Introduction
Endometrial carcinoma (EC), originating from the
endometrial epithelium, is one of the four major reproductive
system tumors that pose a risk to women's health. The main risk
factors for EC are excessive endogenous or exogenous estrogens,
coupled with a lack of sufficient progesterone (1). In addition to conventional surgery and
radiochemotherapy, medroxyprogesterone acetate (MPA) is a principal
conservative treatment for EC. MPA therapy is primarily
administered through medication and intrauterine devices, making it
particularly suitable for patients expecting to preserve fertility.
MPA exerts its anticancer effects through progesterone receptor B
(2). However, due to individual
differences in progesterone receptor B among patients and the
emergence of MPA resistance during treatment, the complete response
rate is <70% for patients with atypical endometrial hyperplasia
or stage I EC, and the recurrence rate is ~25–40% (3,4).
Therefore, further research is needed for the clinical resolution
of the resistance of EC to MPA.
Paris polyphylla is a commonly used antitumor
herb in Traditional Chinese Medicine, and saponins are its main
active component. In addition to anthelmintic and anti-inflammatory
effects, saponins also possess anticancer properties (5). Polyphyllin VII (PPVII), a saponin
extracted from Paris polyphylla, exhibits anti-proliferative
and pro-apoptotic effects on various tumor cells (6), including ovarian (7), breast (8) and liver cancer cells (9). However, the inhibitory effects of
PPVII on EC have been rarely studied.
MicroRNAs (miRNAs) are short chain, non-coding RNAs
that regulate the transcription and translation levels of target
genes through binding to the 3′ untranslated regions (3′ UTRs) of
target genes. Besides, miRNAs participate in the regulation of
multiple biological processes, such as cell proliferation,
apoptosis, differentiation and metabolism (10). A recent review has illustrated that
miRNAs play a significant role in the occurrence and development of
tumors and could serve as biomarkers for tumor diagnosis, prognosis
assessment and treatment (11). As
a member of the miR-33 family, miR-33a-5p is involved in regulating
the proliferation, migration and invasion of various tumor cells
such as breast and colorectal cancer (12,13).
High-throughput genomics has demonstrated that miR-33a-5p may be a
potential molecular regulator in the development of EC (14). In our preliminary, unpublished
experiments, PPVII treatment significantly upregulated miR-33a-5p,
which was associated with increased cell death. Additionally,
miR-33a-5p has been reported to regulate key pathways involved in
cell cycle control and apoptosis, underscoring its potential
therapeutic relevance in EC (15).
Previous studies have suggested that F-box and
leucine rich repeat protein 16 (FBXL16) plays a crucial role in the
regulation of the cell cycle and apoptosis, and is associated with
poor prognosis in EC (16). Given
the connections between miR-33a-5p and cellular regulation, we
hypothesize that miR-33a-5p may influence EC progression by
targeting FBXL16, potentially offering antitumor effects.
PPVII has been proven to possess multiple biological
activities, including anti-inflammatory, antioxidant and antitumor
effects. Besides, PPVII can affect the growth and metastasis of
tumor cells by regulating the expression of miRNAs (17). However, the role and regulatory
mechanisms of PPVII in EC are still unclear. Notably, miR-33a-5p
may be a key molecule in the treatment of EC with PPVII. Hence, the
present study was designed to explore whether PPVII enhanced the
sensitivity of EC to MPA by regulating the expression of
miR-33a-5p. The findings of the present study are expected to
provide new insights and strategies for the clinical treatment of
EC.
Materials and methods
Cell culture, treatment and
transfection
The human EC cell (ECC) line, Ishikawa (cat. no.
CL-0283), was purchased from Wuhan Pricella Biotechnology Co., Ltd.
Briefly, ECCs were cultured in Dulbecco's Modified Eagle
Medium/Nutrient Mixture F-12 (Gibco; Thermo Fisher Scientific,
Inc.; cat. no. 12634010) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. A5669701) and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.;
cat. no. 15140148) and maintained in an incubator at 37°C with 5%
CO2 (Thermo Fisher Scientific, Inc.; cat. no. 51023126).
Then, a progesterone-resistant cell line, referred to as
Ishikawa/MPA-R, was established using MPA (MilliporeSigma). Cells
were cultured in conventional medium with an initial concentration
of 1 µM MPA. Then, the cells were cultured and passaged in 0.02%
ethylenediaminetetraacetic acid and 0.25% trypsin solution. The MPA
concentration was increased by 2.5 µM every 4 weeks. A stable
Ishikawa/MPA-R cell line was established when the MPA concentration
reached 10 µM and the cell proliferation rate was comparable to
that of the original Ishikawa cells using the MTT assay. The cells
were then cultured in 10 µM MPA to maintain resistance. The
inhibition rate and half maximal inhibitory concentration
(IC50) for MPA in both Ishikawa and Ishikawa/MPA-R cells
were determined using the MTT assay (16,18).
In the present study, PPVII was procured from MedChemExpress (cat.
no. HY-N0048).
Based on different treatments, Ishikawa/MPA-R cells
were divided into the following groups: i) The Control group, cells
were cultured with medium only; ii) the MPA group, cells were
cultured with medium containing MPA (30 µM); iii) the PPVII group,
cells were cultured with medium containing PPVII (2 µM); iv) the
MPA + PPVII group, cells were cultured with medium containing both
MPA (30 µM) and PPVII (2 µM); v) the PPVII + negative control (NC)
inhibitor group, Ishikawa/MPA-R cells were transfected with a NC
inhibitor and cultured with PPVII (2 µM); and vi) the PPVII +
miR-33a-5p inhibitor (miR-33a-5p in) group, Ishikawa/MPA-R cells
were transfected with a miR-33a-5p inhibitor and cultured with
PPVII (2 µM).
Before performing the transfection experiment, ECCs
were allocated to different treatment groups according to the
experimental design and seeded in culture dishes at a density of
5×105 cells/well. The transfection experiment was
conducted using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.;
cat. no. 11668500) according to the manufacturer's protocol.
Briefly, 5 µl Lipofectamine 2000 was diluted in 250 µl Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 5
min at room temperature. Separately, 1 µg of miR-33a-5p in or NC
was diluted in 250 µl Opti-MEM and then mixed with the
Lipofectamine 2000 mixture. The final mixture was added to the
cells and incubated at 37°C for 6 h. Following transfection, cells
were incubated for 48 h before proceeding with subsequent
experiments. The sequences for the miR-33a-5p in and negative
control were as follows: 5′-UGCAAUGCAACUACAAUGCAC-3′ and
5′-UUGCUUACGUAGCACUUACGU-3′, respectively (Shanghai GenePharma Co.,
Ltd.).
MTT assay for cell viability
The experiment was conducted according to the MTT
assay kit protocol (Merck KGaA; cat. no. 475989). First, cells were
seeded into a 96-well plate at a density of 5,000 cells/well, with
a culture volume of 100 µl. After treatment of the cells, the
medium was replaced and 10 µl MTT solution was added for 4 h of
incubation. The medium in each well was removed and 100 µl DMSO was
added to dissolve the formazan crystals. The plate was shaken for
10 min to ensure complete dissolution. Subsequently, the optical
density at 570 nm was measured using a microplate reader, and the
cell viability and half maximal inhibitory concentration values for
each group were calculated (19,20).
Clonogenic assay
Cells from each group were grown to the logarithmic
phase, seeded into 6-well plates at a density of 300 cells/well and
cultured under the aforementioned conditions. The observation
lasted for 7–14 days and the medium was replaced every 3 days
during the culture period. At the end of the culture, cells were
fixed with 4% paraformaldehyde for 20 min at room temperature and
then stained with 1% crystal violet dye (Sangon Biotech, Co., Ltd.)
for 5 min at room temperature. A digital camera (Canon, Inc.) was
used to collect images and the number of cell colonies (>50
cells as one colony) was counted using a light microscope (Olympus
Corporation). The experiment was independently performed in
triplicate.
