Introduction
Hyperlactatemia is a serious clinical manifestation
typically categorized into type A, type B and type D (1). Type A hyperlactatemia is characterized
by hyperlactatemia due to inadequate tissue oxygenation, typically
seen in conditions such as shock, sepsis and severe hypoxemia. In
Type A hyperlactatemia, the primary cause is tissue hypoxia leading
to anaerobic metabolism and lactate production. Malignancy-induced
lactic acidosis (Type B lactic acidosis) occurs in the absence of
systemic oxygenation impairment, characterized by a pH below 7.35
and serum lactate concentration exceeding 5–6 mM (2). Lactate is produced anaerobically from
pyruvate, primarily metabolized in the liver through
gluconeogenesis, with a secondary role in renal lactate metabolism
(3). Under normal circumstances,
the production and clearance rates of lactate remain in equilibrium
to maintain physiological acid-base balance. However, in cases of
high tumor burden, anaerobic glycolysis leads to excessive lactate
production, resulting in hyperlactatemia. Type D hyperlactatemia is
related to drug-induced causes, where medications or substances
interfere with lactate metabolism and clearance, leading to
elevated lactate levels in the blood (2,4). Among
these, type B lactic acidosis associated with malignancies is a
rare but life-threatening oncological emergency, most commonly
observed in hematological malignancies and less frequently in solid
tumors. Documented cases (5,6) of
solid tumor-associated Type B lactic acidosis are associated with
various types of cancer such as lung cancer (especially small-cell
lung cancer), breast cancer, sarcoma, cholangiocarcinoma, and
colorectal cancer (7). The
prognosis for patients with solid tumor-induced hyperlactatemia is
typically poor, with ~80% of patients succumbing within 10 weeks
and 55% within the first week of onset (5). Cervical neuroendocrine carcinoma (NEC)
is a rare and highly malignant tumor, accounting for only 1.6% of
all cervical cancer cases. To date, there have been no reported
cases of hyperlactatemia caused by metastatic cervical NEC. Current
treatment guidelines for metastatic NEC recommend use of etoposide
and cisplatin chemotherapy (8). The
current study presents a rare case of type B lactic acidosis in a
patient with metastatic cervical NEC. Notably, the patient's
condition significantly improved after the administration of a
reduced dose of intravenous etoposide and a delayed intraperitoneal
infusion of carboplatin. This case underscores the potential
efficacy of this therapeutic approach in managing this critical
condition and contributes to the limited body of literature on the
subject.
Case report
A 59-year-old female patient with a history of good
health, no surgical history and no chronic diseases experienced
intermittent vaginal bleeding in January 2023, which was left
untreated. In August 2023, the patient developed abdominal
distension and sought medical attention at West China Hospital
(Chengdu, China), where the initial diagnosis by cervical biopsy,
was high-grade NEC, a cervical malignant tumor. CT scan indicated
extensive abdominal metastases. Chemotherapy was recommended, but
the patient refused and opted for palliative care at home. In
September 2023, the patient presented to the Emergency Department
at the People's Liberation Army General Hospital of Western Theater
Command (Chengdu, China) due to a significant worsening of
abdominal distension accompanied by dyspnea. A physical examination
revealed a performance status (PS) score (9) of 3 and a heart rate of 110 bpm. The
patient was wheelchair-bound. Emergency computed tomography showed
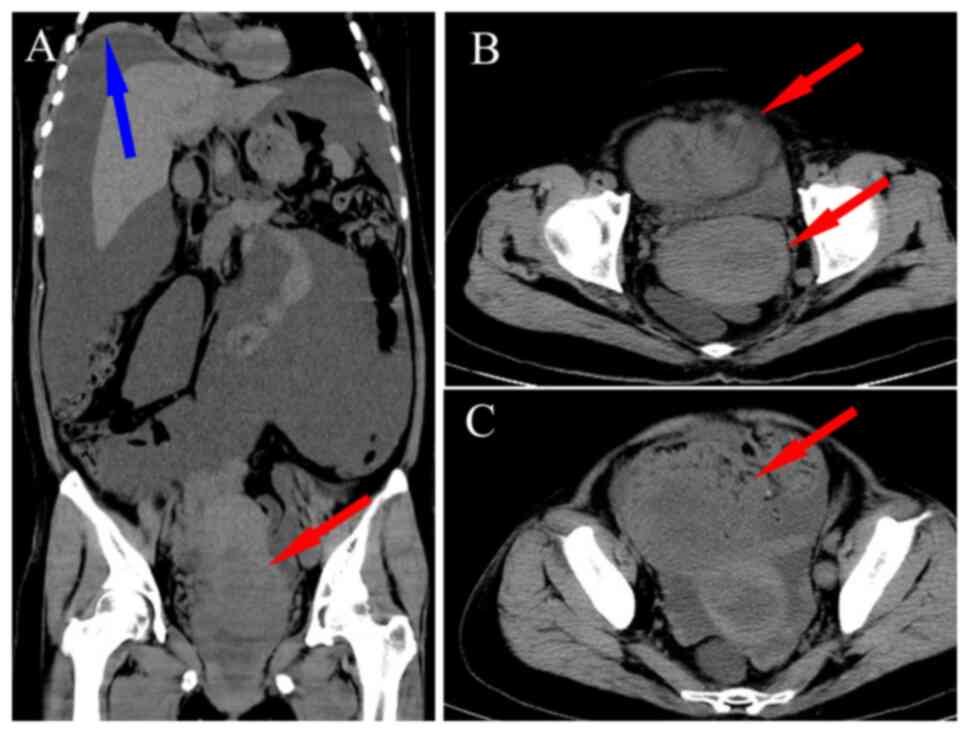
an extremely distended abdomen with an elevated right lung base. A
large amount of ascites was present around the liver, with no
pleural effusion detected (Fig.
1A). A roughly round, heterogeneous density mass was observed
within the cervix, measuring ~6.1×5.6 cm, with unclear boundaries
(Fig. 1B). Patchy and nodular
shadows were seen adjacent to the right side of the uterine body
and in the lower left abdominal cavity, with the largest measuring
~4.5×4.1 cm (Fig. 1C).
