Introduction
Colorectal cancer (CRC), including colon cancer and
rectal cancer, is one of the major cancers that threaten the life
and health of Chinese residents, causing a heavy social burden.
According to the national cancer statistics released by China
National Cancer Center in March 2024, from 2000 to 2018, the
incidence rate of male CRC in China will increase by 2.7% annually,
the incidence rate of female CRC will increase by 1.1% annually,
the male mortality rate will increase by 1.2% annually, and the
female mortality rate will be basically flat, indicating that the
overall incidence rate and mortality rate of CRC in China will show
a significant upward trend (1).
According to the clinical practice guidelines of the European
Society for Medical Oncology (ESMO) (2), rectal cancer can be classified into
very low-, low-, medium-, high- and very high-risk groups according
to the tumor-node (TN) stage, depth of submucosal invasion (SM),
tumor location, mesenteric fascia (MRF) involvement, external
vascular invasion (EMVI) and lymph node metastasis. Different
treatment methods are used for the different risk groups. The very
low-risk and low-risk groups are mainly treated with transanal
endoscopic microsurgery and total mesorectal excision (TME),
respectively. Direct TME surgery is the standard treatment for the
medium-risk group, which may be followed by radiotherapy or
chemotherapy based on the TME surgery quality and local recurrence
rate. Patients with high-risk rectal cancer require short- or
long-term radiation therapy or chemotherapy before undergoing
surgery. Long-term neoadjuvant chemoradiotherapy is required before
surgery in patients with very high-risk rectal cancer (2,3).
Therefore, accurate risk stratification of rectal cancer before
surgery is essential for selecting appropriate clinical treatment
methods. At present, there have been some studies on preoperative
evaluation of lymph node metastasis, tumor infiltration depth, and
EMVI using magnetic resonance imaging (MRI), but the results vary
and its benefit is controversial (4–6).
MRI is commonly used for the preoperative evaluation
of rectal cancer and as an additional biomarker that reflects the
tumor microstructure (5).
Diffusion-weighted imaging (DWI) and the apparent diffusion
coefficient can be used to evaluate benign or malignant tumors,
tumor composition and biological behavior (6). Intravoxel incoherent motion (IVIM) is
a form of DWI that simultaneously quantifies both pure water
molecular- and perfusion-related diffusion information with
multiple b-values (the b-value is a factor that reflects the
strength and timing of the gradients used to generate
diffusion-weighted images) (7).
Dynamic contrast-enhanced MRI (DCE-MRI) is a novel imaging
technique used to assess tumor vascular status, provide data on
tumor aggressiveness and the degree of angiogenesis, and aid the
restaging of rectal cancer (8).
DCE-MRI is used to assess the morphological and hemodynamic
information of tumors and to indirectly reflect the formation of
blood vessels in tumor cells (9).
Functional MRI, including IVIM and DCE-MRI, are the focus of recent
research, with a small number of studies on rectal cancer (10,11).
The accuracy of high-resolution T2 weighted imaging (WI) combined
with DCE-MRI in evaluating the mrT staging (magnetic resonance T
staging) of rectal cancer after neoadjuvant therapy (80.60%) is
higher than that of high-resolution T2 weighted imaging and
high-resolution T2 weighted imaging combined with DWI, which is
highly consistent with pathological T staging (12). A histogram of DCE-MRI parameters can
facilitate the preoperative identification of EMVI in rectal cancer
(13). IVIM and diffusion kurtosis
imaging (DKI) can provide microstructural information of cancer,
such as blood vessels and cells, and have the potential to
accurately grade cancer (14).
Furthermore, IVIM can be used to evaluate the efficacy of
chemoradiotherapy in rectal cancer (15).
However, the use of IVIM and DCE-MRI for
preoperative risk stratification of rectal adenocarcinoma has been
limited to a small number of isolated studies (16,17),
and a consistent conclusion has not been reached. Therefore, the
present study aimed to combine the quantitative parameters of
DCE-MRI and IVIM for preoperative risk stratification of rectal
adenocarcinoma and to analyze the diagnostic potential of the
parameters.
Materials and methods
Participants
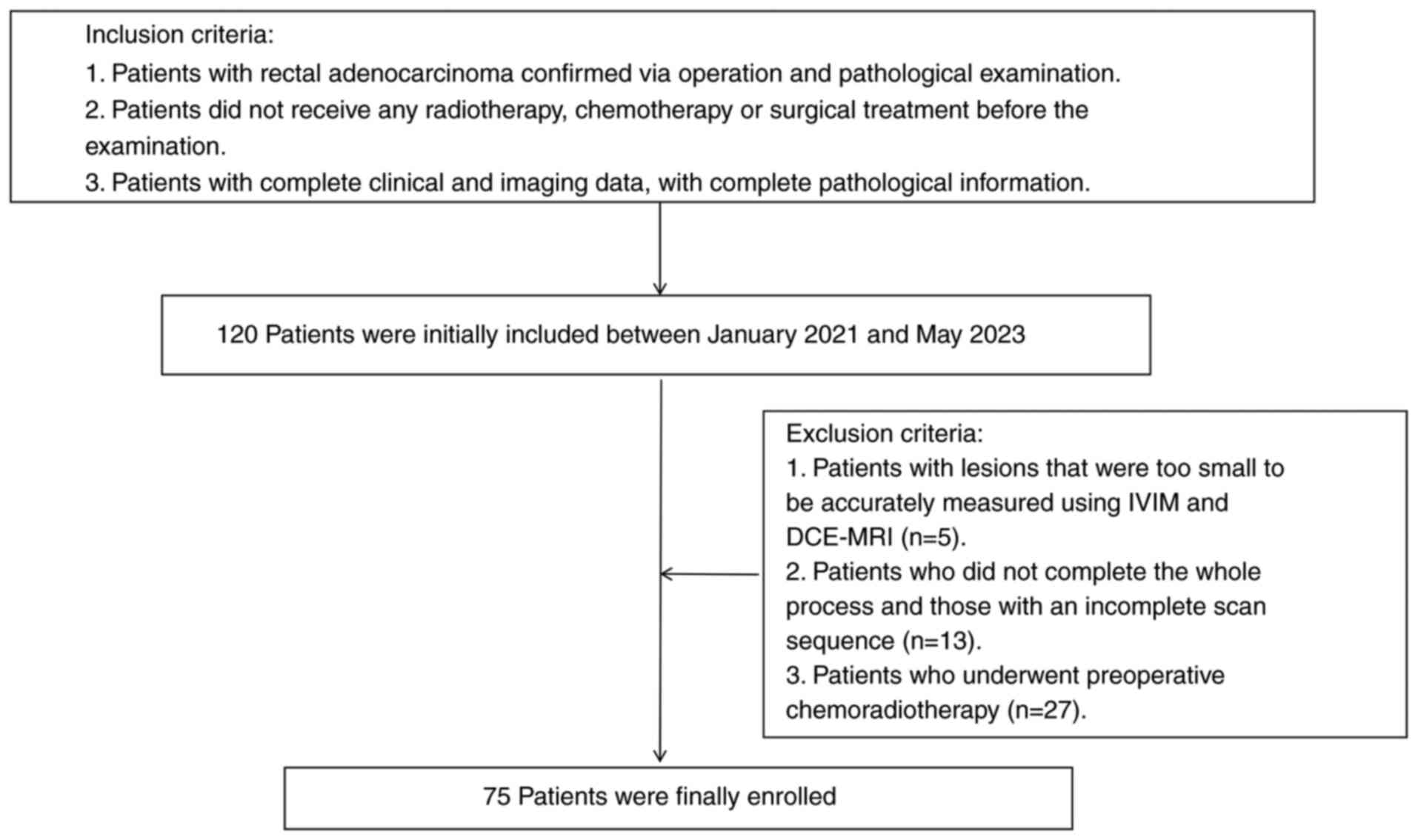
A total of 120 patients with rectal adenocarcinoma
who were admitted to Xinxiang Central Hospital (Xinxiang, China)
between January 2021 and May 2023 were retrospectively enrolled in
the present study. The inclusion criteria were as follows: ⅰ)
Patients with rectal adenocarcinoma confirmed via operation and
pathological examination; ⅱ) patients who did not receive any
radiotherapy, chemotherapy or surgical treatment before the
examination; and ⅲ) patients with complete clinical and imaging
data, with complete pathological information. The exclusion
criteria were as follows: ⅰ) Patients with lesions that were too
small to be accurately measured using IVIM and DCE-MRI (n=5); ⅱ)
patients who did not complete the whole process and those with an
incomplete scan sequence (n=13); and ⅲ) patients who underwent
preoperative chemoradiotherapy (n=27). Finally, 75 patients were
enrolled (Fig. 1). The present
study was approved by the Xinxiang Central Hospital Ethics
Committee (approval no. 2020-098; Xinxiang, China) and all patients
provided written informed consent.