Flow cytometry
Ishikawa/MPA-R cells were cultured in 6-well plates
at a density of 5×105 cells/well for 24 h. Next, cells
in the logarithmic growth phase were collected and washed 2 or 3
times with phosphate-buffered saline (PBS) for the following
experiments. i) Cell cycle analysis: Cells were fixed with 70%
ethanol at −20°C for at least 2 h. Then, the cells were washed with
PBS and centrifuged (300 × g, 5 min, 4°C) to remove the ethanol.
Subsequently, the cells were stained with propidium iodide (Abcam;
cat. no. ab14083) for 30 min at 37°C. Then, flow cytometry
(LSRFortessa; BD Biosciences) was performed to analyze the stained
cells to determine the proportion of cells in different cell cycle
stages (G0/G1, S and G2/M). Lastly, the flow cytometry data were
analyzed using FlowJo v10.0 software (FlowJo LLC). ii) Cell
apoptosis analysis: Early apoptotic cells were labeled with
fluorescein isothiocyanate-conjugated annexin V (Abcam; cat. no.
ab14085) and late apoptotic and necrotic cells were stained with
propidium iodide. The staining was performed at room temperature in
the dark for 15 min. Then, the cells were gently washed with PBS to
remove unbound markers, followed by resuspension in 1X binding
buffer provided in the Annexin V-FITC Apoptosis Detection Kit
(Abcam; cat. no. ab14085). The apoptosis rate of cells was analyzed
using a flow cytometer (LSRFortessa; BD Biosciences) and calculated
with FlowJo software.
Western blotting
Cells from each group were lysed on ice using
radioimmunoprecipitation assay lysis buffer (Biosharp Life
Sciences; cat no. BL504A), and the supernatant was collected to
measure protein concentration using the bicinchoninic acid assay.
Equal amounts of denatured proteins (20 µg per lane) were separated
on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Then, the proteins were transferred onto a polyvinylidene fluoride
membrane. Subsequently, the membrane was blocked with 5% bovine
serum albumin (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
A5256701) at room temperature for 1 h and then incubated with the
primary antibodies on a shaker at 4°C overnight. The next day, the
membrane was washed three times with TBST (containing 0.1% Tween
20), 10 min each time. Subsequently, the membrane was incubated
with HRP-conjugated secondary antibodies for 1–2 h at room
temperature and washed three times for 10 min each with TBST. Then,
bands were developed using an enhanced chemiluminescence reagent
(Abcam; cat. no. ab133406) on the ImageQuant LAS 500 system (GE
Healthcare Life Sciences), and the band intensities were analyzed
using ImageJ software (version 1.54; National Institutes of
Health). Specific information on the antibodies (all from Abcam)
used in this experiment were as follows: B-cell lymphoma 2 (Bcl-2;
cat. no. ab182858; 1:2,000); Bcl-2-associated X (Bax; cat. no.
ab32503; 1:1,000), Cyclin D1 (cat. no. ab134175; 1:1,000);
Cyclin-dependent kinase 4 (CDK4; cat. no. ab108357; 1:400); FBXL16
(cat. no. ab272898; 1:100); GAPDH (cat. no. ab9485; 1:2,500); and
secondary antibody (cat. no. ab288151; 1:10,000).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using the
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The quality and
quantity of the extracted RNA were assessed using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.). Then, 1 µg of
total RNA was reverse-transcribed into cDNA using HiScript III 1st
Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd.; cat. no. R312)
according to the manufacturer's instructions. qPCR was conducted
using Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech Co.,
Ltd.; cat. no. Q712) following the kit protocol. The reaction
conditions were set as follows: Stage 1, 95°C for 30 sec; stage 2,
40 cycles of denaturation at 95°C for 10 sec and annealing at 60°C
for 10 sec; stage 3, extension at 72°C for 30 sec. The results were
calculated using the 2−ΔΔCq method to determine the
relative expression levels of the target genes (21), with U6 or GAPDH serving as the
internal control. The primers for all genes were designed based on