Upon admission, the patient had significant ascites,
and a paracentesis was performed, draining ~2,000 ml daily. The
patient's white blood cell count (16.5×109/l; normal
reference range, 3.50–5.30×109/l) and C-reactive protein
level (119.00 mg/l; normal reference range, 0–3.0 mg/l) were
significantly elevated. The patient presented with a PS score of
3–4 accompanied by severe hyperlactatemia, caused by both tumor-
and non-tumor-related factors. Tumor-related factors include pelvic
and abdominal mass caused by the tumor, extensive ascites, and
excessive lactate production, while non-tumor-related factors
include infection, hypoalbuminemia. The patient presented with
infections, hypoalbuminemia and electrolyte imbalances that all
need to be corrected. Therefore, supportive treatment was
initiated. Cefoperazone-sulbactam (4 g twice a day for 8 days) was
administered for anti-infective therapy and tramadol hydrochloride
extended-release tablets (200 mg twice a day for 2 days) were used
for pain management. Enteral nutrition powder (400 mg daily for 6
days) was provided for nutritional support. Rivaroxaban (10 mg
every day for 13 days) was used for thrombosis prevention. The
patient's neuron-specific enolase (NSE) level was >370.00 ng/ml
(normal reference range, 0–17.00 ng/ml). After anti-infection and
fluid therapy, the infection markers improved. On the 7th day
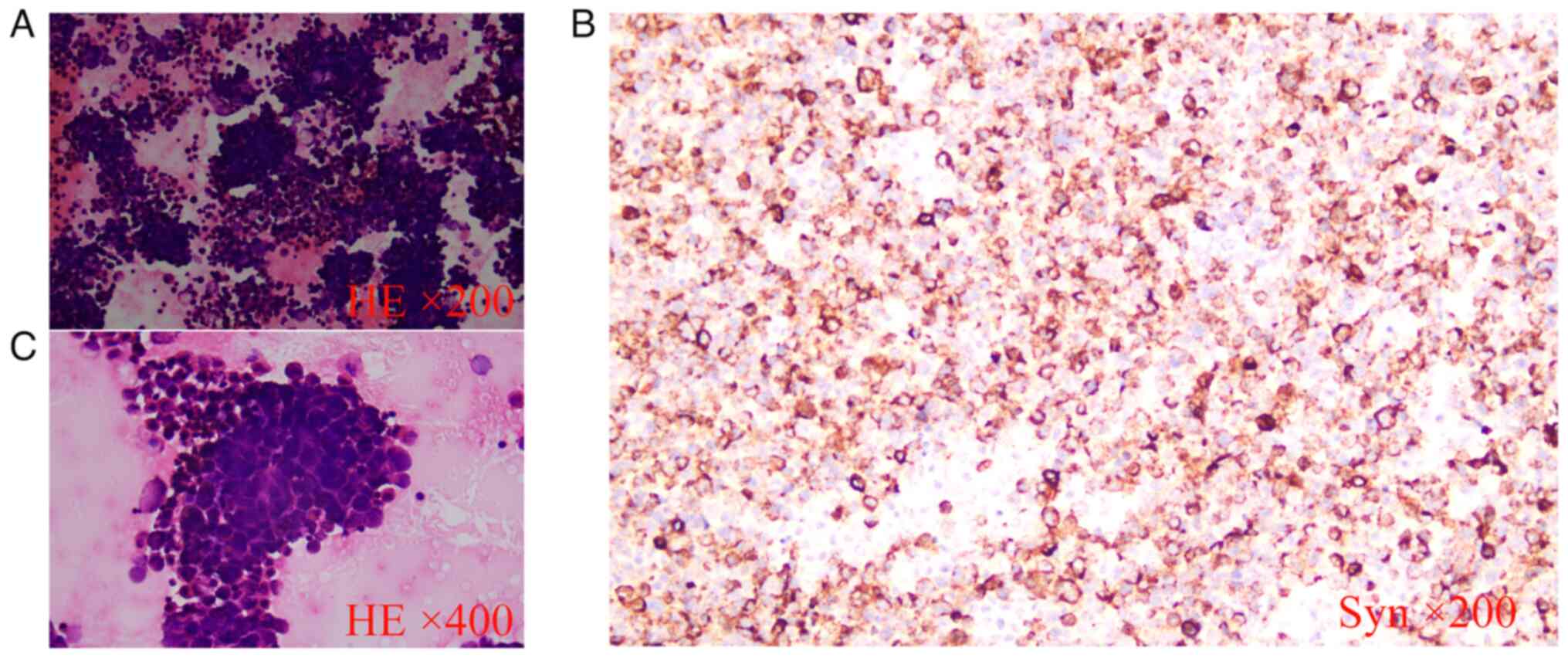
post-admission, a pathological report on the ascitic fluid revealed
a small amount of nuclear heterogeneous cell clusters (Fig. 2), with immunohistochemical markers
suggesting NEC cells (10,11). The immunohistochemical (IHC) marker
results for this patient were: Carcinoembryonic antigen (CEA)(+),
cytokeratin (CK)5/6(−), CK8/18(+), chromogranin A (CgA)(+),
estrogen receptor (ER)(−), Ki-67(+, 80%), NSE(+), p16(+), p63(+),
synaptophysin (Syn)(+), thyroid transcription factor-1 (TFT-1)(+),
vimentin (Vim)(−) and Wilms' tumor-1 (WT-1)(−; data not shown). The
IHC and pathological staining protocols were as follows: A total of
500 ml ascitic fluid was collected in a sterile glass bottle and
allowed to settle for 1 h. The bottom liquid was drawn and
transferred to a plastic centrifuge tube. Next, 5 ml of 10%
formalin solution was added, and the tube was centrifuged at 500 g
at 4°C for 5 min. The supernatant was discarded, and another 5 ml
of 10% formalin solution was added. The tube was centrifuged again,
this process was repeated once more, and then the tube was left to
stand. The cells obtained following centrifugation of asciteswere
fixed in 10% neutral-buffered formalin at room temperature for 24
h. The samples were dehydrated through graded alcohols (70, 80, 95
and 100%) and then cleared in xylene. The samples were embedded in
paraffin and sectioned to 4- to 5-µm thick. The sections were
deparaffinized in xylene and rehydrated through graded alcohols to
water. Antigen retrieval was performed using citrate buffer (pH
6.0) in a microwave or pressure cooker. Endogenous peroxidase
activity was blocked with 3% hydrogen peroxide for 10 min.
Non-specific binding was blocked with 5% normal goat serum at room
temperature for 30 min. The sections were then incubated with
primary antibodies at room temperature for 60 min. The primary
antibodies used were Syn antibody, with a dilution concentration of
1:200 (cat. no. MAB0742), CEA antibody at a dilution of 1:200 (cat.
no. MAB0852), CK5/6 antibody at a dilution of 1:200 (cat. no.
MAB0744), CK8/18 antibody at a dilution of 1:200 (cat. no.
MAB1002), CgA antibody at a dilution of 1:200 (cat. no. MAB0548),
ER antibody ready-to-use (cat. no. Kit 0012), Ki-67 antibody at a
dilution of 1:200 (cat. no. MAB0542), NSE antibody at a dilution of
1:100 (cat. no. MAB0791), p16 at a dilution of 1:200 (cat. no.
