Introduction
Cervical cancer (CC) is the fourth leading cause of
cancer-associated mortalities among women worldwide despite
advancements in diagnosis, prevention and treatment (1,2). The
prognosis of patients with advanced or recurrent CC is poor, with a
1-year survival rate of only 10–20% (3). Chemotherapy is the standard treatment
for patients with advanced or recurrent CC. Although the
chemotherapeutic agent cisplatin (DDP) is the most effective drug
for treating CC (4), resistance to
DDP-based treatment limits the survival of patients with partial
CC, leading to poor prognosis (4).
The mechanisms underlying DDP resistance in CC have
been examined and strategies have been proposed to overcome the
resistance (5–8). Previous studies show that reduced
accumulation of intracellular platinum compounds (5), increased DNA damage repair (6), inactivation of apoptosis (7) and activation of the
epithelial-mesenchymal transition (8) are associated with DDP resistance. In
the previous number of decades, an increasing number of studies
have shown that tumor cells hijack the unfolded protein response to
induce chemotherapy resistance by activating the unfolded response
sensors activated transcription factor 6, inositol-requiring
transmembrane kinase/endoribonuclease 1α and protein kinase R-like
endoplasmic reticulum kinase as well as their master regulator
glucose regulated protein 78 (9–12). The
hypoxia-upregulated 1 (HYOU1) gene encodes a
chaperone protein in the endoplasmic reticulum (ER). Various
stimuli, including hypoxia, impaired ubiquitination, proteasomal
degradation and energy deficiency induce an unfolded protein
response in the presence of ER stress, accompanied by the
expression of ER molecular chaperones such as protein kinase-like
ER kinase, inositol-requiring enzyme 1, and activating
transcription factor 6α (13).
N6-methyladenosine (m6A),
which is among the most prevalent and reversible internal RNA
modifications in eukaryotic RNAs (14), occurs at the consensus motif RRACH
(R is G, A or U; H is U, A or C) and regulates RNA transcription,
splicing, degradation and translation (15). m6A modification of RNA is
catalyzed by the m6A methyltransferase enzyme complexes
(writers), removed by m6A demethylase enzymes (erasers)
and recognized by specific proteins (readers) (16–20).
Previous studies demonstrate that the m6A modification
is involved in promoting the tumorigenesis, metastasis and drug
resistance of different types of cancer (21–23).
However, whether the m6A modification is involved in
regulating DDP resistance in CC remains unclear.
The present study aimed to utilize bioinformatic
methods to identify genes associated with DDP resistance in CC
using various public databases. Using CRISPR data of CC cell lines
and the gene expression profiles of CC samples, key genes
associated with the survival and DDP resistance of CC were
investigated. Furthermore, the association of key genes with the
survival of patients with CC treated with DDP were also
investigated using public datasets and in vitro experiments.
Additionally, the m6A-associated genes involved in
regulating dysregulated genes were investigated.
Materials and methods
Gene expression data of CC
samples
The dataset associated with CC [The Cancer Genome
Atlas (TCGA)-CC] was obtained by searching for the keywords
‘cervical cancer’ in TCGA (https://portal.gdc.cancer.gov/) database. The dataset
(accession no. GSE56363) was obtained from the Gene Expression
Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/) database. The inclusion
criteria were as follows: i) Patients with CC who received DDP; and
ii) survival information or response status to DDP were
recorded.
The expression profile of CC and clinical data were
obtained by searching for ‘cervical cancer’ in the TCGA database
from the Genomic Data Commons (GDC) Data Portal (https://portal.gdc.cancer.gov/), and consisted of
178 CC tissues and three adjacent non-tumor tissues. Based on the
clinical information of the patients in the TCGA-CC dataset, there
were 43 patients with both the response status to DDP and overall
survival (OS) status recorded. These patients were selected as the
discovery set (TCGA-CC1 set; Table I) to identify the genes associated
with DDP resistance. The 40 samples, which only recorded the OS of
patients receiving DDP were used as the validation set
(TCGA-CC2 set; Table I)
to support the association of genes with DDP resistance. To exclude
the prognostic association of the genes, the 95 patients that did
not receive treatment were selected as the control set
(TCGA-CC3 set; Table I)
for survival analysis (24).