sequences obtained from miRBase (Table
I).
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Gene | Primer (5′-3′) |
|---|
| miR-33a-5p | Forward:
TGCAATGCAACTACAATGCAAA |
| (human) | Reverse:
CTCAACTGGTGTCGTGGAGTCG |
|
| GCAATTCAGTTGAG |
| U6 (human) | Forward:
CTCGCTTCGGCAGCACA |
|
| Reverse:
AACGCTTCACGAATTTGCGT |
| FBXL16 | Forward:
TCTGGTATTTCTCGGCCTGC |
| (human) | Reverse:
ACGTTGTAGAGCTCCTTGGC |
| GAPDH | Forward:
ATGGGCAGCCGTTAGGAAAG |
| (human) | Reverse:
ATCACCCGGAGGAGAAATCG |
Dual-luciferase reporter gene
assay
The TargetScan database (https://www.targetscan.org/vert_72/) was used to
predict the potential binding sites of miR-33a-5p in the FBXL16
3′UTR. The sequences of wild-type or mutated miR-33a-5p targeting
the FBXL16 3′UTR were cloned into the pMIR-REPORT luciferase vector
(Shanghai GenePharma Co., Ltd.). Subsequently, the cells were
cultured and co-transfected with the reporter vectors and
miR-33a-5p mimics or NC miRNAs using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. After 48 h, the luciferase activity was measured
using the Dual-Luciferase Reporter Assay System (Promega
Corporation). The results were normalized to Renilla
luciferase activity to control for transfection efficiency
(15). The sequences for the
miR-33a-5p mimics and NC were as follows:
5′-GUGCAUUGUAGUUGCAUUGCA-3′ (sense) and 5′-UGCAAUGCAACUACAAUGCAC-3′
(anti-sense); 5′-UGUACGUAUCGUAGCAUGUCA-3′ (sense) and
5′-ACAUGCUACGAUACGUACAGU-3′ (anti-sense), respectively.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 (Dotmatics) and SPSS 26.0 (IBM Corp.). All data are
presented as the mean ± standard deviation. A two-tailed
independent samples t-test was adopted for comparisons between two
groups. For comparisons among multiple groups, a one-way analysis
of variance followed by Tukey's post-hoc test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
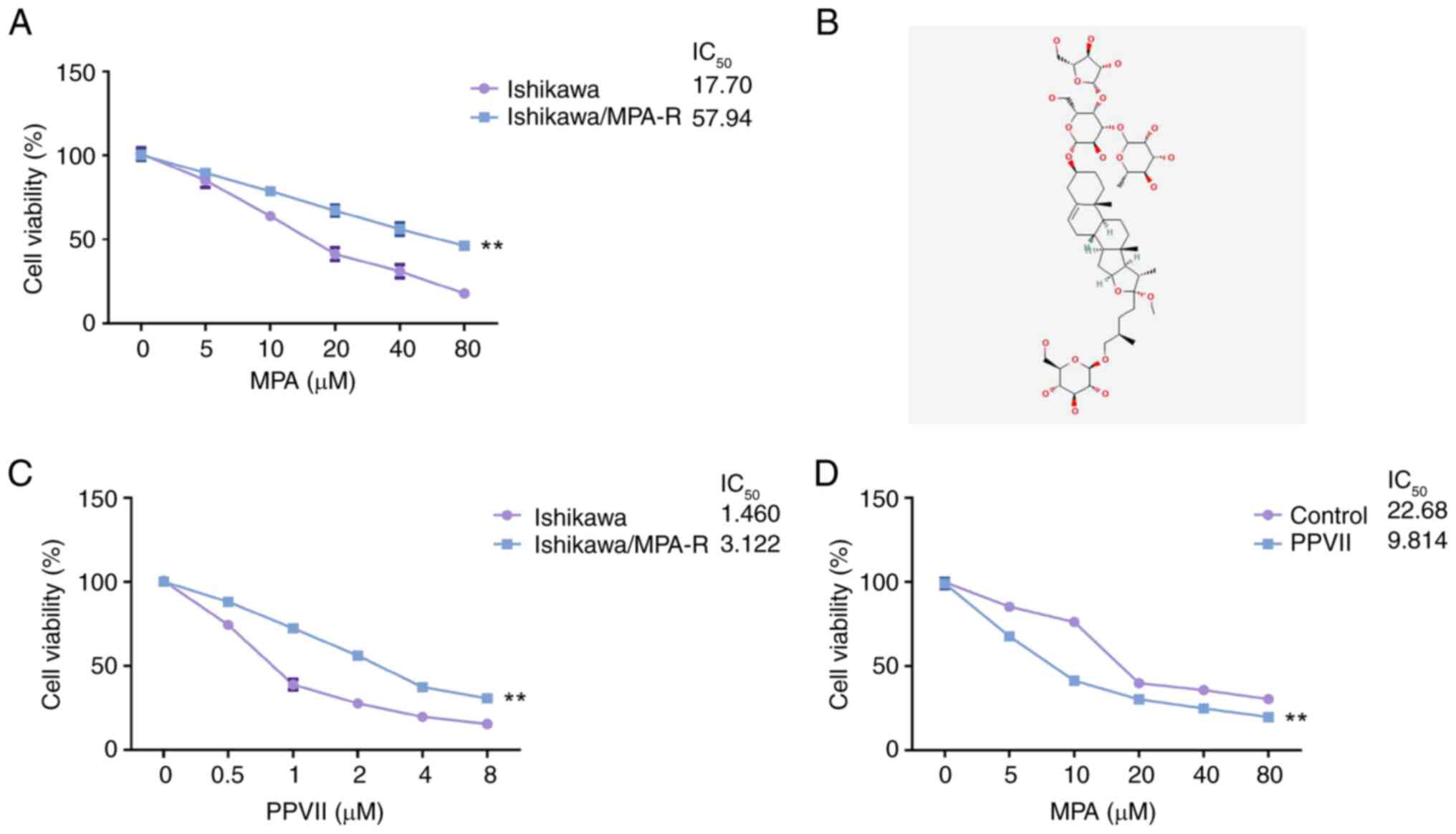
PPVII significantly increases the
sensitivity of ECCs to MPA
The MTT results showed a decrease in both Ishikawa
and Ishikawa/MPA-R cell viability as the concentrations of MPA
increased (0, 5, 10, 20, 40 and 80 µM), with a more notable decline
in the Ishikawa cells. Notably, the higher viability of
Ishikawa/MPA-R cells compared with Ishikawa cells at increasing MPA
concentrations confirmed the successful construction of the
MPA-resistant Ishikawa cell line (P<0.01; Fig. 1A). Following incubation with PPVII
(Fig. 1B), the viability of the
Ishikawa and Ishikawa/MPA-R cells exhibited a downward trend with
increasing concentrations of PPVII (0, 0.5, 1, 2, 4 and 8 µM), with
a more significant reduction in Ishikawa cell viability (P<0.01;
Fig. 1C). Compared with the
untreated Ishikawa/MPA-R control group, Ishikawa/MPA-R cells
treated with PPVII (2 µM) displayed a more notable decrease in cell
viability at various concentrations of MPA (0, 5, 10, 20, 40 and 80
µM; P<0.01; Fig. 1D). These
outcomes indicate that PPVII significantly increased the
sensitivity of ECCs to MPA.
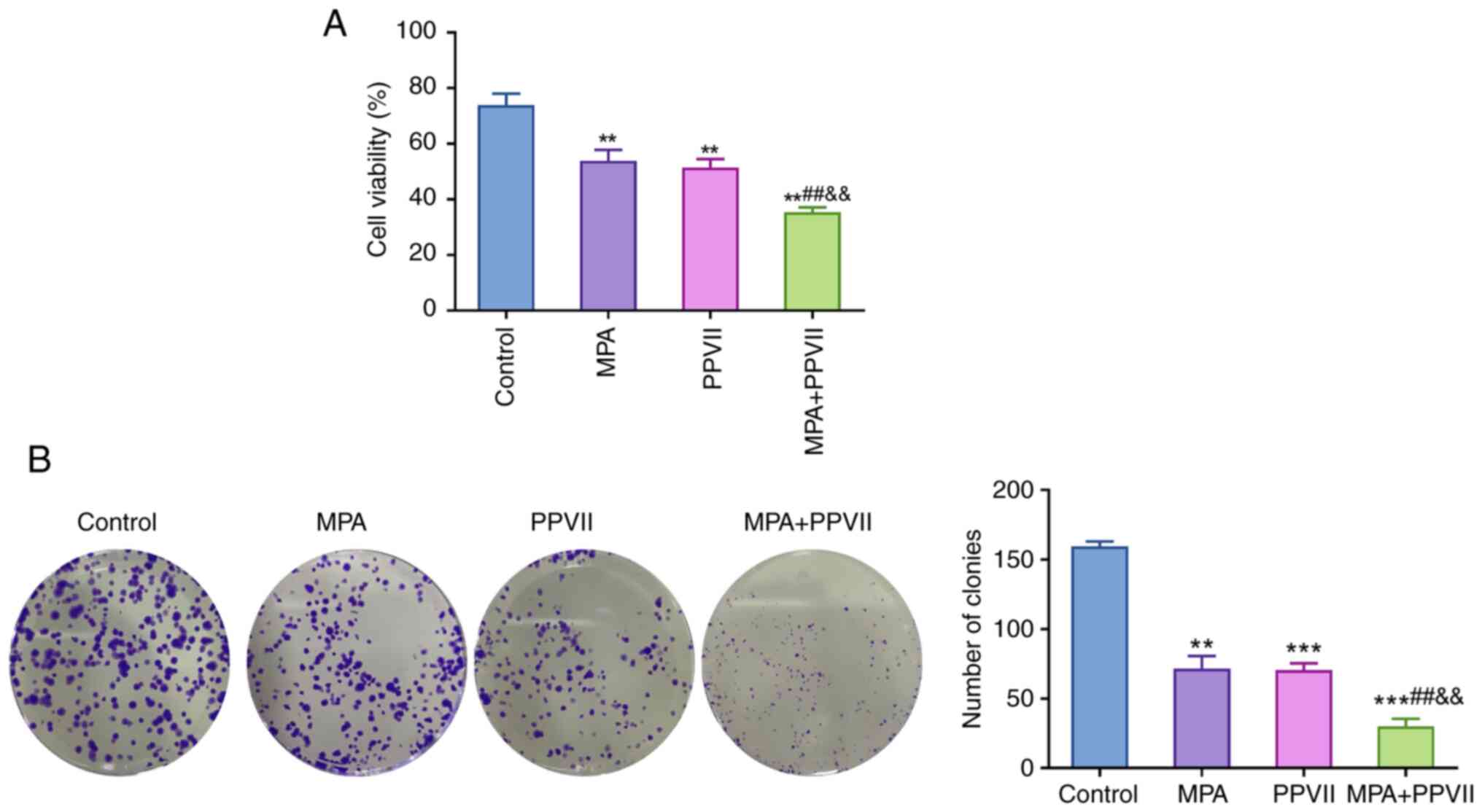
PPVII synergizes with MPA to inhibit
the viability of ECCs
Compared with the control group, a significant
decrease was observed in the viability of Ishikawa/MPA-R cells
treated with MPA and PPVII alone (P<0.01). Furthermore, the
combined treatment of MPA + PPVII resulted in a more notable
reduction in cell viability (P<0.01), indicating a synergistic
effect (Fig. 2A). As shown in
Fig. 2B, the clonogenic assay
further confirmed the synergistic effects of MPA and PPVII in the
Ishikawa/MPA-R cells. The number of colonies formed in the control
group was significantly higher relative to the treatment groups.
Treatment with MPA and PPVII alone markedly reduced the number of
colonies (P<0.01 or P<0.001), while the combined treatment of
MPA + PPVII led to an even greater decrease (P<0.001; Fig. 2B). These results highlight the
potential of the combined treatment to more effectively inhibit ECC
proliferation and colony formation as opposed to the treatment with
either agent alone.