MAB0673), p63 antibody at a dilution of 1:200 (cat. no. MAB0694),
TFT-1 antibody at a dilution of 1:100 (cat. no. MAB0266), Vim
antibody at a dilution of 1:200 (cat. no. Kit 0019), and WT-1
antibody at a dilution of 1:200 (cat. no. MAB0678; all antibodies
from Fuzhou Maixin Biotechnology Development Co., Ltd. The sections
were next incubated with biotinylated secondary antibody for 30 min
at room temperature. The sections were incubated with DAB Detection
Kit (Amplifier Polymer) cat. no. TT0803, Fuzhou Maixin
Biotechnology Development Co., Ltd)for 30 min and then developed
with DAB substrate for 3–5 min. The sections were finally
counterstained with hematoxylin at room temperature for 1–2
minutes, dehydrated, cleared and mounted.
On the 8th day post-admission, blood gas analysis
showed a pH level of 7.44 (normal reference range, 7.35–7.45) and
lactate level of 9.8 mM (normal reference range, 0.4–2.2 mM). Daily
fluid intake was 3,000 ml and a daily injection of 125 ml sodium
bicarbonate was administered. Fluid replacement and sodium
bicarbonate therapy did not significantly reduce the lactate levels
(Fig. 3). The patient's PS score
was 4, and despite anti-infection and electrolyte correction
therapy, the lactate levels continued to rise, with worsening
tachycardia. Given the normal pH, significantly elevated lactate
levels and severe metabolic disturbances, type B lactic acidosis
secondary to the tumor was considered. According to the literature
(1,2), chemotherapy is the only effective
treatment for this condition. Solid tumors with type B lactic
acidosis are rare and critical conditions. Due to the lack of
treatment literature for cervical NECs with type B lactic acidosis,
the chemotherapy treatment method used for lymphoma with type B
lactic acidosis was adopted. The specific chemotherapy regimen was
referenced from the NCCN Guidelines for Cervical NEC (12) and the Chinese Society of Clinical
Oncology (CSCO) Guidelines for Small Cell Lung Cancer (2023)
(13).
According to the Guidelines of the CSCO for Small
Cell Lung Cancer, for patients with non-tumor-related PS scores of
3–4, after improvement with symptomatic supportive treatment, if
the physical condition improves and the PS score reaches ≥2,
treatment can be conducted following the strategy for patients with
PS 0–2. Therefore, supportive therapy, including anti-infection,
fluid replacement, nutritional support, and correction of
electrolyte disturbances, was initially administered. However,
following this treatment, the patient's PS score not only failed to
improve but deteriorated further. However, considering the rapid
tumor progression and risk of further deterioration without
chemotherapy, the patient and their family were repeatedly informed
about the current condition and the risks associated with
chemotherapy. After thorough consideration, they agreed to proceed
with salvage chemotherapy. Considering the patient's condition, a
reduced-dose chemotherapy regimen was administered. This modified
regimen involved one cycle of 100 mg etoposide on days 1, 3 and 4,
and 300 mg carboplatin on day 6. On the 11th day post-admission,
the patient received chemotherapy. By the next day, the patient's
condition had deteriorated, with an ECG showing junctional
tachycardia and a heart rate of 167 bpm. On the 12th day
post-admission esmolol was administered to control the heart rate,a
daily 1 g esmolol hydrochloride injection was infused slowly for
controlling the ventricular rate to keep it below 140 bpm for 70 h.
An ultrasound also revealed a large right pleural effusion, which
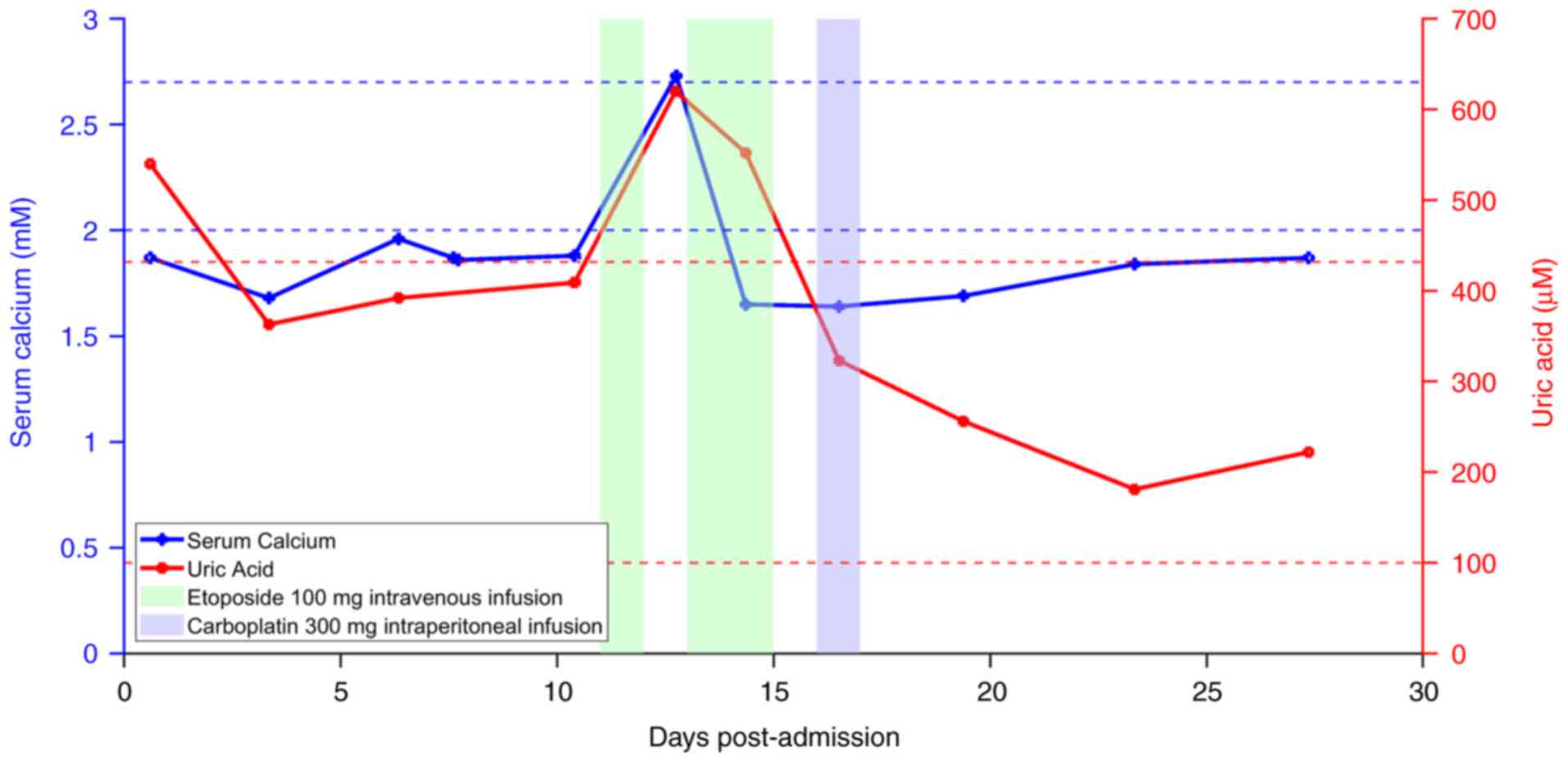
was drained. Blood tests showed a lactate level of 11.2 mM, a uric
acid level of 620 µM (normal reference range, 100–432 µM) and a
sudden increased in serum calcium to 2.73 mM (normal reference
range, 2.00–2.70 mM) (Fig. 4),
suggestive of tumor lysis syndrome (TLS). Fluid replacement and
glucose-insulin therapy were administered over a 20-h period,
consisting of 3,095 ml of liquid, which included 22 IU of insulin
and 160 g of glucose, 125 ml of 5% NaHCO3), 120 ml of 50% glucose
solution (GS), 500 ml of 10% GS, 500 ml of glucose sodium chloride,
250 ml 30% lipid emulsion injection (LE), 500 ml of 11.4% amino
acid, 500 ml of GS, 500 ml 0.9% sodium chloride injection (NS), and
100 ml of 20% human albumin solution (HAS). Blood potassium levels
were monitored during this period. On the 11 and 12th days
post-admission, the total fluid intake was 6,850 ml, excluding oral
fluid intake.