MRI technique and methods
The patients were provided with a liquid diet the
day before the examination, and their intestines were cleared as
required on the examination day. The patients were administered 20
mg of raceanisodamine hydrochloride (Suicheng Pharmaceutical Co.,
Ltd.) intramuscularly 5–10 min before the examination to suppress
intestinal movement. The 3.0T MR scanner (SIGNA™ Pioneer; GE
Healthcare) with a 16-channel phased coil was used. The patients
were placed in a supine position with the head in front and the
magnetic field center was at the superior margin of the pubis. The
scanning sequences included periodically rotated overlapping
parallel lines with enhanced reconstruction (propeller) T2WI, T1WI,
DWI, IVIM and DCE-MRI. The oblique-axis scanning plane was
perpendicular to the long axis of the bowel, where the tumor was
located. For IVIM, 11 groups of b-values (0, 30, 50, 80, 100, 200,
400, 600, 800, 1,200 and 2,000 sec/mm2) were selected
(18–20). The liver acceleration volume
acquisition sequence was used for the DCE-MRI. First, the image was
scanned as a mask, and in five phases, gadolinium
diethylenetriamine pentaacetic acid was injected through the elbow
vein at a flow rate of 3.5 ml/sec at a dose of 0.1 mmol/kg.
Finally, 20 ml of normal saline was flushed into the tube. The
detailed sequence parameters are listed in Table I.
 | Table I.MRI acquisition parameters. |
Table I.
MRI acquisition parameters.
| Parameters | Propeller T2WI | Propeller T1WI | DWI | IVIM | DCE-MRI |
|---|
| TR/TE, msec | 4,729/92.1 | 4,709/91.0 | 4421/70 | 5,000/70 | 2.9/1.2 |
| Field of view,
mm2 | 200×200 | 200×200 | 240×240 | 260×260 | 380×380 |
| Slice thickness,
mm | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| b-values,
sec/mm2 | N/A | N/A | 1,000 | 0, 30, 50, 80, 100,
200, 400, 600, 800, 1,200 and 2,000 | N/A |
| Fat
suppression | No | No | No | No | Yes |
Image analysis and index
measurement
The original DCE-MRI images were transferred to a
post-processing workstation (GenIQ, version AW 4.7; GE Healthcare)
to obtain pseudocolor maps of the volume transfer constant
(Ktrans), rate constant (Kep) and
extravascular extracellular volume fraction (Ve).
Ktrans reflects the local microvascular blood flow state
and its surface penetration area, Kep reflects the rate
constant between the plasma and extravascular extracellular space
(EES) and Ve reflects the volume fraction of the EES
contrast agent (21); thus,
Kep=Ktrans/Ve.
After the original IVIM images were transferred to
the Medical Imaging Interaction Toolkit software (version 2023.04;
German Cancer Research Center), pseudocolor maps of the true
diffusion coefficient (D), false diffusion coefficient (D*) and
perfusion fraction (f) were obtained. The linear fitting equation
was as follows: Sb/S0=(1-f) exp(−b ×
D) + f exp(−b × D*), where Sb is the
MRI signal intensity with the diffusion gradient, S0 is
the MRI signal intensity without a diffusion gradient and exp is
the exponential function.
Measurements were taken in the largest plane of the
tumor (22) by two double-blinded
radiologists (YXC, 7 years of work experience; YCN, 18 years of
work experience). In total, three different regions were selected
to manually draw three regions of interest (ROI) with similar
areas, avoiding the areas of liquefaction, bleeding and necrosis.
The ROIs were drawn on the D and Ktrans images and
copied to the other two corresponding parameter graphs. Each group
of data was measured three times and the mean value was
obtained.
Pathological grouping
Detailed pathological reports were obtained for all
surgical specimens according to protocols published by the College
of American Pathologists (23), and
all sections including the degree of differentiation, tumor
location, MRF, EMVI, lymph node metastasis and subdivision of T1
cancer according to submucosal invasion depth (SM; SM1, upper 1/3;
SM2, middle 1/3; SM3, lower 1/3) were reviewed by a
gastrointestinal pathologist (XYS, 8 years of work experience; SL,
10 years of work experience), and in case of any dispute, assisted
by a more experienced gastrointestinal pathologist (LY, 18 years of
work experience). In accordance with the clinical practice
guidelines of the ESMO (2), all
included tissue specimens were divided into the following groups:
Very low-risk (pathological T1 staging, SM1 and pathological N0
staging), low-risk [pathological T1-T2 staging, medium/high
(distance between tumor and anal margin: <5 cm low, 5–10 cm
medium, >10 cm high) T3a/b, pathological N0 staging or high
pathological N1 staging, MRF- and EMVI-] and medium-risk
(low/medium/high pathological T3a/b staging, without involvement of
the levator ani muscle, pathological N1-N2 staging, not extranodal,
MRF- and EMVI-) groups. Due to the fact that patients in high- and
very high-risk groups need to receive routine radiotherapy and
chemotherapy before surgery, this study used it as an exclusion
criterion and only included patients with rectal adenocarcinoma who
can be directly resected.
Statistical analysis
SPSS (version 25.0; IBM Corp.) and MedCalc (version
15.2; MedCalc Software, Ltd.) statistical software were used for
analysis. The interclass correlation coefficient was used to
evaluate the consistency of measurement results of the two
radiologists (r ≥0.75, excellent; 0.60≤ r <0.75, good; 0.40≤ r
<0.60, moderate; r <0.40, poor). The measurement data
consistent with a normal distribution and homogeneity of variance
were expressed as the mean ± standard deviation, while data that
were inconsistent were expressed as the median (interquartile
range). The differences in DCE-MRI and IVIM parameters between the
different risk-stratification groups were analyzed using analysis
of variance or the Kruskal-Wallis H-test, followed by multiple
comparisons using the least-significant difference (LSD) or
Bonferroni tests. Spearman's or Pearson's tests (Pearson's test was
used for variables with a normal distribution and Spearman's test
was used for variables without a normal distribution) were used to
analyze the correlations between all parameters and rectal
adenocarcinoma risk stratification groups. Receiver operating
characteristic (ROC) curves were used to analyze diagnostic
efficiency, and the Delong test was used to compare the difference
in the area under the curve (AUC) between the different risk
stratification groups.
Results
Patient clinical and pathological
characteristics
The study included 19 cases (25.33%), 29 cases
(38.67%) and 27 cases (36.00%) in the very low-risk, low-risk and
medium-risk groups, respectively. There were 49 males and 26
females, aged 36–92 years, with a mean age of 66.03±12.33 years. In
the very low-risk group, 6 patients were female (31.57%) and 13
were male (68.42%), aged 48–91 years, with a mean age of
67.89±10.91 years. In the low-risk group, 12 patients were female
(41.38%) and 17 were male (58.62%), aged 36–92 years, with a mean
age of 64.48±14.19 years. In the medium-risk group, 8 patients were
female (29.63%) and 19 were male (70.37%), aged 38–88 years, with a
mean age of 66.37±11.31 years. There was no significant difference
in the distribution of age and sex (Table II). The clinical features, TN
stages, EMVI, MRF, SM and distance are summarized in Table III. Typical cases from different
risk groups are shown in Fig. 2,
Fig. 3, Fig. 4. A 72 year-old-male patient with
rectal adenocarcinoma, pT3aN1, medium-risk group, is presented in
Fig. 2. A 72 year-old-male patient
with rectal adenocarcinoma, pT2N0, low-risk group, is shown in
Fig. 3. A 54-year-old male patient
with rectal adenocarcinoma, pT1N0, very low-risk group, is
presented in Fig. 4.