PPVII combined with MPA increases
apoptosis and inhibits the cell cycle in ECCs
Flow cytometry analysis showed a significant
increase in the apoptosis rates of Ishikawa/MPA-R cells treated
with MPA and PPVII alone (P<0.01; Fig. 3A). The combined treatment of MPA +
PPVII further enhanced the apoptosis levels compared with the MPA
and PPVII alone groups (P<0.01; Fig.
3A). These findings indicated that the synergistic effect
involved the promotion of apoptotic cell death. Notably, the
observed 10% apoptotic cells in the Control group without treatment
could be attributed to baseline apoptosis, which is common in cell
cultures due to natural cell turnover and stress conditions.
Moreover, the cell cycle analysis displayed in Fig. 3B demonstrated the impact of MPA and
PPVII on cell cycle distribution. Both MPA and PPVII treatments led
to significant cell cycle arrest at the G0/G1 phase compared with
the Control group (P<0.05). The combined treatment resulted in
an even greater accumulation of cells in the G0/G1 phase and a
reduction of cells in the S phase (P<0.01), indicating enhanced
cell cycle arrest. This suggests that the combination treatment of
MPA and PPVII effectively halted cell cycle progression.
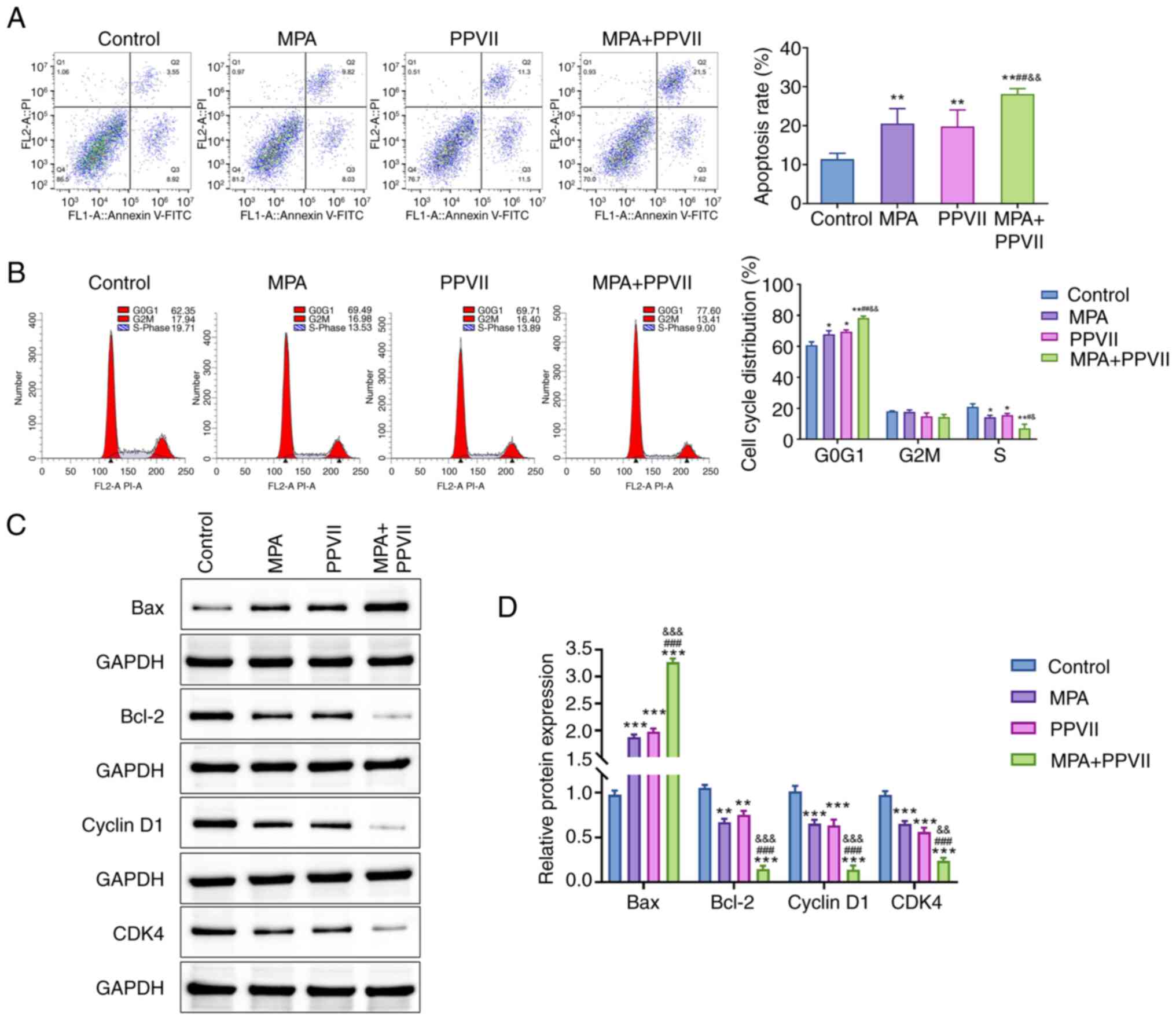 | Figure 3.PPVII combined with MPA promotes the
apoptosis of endometrial carcinoma cells. (A) The apoptosis level
of Ishikawa/MPA-R cells in each group, as determined using flow
cytometry. (B) The cell cycle changes in Ishikawa/MPA-R cells in
each group, as determined using flow cytometry. (C) Western
blotting was used to detect the protein expression of Bax, Bcl-2,
Cyclin D1 and CDK4 in Ishikawa/MPA-R cells in each group, which was
(D) semi-quantified. Data are presented as the mean ± standard
deviation (n=3); *P<0.05, **P<0.01, ***P<0.001 vs. Control
group; #P<0.05, ##P<0.01,
###P<0.01 vs. MPA group; &P<0.05,
&&P<0.01,
&&&P<0.001 vs. PPVII group. PPVII,
polyphyllin VII; MPA, medroxyprogesterone acetate; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X; CDK4, Cyclin-dependent kinase
4. |
In addition, western blot analysis was performed to
investigate the synergistic effects of MPA and PPVII on cell
apoptosis and cell cycle regulation (Fig. 3C and D). The results revealed that,
compared with the Control group, the expression levels of Bax
protein were significantly higher in the treatment groups
(P<0.001), while the expression levels of Bcl-2, Cyclin D1 and
CDK4 were significantly lower (P<0.01 or P<0.001). Notably,
the changes in the MPA + PPVII group were the most notable
(P<0.01 or P<0.001). These results indicate that the
combination of PPVII and MPA enhanced apoptosis and inhibited the
cell cycle in ECCs.
