Introduction
As one of the most common gastrointestinal tumors,
gastric cancer (GC) has the 4th and 5th highest mortality and
morbidity rates, respectively. A worldwide estimate of 769,000
fatalities from GC and >1 million new cases were reported in
2020 (1). Among malignant tumors in
the Chinese population, the incidence and death rates of GC rank
second and third, respectively (2,3). GC
development is a complex process influenced by a variety of
factors, including malnutrition, infections and genetics. Viruses
(Epstein-Barr virus), bacteria (Helicobacter pylori) and
inherited mutations in specific genes (GSTM1-null or CDH1 gene) may
be the main risk factors for GC (4). In addition, lifestyle factors, such as
nitroso-rich diets, alcohol consumption and smoking, are also
associated with GC development (5).
Currently, the basic treatment for GC is chemotherapy (6), but it has limited efficacy and serious
side effects (6). Thus, identifying
safe and available therapeutic drugs for GC treatment is
urgent.
Natural herbs and their active ingredients, which
have low toxicity and multiple targets, have recently demonstrated
considerable potential as antitumor agents (7,8).
Luteoloside, a natural flavonoid with diverse biological
activities, is a main component of Ecliptae herba (9), also named ‘Mo-Han-Lian’, which is the
dried aerial portion of Eclipta prostrata L. This plant is
distributed throughout China (10)
and possesses hypolipidemic (11),
antitumor (12) and
anti-inflammatory (13) properties
due to its rich composition of bioactive compounds (14). Luteoloside also exerts
pharmacological effects on the cardiovascular system and protective
effects on the neurological system (15), and possesses anti-inflammatory
(16), antiviral (17) and anti-tumor properties (18). Furthermore, it has been demonstrated
to block the proliferation and migration of human oral cancer cells
by decreasing p38 phosphorylation and downregulating MMP-2
expression (19). Thus, luteoloside
is of significant medical importance in cancer treatment.
To date, the influence of luteoloside on the
proliferation, invasion and migration ability of GC cells has not
been reported, to the best of our knowledge. In the present study,
a network pharmacology-based strategy was used to determine the
targets of the luteoloside associated with GC development. A
protein-protein interaction (PPI) network was established and
subsequently, a network topology was developed and functional
enrichment analyses were performed. Finally, the mechanism of
action of luteoloside was investigated using in vitro
experiments, which validated the bioinformatics predictions by
demonstrating the biological effects of luteoloside on GC cells,
such as inhibiting their proliferation, migration and invasion.
These results were consistent with the predicted involvement of the
p53/p21 pathway and other molecular targets, thereby providing a
theoretical reference for further experiments and the clinical use
of luteoloside for GC treatment.
Materials and methods
Acquisition of GC target genes
The key word ‘gastric cancer’ was entered into the
Online Mendelian Inheritance in Man (OMIM; http://omim.org/) and GeneCards (https://www.genecards.org/) databases to search for GC
target genes. After compiling the results and removing duplicates,
the remaining targets were uploaded to the UniProt database
(https://www.uniprot.org/) to retrieve the names
of the corresponding genes that are implicated in GC. After
confirming the gene names in UniProt, they were reuploaded into the
GeneCards database to cross-verify their specific roles and
relevance in GC.
Acquisition of luteoloside target
genes
The relevant targets and 3D structures of
luteoloside were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/), and potential
targets from compound structures were predicted using
SwissTargetPrediction (http://swisstargetprediction.ch/) and SuperPred
(https://prediction.charite.de/index.php). In addition,
the STITCH (http://stitch.embl.de/) database was
used to predict genes that interact with the compounds.
Determination of the common targets
between luteoloside and GC
The potential targets of luteoloside and the targets
of GC were uploaded to Venny v2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html)
to create a Venn diagram to identify the common targets and the
compounds corresponding to the common targets. The intersecting
targets indicated those that luteoloside may act on GC.
Enrichment analyses
The Database for Annotation, Visualization and
Integrated Discovery (DAVID; http://david.ncifcrf.gov/) was used to perform Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis and Gene Ontology (GO) functional enrichment analysis of
the common targets between luteoloside and GC. The GO functional
enrichment included the following categories: Molecular function
(MF), cellular component (CC) and biological process (BP). The
screening criteria were P<0.01, overlap ≥3 and enrichment ≥1.5.
The enrichment results in the categories BP, CC and MF were plotted
as bar graphs and the top 20 KEGG analysis results were plotted as
a bubble chart.
Establishment of core target and PPI
networks
The common targets between luteoloside and GC were
uploaded to the STRING database (https://string-db.org/) and Cytoscape v3.8.0 software
was used for visualization, where the option of ‘Multiple protein’
was selected, the protein species was set as ‘Homo sapiens’,
the confidence was set as ‘highest confidence (≥0.900)’ and the
other parameters were kept at the default settings, resulting in
the generation of a network diagram and data on the protein
interactions with the highest correlation. The data were saved in
TSV format. The Analyze Network tool in Cytoscape v3.8.0 software
was used to construct the PPI network of luteoloside in the
treatment of GC. Network analysis identified the pathways with the
greatest involvement and these were considered the main signaling
pathways of luteoloside in the treatment of GC.
Molecular docking
3D structures of proteins were obtained from the
Protein Data Bank (https://www.rcsb.org/). In addition, PyMOL version 2.4
(https://pymol.org/2/) was used for eliminating
hydrogenated and excess ligands and water molecules, AutoDock
version 4.2.6 (http://autodock.scripps.edu/) for molecular docking
and Discovery Studio version 4.5 (https://discover.3ds.com/discovery-studio-visualizer-download)
for visualizing the docking results. Binding energies <0 are
generally considered to indicate the spontaneous binding of two
molecules, with smaller binding energies indicating a more stable
conformation.
Cell culture and grouping
The human GC cell line NCI-N87 (CRL-5822) was
purchased from the American Type Culture Collection and incubated
in RPMI-1640 medium (cat. no. R8758; Sigma-Aldrich; Merck KGaA)
with 10% fetal bovine serum (cat. no. 16000-044; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin at 37°C in
a humidified atmosphere containing 5% CO2. Upon reaching
70–80% confluence, the cultured cells were partitioned into four
groups as follows: i) Control group: NCI-N87 cells cultured under
normal conditions; ii) low-concentration group: 25 µM luteoloside
added to the NCI-N87 cell culture; iii) medium-concentration group:
50 µM luteoloside added to the NCI-N87 cell culture; and iv)
high-concentration group: 100 µM luteoloside added to the NCI-N87
cell culture. The concentrations were chosen based on preliminary
dose-response experiments and luteoloside (>98% purity) was
purchased from Sigma-Aldrich (Merck KGaA).
Determination of cell
proliferation
A Cell Counting Kit-8 (CCK-8; cat. no. 96992;
Sigma-Aldrich; Merck KGaA) assay was used to determine the level of
cell proliferation as per the manufacturer's instructions. In
brief, cells from the different treatment groups were inoculated in
a 96-well plate (5×103 cells/well) and complete medium
(100 µl) was added to each well. In the Cell Counting Kit-8 (CCK-8)
assay, luteoloside was added to the culture medium at varying
concentrations (25, 50 and 100 µM) before the 24-h incubation
period, ensuring its presence during the entire experiment.