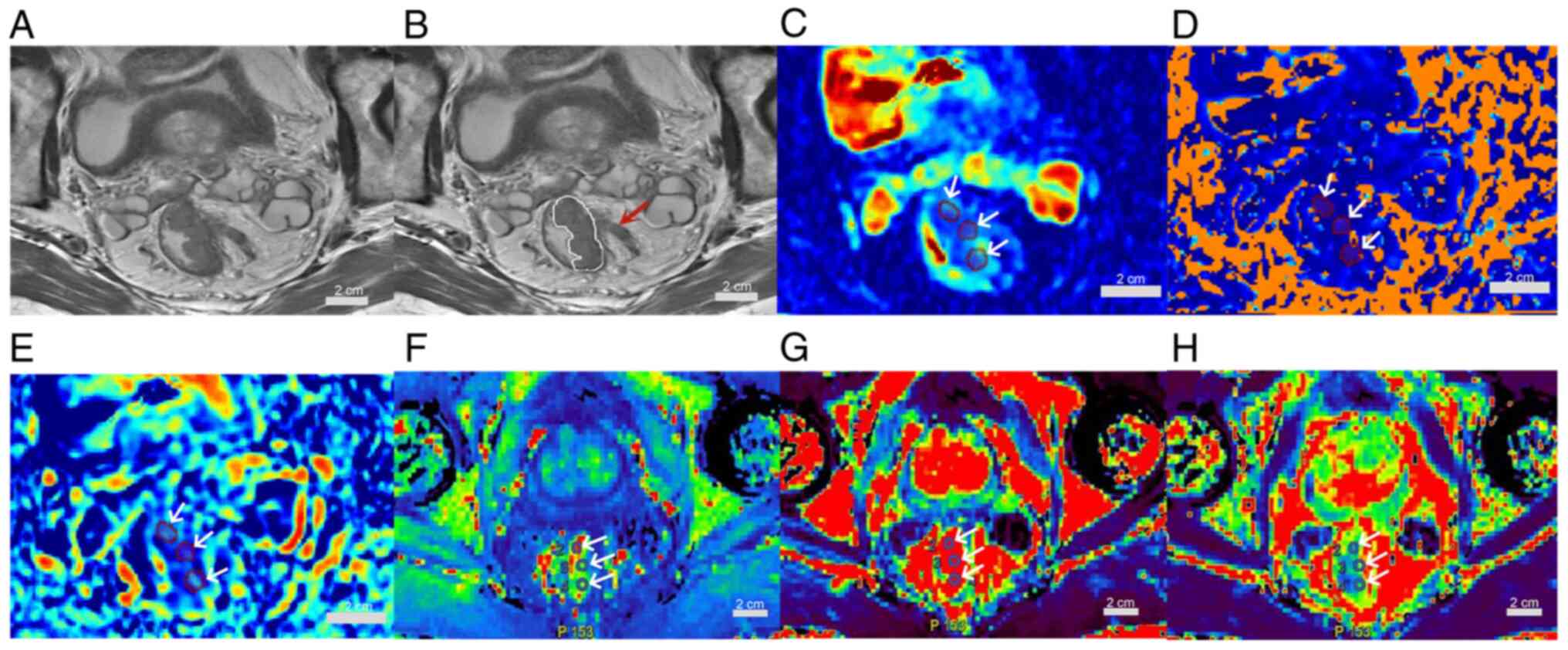 | Figure 2.A 72-year-old male patient with
rectal adenocarcinoma, pT3aN1, medium-risk group. (A) Oblique axial
T2-weighted image shows a mass with slightly high intensity signal
in the rectum. (B) The white line indicates the tumor area; the
lesion breaks through the musculi propria and burrs into the
surrounding adipose space (red arrow). (C) D, (D) D* and (E) f maps
show the mass with mean values of 0.98×10−3
mm2/sec, 8.39×10−3 mm2/sec and
0.23, respectively. (F) Kep, (G) Ktrans and
(H) Ve maps show the mass with mean values of 1.73
1/min, 1.46 1/min and 0.98, respectively (scale bars, 2 cm; white
arrows indicate regions of interest). Ktrans, volume
transfer constant; Kep, rate constant; Ve,
extravascular extracellular volume fraction; D, true diffusion
coefficient; D*, false diffusion coefficient; f, perfusion
fraction; EMVI, external vascular invasion; -, negetive. |
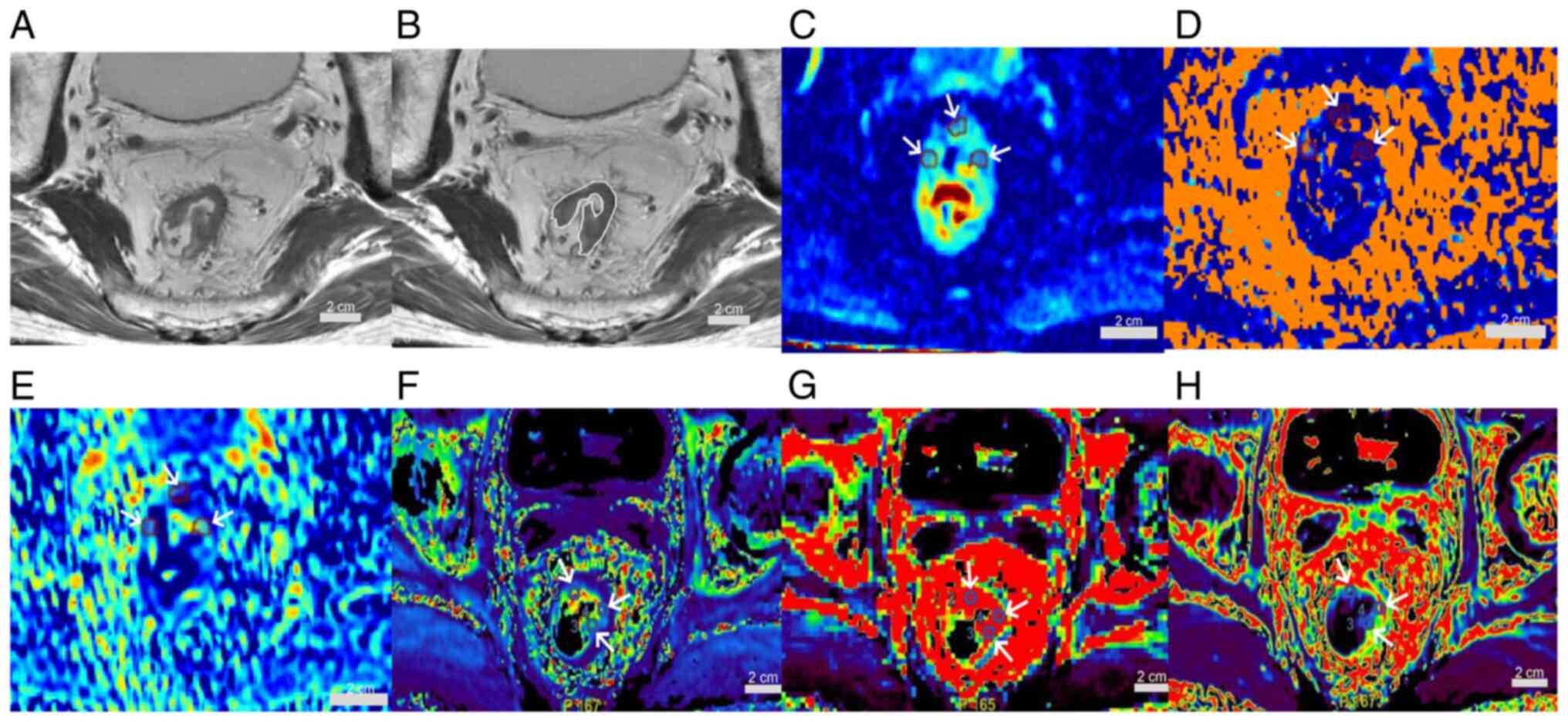 | Figure 3.A 72-year-old male patient with
rectal adenocarcinoma, pT2N0, low-risk group. (A) Oblique axial
T2-weighted image shows a mass with slightly high intensity signal
in the rectum. (B) The white line indicates the tumor area. (C) D,
(D) D*and (E) f maps show the mass with mean values of
0.51×10−3 mm2/sec, 7.53×10−3
mm2/sec and 0.11, respectively. (F) Kep, (G)
Ktrans and (H) Ve maps show the mass with
mean values of 1.41 1/min, 0.83 1/min and 0.37, respectively (scale
bars, 2 cm; white arrows indicate regions of interest).