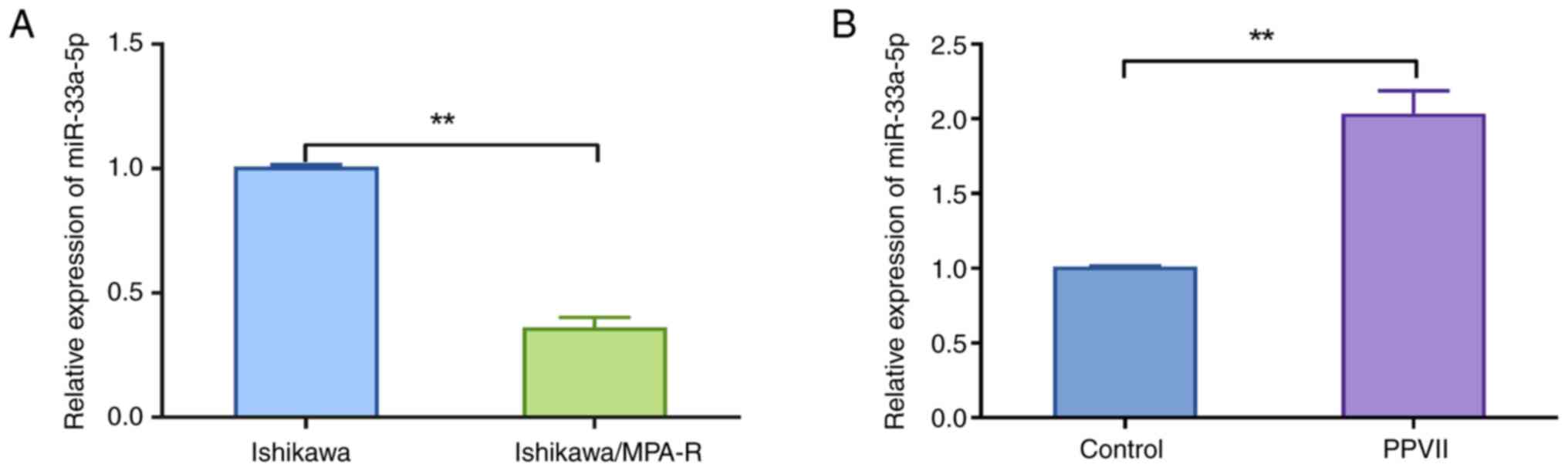
PPVII increases the sensitivity of
ECCs to MPA by upregulating miR-33a-5p
Next, the mechanisms by which PPVII regulates the
expression of miR-33a-5p were investigated. miR-33a-5p was chosen
for investigation due to its known role in regulating cell
proliferation, apoptosis, and drug sensitivity in various cancers.
Given PPVII's antitumor properties, it was hypothesized that it
might influence miR-33a-5p expression as a potential mechanism for
enhancing MPA sensitivity in endometrial cancer cells. Quantitative
analysis revealed that the miR-33a-5p expression was significantly
decreased in Ishikawa/MPA-R cells compared with Ishikawa cells
(P<0.01; Fig. 4A). Additionally,
treatment with PPVII significantly increased the expression of
miR-33a-5p in Ishikawa/MPA-R cells compared with the Control group
(P<0.01; Fig. 4B).
To further elucidate the role of miR-33a-5p in ECCs,
the expression of miR-33a-5p was manipulated and its effects were
assessed. Transfection of Ishikawa/MPA-R cells with miR-33a-5p in
was confirmed to be successful, as evidenced by a significant
downregulation of miR-33a-5p expression compared with the in NC
group (P<0.01; Fig. S1A). As
showed in Fig. 5A, cells
transfected with miR-33a-5p inhibitor resulted in a notable
downregulation of miR-33a-5p expression compared with the PPVII +
in NC group (P<0.01). This inhibition of miR-33a-5p led to a
significant elevation in cell viability (P<0.01; Fig. 5B) and clonogenic capacity
(P<0.01; Fig. 5C), a reduction
in apoptosis levels (P<0.05; Fig.
5D), accompanied by an increase in the proportion of cells in
the G1 phase and a decrease in the proportion of cells in the S
phase compared with the PPVII + in NC group (P<0.05; Fig. 5E). Western blot analysis further
supported these findings, showing that the PPVII + miR-33a-5p in
group exhibited a significant reduction in the Bax protein level
(P<0.001) and a notable elevation in the protein expression
levels of Bcl-2, Cyclin D1, and CDK4 compared with the PPVII + in
NC group (P<0.01; Fig. 5F).
These findings suggest that PPVII enhanced the sensitivity of ECCs
to MPA, and the inhibition of miR-33a-5p reversed this effect,
which indicated the critical role of miR-33a-5p in mediating the
response of ECCs to PPVII and MPA.
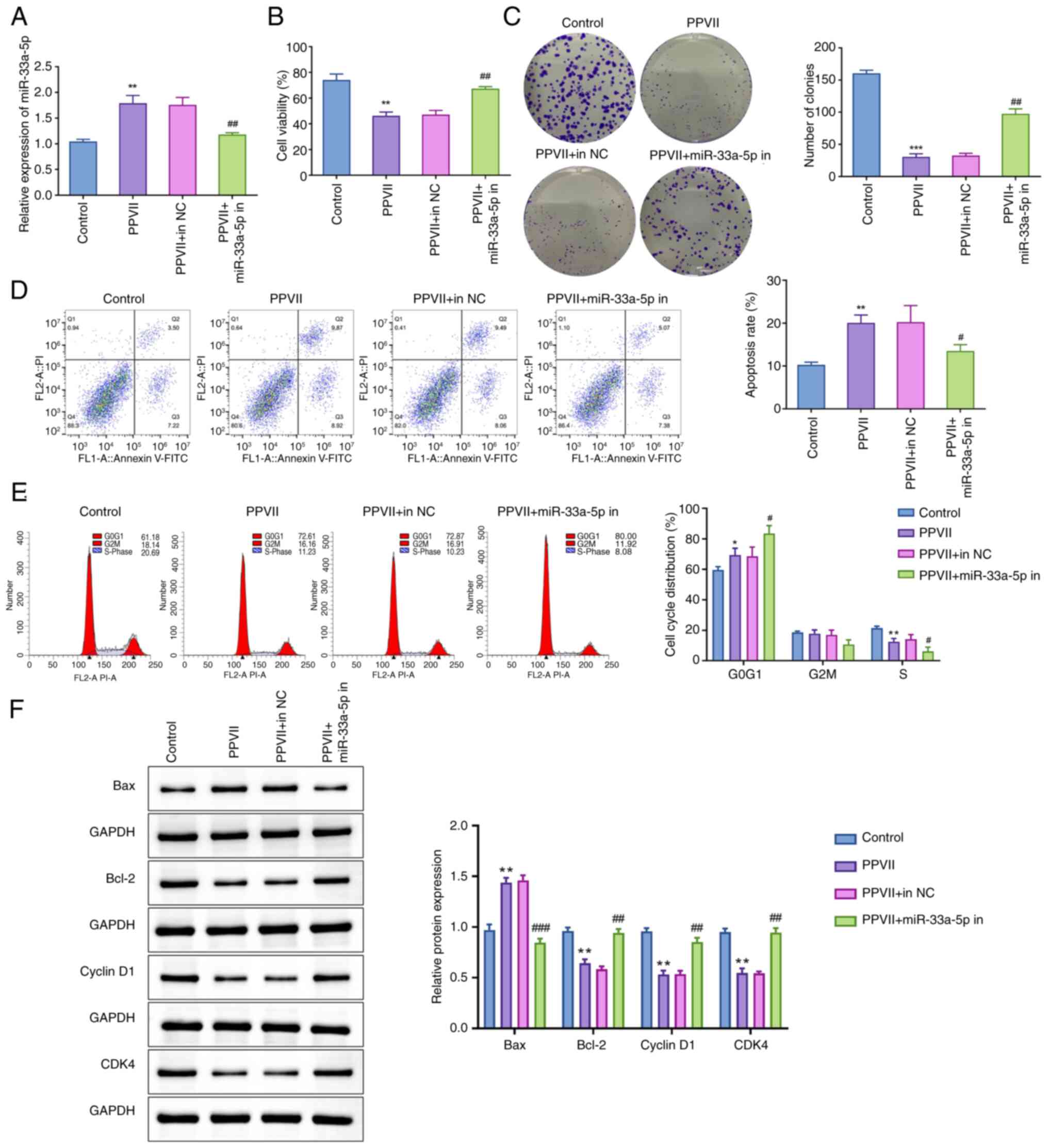 | Figure 5.Inhibition of miR-33a-5p suppresses
the anticancer effect of PPVII on endometrial carcinoma cells. (A)
The detection of miR-33a-5p in Ishikawa/MPA-R cells in each group
using reverse transcription-quantitative polymerase chain reaction.