Finally, the optical density of each well at 450 nm was measured
using a microplate plate reader.
Determination of cell invasion
Transwell inserts (cat. no. 3422; Corning, Inc.)
placed in 24-well plates and Matrigel® (cat. no. 354230;
Corning, Inc.) were used to determine the levels of cell invasion.
As per the manufacturer's instructions, Matrigel® (1:30)
was applied to the porous membrane of the upper chamber and
incubated for 1 h at 37°C for solidification. Next, cell
suspensions were inoculated in the upper chamber (5×104
cells/well), while 600 µl of complete medium was introduced into
the lower chamber, and the cells were cultured for 24–48 h. The
cells were then fixed with pre-cooled methanol at 4°C for 15 min
and rinsed with PBS. After this, the cells on the upper side of the
membrane were carefully wiped off using a cotton swab, ensuring
only the cells that had invaded to the lower side remained. Next,
0.1% crystal violet staining solution was added to the lower side
of the membrane and incubated at room temperature for 30 min. The
chambers were rinsed with PBS to remove the staining solution. The
lower side of the membrane was photographed so that the number of
cells that had passed through the Matrigel and the porous Transwell
membrane could be counted. The results were analyzed using ImageJ
software (version 1.53; National Institute of Health).
Determination of cell migration
A scratch-wound assay was performed to determine the
extent of cell migration. First, cells were seeded in a 6-well
plate at a density of 5×105 cells/well. Once a monolayer
had formed, a sterile 200-µl pipette tip was used to make a linear
scratch on the monolayer. The cell surface was then washed with PBS
to remove any cell debris generated from the scratch. After adding
an appropriate amount of complete medium (with serum) (20), the culture was continued. After
incubation for 24 h, the width of the scratch was photographed
using an inverted microscope. The cell migration rate was
calculated by comparing the initial width of the scratch with the
width at the end of the incubation period. The results were
analyzed using ImageJ software (version 1.53).
Alkaline comet assay
In brief, cells at a density of
1×105/well were digested with 0.25% trypsin (Gibco;
Thermo Fisher Scientific, Inc.). After centrifugation (2,000 × g,
4°C, 5 min), the single-cell suspension was collected, mixed at a
ratio of 1:10 with 1% low-melting-point agarose (Sigma-Aldrich;
Merck KGaA) (37°C) and the suspension (30 µl) was immediately
transferred to a clean slide. The slides were placed flat in a
light-proof environment at 4°C for 10 min and then immersed in
precooled 1X mammalian lysis buffer (Gibco; Thermo Fisher
Scientific, Inc.) to completely cover the cells and gel and
incubated overnight at 4°C. The slides were then immersed in sodium
alcohol ether sulfates (AES) for 1 h at 4°C to unwind the DNA.
Pre-cooled AES was added to the electrophoresis gel tray and the
slides were immersed in the AES and electrophoresed at 30 V and 400
mA for 30 min. Subsequently, the slides were dried at 37°C for 15
min and stained using 50 µl of red fluorescent nucleic acid
staining solution (Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature for 15 min in the dark. Finally, the slides were
observed using fluorescent microscopy, and images were recorded and
quantitatively analyzed using ImageJ software (version 1.53). The
tail moment was calculated as the product of the tail length and
Tail%DNA divided by 100. Tail%DNA refers to the percentage of DNA
present in the comet tail relative to the total DNA in the comet,
which includes both the tail and the head.
Western blot analysis of key signaling
pathway-related proteins
Total protein of each group of cells was extracted
using RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific,
Inc.) and quantified using a bicinchoninic acid assay kit
(P1250-50; Applygen Technologies, Inc.) according to the
manufacturer's instructions. Subsequently, 30 µg of the extracted
total protein was separated by SDS-PAGE (10% polyacrylamide gel).
Next, the gel was transferred to a polyvinylidene difluoride (PVDF)
membrane (MilliporeSigma) and blocked for 1 h using a 5% solution
of skimmed milk powder (Sigma-Aldrich; Merck KGaA). The membrane
was incubated at 4°C overnight with the following primary
antibodies: γ-H2A histone family member X (γH2AX; cat. no. ab26350;
1:1,000 dilution; Abcam), p53 (cat. no. ab26; 1:1,000 dilution;
Abcam), p21 (cat. no. ab109520; 1:1,000 dilution; Abcam), Bcl-2
(cat. no. ab182858; 1:1,000 dilution; Abcam) and β-actin (cat. no.
ab8226; 1:5,000 dilution; Abcam). After rinsing in PBS, the
membranes were incubated at ambient temperature for 1 h with the
corresponding horseradish peroxidase (HRP)-labeled secondary
antibodies; either HRP-labeled anti-rabbit IgG (cat. no. 7074; Cell
Signaling Technology, Inc.) or HRP-labeled anti-mouse IgG (cat. no.
7076; Cell Signaling Technology, Inc.). The immunoblotting signals
were developed using an enhanced chemiluminescence substrate (cat.