Intravenous etoposide (0.1 g) administration was
continued on the 13th day and the 14th day post-admission.
Post-chemotherapy, the lactate levels significantly decreased, the
heart rate improved and the general condition of the patient also
improved. On the 16th day post-admission, the lactate levels were
2.4 mM and the patient had a PS score of 3. The patient's daily
drainage of ascites ranged from 1,600 to 2,600 ml. To further
reduce the ascites, 300 mg carboplatin was administered
intraperitoneally on day 6. During the course of the disease, the
patient developed bilateral lower extremity edema and recurrent
hypoalbuminemia, requiring albumin supplementation (intravenous
infusion of 100 ml of 20% human albumin daily for a total of 18
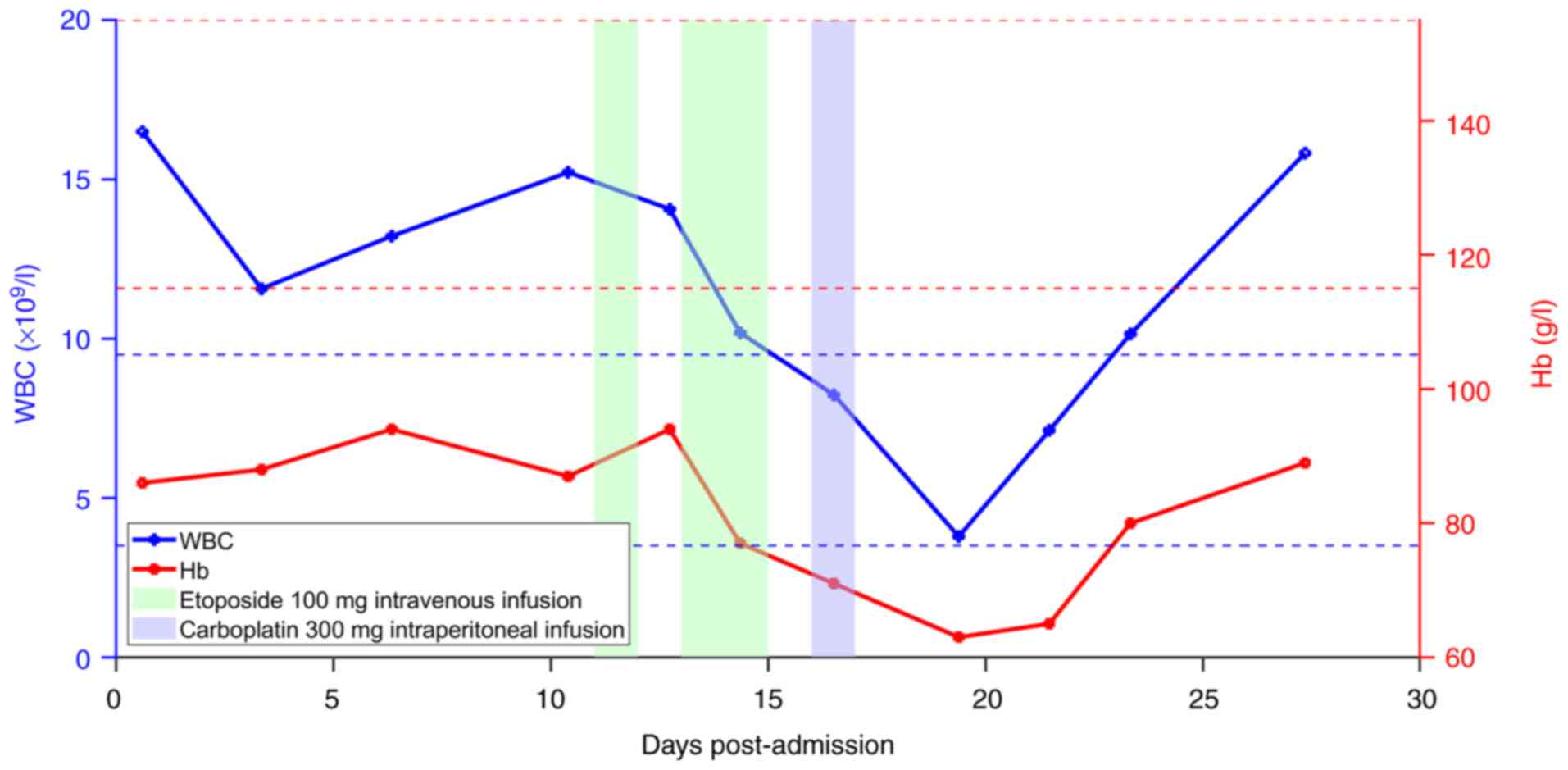
days. On the 20th day post-admission, the patient's hemoglobin (Hb)
concentration dropped from a pre-chemotherapy level of 89 g/l to 63
g/l (normal reference range, 115–150 g/l). A total of 1.5 units of
red blood cells were administered, which increased the Hb level to