Ktrans, volume transfer constant; Kep, rate
constant; Ve, extravascular extracellular volume
fraction; D, true diffusion coefficient; D*, false diffusion
coefficient; f, perfusion fraction. |
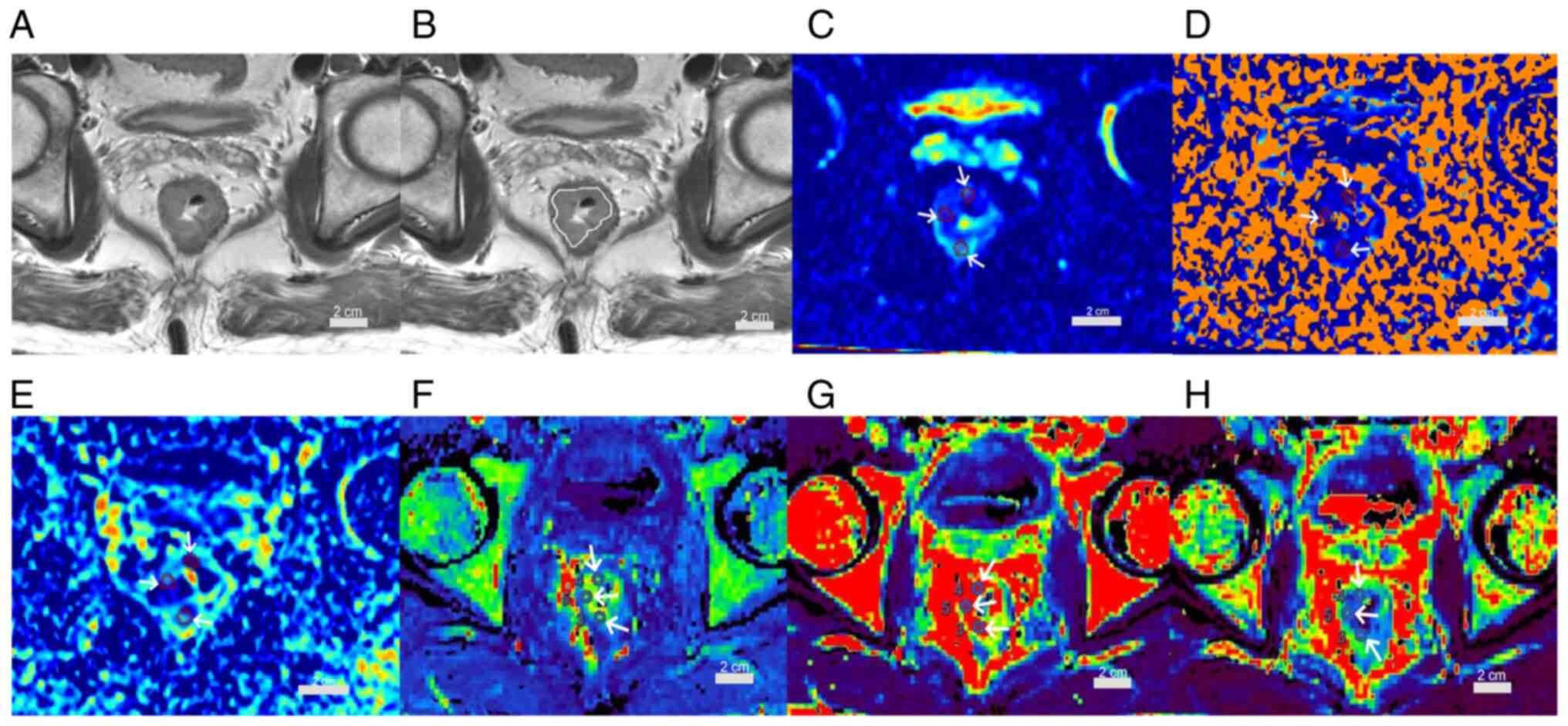 | Figure 4.A 54-year-old male patient with
rectal adenocarcinoma, pT1N0, very low-risk group. (A) Oblique
axial T2-weighted image shows a mass with slightly high intensity
signal in the rectum. (B) The white line indicates the tumor area.
(C) D, (D) D*and (E) f maps show the mass with mean values of
0.48×10−3 mm2/sec, 4.99×10−3
mm2/sec and 0.09, respectively. (F) Kep, (G)
Ktrans and (H) Ve maps show the mass with
mean values of 0.77 1/min, 0.25 1/min and 0.33, respectively (scale
bars, 2 cm; white arrows indicate regions of interest).
Ktrans, volume transfer constant; Kep, rate
constant; Ve, extravascular extracellular volume
fraction; D, true diffusion coefficient; D*, false diffusion
coefficient; f, perfusion fraction. |
 | Table II.Comparison of age and sex
distribution differences among different risk stratification
groups. |
Table II.
Comparison of age and sex
distribution differences among different risk stratification
groups.
|
| Age | Sex |
|---|
|
|
|
|
|---|
| Groups | Mean difference
(I-J) | P | Mean difference
(I-J) | P |
|---|
| Very low- vs.
low-risk | 3.412 | 0.355 | 0.098 | 0.494 |
| Low- vs.
medium-risk | −1.888 | 0.572 | −0.117 | 0.366 |
| Very low- vs.
medium-risk | −1.524 | 0.683 | −0.019 | 0.893 |
| F | 0.449 |
| 0.466 |
|
| P | 0.640 |
| 0.629 |
|
 | Table III.Clinical and pathologic
characteristics of the study participants. |
Table III.
Clinical and pathologic
characteristics of the study participants.
| Characteristic | Value |
|---|
| Sex |
|
|
Male | 49 (65.33) |
|
Female | 26 (34.67) |
| Age, years |
|
| Mean ±
standard deviation | 66.03±12.33 |
|
Range | 36-92 |
| Pathology |
|
| Rectal
adenocarcinoma | 75 (100.00) |
| T stage |
|
| T1 | 20 (26.67) |
| T2 | 28 (37.33) |
|
T3a/b | 27 (36.00) |
| N stage |
|
| N0 | 41 (54.67) |
| N1 | 19 (25.33) |
| N2 | 15 (20.00) |
| EMVI- | 75 (100.00) |
| MRF- | 75 (100.00) |
| SM (T1) |
|
| Upper
1/3 | 19 (95.00) |
| Middle
1/3 | 0 (0.00) |
| Lower
1/3 | 1 (5.00) |
| Distance |
|
| Very
low | 13 (17.33) |
|
Medium | 46 (61.33) |
|
High | 16 (21.33) |
Intraclass correlation coefficient
test results
The Ve, Kep,
Ktrans, f, D and D*-values measured by two radiologists
showed excellent consistency, with values of 0.981, 0.968, 0.920,
0.917, 0.912, and 0.862, respectively (Table IV). The data obtained by senior
physicians were used in the present study.
 | Table IV.ICC test for measuring IVIM and DCE
parameters by two radiologists. |
Table IV.
ICC test for measuring IVIM and DCE
parameters by two radiologists.
|
|
| 95% Confidence
Interval |
|
|---|
| Parameter | Intraclass
correlation |
|
|
|---|
| Lower | Upper | P |
|---|
| Ve | 0.981 | 0.971 | 0.988 | <0.001 |
| Kep | 0.968 | 0.950 | 0.980 | <0.001 |
|
Ktrans | 0.920 | 0.877 | 0.949 | <0.001 |
| f | 0.917 | 0.872 | 0.947 | <0.001 |
| D | 0.912 | 0.875 | 0.948 | <0.001 |
| D* | 0.862 | 0.820 | 0.924 | <0.001 |
Comparison of DCE-MRI parameters among
the risk stratification groups
The differences in Kep-values among the
very low-risk, low-risk and medium-risk groups were statistically
significant (P=0.037, P<0.05), whereas there were no significant
differences in Ve and Ktrans-values (Table V).
 | Table V.Comparison of DCE-MRI parameter
values in different risk stratification groups. |
Table V.
Comparison of DCE-MRI parameter
values in different risk stratification groups.
| Group | Ve | Kep,
1/min | Ktrans,
1/min |
|---|
| Very low-risk | 0.59±0.23 | 1.51±0.89 | 0.92±0.44 |
| Low-risk | 0.59±0.23 | 1.88±0.87 | 0.93±0.44 |
| Medium-risk | 0.62±0.19 | 2.17±0.79 | 1.16±0.63 |
| F | 0.189 | 3.446 | 1.801 |
| P-value | 0.828 | 0.037 | 0.173 |
The Kep-value differences between the
very low- and medium-risk groups were statistically significant
(P=0.01, P<0.05) according to an LSD pairwise comparison,
whereas the differences between the other groups were not
statistically significant.
Comparison of IVIM parameters among
different risk stratification groups
The differences in D* and f-values among the three
groups were statistically significant (P=0.014, 0.042, P<0.05,
respectively), and the values increased with an increase in the
risk grade. There were no statistically significant differences in
D-values among the different risk groups (P=0.929, P>0.05)
(Table VI).
 | Table VI.Comparison of IVIM parameter values
of different risk stratification groups. |
Table VI.
Comparison of IVIM parameter values
of different risk stratification groups.
| Group | f (range) | D, ×10−3
mm2/sec (range) | D*,
×10−3 mm2/sec (range) |
|---|
| Very low-risk | 0.094
(0.07–0.12) | 0.73
(0.53–1.55) | 34.75
(27.77–41.64) |
| Low-risk | 0.125
(0.09–0.15) | 0.83
(0.59–1.14) | 53.81
(24.52–77.57) |
| Medium-risk | 0.131
(0.11–0.17) | 0.82
(0.67–1.04) | 56.59
(34.95–78.05) |
| H-value | 6.344 | 0.147 | 8.501 |
| P-value | 0.042 | 0.929 | 0.014 |
The f-values between the very low- and medium-risk
groups (P=0.044, P<0.05), the D* values between the very low-
and low-risk groups (P=0.0038, P<0.05) and D*-values between the
very low- and medium-risk groups (P=0.004, P<0.05) were
significantly different according to Bonferroni's pairwise
comparison. However, the differences between the other groups were
not statistically significant (P>0.05).