no. 32106; Thermo Fisher Scientific, Inc.). Finally, the PVDF
membrane were scanned with a chemiluminescence imaging system
(ChemiDoc™ XRS+ System; Bio-Rad Laboratories, Inc.) and
ImageJ software (version 1.53) was used to analyze the grayscale
value of each protein band.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.). Quantitative data were expressed as the mean
± standard deviation. One-way analysis of variance followed by
Tukey's post-hoc test was used to compare multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Screening of targets related to
luteoloside and GC and analyses of GO functional enrichment and
KEGG pathway enrichment
The target genes of luteoloside and GC-related genes
obtained from relevant databases were screened to explore the
mechanism of action of luteoloside in treating GC. A total of 3,514
and 10,778 GC-related targets were obtained from GeneCards and
OMIM, respectively. After removing duplicates, a total of 11,482
GC-related genes remained. Potential targets of luteoloside were
predicted using PubChem, SwissTargetPrediction and SuperPred, and
genes interacting with luteoloside were predicted using the STITCH
database, resulting in a total of 224 targets in the dataset after
removing duplicates. Venn diagram analysis of the intersection of
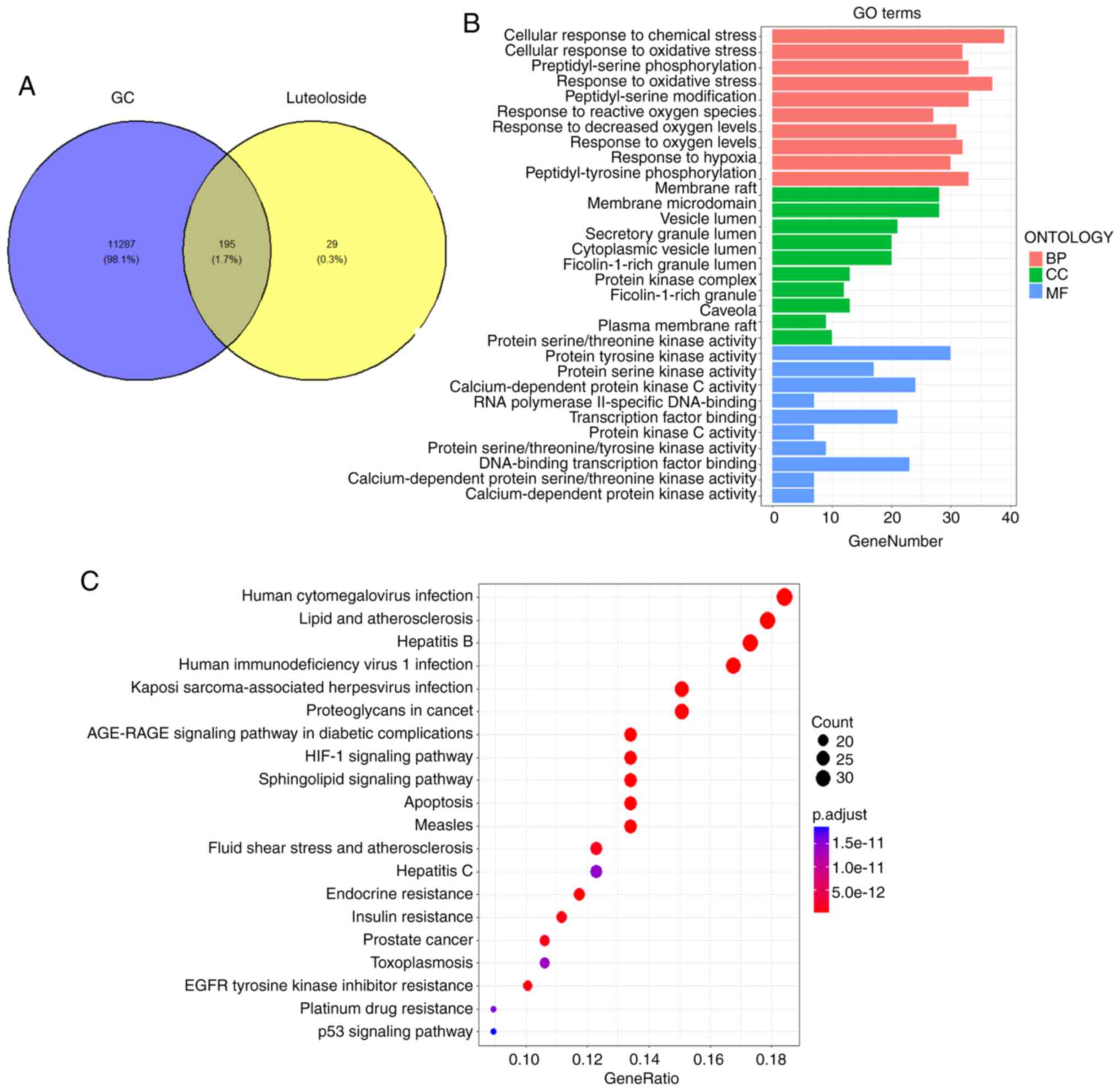
luteoloside target genes and GC-related genes revealed 195 common
targets of luteoloside in the treatment of GC (Fig. 1A), suggesting that they may be
related to the treatment of GC with luteoloside.
Using the DAVID online database, the 195 common
target genes underwent GO functional enrichment and KEGG pathway
enrichment analyses. Fig. 1B shows
the top 10 GO enrichment results. Enriched functional terms in the
category BP mainly included ‘negative regulation of apoptosis’,
‘protein phosphorylation’ and ‘signal transduction’; in the
category CC, enriched functional terms were mainly cell structures
such as the ‘plasma membrane’, ‘cytoplasm’ and ‘cytosol’; and
enriched functional terms in the category MF mainly included ‘ATP
binding’, ‘protein serine/threonine/tyrosine kinase activity’ and
‘protein kinase activity’ (Fig.
1B). KEGG enrichment analysis revealed that the 195 common
target genes were mostly enriched in pathways including
hypoxia-inducible factor (HIF)-1 signaling, apoptosis, lipids and
atherosclerosis, and p53 signaling (Fig. 1C). Overall, luteoloside may exert a
therapeutic effect on GC by regulating these signaling
pathways.
PPI network construction and core
target gene screening
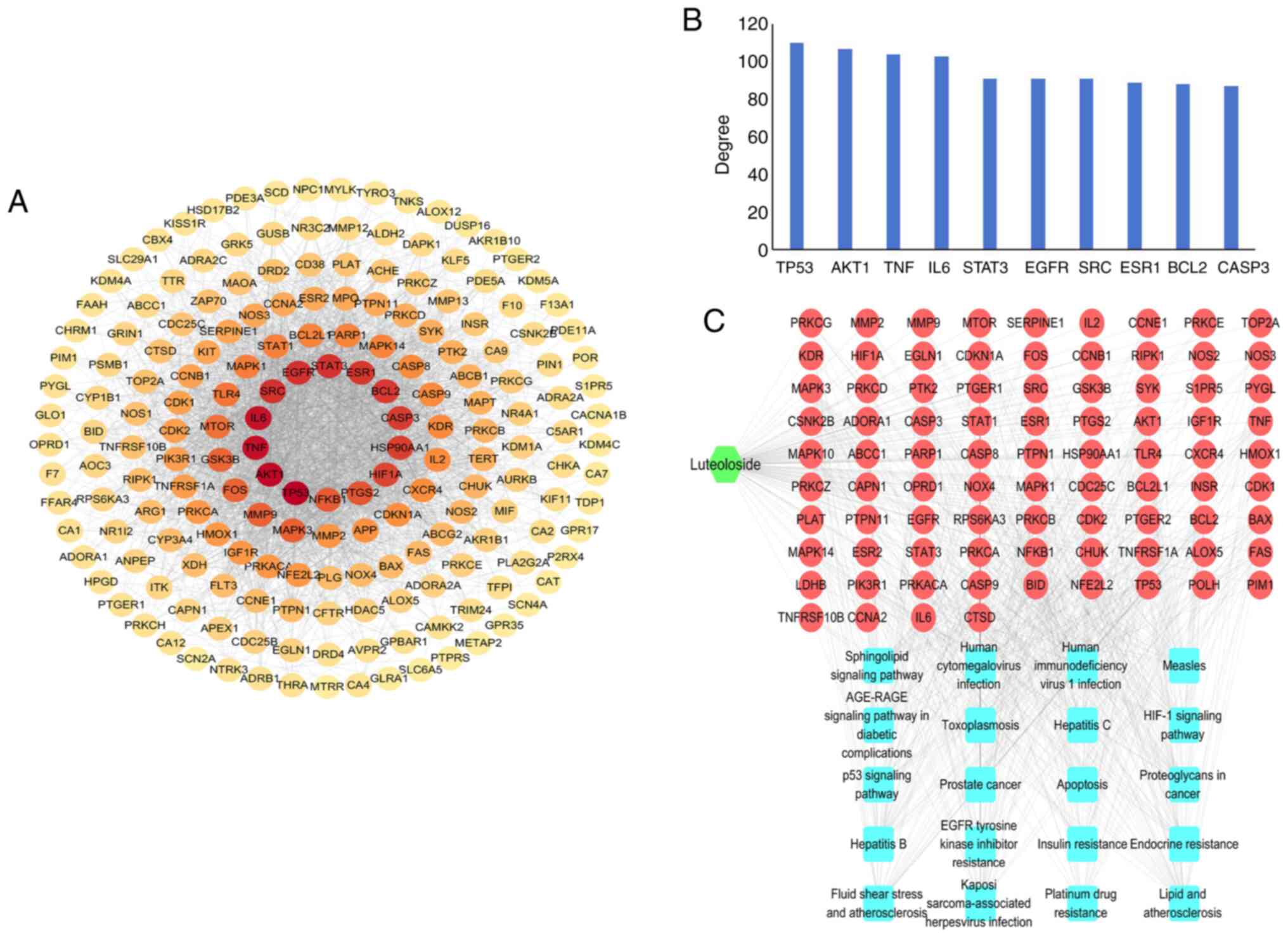
PPI analysis of the 195 common target genes of
luteoloside and GC was performed using the STRING database, and the
PPI network of luteoloside in the treatment of GC was then
constructed (Fig. 2A). The top 10
genes ranked by degree value were, in order, tumor protein
(TP)53, threonine kinase 1 (AKT1), TNF,
IL6, STAT3, EGFR, SRC, ESR1, BCL2 and caspase
(CASP)3 (Fig. 2B).