80 g/l, and it later stabilized at 90 g/l (Fig. 5). Leukopenia occurred on the 8th day
after the start of chemotherapy. The patient's white blood cell
(WBC) count was 3.89×109/l (normal reference range,
3.50–5.30×109/l), necessitating the administration of
300 mg granulocyte colony-stimulating factor daily for 6 days. The
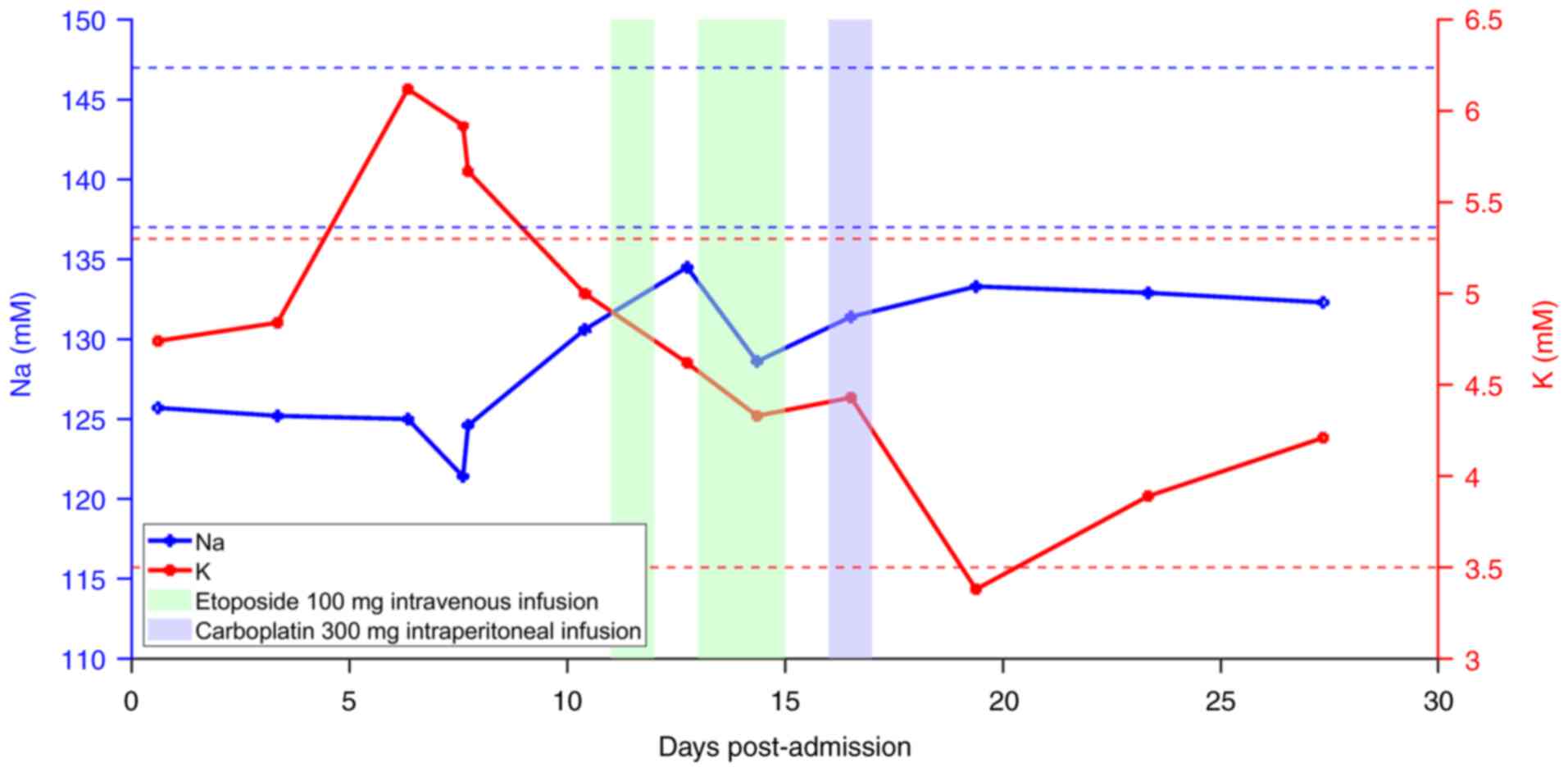
patient's high blood serum potassium level of 5.92 mM (normal
reference range, 3.50–5.30 mM) before chemotherapy shifted to a low
blood potassium level of 3.38 mM, and potassium supplementation
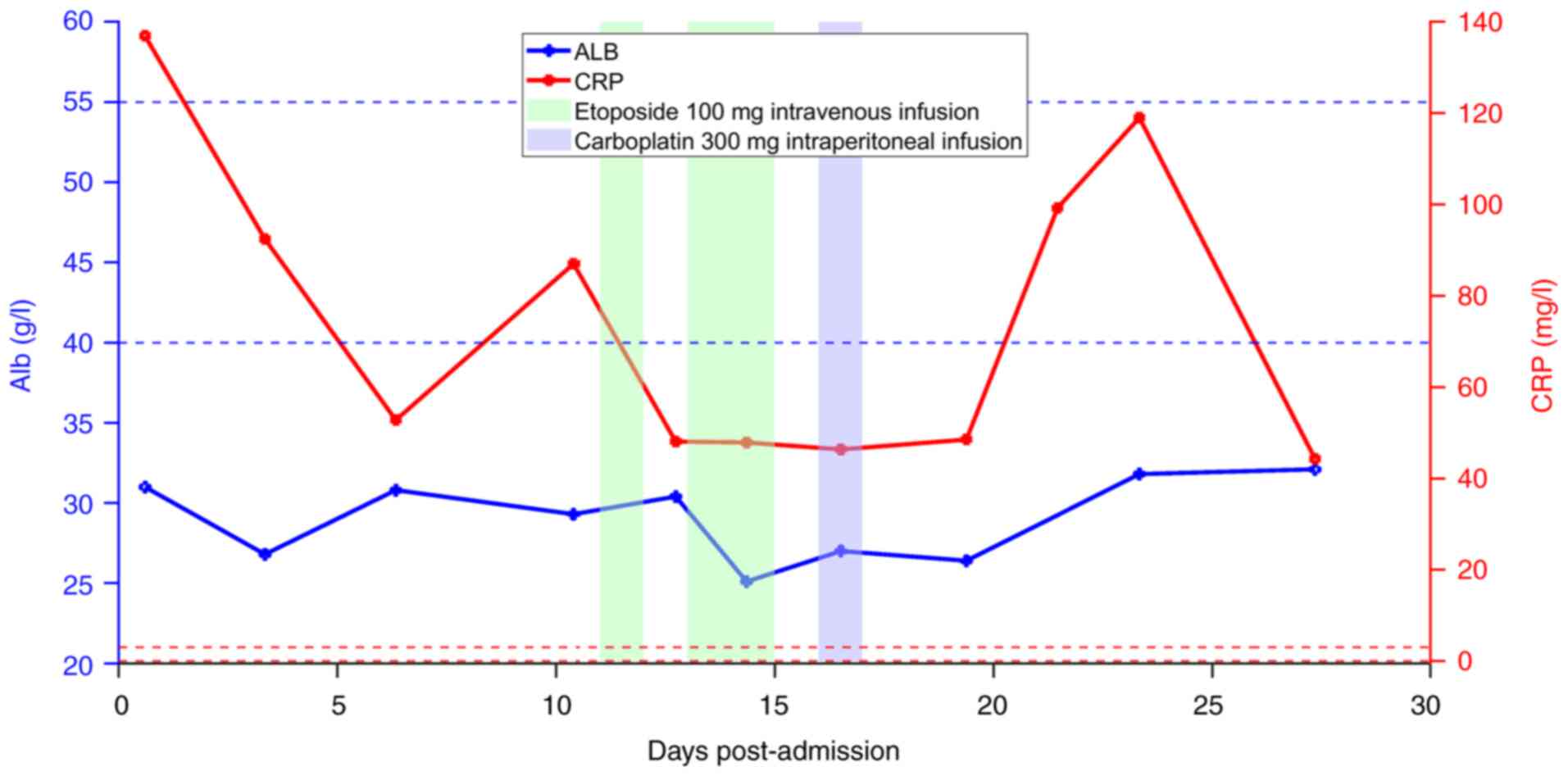
began on the 8th day after chemotherapy (Fig. 6). Due to elevated C-reactive protein
levels (Fig. 7), 4 g
cefoperazone-sulbactam was administered twice a day for 15 days for
anti-infection treatment, which subsequently alleviated the
infection. Additionally, the patient experienced poor appetite
post-chemotherapy and was provided with intravenous nutritional
support; specifically, an 11.4% amino acid injection (250 ml twice
a day) and a 30% lipid emulsion injection (250 ml every day).