Correlation between DCE-MRI, IVIM
parameters and risk stratification
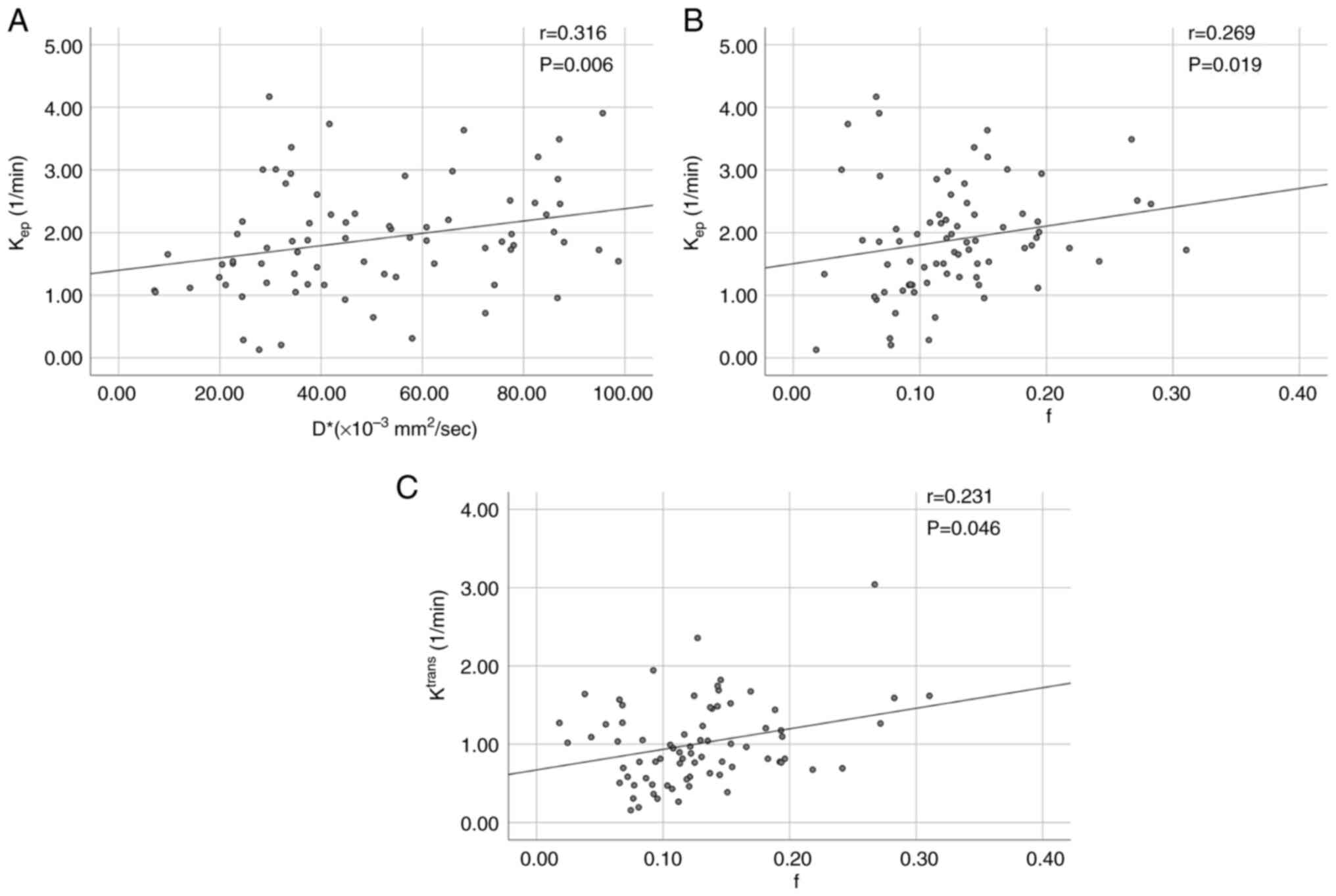
The Kep (r=0.307), f (r=0.270) and D*
(r=0.323) values were all positively correlated with the risk
stratification groups and the D*-value had the highest correlation.
No other significant correlation was found, as shown in Table VII. Kep was positively
correlated with D*-value (r=0.316; P=0.006, <0.05),
Kep and f-values were also positively correlated
(r=0.269; P=0.019, <0.05). Ktrans was positively
correlated with the f-value (r=0.231; P=0.046, <0.05; Fig. 5).
 | Table VII.Correlation between parameters and
risk stratification groups. |
Table VII.
Correlation between parameters and
risk stratification groups.
| Parameter | r value | P-value |
|---|
| Kep | 0.307 | 0.007 |
|
Ktrans | 0.130 | 0.268 |
| Ve | 0.057 | 0.629 |
| f | 0.270 | 0.019 |
| D | 0.037 | 0.753 |
| D* | 0.323 | 0.005 |
Diagnostic efficacy of Kep,
D* and f-values for risk stratification
In the comparison of the very low-risk group with
the low-risk group, there were statistically significant
differences in the AUCs for D*, f, D* + f and Kep + D* +
f-values (Z=2.042, 2.100, 2.955 and 2.919; P<0.05). D* +
f had the highest diagnostic efficacy (AUC=0.719), with significant
discriminatory ability, sensitivity of 100% and specificity of
51.70%. In the comparison of the low-risk group with the
medium-risk group, there was a significant statistical difference
in the AUC of Kep (Z=1.340; P<0.05),
indicating a significant discriminatory ability (AUC=0.602) with a
sensitivity of 72.41% and specificity of 55.56%. In the comparison
of the very low-risk group with the medium-risk groups, there were
statistically significant differences in the AUC for
Kep, D*, f, D* + f and Kep + D* + f-values
(Z=2.910, 3.832, 2.733, 5.131 and 7.936; P<0.05) and
Kep + D* + f has the highest discriminative ability
(AUC=0.887), with a sensitivity of 100% and a specificity of 70.40%
(Table VIII; Fig. 6).
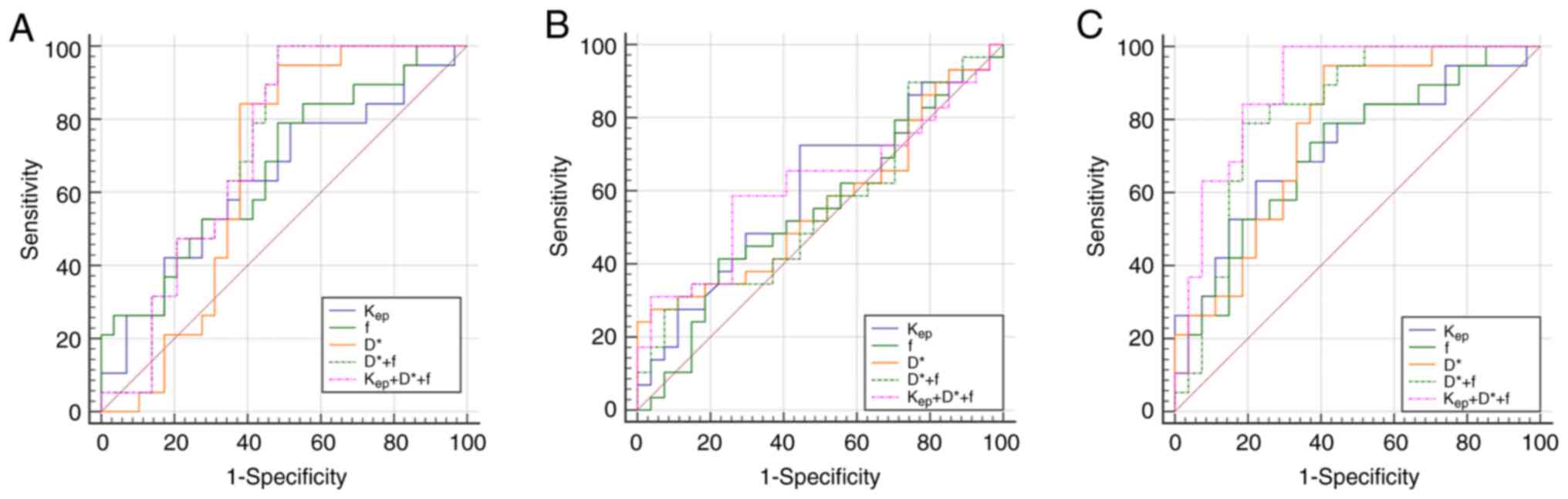 | Figure 6.(A) ROC curves for the
Kep, D*, f, D* + f and Kep + f + D* values
for discrimination between very low- and low-risk groups. (B) ROC
curves for Kep, D*, f, D* + f and Kep + f +
D* values for discrimination between low- and medium-risk groups.