The high degree values suggested that these genes have key roles in
the PPI network. A luteoloside-target-signaling pathway network was
also constructed (Fig. 2C); the 20
most significant pathways from the KEGG enrichment analysis were
obtained, and the 10 genes with the largest degree among them were
analyzed. After intersection, four common genes (TP53, AKT1,
BCL2 and CASP3) were identified.
Molecular docking of p53, Akt1, Bcl-2
and Casp3 with luteoloside
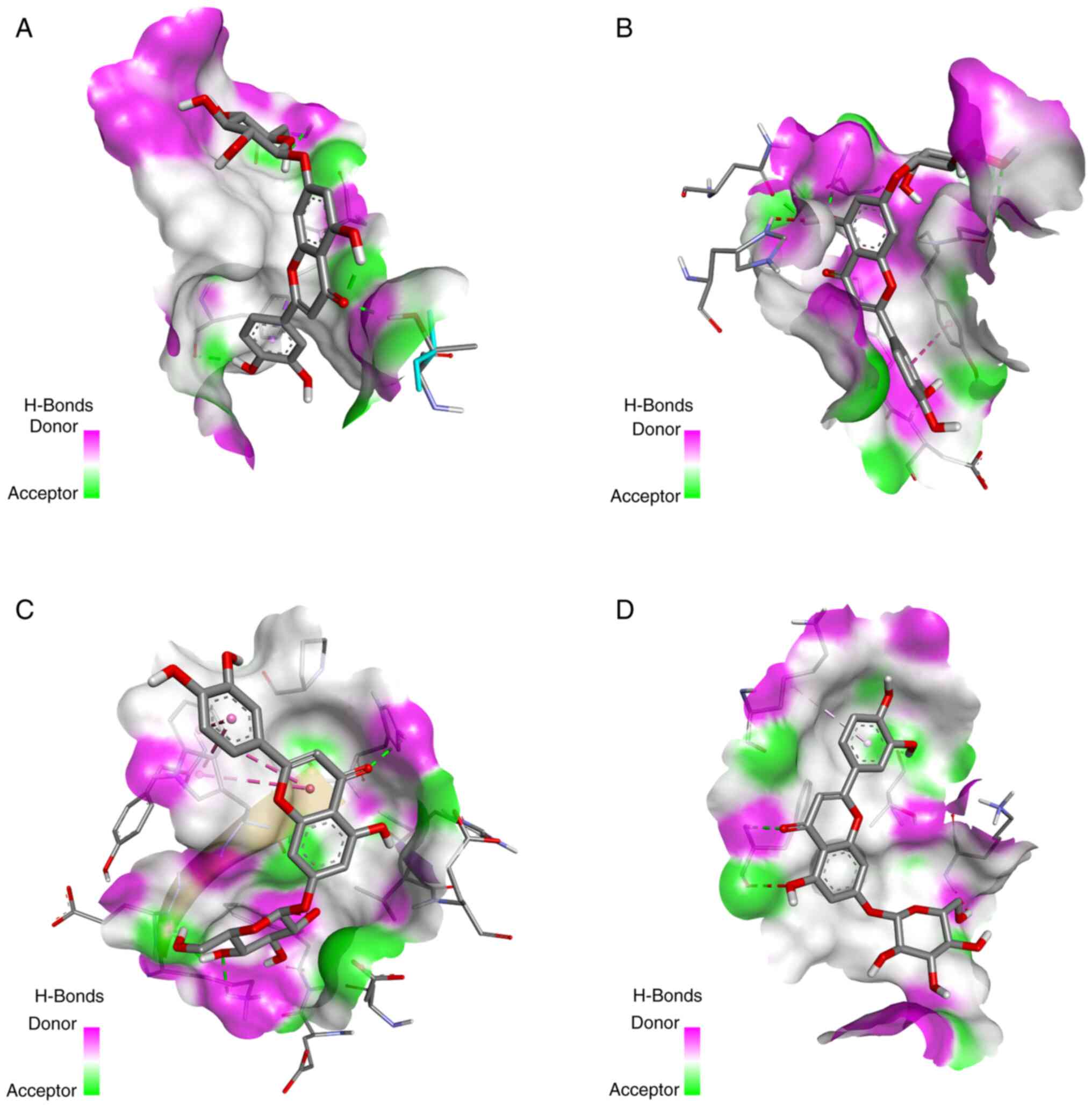
AutoDock was used for the molecular docking of
luteoloside and key targets. Luteoloside was docked with the top
four targets: p53, Akt1, Bcl-2 and Casp3. Table I shows the intermolecular docking
binding energies and the molecular docking conformation of
luteoloside is presented in Fig. 3.
The results were visualized using PyMOL and showed good matching.
The optimal conformation of compound-target binding was presented
in the form of hydrogen bonding and the binding energy was <-6.0
kcal/mol, indicating high binding activity.
 | Table I.Molecular docking binding energies of
active ingredients of luteoloside and key targets. |
Table I.