Esomeprazole sodium injection (40 mg) was used for treating
chemotherapy-induced nausea and vomiting. As the albumin level was
30.8 g/l (normal reference range, 40.0–55.0 g/l), 20% human albumin
solution was injected at 100 ml daily for correcting
hypoalbuminemia. On the 19th day post-admission, the lactate levels
were 2.2 mmol/l and the amount of ascites was reduced compared with
previously. Symptoms such as bilateral lower extremity edema,
recurrent hypoalbuminemia, infection and electrolyte imbalances
were attributed to the cancer. Adverse effects caused by the
therapy included decreased Hb and WBC counts, as well as the poor
appetite experienced by the patient. Detailed medication
information can be found in Fig.
8.
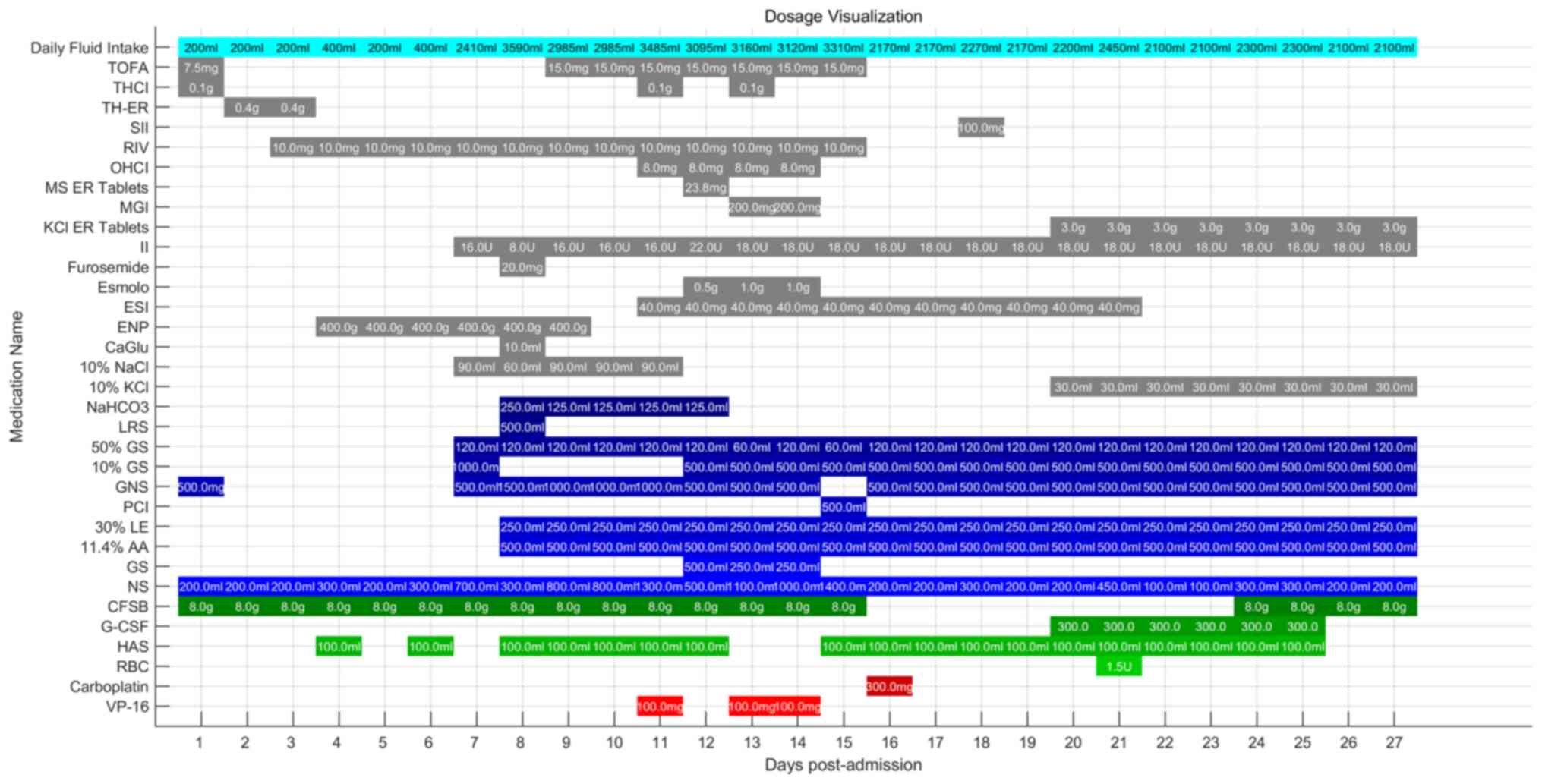 | Figure 8.Detailed medication information. CFSB
was administered twice a day, TH-ER was administered twice a day,
NS and GS were administered multiple times, 10% KCl and 10% NaCl
were slowly infused into NS or GS, and the remaining medications
were administered once daily. All pharmacological agents and
solutions are commonly used in medical treatment and therapy.