(B) The viability of Ishikawa/MPA-R cells, as determined using MTT
assay. (C) Clonogenic assay to assess the clonogenic capacity of
Ishikawa/MPA-R cells in each group. (D) Flow cytometry to measure
the apoptosis level of Ishikawa/MPA-R cells in each group. (E) Flow
cytometry to analyze the cell cycle phase proportions of
Ishikawa/MPA-R cells in each group. (F) Western blotting to assess
the protein expression levels of Bax, Bcl-2, Cyclin D1 and CDK4 in
Ishikawa/MPA-R cells in each group. Data are presented as the mean
± standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001
vs. Control group; #P<0.05, ##P<0.01,
###P<0.001 vs. PPVII + in NC group. PPVII,
polyphyllin VII; MPA, medroxyprogesterone acetate; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X; CDK4, Cyclin-dependent kinase
4; miR, microRNA; NC, negative control; in, inhibitor. |
miR-33a-5p affects the viability and
proliferation of ECCs through targeted regulation of FBXL16
Preliminary bioinformatics analysis suggested that
miR-33a-5p might regulate FBXL16, guiding our investigation into
its functional role in ECCs. As shown in Fig. S2A, publicly available miRNA
sequencing data revealed a distinct miRNA expression profile in
PPVII-treated Ishikawa/MPA-R cells, with miR-33a-5p significantly
upregulated among other miRNAs. Pathway enrichment analysis
indicated that miR-33a-5p was involved in critical cellular
processes, including cell cycle regulation and mitotic progression
(Fig. S2B). Furthermore,
miR-33a-5p was predicted to target the FBXL16 gene as illustrated
in Fig. S2C.
To experimentally confirm whether miR-33a-5p
directly targets FBXL16, dual-luciferase reporter assays were
conducted. Firstly, the transfection efficiency of Ishikawa/MPA-R
cells with miR-33a-5p mimic was verified using RT-qPCR. As shown in
Fig. S1B, cells transfected with
the miR-33a-5p mimic exhibited a significant increase in miR-33a-5p
levels compared with the NC mimic group (P<0.01), which the
confirmed successful transfection with high efficiency. Next, the
dual-luciferase reporter assay results confirmed that miR-33a-5p
bound to a specific sequence within the 3′UTR of FBXL16 (Fig. 6A). Specifically, miR-33a-5p
significantly reduced the activity of the wild-type FBXL16 3′UTR
reporter gene (P<0.01), indicating direct binding and regulation
by miR-33a-5p. In contrast, cells with a mutated FBXL16 3′UTR
showed no significant change in luciferase activity, confirming the
specificity of miR-33a-5p binding to the wild-type sequence
(Fig. 6B).
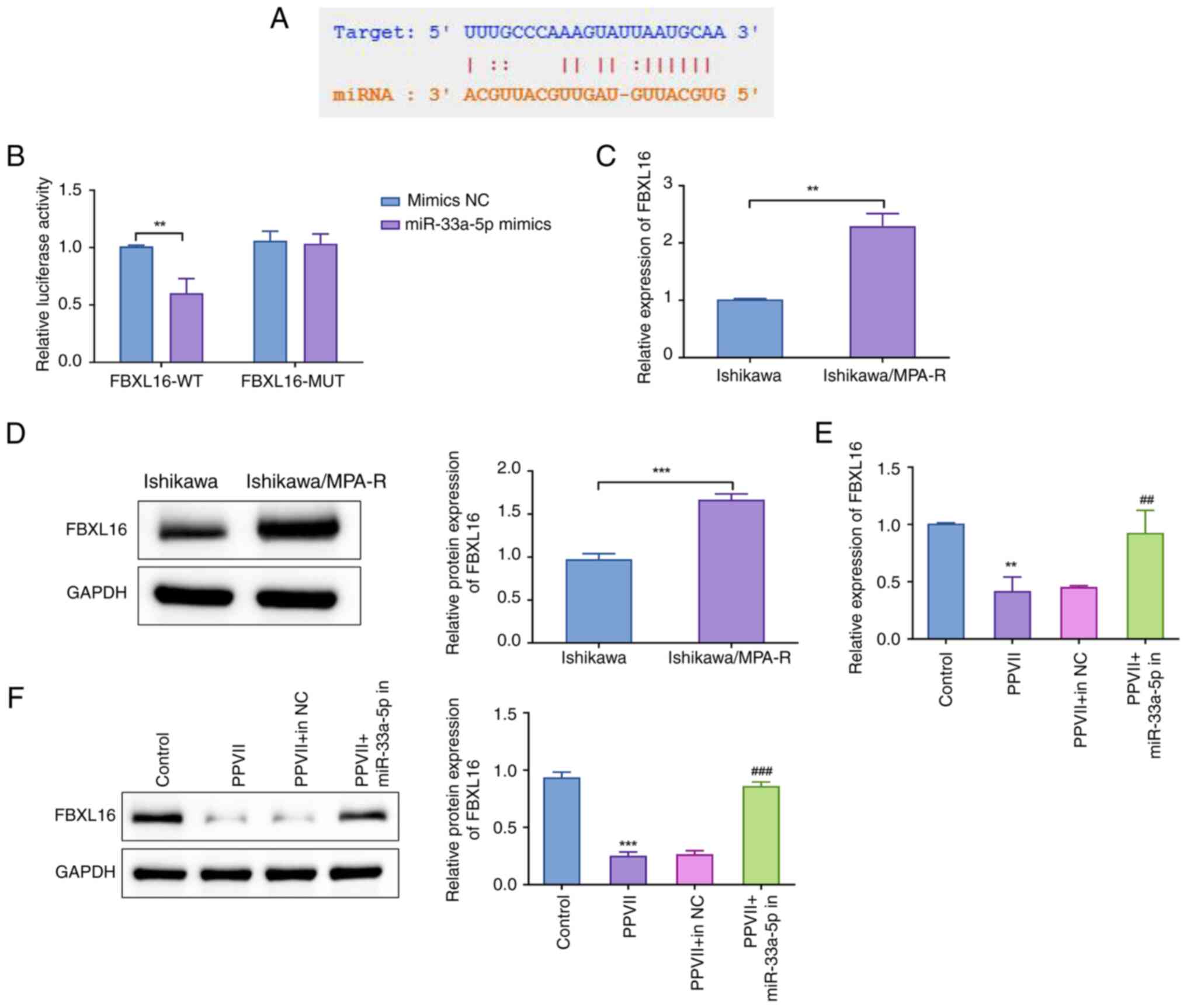 | Figure 6.Negative regulation of FBXL16 by
miR-33a-5p in endometrial carcinoma cells. (A) Prediction of the
potential binding site of miR-33a-5p and the FBXL16 3′ untranslated
region using the TargetScan database. (B) Dual-luciferase reporter
assay to investigate the targeting relationship between miR-33a-5p
and FBXL16; **P<0.01 vs. mimics NC group. Detection of the (C)
mRNA and (D) protein expression levels of FBXL16 in Ishikawa and
Ishikawa/MPA-R cells using RT-qPCR and western blotting. Detection
of the (E) mRNA and (F) protein expression levels of FBXL16 in
Ishikawa/MPA-R cells in each group using RT-qPCR and western
blotting. Data are presented as the mean ± standard deviation
(n=3). **P<0.01, ***P<0.001 vs. Ishikawa or Control group;
##P<0.01, ###P<0.001 vs. PPVII + in NC
group. PPVII, polyphyllin VII; FBXL16, F-box and leucine rich
repeat protein 16; MPA, medroxyprogesterone acetate; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; miR,
microRNA; NC, negative control; in, inhibitor; WT, wild-type; MUT,
mutated. |
The expression of FBXL16 in Ishikawa and
Ishikawa/MPA-R cells was then further examined. The expression of
FBXL16 was significantly higher in Ishikawa/MPA-R cells compared
with Ishikawa cells (P<0.01 or P<0.001; Fig. 6C and D). This indicated that FBXL16
was upregulated in MPA-resistant cells. To assess the effect of
PPVII and miR-33a-5p on FBXL16 expression, the mRNA and protein
levels of FBXL16 were measured in Ishikawa/MPA-R cells under
different treatment conditions. In the presence of PPVII, the mRNA
(P<0.01) and protein expression levels of FBXL16 (P<0.001)
were significantly downregulated compared with the control group
(Fig. 6E and F). Conversely, in the
PPVII + miR-33a-5p in group, there was a notable elevation in both
the mRNA (P<0.01) and protein expression levels of FBXL16
(P<0.001) compared with the PPVII + in NC group (Fig. 6E and F). These results suggest that
PPVII and miR-33a-5p inhibited the expression of FBXL16, whereas
the inhibition of miR-33a-5p led to the upregulation of FBXL16.