Molecular docking binding energies of
active ingredients of luteoloside and key targets.
| Protein (PDB
structural identifier) | Affinity,
kcal/mol |
|---|
| TP53 (1AIE) | −7.5 |
| AKT1 (1H10) | −6.9 |
| BCL2 (1G5M) | −8.5 |
| CASP3 (1NME) | −6.9 |
Luteoloside inhibits the
proliferation, invasion and migration of NCI-N87 cells
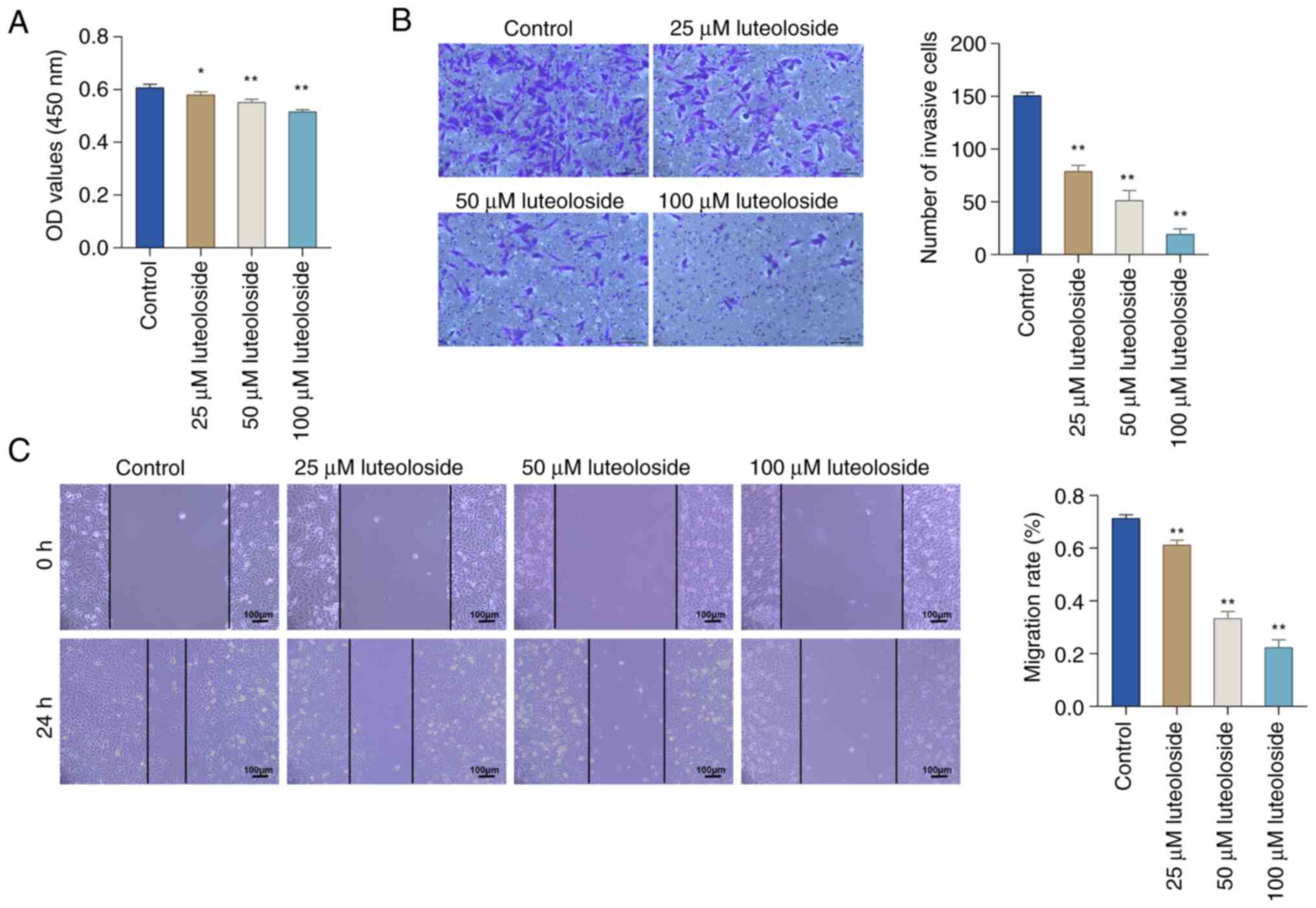
The CCK-8 assay was used to explore the role of
luteoloside in NCI-N87 cells and the impact of different
concentrations on cell proliferation (Fig. 4A). Compared with the control cells,
the proliferation of NCI-N87 cells treated with luteoloside showed
a dose-dependent decrease as the luteoloside concentration
increased. The invasion ability of NCI-N87 cells in each group was
explored using the Transwell assay (Fig. 4B). Luteoloside treatment visibly
inhibited the invasive ability of NCI-N87 cells. The influence of
various concentrations of luteoloside on the migration of NCI-N87
cells was investigated using the scratch-wound assay (Fig. 4C). Compared with the control group,
luteoloside treatment significantly decreased the migration ability
of NCI-N87 cells. The above results indicated the potential of
luteoloside to inhibit the proliferation, invasion and migration of
NCI-N87 cells.
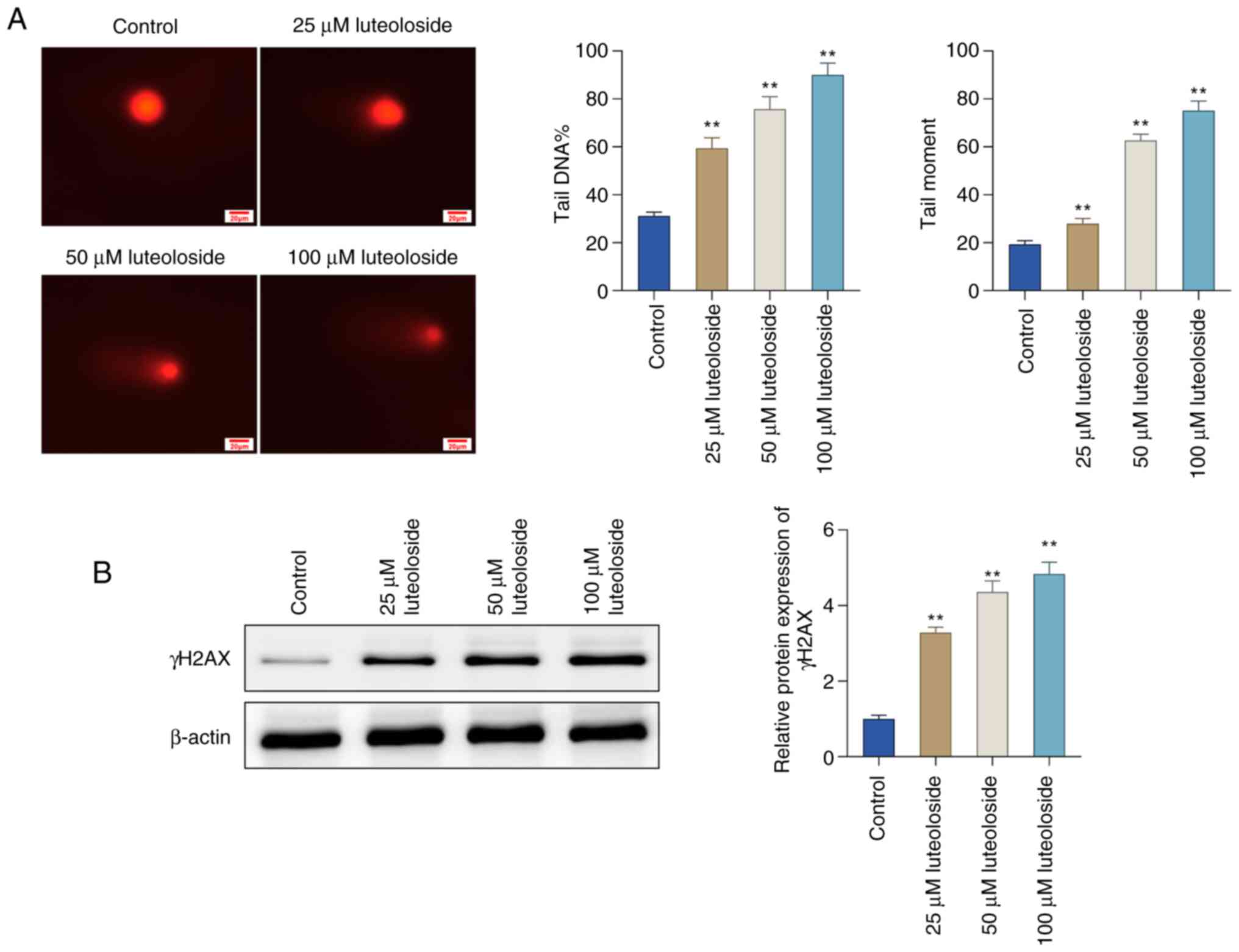
Luteoloside promotes DNA damage in
NCI-N87 cells
The comet assay was performed to determine whether
luteoloside is able to induce DNA double-strand breaks (DSBs).