(C) ROC curves of Kep, D*, f, D* + f and Kep
+ f + D* values for the discrimination between very low- and
medium-risk groups. Kep, rate constant; D, true
diffusion coefficient; D*, false diffusion coefficient; f,
perfusion fraction; ROC, receiver operating characteristic. |
 | Table VIII.Performance comparison of parameters
among different risk stratification groups. |
Table VIII.
Performance comparison of parameters
among different risk stratification groups.
| A, Very low- vs.
low-risk group |
|---|
|
|---|
| Parameters | Cut-off value | AUC | Sensitivity, % | Specificity, % | Z | P-value |
|---|
| Kep,
mm−1 | 0.272 | 0.639 | 78.95 | 48.28 | 1.663 | 0.094 |
| D*,
×10−3 mm2/sec | 0.465 | 0.662 | 94.74 | 51.72 | 2.042 | 0.041 |
| f | 0.307 | 0.668 | 78.95 | 51.72 | 2.100 | 0.036 |
| D* + f | 0.517 | 0.719 | 100.00 | 51.70 | 2.955 | 0.003 |
| Kep + D*
+ f | 0.517 | 0.717 | 100.00 | 51.72 | 2.919 | 0.004 |
|
| B, Low- vs.
medium-risk group |
|
|
Parameters | Cut-off
value | AUC | Sensitivity,
% | Specificity,
% |
Z | P-value |
|
| Kep,
1/mm | 0.279 | 0.602 | 72.41 | 55.56 | 1.34 | 0.018 |
| D*,
×10−3 mm2/sec | 0.241 | 0.563 | 67.50 | 87.20 | 0.831 | 0.413 |
| f | 0.191 | 0.547 | 44.38 | 77.78 | 0.598 | 0.550 |
| D* + f | 0.556 | 0.202 | 27.60 | 92.60 | 0.706 | 0.480 |
| Kep + D*
+ f | 0.327 | 0.616 | 58.62 | 74.07 | 1.497 | 0.134 |
|
| C, Very low- vs.
medium-risk group |
|
|
Parameters | Cut-off
value | AUC | Sensitivity,
% | Specificity,
% |
Z | P-value |
|
| Kep,
1/mm | 0.409 | 0.727 | 63.16 | 77.78 | 2.910 | 0.003 |
| D*,
×10−3 mm2/sec | 0.540 | 0.766 | 94.74 | 59.26 | 3.832 | 0.001 |
| f | 0.382 | 0.712 | 78.95 | 59.26 | 2.733 | 0.006 |
| D* + f | 0.604 | 0.823 | 78.90 | 81.50 | 5.131 | <0.001 |
| Kep + D*
+ f | 0.704 | 0.887 | 100.00 | 70.40 | 7.936 | <0.001 |
Discussion
DCE-MRI is a combination of morphological and
hemodynamic imaging technology that reflects tissue perfusion
information by analyzing the flow of contrast agents in and out of
cells and blood vessels (24).
Horvat et al (25) showed
that DCE-MRI can be used to assess the proliferation and invasion
of malignant rectal tumors. DCE-MRI sequences can be used to
quantify the microscopic structure of blood vessels and display
perfusion and metabolic information of tumor lesions, such as
Ktrans, Ve and Kep, among which
Ktrans and Kep mainly reflect the
permeability and blood volume of the blood vessels. Ve
refers to the volume fraction of contrast agents within the EES,
which is associated with tumor cell proliferation (26).
Based on the pathological results, patients were
divided into very low-, low- and medium-risk groups according to
the clinical guidelines of the ESMO (2). The difference in Kep-values
among the different risk stratification groups was statistically
significant, whereas the differences in Ktrans and
Ve-values among the groups were not statistically
significant. An increase in tumor malignancy is often accompanied
by an increase in the secretion of microvascular endothelial
factors, which accelerates the loss of function of intercellular
adhesion molecules, resulting in increased vascular permeability
and consequent hyperperfusion (27). Kep is an indicator of
tissue microvascular density and permeability, and thus increases.
A higher Kep-value is associated with a longer time for
the blood to return to the vasculature. Kep is only
affected by the contrast agent concentration and fractional volume
in the extracellular space outside the tumor's blood vessels, and
may therefore more accurately reflect the status of tumor
capillaries (28). T, N, MRF, EMVI,
CRM and metastasis are all indicators of tumor malignancy, which
reflect the depth of tumor invasion and malignant state, and are
important factors in tumor risk stratification (22). Wang et al (29) reported that Ktrans and
Kep could predict risk stratification for early
endometrial cancer, which differs from the results of the present
study. Sun et al (30)
demonstrated that the Ktrans-value does not correlate
with the pathological stage of rectal cancer, which is consistent
with the results of the present study. The authors considered that
Ktrans refers to the passage rate of the contrast agent
from the EES into the blood vessel, which mainly reflects vascular
permeability. However, this permeability is affected by the
patient's blood pressure, cardiac output, contrast agent injection
speed and other factors, resulting in a difference in the value of
this parameter. The present study showed no significant differences
in the Ve-values between the different risk
stratification groups. Ao et al (31) showed no statistically significant
difference in the Ve-value between the EMVI+ and EMVI-
groups of rectal cancer, which was similar to the results of the
present study. This may be related to the poor stability of
Ve and its susceptibility to pathological edema,
microcystic degeneration, complex microenvironment and tumor
heterogeneity (32).
In the past 20 years, MRI has served an increasingly
important role in the evaluation of rectal cancer, including TNM
staging, EMVI and treatment efficacy (4,33–35).
Functional MRI techniques, such as DWI, IVIM and DKI, have served
important roles (36). As a form of
DWI, IVIM can be used to quantify complex signals, such as cell
structure, vascular structure and microenvironment, where the
D-value is the true diffusion state of water molecules related to
the cell structure. The value of D* is perfusion-related diffusion
and f is the perfusion fraction, both of which are related to
vascular distribution, length and structure (7). IVIM reflects more of the behavioral
characteristics of tumors by analyzing the tumor perfusion and
diffusion information (37). IVIM
can improve the diagnostic performance of nodal staging of rectal
cancer (38). Early changes in the
D* and D-values could be used to predict the efficacy of
chemoradiotherapy for rectal cancer (39). The D-value of IVIM-DWI is also the
most sensitive parameter for the histological classification of
rectal cancer (14).
In the present study, the differences in D* and
f-values among the different risk-stratification groups of rectal
adenocarcinomas were statistically significant and increased with
an increase in the risk grade. Both the D* and f-values were
correlated with tumor blood vessels, which indicated that the
higher the risk stratification, the more abundant the blood
perfusion. A previous study has shown that D-values can provide
valuable information regarding whether rectal cancer cells have
EMVI, whereas f and D*-values do not correlate with EMVI (40), which differs from the results of the
present study. Potentially, the different selection of b-values,
the absence of patients with EMVI+ in the present study and the
disorder, imperfect function and abnormal leakage of blood vessels
in tumor cells complicate tumor tissue perfusion. It has been
reported that D* can improve the performance of prostate cancer
risk prediction (41). High-risk
lesions tend to have a high blood supply, high cell density, longer
capillary segment length, faster average blood drug velocity and,
therefore, higher D*-values (11).
The perfusion fraction f is another parameter that can convey
perfusion information, reflecting the density of the blood vessels
in tumor cells (42). A higher
degree of malignancy of the lesion is associated with a higher
f-value. Meng et al (43)
showed that the f-value in the low-risk group of endometrial cancer
was lower compared with that in the non-low-risk group, which is
consistent with the results of the present study. Previous studies
have shown that IVIM helps evaluate the tumor grade for
intracranial and liver tumors and that D-values are negatively
correlated with the tumor grade (44,45).
In patients with locally advanced cervical cancer, the D-value was
significantly higher in those who responded well to neoadjuvant
chemotherapy (46). A decrease in
the degree of tumor differentiation and increase in malignancy and
aggressiveness lead to the active proliferation of cancer cells, an
increase in the proportion of the nucleus and plasma and
restriction of water molecule movement, ultimately leading to a
decrease in the D-value (46). In
the present study, there was no correlation between the D-value and
rectal adenocarcinoma risk stratification. It may be considered
that the density of cells may differ between study participants;
therefore, the trend of the D-value may be different. Additionally,
malignant tumors are often accompanied by liquefaction necrosis.