VP-16, etoposide; RBC, red blood cell suspension; HAS, 20% human
albumin solution for injection; G-CSF, granulocyte
colony-stimulating factor; CFSB, cefoperazone-sulbactam; NS, 0.9%
NaCl injection; GS, 5% glucose injection; 11.4% AA, 11.4% amino
acid injection; 30% LE, 30% lipid emulsion injection; PCI,
polypeptide collagen injection; GNS, glucose sodium chloride
injection; GS, glucose solution for injection; LRS, lactated
Ringer's injection; NaHCO3, sodium bicarbonate injection; 10% KCl,
10% potassium chloride injection; 10% NaCl, 10% concentrated sodium
chloride solution for injection; Esmolo, esmolo hydrochloride
injection; CaGlu, 10% calcium gluconate; ENP, enteral nutrition
powder; ESI, esomeprazole sodium for injection; II, insulin
injection; KCl ER tablets, potassium chloride extended-release
tablets; MGI, magnesium isoglycyrrhizinate injection; MS ER
tablets, metoprolol succinate extended-release tablets; OHCI,
ondansetron hydrochloride injection; RIV, rivaroxaban; SII, sucrose
iron injection; TH-ER, tramadol hydrochloride extended-release
tablets; THCI, tramadol hydrochloride injection; TOFA, tofacitinib
tablet. |
As the patient condition had improved, they were
discharged from hospital on the 28th day post-admission, with a PS
score of 2. The daily drainage of ascites had decreased from
2,000-3,000 ml before chemotherapy to 800–1,000 ml at discharge.
The patient was discharged home for palliative care treatment, and
declined re-hospitalization for chemotherapy. Recurrent abdominal
distension was noted 20 days after discharge, and the patient
passed away 47 days post-discharge. However, the patient did not
receive hospital treatment after the onset of abdominal distension
until the time of death, which spanned 27 days, and the specific
management measures are unclear. Through communication with the
family, it was understood that the patient did not undergo
chemotherapy or other treatment but only received symptomatic
supportive treatment at home, including pain relief.
Discussion
Malignancy-associated type B lactic acidosis (MA-LA)
is a rare but life-threatening oncological emergency. The exact
pathophysiology of MA-LA remains unclear. One prominent hypothesis,
known as the Warburg effect, describes a phenomenon in which tumor
cells switch their metabolic machinery towards a glycolytic state
even in the presence of normal oxygen concentrations, leading to
excess lactate production (6,14). The
condition of the present patient deteriorated rapidly, with lactate
levels rising precipitously. On the 8th day post-admission, 2023,
the lactate levels had reached 9.8 mmol/l. Despite symptomatic
treatments such as sodium bicarbonate infusion and rehydration, the
lactate levels did not decrease, remaining at 8.8 mmol/l 2 days
later. This aligns with literature reports that MA-LA is difficult
to alleviate with symptomatic treatment alone (4). Effective chemotherapy appears to be
the only hope for survival (1).
Traditional chemotherapy can be overly aggressive for patients with
compromised metabolic states, and the reported success rates for
such interventions are generally low (15,16).
Cervical NEC is a rare malignancy, accounting for only 0.9–1.5% of
all cervical malignancies. Cervical NEC represents a significant
challenge in treatment, given the small number of patients and
limited clinical experience. Current therapeutic modalities are
mainly based on experience in treating small cell NEC of the lung,
given the great histological similarities between these two
diseases (17). According to the
Guidelines of the CSCO for Small Cell Lung Cancer (2023) (13), treatment for patients with PS scores
of 3–4 due to the tumor, various factors should be fully considered
when selecting a treatment plan, such as chemotherapy (single-agent
regimen or reduced combination regimen). For patients with
non-tumor-related PS scores of 3–4, after improvement with
symptomatic supportive treatment, if physical condition improves
and the PS score reaches ≥2, treatment can be conducted following
the strategy for patients with PS 0–2. The standard regimen
includes carboplatin with an area under the curve (AUC) of 5–6 on
day 1, and etoposide at 100 mg/m2 on days 1, 2 and 3.
The present patient had a creatinine level of 37 µM due to
malnutrition (normal reference range, 44–133 µM), a height of 156
cm, a weight of 65 kg and a body surface area of 1.58
m2. Therefore, the standard regimen would require
etoposide to be administered at 158 mg on days 1, 2 and 3, and
carboplatin to be administered at 594 mg with an AUC of 5 on day 1.
The modified regimen actually used involved administering etoposide
at 100 mg on days 1, 3 and 4, and carboplatin at 300 mg on day 6,
and was thus considered a ‘reduced-dose’ regimen as per the CSCO
guidelines for Small Cell Lung Cancer. Dose-reduced chemotherapy
aims to decrease tumor burden and lactate production while
minimizing the risk of severe side effects. Although the literature
on this approach is limited, there are precedents in oncological
practice where dosages are adjusted based on patient tolerance. For
instance, certain chemotherapeutic regimens are modified for
patients with reduced organ function or those who have experienced
significant toxicities from standard doses. Lowering lactate levels
and alleviating symptoms can significantly enhance the quality of
life and potentially extend survival for patients in acute
distress. The immediate stabilization achieved through dose-reduced
chemotherapy provides an opportunity for further therapeutic
interventions, whether they involve more aggressive
chemotherapeutic regimens or supportive care measures. However,
considering the present patient's PS score of 4, the risks were
significant. After comprehensive evaluation, 100 mg etoposide was
administered intravenously on the 11th day post-admission. The
patient subsequently developed tachycardia and dyspnea. Blood tests
revealed a lactate level of 11.2 mmol/l and a uric acid level of
620 mM, and that the serum calcium level had suddenly increased to
2.73 mM, with a potassium level of 4.2 mM. TLS is an oncological
emergency characterized by severe electrolyte imbalances, typically
occurring when patients with hematological malignancies begin
systemic chemotherapy (18). Given
the patient's high tumor burden and rapid proliferation rate, TLS
was considered as the diagnosis. Etoposide treatment was paused,
and symptomatic treatments, including hydration, diuresis and
potassium restriction were administered, leading to symptom relief
(19,20).
On the 12th day post-admission, the lactate levels
had dropped to 5.9 mmol/l. Etoposide treatment was resumed over the
next 2 days, with lactate levels decreasing to 2.4 mmol/l by the
third day. Additionally, carboplatin is typically administered
intravenously as part of the standard regimen. However, due to the
patient's significant ascites volume, the choice was made to
administer an intraperitoneal carboplatin perfusion to enhance the
local drug concentration within the ascites and minimize any
systemic toxic effects. Following intraperitoneal infusion of 300
mg carboplatin, the lactate levels had further decreased to 2.2
mmol/l on the 19th day post-admission. Throughout the course of
treatment, the patient's pH levels remained above 7.35, which
differs from literature reports of low pH in similar cases,
suggesting that the specific mechanisms warrant further
investigation (6,21,22).
During the disease course, the patient experienced severe
infections, hypoproteinemia, anemia and chemotherapy-induced
myelosuppression. Benefiting from previous literature reports
(23,24) and our clinical experience, these
complications were anticipated, and appropriate treatments were
administered promptly. The symptoms of bilateral lower extremity
edema, recurrent hypoalbuminemia, infection and electrolyte
imbalances were attributed to the cancer. Adverse effects caused by
the therapy included decreased Hb and WBC counts, as well as the
poor appetite experienced by the patient. This proactive approach
allowed the patient to overcome the most critical periods and
achieve clinical remission, leading to discharge. The utilization
of reduced-dose intravenous etoposide in combination with
intraperitoneal carboplatin deviates from standard protocols,
providing a novel approach for addressing critical oncological
emergencies in patients with a poor PS.