Discussion
EC ranks as the second most common gynecological
malignant tumor after cervical cancer, accounting for 20–30% of
gynecological malignant tumors. In some developed cities, the
incidence of EC has surpassed that of other gynecological malignant
tumors (22). PPVII is one of the
main active components with anticancer properties derived from
plants in the Paris polyphylla family. Generally, PPVII exerts its
anticancer effects by promoting apoptosis and necrosis, inhibiting
proliferation and migration, inducing autophagy and increasing drug
sensitivity in cancer cells. PPVII has been reported to treat
various tumors, such as gastric and small cell lung cancer
(23,24). It is also worth noting that PPVII
may exert anticancer effects through multiple pathways (25). For instance, PPVII can not only
reduce pro-inflammatory cytokines by inhibiting nuclear factor-κB
and mitogen-activated protein kinase (26), but also activate the c-Jun
N-terminal kinase pathway by inhibiting the phosphorylation of
phosphoinositide 3-kinase/protein kinase B/mechanistic target of
rapamycin, thereby inducing the autophagy of tumor cells (23,27).
In addition, PPVII can lead to cancer cell death by inducing
mitochondrial dysfunction (28).
Furthermore, PPVII may synergize with chemotherapeutic drugs to
increase the expression of p52 and activate the signaling pathways
such as mitogen-activated protein kinase, thereby inducing cancer
cell apoptosis (27) and inhibiting
cancer cell growth (29). At
present, the studies on the anticancer effects of PPVII on EC are
limited. In the present study, PPVII could effectively inhibit ECC
viability, and PPVII combined with MPA could inhibit the
proliferation of ECCs and promote apoptosis.
Epithelial-mesenchymal transition (EMT) refers to
the transformation of epithelial cells into mesenchymal cells to
acquire the differentiation and invasive abilities of mesenchymal
cells. EMT is also a crucial mechanism in the development of EC
(30). PPVII can attenuate EMT by
inhibiting the cancerous inhibitor of protein phosphatase
2A/protein kinase B/mechanistic target of the rapamycin axis,
thereby increasing the sensitivity of tumor cells to
chemotherapeutic agents (31). The
long non-coding RNA just proximal to the X-inactive specific
transcript/miR-33a-5p/twist-related protein 1 axis affects the EMT
process by activating the wingless-related integration
site/β-catenin signaling pathway, thus inhibiting cancer cell
metastasis (32). Hypoxia-inducible
factor expression is activated in cancer cells under hypoxic
conditions, which in turn upregulates miR-33a-5p expression,
affecting the EMT process and further inhibiting the invasive
ability of cancer cells (33). To
the best of our knowledge, no studies have thus far directly linked
PPVII and miR-33a-5p. However, in the present study, PPVII and MPA
treatments elevated the expression of miR-33a-5p in ECCs,
suggesting a potential regulatory relationship. Hence, more
detailed mechanistic studies, including validation of key pathways
and in vivo studies, are necessary to provide comprehensive
mechanistic insights into the synergistic effects of PPVII and MPA
in inhibiting EC growth. Furthermore, in the present study, the
inhibition of miR-33a-5p function weakened the anticancer effects
of PPVII and MPA in ECCs, indicating that miR-33a-5p might act as a
tumor suppressor and could play a key role in EC treatment.
FBXL16 is an important target gene of miR-33a-5p
(34,35), and its high expression is associated
with MPA resistance and poor prognosis in EC (16). FBXL16 promotes the dephosphorylation
of Cyclin D1 via the AKT serine/threonine kinase 1/glycogen
synthase kinase 3β/Cyclin D1 pathway, thus enhancing MPA resistance
in ECCs (16). Based on the studies
of FBXL16 in other tumor cells (16,36),
it can be speculated that FBXL16 may be involved in key biological
processes in ED, such as apoptosis, invasion and metastasis. The
present study demonstrated that FBXL16 was negatively regulated by
miR-33a-5p, which was consistent with the previous findings.
However, in addition to FBXL16, miR-33a-5p may regulate multiple
downstream target genes. Therefore, it is of significance to expand
future studies to other downstream targets of miR-33a-5p, providing
a more comprehensive understanding of the role of miR-33a-5p in the
biological pathways under investigation.
Despite the significant findings of the present
study, several limitations should be acknowledged. First, although
miR-33a-5p and its target gene, FBXL16, play a role in regulating
ECC proliferation, the precise regulatory mechanisms remain to be
explored. Specifically, the pathway through which miR-33a-5p
modulates FBXL16 expression and the biological significance of this
modulation warrant further investigation. Second, the present study
primarily focused on in vitro experiments. Future research
should incorporate in vivo studies to validate the findings
in a more complex biological context. These studies should involve
the use of animal models to evaluate the efficacy and safety of the
combined treatment of PPVII and MPA in EC. Moreover, the
implications of the present study suggest that PPVII could enhance
the sensitivity of ECCs to MPA, potentially offering a novel
therapeutic strategy. Therefore, it is imperative to conduct
clinical trials to confirm the effectiveness and safety of this
combination therapy for patients. In addition, previous studies
have shown that miR-33a-5p often collaborates with other miRNAs to
modulate key signaling pathways involved in cell growth and
survival (37,38). Notably, miR-33a-5p interacts with
miR-128-3p in lung cancer (37),
influencing processes such as cell proliferation, apoptosis and
metastasis. This interaction suggests a complex regulatory network
that may contribute to the resistance mechanisms observed in EC.
Thus, additional experiments are necessary to investigate the
relationship between miR-33a-5p and other miRNAs in EC, providing a
more comprehensive understanding of the therapeutic potential of
targeting miR-33a-5p alongside other miRNAs.
In conclusion, the present study demonstrated that
PPVII, alone and in combination with MPA, effectively inhibited ECC
proliferation by promoting apoptosis and inducing cell cycle
arrest. The upregulation of miR-33a-5p by PPVII enhanced the
sensitivity of MPA-resistant ECCs. The miR-33a-5p/FBXL16 axis may
play a crucial role in this regulatory mechanism. While the
findings of the present study provide significant insights, further
research is needed to explore the detailed mechanisms and validate
these results in in vivo and clinical settings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and Educational
Collaborative Innovation Fund of Jiangsu University (grant no.
JDYY2023073).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conception and design of the research and drafting
of the manuscript was conducted by HL; acquisition of data and
revision of the manuscript for important intellectual content was
conducted by YP and XZ; analysis and interpretation of data was
conducted by HL and XZ; statistical analysis was conducted by YP.
All authors contributed to editorial changes in the manuscript. All
authors read and approved the final version of the manuscript. All
authors have participated sufficiently in the work and agreed to be
accountable for all aspects of the work. HL and XZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Passarello K, Kurian S and Villanueva V:
Endometrial cancer: An overview of pathophysiology, management, and
care. Semin Oncol Nurs. 35:157–165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai D, Wolf DM, Litman ES, White MJ and
Leslie KK: Progesterone inhibits human endometrial cancer cell
growth and invasiveness: Down-regulation of cellular adhesion
molecules through progesterone B receptors. Cancer Res. 62:881–886.