While cell proliferation restricts the movement of water molecules,
liquefaction necrosis relieves the restriction of water molecule
movement and the two can interact, leading to instability of the
D-value (8,11).
In the present study, the Kep, f and
D*-values were positively correlated with the risk stratification
of rectal adenocarcinoma. In addition, Kep was
positively correlated with the f and D*-values, and
Ktrans was positively correlated with the f-value,
indicating that the two methods have a certain correlation in the
evaluation of rectal cancer perfusion. A study have shown a
significant correlation between the f-value and DCE-MRI parameters
in patients with prostate cancer in the transition zone (47). After the Delong test, the
differences in the AUC of the D*, f, D* + f and Kep + D*
+ f were statistically significant when the very low-risk and
low-risk groups were compared, confirming that the AUC of the f +
D*-value was the highest and had the highest discriminatory
ability; this indicates that IVIM can be used to distinguish
between very low- and low-risk groups of rectal adenocarcinoma, and
its diagnostic efficacy was higher compared with that of DCE-MRI,
IVIM combined with DCE-MRI. When the low-risk group was compared
with the medium-risk group, a statistically significant difference
in the AUC values of the Kep-value was observed,
confirming that Kep has the ability to distinguish
between low- and medium-risk groups of rectal adenocarcinoma, with
a diagnostic sensitivity of 72.41% and specificity of 55.56%. IVIM
had a limited value in distinguishing these two risk groups. The
differences in the AUC of Kep, f, D*, D* + f and
Kep + D* + f in the very low-risk group compared with
the medium-risk group were statistically significant, indicating
that the Kep + D* + f-value has the highest
discriminatory ability, further demonstrating that that DCE-MRI
with IVIM could significantly improve the diagnostic efficiency.
The Ktrans, Ve and f-values were independent
predictors of early endometrial cancer in the low- and
medium-high-risk groups (29). This
is inconsistent with the results of the present study, which may be
due to differences in the participants of the present study. In
addition, invasive tumors grow rapidly and have insufficient new
blood vessels, leading to tissue hypoxia and necrosis, formation of
a low-perfusion area and eventual variability in overall tumor
perfusion (48). Arian et al
(20) showed that IVIM with DCE had
the highest accuracy in the diagnosis of benign and malignant types
of breast cancer. Zhao et al (17) showed that risk assessment based on
MRI staging is essential for the clinical treatment of advanced
rectal cancer. Radiomics features based on IVIM and DCE-MRI can
improve the predictive efficiency of high aquaporin-1 expression
levels in rectal cancer (49). In
the present study, IVIM and DCE-MRI were effective in assessing
risk stratification for resectable rectal adenocarcinoma and
Kep, f and D* were potential imaging indicators for the
risk stratification of resectable rectal adenocarcinoma.
The number of cases included in the present study
was small and the results may be biased, thus requiring further
research for verification. In the post-processing of the present
study, only the ROI of the largest tumor level was selected, which
is highly operable; however, the results may not represent the
overall state of the tumor. The present study was conducted by
taking multiple measurements of different parts of the maximum
cross-sectional area and averaging them to minimize errors. IVIM
was based on plane-echo imaging; therefore, the selection of the
b-value is important, but currently, it has not been recommended by
any guidelines. In the present study, the selection of the b-value
was based on several authoritative studies (18–20).
The present study lacks follow-up data on the long-term prognosis
of patients, and therefore, future work includes the exploration
and investigation of the long-term predictive value of DCE-MRI and
IVIM parameters for rectal cancer on patient prognosis and survival
analysis.
In conclusion, DCE-MRI and IVIM were found to be
useful in the non-invasive assessment for risk stratification of
resectable rectal adenocarcinoma. Kep, f and D*-values
may be used as imaging indicators for risk stratification of
resectable rectal adenocarcinoma, which could potentially improve
the diagnostic efficiency when combined.
Acknowledgements
Not applicable .
Funding
The present study received funding from the Project of Henan
Province Medical Science and Technology Project (grant no.
LHGJ20210902) and Henan Roentgen Image Research Project (grant no.
HN-20201017-007).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YXC and JBG designed the study. YXC drafted the
manuscript. YXC and YCN performed experiments and analyzed data.
RXC performed experiments.. YXC and JBG interpreted the data and
edited the manuscript. JBG critically reviewed the manuscript and
revised it. YXC and JBG checked and confirmed the authenticity of
all the raw data. All authors made a substantial contribution to
researching data, discussion of content, and reviewing and editing
the manuscript before submission. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Xinxiang
Central Hospital Ethics Committee (approval no. 2020-098; Xinxiang,
China), with all patients providing written informed consent.
Patient consent for publication
Consent for publication was obtained from patients
whose images were contained in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel C, Cervantes A and Arnold D; ESMO Guidelines Committee, :
Rectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28:iv22–iv40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel C, Cervantes A and Arnold D; ESMO Guidelines Committee, :
Rectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 29 (Suppl 4):iv2632018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bates DDB, Homsi ME, Chang KJ, Lalwani N,
Horvat N and Sheedy SP: MRI for rectal cancer: Staging, mrCRM,
EMVI, lymph node staging and post-treatment response. Clin
Colorectal Cancer. 21:10–18. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer HJ, Höhn AK, Woidacki K, Andric M,
Powerski M, Pech M and Surov A: Associations between IVIM histogram
parameters and histopathology in rectal cancer. Magn Reson Imaging.
77:21–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nougaret S, Jhaveri K, Kassam Z, Lall C
and Kim DH: Rectal cancer MR staging: Pearls and pitfalls at
baseline examination. Abdom Radiol (NY). 44:3536–3548. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Surov A, Meyer HJ, Höhn AK, Behrmann C,
Wienke A, Spielmann RP and Garnov N: Correlations between
intravoxel incoherent motion (IVIM) parameters and histological
findings in rectal cancer: Preliminary results. Oncotarget.
8:21974–2198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arian A, Taher HJ, Alareer HS and Aghili
M: Value of conventional MRI, DCE-MRI, and DWI-MRI in the
discrimination of metastatic from non-metastatic lymph nodes in
rectal cancer: A systematic review and meta-analysis study. Asian
Pac J Cancer Prev. 24:401–410. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reynolds HM, Parameswaran BK, Finnegan ME,
Roettger D, Lau E, Kron T, Shaw M, Chander S and Siva S: Diffusion
weighted and dynamic contrast enhanced mri as an imaging biomarker
for stereotactic ablative body radiotherapy (Sabr) of primary renal
cell carcinoma. PLoS One. 13:e02023872018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia H, Jiang X, Zhang K, Shang J, Zhang Y,
Fang X, Gao F, Li N and Dong J: A nomogram of combining IVIM-DWI
and MRI radiomics from the primary lesion of rectal adenocarcinoma
to assess nonenlarged lymph node metastasis preoperatively. J Magn
Reson Imaging. 56:658–667. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan J, Gong Z, Liu K, Song J, Wen Q, Tan
W, Zhan S and Shen Q: Correlation between diffusion kurtosis and
intravoxel incoherent motion derived (IVIM) parameters and tumor
tissue composition in rectal cancer: A pilot study. Abdom Radiol
(NY). 47:1223–1231. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu B, Sun C, Zhao X, Liu L, Liu S and Ma
H: The value of multimodality MR in T staging evaluation after
neoadjuvant therapy for rectal cancer. Technol Health Care.
32:615–627. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang KX, Yu J and Xu Q: Histogram analysis
of dynamic contrast-enhanced magnetic resonance imaging to predict
extramural venous invasion in rectal cancer. BMC Med Imaging.
23:772023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geng Z, Zhang Y, Yin S, Lian S, He H, Li
H, Xie C and Dai Y: Preoperatively grading rectal cancer with the
combination of intravoxel incoherent motions imaging and diffusion
kurtosis imaging. Contrast Media Mol Imaging. 2020:21645092020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu H, Jiang H, Wang S, Jiang H, Zhao S and
Pan W: 3.0 T MRI IVIM-DWI for predicting the efficacy of
neoadjuvant chemoradiation for locally advanced rectal cancer.