Luteoloside treatment increased the length of comet tails in
NCI-N87 cells, which was further enhanced with increasing
concentrations, suggesting that luteoloside contributed to the
induction of cellular DNA damage (Fig.
5A). The effect of luteoloside on DNA damage marker γH2AX in
NCI-N87 cells was also evaluated. Western blot analysis showed that
the protein level of γH2AX was significantly elevated in NCI-N87
cells in a concentration-dependent manner relative to the control
group (Fig. 5B). Collectively,
these findings indicated that luteoloside induced DNA DSBs, as
evidenced by elevated γH2AX protein levels and enhanced comet tail
moments.
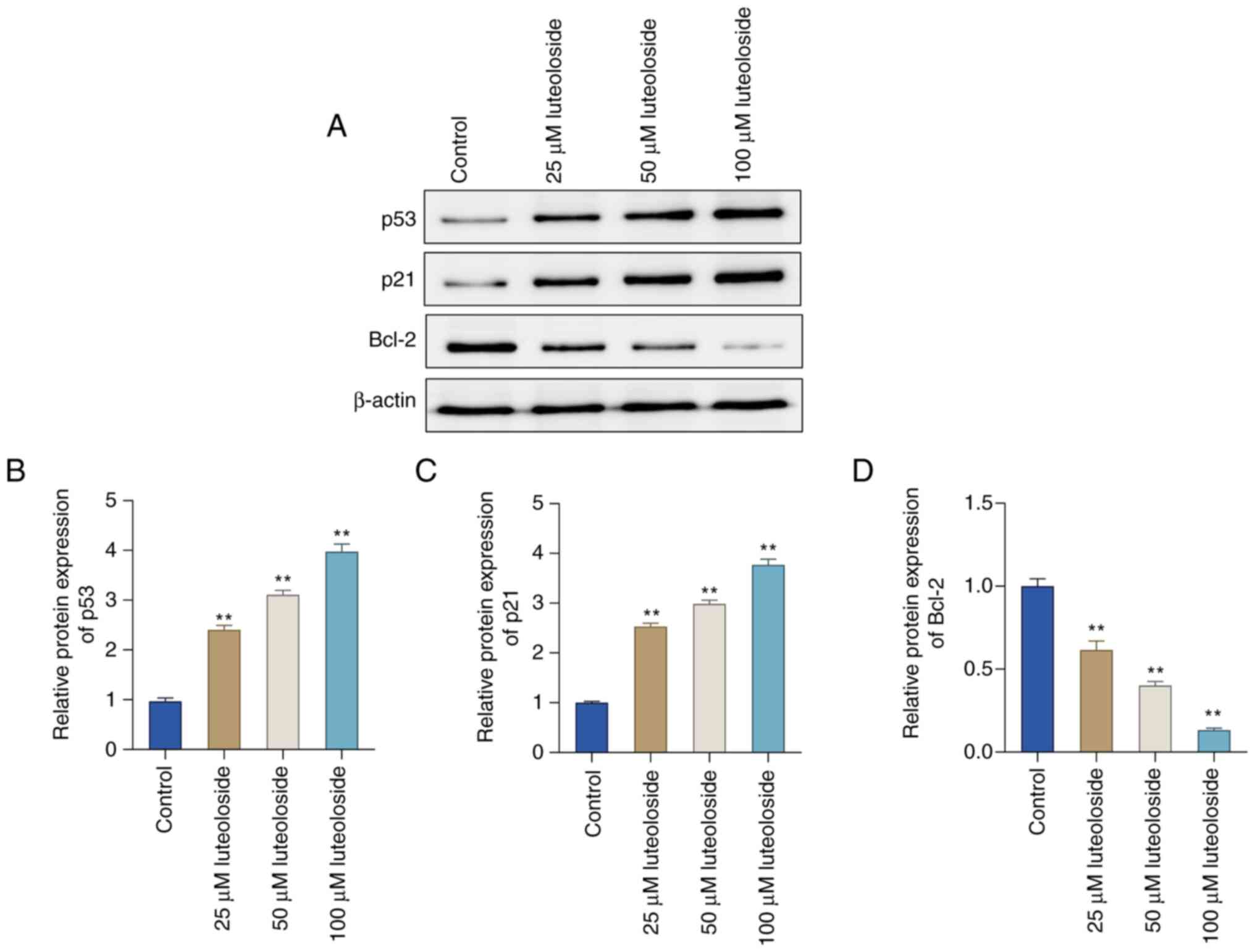
Luteoloside activates the p53/p21
pathway in NCI-N87 cells
The expression of p53/p21 signaling pathway-related
proteins was analyzed in conjunction with the preceding
bioinformatics results. Western blot analysis showed that, compared
with the control group, p53 and p21 levels increased with
increasing concentrations of luteoloside. However, Bcl-2 levels
showed a concentration-dependent decrease after luteoloside
treatment (Fig. 6A-D).
Collectively, these results indicated that the p53/p21 signaling
pathway has a key role in luteoloside-induced inhibition of GC cell
invasion, migration and proliferation.