2002.PubMed/NCBI
|
|
3
|
Baker J, Obermair A, Gebski V and Janda M:
Efficacy of oral or intrauterine device-delivered progestin in
patients with complex endometrial hyperplasia with atypia or early
endometrial adenocarcinoma: A meta-analysis and systematic review
of the literature. Gynecol Oncol. 125:263–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chae-Kim J, Garg G, Gavrilova-Jordan L,
Blake LE, Kim TT, Wu Q and Hayslip CC: Outcomes of women treated
with progestin and metformin for atypical endometrial hyperplasia
and early endometrial cancer: A systematic review and
meta-analysis. Int J Gynecol Cancer. 31:1499–1505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu XH, Li T, Fong CM, Chen X, Chen XJ,
Wang YT, Huang MQ and Lu JJ: Saponins from Chinese medicines as
anticancer agents. Molecules. 21:13262016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Jia J, Zhu W, Chen J, Zheng Q and Li
D: Therapeutic effects on cancer of the active ingredients in
rhizoma paridis. Front Pharmacol. 14:10957862023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao L, Liu Z, Deng X, Wang J, Sun L, Fan
L and Zhang Y: Polyphyllin VII induces mitochondrial apoptosis by
regulating the PP2A/AKT/DRP1 signaling axis in human ovarian
cancer. Oncol Rep. 45:513–522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du SG, Zhang HM, Ji YX, Tian YL, Wang D,
Zhu K, Zhang QG and Liu SP: Polyphyllin VII promotes apoptosis in
breast cancer by inhibiting MAPK/ERK signaling pathway through
downregulation of SOS1. Am J Chin Med. 52:885–904. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Chen Z, Wang Y, Li Y, Wang Z, Li F
and Xi X: Polyphyllin VII as a potential drug for targeting
stemness in hepatocellular cancer via STAT3 signaling. Curr Cancer
Drug Targets. 23:325–331. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Xie S, Yuan S, Zhang Y, Bai Y,
Chu L, Wu Z, Guo N, Wang Q and Zhang J: Metabotropic glutamate
receptor 8 is regulated by miR-33a-5p and functions as an oncogene
in breast cancer. J Oncol. 14:80020872021.PubMed/NCBI
|
|
13
|
Yan Y, Zhang D, Lei T, Zhao C, Han J, Cui
J and Wang Y: MicroRNA-33a-5p suppresses colorectal cancer cell
growth by inhibiting MTHFD2. Clin Exp Pharmacol Physiol.
46:928–936. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao C, Wei J, Tang T and Huang Z: Role of
microRNA-33a in malignant cells. Oncol Lett. 20:2537–2556. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing W, Li T, Wang Y, Qiang Y, Ai C and
Tang H: MiR-33a-5p targets NOMO1 to modulate human cardiomyocyte
progenitor cells proliferation and differentiation and apoptosis. J
Recept Signal Transduct Res. 41:476–487. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Han L, Zhong L, Zhuang X and Peng
Y: FBXL16 promotes endometrial progesterone resistance via
PP2A(B55α)/Cyclin D1 axis in Ishikawa. J Immunol Res.
2022:73722022022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao F, Pan C, Zhang Y, Yang J and Xing X:
Polyphyllin VII alleviates pulmonary hypertension by inducing
miR-205-5p to target the β-catenin pathway. Biomed Pharmacother.
167:1155162023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Zhang L, Che X, Li W, Liu Z and
Jiang J: Roles of SIRT1/FoxO1/SREBP-1 in the development of
progestin resistance in endometrial cancer. Arch Gynecol Obstet.
298:961–969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui Y, Wu H, Yang L, Huang T, Li J, Gong
X, Li L, Sun X, Mao F and Wang Y: Chlorpromazine sensitizes
progestin-resistant endometrial cancer cells to MPA by upregulating
PRB. Front Oncol. 11:6658322021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murakami H, Hayashi M, Terada S and
Ohmichi M: Medroxyprogesterone acetate-resistant endometrial cancer
cells are susceptible to ferroptosis inducers. Life Sci.
325:1217532023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Que Z, Luo B, Yu P, Qi D, Shangguan W,
Wang P, Liu J, Li Y, Li H, Ke R, et al: Polyphyllin VII induces CTC
anoikis to inhibit lung cancer metastasis through EGFR pathway
regulation. Int J Biol Sci. 19:5204–5217. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiang Y, Wan F, Ren Y, Yang D, Xiang K,
Zhu B, Ruan X, Li S, Zhang L, Liu X, et al: Polyphyllin VII induces
autophagy-dependent ferroptosis in human gastric cancer through
targeting T-lymphokine-activated killer cell-originated protein
kinase. Phytother Res. 37:5803–5820. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song C, Pan B, Yang X and Tang W:
Polyphyllin VII suppresses cell proliferation, the cell cycle and
cell migration in colorectal cancer. Oncol Lett.
21:252021.PubMed/NCBI
|
|
26
|
Zhang C, Li C, Jia X, Wang K, Tu Y, Wang
R, Liu K, Lu T and He C: In Vitro and in vivo anti-inflammatory
effects of polyphyllin VII through downregulating MAPK and NF-κB
pathways. Molecules. 24:8752019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Jia X, Wang K, Bao J, Li P, Chen
M, Wan JB, Su H, Mei Z and He C: Polyphyllin VII induces an
autophagic cell death by activation of the JNK pathway and
inhibition of PI3K/AKT/mTOR pathway in HepG2 cells. PLoS One.
11:e01474052016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee MS, Yuet-Wa JC, Kong SK, Yu B,
Eng-Choon VO, Nai-Ching HW, Chung-Wai TM and Fung KP: Effects of
polyphyllin D, a steroidal saponin in Paris polyphylla, in growth
inhibition of human breast cancer cells and in xenograft. Cancer
Biol Ther. 4:1248–1254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang W, Zhang D, Ma X, Liu Z, Li F and Wu
D: Paris saponin VII suppressed the growth of human cervical cancer
Hela cells. Eur J Med Res. 19:412014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cassier PA, Navaridas R, Bellina M, Rama
N, Ducarouge B, Hernandez-Vargas H, Delord JP, Lengrand J, Paradisi
A, Fattet L, et al: Netrin-1 blockade inhibits tumour growth and
EMT features in endometrial cancer. Nature. 620:409–416. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ashrafizadeh M, Zarrabi A, Hushmandi K,
Kalantari M, Mohammadinejad R, Javaheri T and Sethi G: Association
of the epithelial-mesenchymal transition (EMT) with cisplatin
resistance. Int J Mol Sci. 21:40022020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan J, Fang S, Tian H, Zhou C, Zhao X,
Tian H, He J, Shen W, Meng X, Jin X and Gong Z: lncRNA
JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis
of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer.
19:92020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo XF, Wang AY and Liu J:
HIFs-MiR-33a-Twsit1 axis can regulate invasiveness of
hepatocellular cancer cells. Eur Rev Med Pharmacol Sci.
20:3011–3016. 2016.PubMed/NCBI
|
|
34
|
Cao D, Wang J, Ji Z, Shangguan Y, Guo W,
Feng X, Xu K and Yang J: Profiling the mRNA and miRNA in peripheral
blood mononuclear cells in subjects with active tuberculosis.
Infect Drug Resist. 13:4223–4234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghosh N, Saha I, Sharma N and Sarkar JP:
Human miRNAs to identify potential regions of SARS-CoV-2. ACS
Omega. 7:21086–21101. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim YJ, Zhao Y, Myung JK, Yi JM, Kim MJ
and Lee SJ: Suppression of breast cancer progression by FBXL16 via
oxygen-independent regulation of HIF1α stability. Cell Rep.
37:1099962021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan J, Zhou C, Zhao X, He J, Tian H, Shen
W, Han Y, Chen J, Fang S, Meng X, et al: A two-miRNA signature
(miR-33a-5p and miR-128-3p) in whole blood as potential biomarker
for early diagnosis of lung cancer. Sci Rep. 8:166992018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Costa V, De Fine M, Carina V, Conigliaro
A, Raimondi L, De Luca A, Bellavia D, Salamanna F, Alessandro R,
Pignatti G, et al: How miR-31-5p and miR-33a-5p regulates SP1/CX43
expression in osteoarthritis disease: Preliminary insights. Int J
Mol Sci. 22:24712021. View Article : Google Scholar : PubMed/NCBI
|