Abdom Radiol (NY). 46:134–143. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bogveradze N, El Khababi N, Schurink NW,
van Griethuysen JJM, de Bie S, Bosma G, Cappendijk VC, Geenen RWF,
Neijenhuis P, Peterson G, et al: Evolutions in rectal cancer MRI
staging and risk stratification in The Netherlands. Abdom Radiol
(NY). 47:38–47. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao M, Feng L, Zhao K, Cui Y, Li Z, Ke C,
Yang X, Qiu Q, Lu W, Liang Y, et al: An MRI-based scoring system
for pretreatment risk stratification in locally advanced rectal
cancer. Br J Cancer. 129:1095–1104. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao L, Li Y, Cui B, Lu L, Dou W, Pylypenko
D, Zhu J and Li H: Multiparametric MRI for staging of bowel
inflammatory activity in Crohn's disease with MUSE-IVIM and
DCE-MRI: A preliminary study. Acad Radiol. 31:880–888. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tao J, Yin Z, Li X, Zhang Y, Zhang K, Yang
Y, Fang S and Wang S: Correlation between IVIM parameters and
microvessel architecture: Direct comparison of MRI images and
pathological slices in an orthotopic murine model of
rhabdomyosarcoma. Eur Radiol. 33:8576–8584. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arian A, Seyed-Kolbadi FZ, Yaghoobpoor S,
Ghorani H, Saghazadeh A and Ghadimi DJ: Diagnostic accuracy of
intravoxel incoherent motion (IVIM) and dynamic contrast-enhanced
(DCE) MRI to differentiate benign from malignant breast lesions: A
systematic review and meta-analysis. Eur J Radiol. 167:1110512023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Bihan D, Breton E, Lallemand D, Aubin
ML, Vignaud J and Laval-Jeantet M: Separation of diffusion and
perfusion in intravoxel incoherent motion MR imaging. Radiology.
168:497–505. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Gao X, Nickel MD, Cheng J and Zhu J:
Native T1 mapping for differentiating the histopathologic type,
grade, and stage of rectal adenocarcinoma: A pilot study. Cancer
Imaging. 22:302022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Washington MK, Berlin J, Branton P,
Burgart LJ, Carter DK, Fitzgibbons PL, Halling K, Frankel W, Jessup
J, Kakar S, et al: Protocol for the examination of specimens from
patients with primary carcinoma of the colon and rectum. Arch
Pathol Lab Med. 133:1539–1551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Huang W and Holmes JH: Dynamic
contrast-enhanced (DCE) MRI. Magn Reson Imaging Clin N Am.
32:47–61. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horvat N, Rocha CC, Oliveira BC, Petkovska
I and Gollub MJ: MRI of rectal cancer: Tumor staging, imaging
techniques, and management. Radiographics. 39:367–387. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonekamp D and Macura KJ: Dynamic
contrast-enhanced magnetic resonance imaging in the evaluation of
the prostate. Top Magn Reson Imaging. 19:273–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Yang X, Wen Z, Liu Y, Lu B, Yu S
and Xiao X: Association between high-resolution MRI-detected
extramural vascular invasion and tumour microcirculation estimated
by dynamic contrast-enhanced MRI in rectal cancer: Preliminary
results. BMC Cancer. 19:4982019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim YE, Lim JS, Choi J, Kim D, Myoung S,
Kim MJ and Kim KW: Perfusion parameters of dynamic
contrast-enhanced magnetic resonance imaging in patients with
rectal cancer: Correlation with microvascular density and vascular
endothelial growth factor expression. Korean J Radiol. 14:878–885.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Yan R, Li Z, Wang B, Jin X, Guo Z,
Liu W, Zhang M, Wang K, Guo J and Han D: Quantitative dynamic
contrast-enhanced parameters and intravoxel incoherent motion
facilitate the prediction of TP53 status and risk stratification of
early-stage endometrial carcinoma. Radiol Oncol. 57:257–269. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun D, Wu X, Wang L, Li G, Huang J and Li
Y: Distinguishing T1-2 and T3a tumors of rectal cancer with texture
analysis and functional MRI parameters. Diagn Interv Radiol.
28:200–207. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ao W, Zhang X, Yao X, Zhu X, Deng S and
Feng J: Preoperative prediction of extramural venous invasion in
rectal cancer by dynamic contrast-enhanced and diffusion weighted
MRI: A preliminary study. BMC Med Imaging. 22:1–12. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho N, Im SA, Park IA, Lee KH, Li M, Han
W, Noh DY and Moon WK: Breast cancer: Early prediction of response
to neoadjuvant chemotherapy using parametric response maps for MR
imaging. Radiology. 272:385–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moreno CC, Sullivan PS, Kalb BT, Tipton
RG, Hanley KZ, Kitajima HD, Dixon WT, Votaw JR, Oshinski JN and
Mittal PK: Magnetic resonance imaging of rectal cancer: Staging and
restaging evaluation. Abdom Imaging. 40:2613–2629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lord AC, D'Souza N, Shaw A, Rokan Z, Moran
B, Abulafi M, Rasheed S, Chandramohan A, Corr A, Chau I and Brown
G: MRI-diagnosed tumor deposits and EMVI status have superior
prognostic accuracy to current clinical TNM staging in rectal
cancer. Ann Surg. 276:334–344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin J, Seo N, Baek SE, Son NH, Lim JS,
Kim NK, Koom WS and Kim S: MRI radiomics model predicts pathologic
complete response of rectal cancer following chemoradiotherapy.
Radiology. 303:351–358. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernandes MC, Gollub MJ and Brown G: The
importance of MRI for rectal cancer evaluation. Surg Oncol.
43:1017392022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu B, Zeng Q, Huang J, Zhang J, Zheng Z,
Liao Y, Deng K, Zhou W and Xu Y: IVIM using convolutional neural
networks predicts microvascular invasion in HCC. Eur Radiol.
32:7185–7195. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao L, Liang M, Yang Y, Zhao X and Zhang
H: Histogram models based on intravoxel incoherent motion
diffusion-weighted imaging to predict nodal staging of rectal
cancer. Eur J Radiol. 142:1098692021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Zhou G, Rao S and Zeng M: Early
changes in intravoxel incoherent motion MRI parameters can
potentially predict response to chemoradiotherapy in rectal cancer:
An animal study. Magn Reson Imaging. 78:52–57. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Lin L, Gao X, Li S and Cheng J:
Amide proton transfer weighted and intravoxel incoherent motion
imaging in evaluation of prognostic factors for rectal
adenocarcinoma. Front Oncol. 11:7835442022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang CB, Lin YC, Wong YC, Lin SN, Lin CY,
Lin YH, Sheng TW, Huang CC, Yang LY and Wang LJ: IVIM parameters on
MRI could predict ISUP risk groups of prostate cancers on radical
prostatectomy. Front Oncol. 11:6590142021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iima M: Perfusion-driven intravoxel
incoherent motion (IVIM) MRI in oncology: Applications, challenges
future trends. Magn Reson Med Sci. 20:125–138. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meng N, Fang T, Feng P, Huang Z, Sun J,
Wang X, Shang J, Wang K, Han D and Wang M: Amide proton
transfer-weighted imaging and multiple models diffusion-weighted
imaging facilitates preoperative risk stratification of early-stage
endometrial carcinoma. J Magn Reson Imaging. 54:1200–1211. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kikuchi K, Hiwatashi A, Togao O, Yamashita
K, Kamei R, Momosaka D, Hata N, Iihara K, Suzuki SO, Iwaki T and
Honda H: Intravoxel incoherent motion MR imaging of pediatric
intracranial tumors: Correlation with histology and diagnostic
utility. AJNR Am J Neuroradiol. 40:878–884. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang D, She H, Wang X, Yang Z and Wang Z:
Diagnostic accuracy of quantitative diffusion parameters in the
pathological grading of hepatocellular carcinoma: A meta-analysis.
J Magn Reson Imaging. 51:1581–1593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dolciami M, Capuani S, Celli V, Maiuro A,
Pernazza A, Palaia I, Di Donato V, Santangelo G, Rizzo SMR, Ricci
P, et al: Intravoxel incoherent motion (IVIM) MR quantification in
locally advanced cervical cancer (LACC): Preliminary study on
assessment of tumor aggressiveness and response to neoadjuvant
chemotherapy. J Pers Med. 12:6382022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kooreman ES, van Pelt V, Nowee ME, Pos F,
van der Heide UA and van Houdt PJ: Longitudinal correlations
between intravoxel incoherent motion (IVIM) and dynamic
contrast-enhanced (DCE) MRI during radiotherapy in prostate cancer
patients. Front Oncol. 12:8971302022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ye Z, Ning G, Li X, Koh TS, Chen H, Bai W
and Qu H: Endometrial carcinoma: Use of tracer kinetic modeling of
dynamic contrast-enhanced MRI for preoperative risk assessment.
Cancer Imaging. 22:142022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen Y, Li B, Jiang Z, Li H, Dang Y, Tang
C, Xia Y, Zhang H, Song B and Long L: Multi-parameter diffusion and
perfusion magnetic resonance imaging and radiomics nomogram for
preoperative evaluation of aquaporin-1 expression in rectal cancer.
Abdom Radiol (NY). 47:1276–1290. 2022. View Article : Google Scholar : PubMed/NCBI
|