Discussion
Gastric cancer (GC) is a leading cause of
cancer-related deaths globally, with limited treatment options due
to the severe side effects and limited efficacy of existing
chemotherapies. In the present study, the intersection of 195
common targets of luteoloside and GC was identified. PPI network
analysis showed that p53, Akt1, Bcl-2 and Casp3 were the core
targets for luteoloside treatment of GC, and they all showed good
binding activity with luteoloside. GO functional enrichment and
KEGG pathway enrichment analyses revealed that luteoloside exerted
anti-GC effects through various signals, including ‘negative
regulation of apoptosis’, ‘lipids’ and ‘atherosclerosis’, ‘HIF-1
signaling’ and ‘p53 signaling’. These signaling pathways have been
reported to inhibit GC growth by regulating several tumor
development processes, including angiogenesis, proliferation,
apoptosis, metastasis and invasion of human GC cells (21–23).
Among them, apoptosis is one of the most important, and triggering
apoptosis in cancer cells has received attention as a potential
treatment for GC (21,24–26).
On the basis of the present networks pharmacology
findings, in vitro experiments were first performed to
investigate the effects of different concentrations of luteoloside
on GC cells. The CCK-8 assay results revealed that luteoloside
significantly decreased the rate of proliferation of GC cells. The
Transwell and scratch-wound assay results indicated that
luteoloside considerably reduced the invasion and migration
capabilities of GC cells, respectively. Therefore, it may be
suggested that luteoloside may serve as a therapeutic agent for GC
by successfully preventing the proliferation, migration and
invasion of GC cells. Next, the mechanism of action of luteoloside
in GC cells was further explored. p53 is a tumor suppressor protein
and its most important pathway for tumor suppression is the
induction of apoptosis and cell cycle arrest (27). p53 significantly promotes the
transcriptional activity of the cell cycle regulator p21, and the
p53/p21 signaling pathway is a central regulatory pathway that
controls cell-cycle progression and regulates apoptosis in tumor
cells (28,29). Bcl-2 is an anti-apoptotic protein.
p53 binds to Bcl-2 family proteins to release Bax, which exerts
anti-invasive or proapoptotic effects, depending on the stress
environment (30). In the in
vitro experiments of the present study, p53 and p21 protein
expression levels were markedly upregulated and Bcl-2 protein
expression was notably downregulated in GC cells after luteoloside
treatment in a concentration-dependent manner. These findings
suggest that luteoloside promotes GC-cell apoptosis by activating
the p53/p21 signaling pathway, thereby suppressing GC-cell
invasion, proliferation and migration. It is important to note that
the NCI-N87 cell line possesses a p53 missense mutation
(p.Arg248Gln), which is pathogenic and common in gastric cancer.
Despite this mutation, luteoloside was still able to activate the
p53/p21 signaling pathway, indicating that the inhibitory effects
on cell proliferation, migration and invasion are relevant even in
the presence of such mutations.
Signaling pathways of research significance were
screened and it was verified that luteoloside effectively hindered
‘GC-cell proliferation’, migration and invasion. However, the
impact on proliferation was not as pronounced as its effects on
migration and invasion. This could potentially be attributed to the
doubling time of the cell line, which may influence the observed
effects within the 24-h period. In addition, apoptosis, which
reduces the number of viable cells, tends to occur after 24 h,
suggesting that more pronounced effects on proliferation may become
evident over longer incubation periods. In additionally, there are
certain limitations to the present study. Previous in vivo
studies have demonstrated that luteoloside exhibits various
beneficial effects, such as analgesic and neuroprotective
properties, although these outcomes were achieved at different
doses than those applied in the present study (31,32).
For instance, luteoloside has been shown to reduce pain and protect
against neuronal damage in animal models, indicating its potential
therapeutic applications. However, to translate these findings into
the context of gastric cancer, in vivo experiments are
essential to verify whether luteoloside can exert similar
therapeutic effects on gastric cancer at the appropriate doses.
First, in vivo experiments are necessary to verify the
therapeutic effect of luteoloside on GC. Furthermore, the
involvement of p53/p21 signaling in the luteoloside-induced
inhibition of GC cells requires verification by gene knockout or a
similar process. Alternatively, an in vitro rescue
experiment, where p53/p21 is silenced or inhibited using small
inhibitory RNA or specific inhibitors, followed by luteoloside
treatment, could assess whether the inhibition of GC cell
proliferation, migration and invasion is reversed. This would
provide functional insights and be less time-consuming than gene
knockout, making it a practical approach to validate the pathway's
role.
In this study, the NCI-N87 gastric cancer cell line
was utilized, which harbors a p53 missense mutation (p.Arg248Gln)
known to be pathogenic and common in gastric cancer. Of note,
certain p53 mutations may not lead to a complete loss of function
and the present findings demonstrate that luteoloside can still
activate the p53/p21 signaling pathway in these cells. This
suggests that even in the presence of a p53 mutation, luteoloside
may exert its inhibitory effects on cell proliferation, migration
and invasion through this pathway. This discovery highlights the
potential of luteoloside as a therapeutic agent and indicates that
the p53 pathway can be effectively activated even in gastric cancer
cells with specific p53 mutations. Future research should further
explore the mechanism of luteoloside in the context of different
p53 mutation backgrounds.
The present study demonstrated that luteoloside
significantly enhances cell migration in a scratch-wound assay,
suggesting its potential role in wound healing. Previous studies
have shown that luteoloside can inhibit the NF-κB pathway and
induce apoptosis in cancer cells (33,34),
which may suggest that its effects are dose-dependent and
context-specific. However, the scratch-wound assay has limitations,
as it does not fully replicate the complex in vivo
environment. Although apoptosis was not observed in the present
study within the 24-h incubation period, it is possible that
apoptosis may occur with prolonged exposure. Future research should
explore longer incubation times and investigate the underlying
molecular mechanisms, particularly the potential involvement of
NF-κB.
In conclusion, network pharmacology combined with
in vitro experiments were used to confirm that luteoloside
inhibits the proliferation, invasion and migration of GC cells by
activating the p53/p21 signaling pathway. Thus, luteoloside is
expected to be a promising therapeutic drug for GC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XXL and PQY designed the study and drafted the
manuscript. XJL, ZZX, SMH and HTW carried out the statistical
analysis. XLY, YD and WZY participated in the study design,
contributed to data collection and analysis, and confirmed the
authenticity of all the raw data. SMH and XLY contributed to the
discussion section and revised it critically for important
intellectual content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao MM, Li H, Sun DQ, He SY, Lei L, Peng J
and Chen WQ: Epidemiological trend analysis of gastric cancer in
China from 2000 to 2019. Chin J Dig Surg. 20:102–109. 2021.
|
|
3
|
Cao M and Chen W: Epidemiology of cancer
in China and the current status of prevention and control. Chin J
Clin Oncol. 46:145–149. 2019.
|
|
4
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI
|
|
5
|
Kobayashi J: Effect of diet and gut
environment on the gastrointestinal formation of N-nitroso
compounds: A review. Nitric Oxide. 73:66–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ilson DH: Advances in the treatment of
gastric cancer: 2019. Curr Opin Gastroenterol. 35:551–554. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Wang MM, Guo XW, Wu CY, Li DR, Zhang
X and Zhang PT: Different survival benefits of Chinese medicine for
pancreatic cancer: How to choose? Chin J Integr Med. 24:178–184.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia W and Wang L: Using traditional
Chinese medicine to treat hepatocellular carcinoma by targeting
tumor immunity. Evid Based Complement Alternat Med.
