.
50:1079682024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ercan S, Rice TW, Murthy SC, Rybicki LA
and Blackstone EH: Does esophagogastric anastomotic technique
influence the outcome of patients with esophageal cancer? J Thorac
Cardiovasc Surg. 129:623–631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugimura K, Miyata H, Matsunaga T, Asukai
K, Yanagimoto Y, Takahashi Y, Tomokuni A, Yamamoto K, Hirofumi A,
Nishimura J, et al: Comparison of the modified Collard and
hand-sewn anastomosis for cervical esophagogastric anastomosis
after esophagectomy in esophageal cancer patients: A propensity
score-matched analysis. Ann Gastroenterol Surg. 3:104–113. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Wei Y, Liu X, Li Z, Zhu G, Li Y
and Wang K: Application value of hand-sewn anastomosis in totally
laparoscopic total gastrectomy for gastric cancer. World J Surg
Oncol. 19:2292021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Wang D, Zhao Q, Yang P, Ding P,
Fan H, Dong T, Liu Z, Yang X, Ren L and Li Y: A case series of 10
patients undergone linear cutter/stapler guiding device-led
overlapped esophagojejunostomy: A preliminary study. J Gastrointest
Oncol. 14:617–625. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrin G, Ruol A, Battaglia G, Buin F,
Merigliano S, Constantini M, Pavei P, Cagol M, Scappin S and Ancona
E: Anastomotic stenoses occurring after circular stapling in
esophageal cancer surgery. Surg Endosc. 14:670–674. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orringer MB, Marshall B and Iannettoni MD:
Eliminating the cervical esophagogastric anastomotic leak with a
side-to-side stapled anastomosis. J Thorac Cardiovasc Surg.
119:277–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kondra J, Ong SRY, Clifton J, Evans K,
Finley RJ and Yee J: A change in clinical practice: A partially
stapled cervical esophagogastric anastomosis reduces morbidity and
improves functional outcome after esophagectomy for cancer. Dis
Esophagus. 21:422–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singhal S, Kailasam A, Akimoto S, Masuda
T, Bertellotti C and Mittal SK: Simple technique of circular
stapled anastomosis in Ivor Lewis esophagectomy. J Laparoendosc Adv
Surg Tech A. 27:288–294. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh D, Maley RH, Santucci T, Macherey
RS, Bartley S, Weyant RJ and Landreneau RJ: Experience and
technique of stapled mechanical cervical esophagogastric
anastomosis. Ann Thorac Surg. 71:419–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang C, Zhao J, Liu Z, Huang J and Zhu Z:
Esophageal suspension method for hand-sewn esophagojejunostomy
after totally laparoscopic total gastrectomy: A simple, safe, and
feasible suturing technique. Front Oncol. 10:5752020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sannohe Y, Hiratsuka R, Doki K, Inutsuka S
and Hirano M: Mechanical suture methods in
Esophago-gastrointestinal anastomosis. Jpn J Surg. 9:313–321. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et
al: Esophageal and esophagogastric junction cancers, version
2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr
Canc Netw. 17:855–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li K, Lu S, Li C, He W, Du K, Liu K, Wang
C, Li J, Wang Z, Zhou Y, et al: Long-Term outcomes of smoker and
drinker with oesophageal squamous cell carcinoma after
oesophagectomy: A Large-scale propensity score matching analysis.
BMJ Open Gastroenterol. 11:e0014522024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kitagawa Y, Ishihara R, Ishikawa H, Ito Y,
Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, et al:
Esophageal cancer practice guidelines 2022 edited by the Japan
Esophageal Society: Part 1. Esophagus. 20:343–372. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitagawa Y, Ishihara R, Ishikawa H, Ito Y,
Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, et al:
Esophageal cancer practice guidelines 2022 edited by the Japan
Esophageal Society: Part 2. Esophagus. 20:373–389. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C, Zhao S, Zheng Y, Han Y, Chen X,
Cheng Z, Wu Y, Feng X, Qi W, Chen K, et al: Preoperative
pembrolizumab combined with chemoradiotherapy for oesophageal
squamous cell carcinoma (PALACE-1). Eur J Cancer. 144:232–241.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu YK, Meng FY, Wei XF, Chen XK, Li HM,
Liu Q, Li CJ, Xie HN, Xu L, Zhang RX, et al: Neoadjuvant
chemotherapy combined with immunotherapy versus neoadjuvant
chemoradiotherapy in patients with locally advanced esophageal
squamous cell carcinoma. J Thorac Cardiovasc Surg. 168:417–428.e3.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou Y, He W, Guo P, Zhou C, Luo M and Liu
Y, Yang H, Qin S, Leng X, Huang Z and Liu Y: Development and
validation of a recurrence-free survival prediction model for
locally advanced esophageal squamous cell carcinoma with
neoadjuvant chemoradiotherapy. Ann Surg Oncol. 31:178–191. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He W, Leng X, Mao T, Luo X, Zhou L, Yan J,
Peng L, Fang Q, Liu G, Wei X, et al: Toripalimab plus paclitaxel
and carboplatin as neoadjuvant therapy in locally advanced
resectable esophageal squamous cell carcinoma. Oncologist.
27:e18–e28. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li K, Du K, Liu K, Nie X, Li C, He W, Li
K, Wang C, Li Z, Zheng K, et al: Impact of two-field or three-field
lymphadenectomy on overall survival in middle and lower thoracic
esophageal squamous cell carcinoma: A single-center retrospective
analysis. Oncol Lett. 25:1892023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li B, Zhang Y, Miao L, Ma L, Luo X, Zhang
Y, Ye T, Li H, Zhang J, Li Y, et al: sophagectomy with three-field
versus two-field lymphadenectomy for middle and lower thoracic
esophageal cancer: Long-term outcomes of a randomized clinical
trial. J Thorac Oncol. 16:310–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oshikiri T, Numasaki H, Oguma J, Toh Y,
Watanabe M, Muto M, Kakeji Y and Doki Y: Prognosis of patients with
esophageal carcinoma after routine thoracic duct resection: A
propensity-matched analysis of 12,237 patients based on the
comprehensive registry of esophageal cancer in Japan. Ann Surg.
277:e1018–e1025. 2023. View Article : Google Scholar : PubMed/NCBI
|