In conclusion, type B lactic acidosis is a rare but
fatal complication of malignancy. The condition is typically
associated with hematological malignancies and has also been
reported in NEC, but to the best of our knowledge, there are no
previous reports of its occurrence in cervical NEC. When a patient
with advanced cancer presents with severe hyperlactatemia and
normal pH levels, malignancy-induced lactic acidosis should be
strongly considered. Chemotherapy may be the only effective
treatment. The present case demonstrates the administration of a
reduced dose of intravenous etoposide and a delayed intraperitoneal
infusion of carboplatin, along with proactive management of TLS and
other complications, leading to patient remission and discharge.
This case highlights the potential efficacy of reduced-dose
chemotherapy in critically ill patients with MA-LA, and provides a
reference for managing similar oncological emergencies in clinical
practice.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BT, PM, JX and YW were responsible for the research
design and data interpretation, the data acquisition, selection and
analysis, as well as the clinical interpretation of the data. XS
and BT designated the clinical treatment plan for the patient. BT
and XS read, revised and approved the final draft. BT and XS
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient's authorized relative (the patient's
son) provided written informed consent allowing for the publication
of the patient's data and related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang C, Lv Z and Zhang Y: Type B lactic
acidosis associated with diffuse large B-cell lymphoma and the
Warburg effect. J Int Med Res. 50:30006052110677492022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abaleka FI, Bedanie G, Olavarria Bernal D,
Yewedalsew SF and Seen T: Type B Lactic acidosis: A very rare but
fatal complication of gastrointestinal solid tumor. Cureus.
16:e567882024.PubMed/NCBI
|
|
3
|
Vavricka J, Broz P, Follprecht D, Novak J
and Krouzecky A: Modern perspective of lactate metabolism. Physiol
Res. 73:499–514. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Claudino WM, Dias A, Tse W and Sharma VR:
Type B lactic acidosis: A rare but life threatening hematologic
emergency. A case illustration and brief review. Am J Blood Res.
5:25–29. 2015.PubMed/NCBI
|
|
5
|
Mangala YO and Freeman NJ: Malignancy
associated type B lactic acidosis: A rare, yet fascinating
oncological emergency. R I Med J (2013). 107:10–12. 2024.
|
|
6
|
Heneberg P: Lactic acidosis in patients
with solid cancer. Antioxid Redox Signal. 37:1130–1152. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Espinoza AM and Venook AP: Lactic acidosis
and colon cancer: Oncologic emergency? Clin Colorectal Cancer.
10:194–197. 2011.PubMed/NCBI
|
|
8
|
Ren X, Wu W, Li Q, Li W and Wang G:
Advances in research, diagnosis, and treatment of neuroendocrine
cervical carcinoma: A review. Oncol Rev. 17:117642023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prodromidou A, Phelps DL, Pergialiotis V,
Cunnea P, Thomakos N, Rodolakis A, Fotopoulou C and Haidopoulos D:
Clinicopathological characteristics and survival outcomes of
patients with large cell neuroendocrine carcinoma of the uterine
cervix: A systematic review and meta-analysis. Eur J Obstet Gynecol
Reprod Biol. 270:212–220. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Razvi H, Tsang JY, Poon IK, Chan SK,
Cheung SY, Shea KH and Tse GM: INSM1 is a novel prognostic
neuroendocrine marker for luminal B breast cancer. Pathology.
53:170–178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abu-Rustum NR, Yashar CM, Arend R, Barber
E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA,
et al: NCCN guidelines® insights Cervical cancer,
version 1.2023. J Natl Compr Canc Netw. 21:1224–1233. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chinese Society of Clinical Oncology, .
Guidelines for Diagnosis and Treatment of Small Cell Lung Cancer.
2023.(In Chinese).
|
|
14
|
Daverio Z, Balcerczyk A, Rautureau GJP and
Panthu B: How warburg-associated lactic acidosis rewires cancer
cell energy metabolism to resist glucose deprivation. Cancers
(Basal). 15:14172023. View Article : Google Scholar
|
|
15
|
Rezar R, Mamandipoor B, Seelmaier C, Jung
C, Lichtenauer M, Hoppe UC, Kaufmann R, Osmani V and Wernly B:
Hyperlactatemia and altered lactate kinetics are associated with
excess mortality in sepsis: A multicenter retrospective
observational study. Wien Klin Wochenschr. 135:80–88. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erdur A, Guven R, Can D, Gurkan TT, Ak E
and Avci A: Prognostic importance of lactate and blood gas
parameters in predicting mortality in patients with critical
malignancies. Ethiop J Health Sci. 33:255–262. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mandic A, Maksimovic T, Nadj GS, Maricic S
and Celebic A: Neuroendocrine cervical cancer: Have we made any
steps forward in its management? Eur J Surg Oncol. 1085702024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao L and Zhang W: Tumor lysis syndrome in
a patient with advanced lung squamous cell carcinoma undergoing
combined therapy with a programmed cell death protein 1 inhibitor
and first-line chemotherapy: A case report. Oncol Lett. 28:3802024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong J, Cao T, Tanner N and Kundranda M:
When the tumor lyses: A case report on spontaneous tumor lysis
syndrome. Case Rep Oncol. 13:979–984. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chhabra R, Vidushi A, Rajpurohit S, Singh
J and Osama MA: Spontaneous tumor lysis syndrome in a case of
hepatocellular carcinoma with sarcomatoid differentiation. Indian J
Surg Oncol. 15:370–374. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohammad Khuzaini A, Mohd Baharudin JA, Md
Fauzi A, Zulkeflee HA, Abdul Halim H, Mazli SK and Osman NFB:
Tumour lysis syndrome in a neonate with transient abnormal
myelopoiesis. J Neonatal Perinatal Med. 17:269–273. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Busse WW and Szefler SJ: Digital health in
difficult-to-treat severe asthma. Lancet Respir Med. 11:578–579.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nzenwa IC, Berquist M, Brenner TJ, Ansari
A, Al-Fadhl HD, Aboukhaled M, Patel SS, Peck EE, Al-Fadhl MD,
Thomas AV, et al: Type B lactic acidosis in a patient with mantle
cell lymphoma. Case Rep Crit Care. 2023:70211232023.PubMed/NCBI
|
|
24
|
van den Haak DAC, Otten LS, Koenen H,
Smeets RL, Piet B, Pickkers P, Kox M and Heine RT: Evidence-based
rationale for low dose nivolumab in critically ill patients with
sepsis-induced immunosuppression. Clin Transl Sci. 16:978–986.
2023. View Article : Google Scholar : PubMed/NCBI
|