2020:98434862020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bouyahya A, Taha D, Benali T, Zengin G, El
Omari N, El Hachlafi N, Khalid A, Abdalla AN, Ardianto C, Tan CS,
et al: Natural sources, biological effects, and pharmacological
properties of cynaroside. Biomed Pharmacother. 161:1143372023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiao GY, Li SK, Deng Y, Yin J, Chen WS,
Chen JF and Zhang F: Review of pharmacological effects, metabolism
and quality control of Eclipta prostrata L. and its chemical
components. J Pharm Res. 40:673–677. 6832021.
|
|
11
|
Gupta A, Kumar A, Kumar D, Nandan S,
Shankar K, Varshney S, Rajan S, Srivastava A, Gupta S, Kanojiya S,
et al: Ethyl acetate fraction of Eclipta alba: A potential
phytopharmaceutical targeting adipocyte differentiation. Biomed
Pharmacother. 96:572–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yadav NK, Arya RK, Dev K, Sharma C,
Hossain Z, Meena S, Arya KR, Gayen JR, Datta D and Singh RK:
Alcoholic Extract of Eclipta alba shows in vitro antioxidant and
anticancer activity without exhibiting toxicological effects. Oxid
Med Cell Longev. 2017:90946412017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryu S, Shin JS, Jung JY, Cho YW, Kim SJ,
Jang DS and Lee KT: Echinocystic acid isolated from Eclipta
prostrata suppresses lipopolysaccharide-induced iNOS, TNF-α,
and IL-6 expressions via NF-κB inactivation in RAW 264.7
macrophages. Planta Med. 79:1031–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Timalsina D and Devkota HP: Eclipta
prostrata (L.) L. (asteraceae): Ethnomedicinal uses, chemical
constituents, and biological activities. Biomolecules. 11:17382021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rehfeldt SCH, Silva J, Alves C, Pinteus S,
Pedrosa R, Laufer S and Goettert MI: Neuroprotective effect of
luteolin-7-O-glucoside against 6-OHDA-induced damage in
undifferentiated and RA-differentiated SH-SY5Y cells. Int J Mol
Sci. 23:29142022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caporali S, De Stefano A, Calabrese C,
Giovannelli A, Pieri M, Savini I, Tesauro M, Bernardini S, Minieri
M and Terrinoni A: Anti-inflammatory and active biological
properties of the plant-derived bioactive compounds luteolin and
luteolin 7-glucoside. Nutrients. 14:11552022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schonhofer C, Yi J, Sciorillo A,
Andrae-Marobela K, Cochrane A, Harris M, Brumme ZL, Brockman MA,
Mounzer K, Hart C, et al: Flavonoid-based inhibition of
cyclin-dependent kinase 9 without concomitant inhibition of histone
deacetylases durably reinforces HIV latency. Biochem Pharmacol.
186:1144622021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji J, Wang Z, Sun W, Li Z, Cai H, Zhao E
and Cui H: Effects of cynaroside on cell proliferation, apoptosis,
migration and invasion though the MET/AKT/mTOR axis in gastric
cancer. Int J Mol Sci. 22:121252021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Velmurugan BK, Lin JT, Mahalakshmi B,
Chuang YC, Lin CC, Lo YS, Hsieh MJ and Chen MK:
Luteolin-7-O-glucoside inhibits oral cancer cell migration and
invasion by regulating matrix metalloproteinase-2 expression and
extracellular signal-regulated kinase pathway. Biomolecules.
10:5022020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arora M: Cell culture media: A review.
Mater Methods. 3:1752013. View Article : Google Scholar
|
|
21
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y
and Song F: Salidroside induces apoptosis and protective autophagy
in human gastric cancer AGS cells through the PI3K/Akt/mTOR
pathway. Biomed Pharmacother. 122:1097262020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji H and Zhang X: RPL38 regulates the
proliferation and apoptosis of gastric cancer via miR-374b-5p/VEGF
signal pathway. Onco Targets Ther. 13:6131–6141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pei J, Wei D and Jiang L: Effects of Yiqi
huayu sanjie decoction on PI3K-AKT-mTOR signal pathway of gastric
cancer cell line of SGC-7901. World Chin Med. 15:2686–2689.
26952020.
|
|
24
|
Jeong S, Jo MJ, Yun HK, Kim DY, Kim BR,
Kim JL, Park SH, Na YJ, Jeong YA, Kim BG, et al: Cannabidiol
promotes apoptosis via regulation of XIAP/Smac in gastric cancer.
Cell Death Dis. 10:8462019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang JR and Yang H: Ginkgolic acid (GA)
suppresses gastric cancer growth by inducing apoptosis and
suppressing STAT3/JAK2 signaling regulated by ROS. Biomed
Pharmacother. 125:1095852020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao S, Tan H and Li D: Oridonin suppresses
gastric cancer SGC-7901 cell proliferation by targeting the
TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J
Cell Mol Med. 27:2661–2674. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao Y and Jiang P: The crisscross between
p53 and metabolism in cancer. Acta Biochim Biophys Sin (Shanghai).
55:914–922. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu X, Wang Z, Gao J, Han D, Zhang L, Chen
P, Luo G and Han B: SIRT1 suppresses p53-dependent apoptosis by
modulation of p21 in osteoblast-like MC3T3-E1 cells exposed to
fluoride. Toxicol In Vitro. 57:28–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuang C, Zhao J, Zhang S and Shahid M:
Escherichia coli infection mediates pyroptosis via activating
p53-p21 pathway-regulated apoptosis and cell cycle arrest in bovine
mammary epithelial cells. Microb Pathog. 184:1063382023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EM, Jung CH, Kim J, Hwang SG, Park JK
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nabavi SF, Braidy N, Gortzi O,
Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K and Nabavi SM:
Luteolin as an anti-inflammatory and neuroprotective agent: A brief
review. Brain Res Bull. 119:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Q, Tian Z, Wang M, Kou J, Wang C, Rong
X, Li J, Xie X and Pang X: Luteoloside attenuates neuroinflammation
in focal cerebral ischemia in rats via regulation of the
PPARγ/Nrf2/NF-κB signaling pathway. Int Immunopharmacol.
66:309–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park CM and Song YS: Luteolin and
luteolin-7-O-glucoside inhibit lipopolysaccharide-induced
inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt
signaling cascades in RAW 264.7 cells. Nutr Res Pract. 7:423–429.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin J, Chen J, Zhang Z, Xu T, Shao Z, Wang
X, Ding Y, Tian N, Jin H, Sheng S, et al: Luteoloside inhibits
IL-1β-induced apoptosis and catabolism in nucleus pulposus cells
and ameliorates intervertebral disk degeneration. Front Pharmacol.
10:8682019. View Article : Google Scholar : PubMed/NCBI
|