GSE56363 consisted of 12 CC samples with complete response to DDP
and 9 CC samples with non-complete response to DDP.
 | Table I.Sample data of TCGA. |
Table I.
Sample data of TCGA.
| Characteristic |
TCGA-CC1 |
TCGA-CC2 |
TCGA-CC3 | Total |
|---|
| Sex, female | 43 | 40 | 95 | 178 |
| Age, years [mean
(SD)] | 48.14 (13.05) | 47.10 (14.08) | 47.60 (13.56) | - |
| Stage |
|
|
|
|
| I | 10 | 21 | 63 | 94 |
| II | 20 | 8 | 18 | 46 |
|
III | 5 | 7 | 9 | 21 |
| IV | 6 | 2 | 4 | 12 |
|
Unknown | 2 | 2 | 1 | 5 |
| Distant
metastasis |
|
|
|
|
|
Yes | 2 | 0 | 0 | 2 |
| No | 13 | 16 | 42 | 71 |
|
Unknown | 28 | 24 | 53 | 105 |
| Lymph node
metastasis |
|
|
|
|
|
Yes | 7 | 10 | 16 | 33 |
| No | 13 | 11 | 59 | 83 |
|
Unknown | 23 | 19 | 20 | 62 |
| Response
status |
|
|
|
|
|
Yes | 37 | 0 | - | 37 |
| No | 6 | 0 | - | 6 |
|
Unknown | 0 | 40 | - | 40 |
RNA-sequencing data were downloaded from TCGA via
the GDC Data Portal (https://portal.gdc.cancer.gov/), which had been
detected using the Illumina HiSeq 2000 platform. The fragments per
kilobase of transcript per million mapped read values were
log2-scaled plus 1 for gene expression level measurements.
Database
To identify key genes associated with CC cell
survival, the CRISPR-Cas9 screening data of CC cell lines were
downloaded from the DepMap portal (https://depmap.org/portal/) by selecting ‘Version:
DepMap Public 21Q2’ and ‘CRISPR_gene_effect’ sections. The database
recorded the gene essentiality scores [CRISPR-Cas9 gene knockout
scores (CERES)] of genes in CC cell lines, which indicated the
influence of knockout genes on the proliferation in CC cell lines
(25,26). The lower the CERES score, the
greater the effect after the gene knockout.
To validate the association of genes with DDP
resistance, the gene expression profiles of CC cell lines and their
half-maximal inhibitory concentration (IC50) values for
DDP drugs were acquired from the Genomics of Drug Sensitivity in
Cancer (GDSC; https://www.cancerrxgene.org; release-8.2) database
(27) by selecting the ‘Cell Line
Gene Expression Data’ and ‘Drug Sensitivity Data’ sections.
Relevant literature was used to identify 30
m6A-associated genes (28–31),
including 11 methyltransferases, two demethylases and 17 reader
proteins (Table II).
 | Table II.R, P and FDR values for
N6-methyladenosine-associated genes. |
Table II.
R, P and FDR values for
N6-methyladenosine-associated genes.
| Type | Genes | R | P-value | FDR |
|---|
|
Methyltransferases | ZC3H13 | 0.4566 | 0.0021 | 0.0156 |
|
| RBM15B | 0.4141 | 0.0058 | 0.0247 |
|
| VIRMA | 0.3708 | 0.0144 | 0.0479 |
|
| ZCCHC4 | 0.3594 | 0.0179 | 0.0538 |
|
| CBLL1 | 0.3150 | 0.0396 | 0.0914 |
|
| METTL16 | −0.2918 | 0.0576 | 0.1234 |
|
| METTL3 | 0.2553 | 0.0985 | 0.1739 |
|
| METTL14 | 0.2313 | 0.1356 | 0.2034 |
|
| METTL5 | −0.1695 | 0.2773 | 0.3618 |
|
| WTAP | 0.0096 | 0.9511 | 0.9908 |
|
| RBM15 | −0.0079 | 0.9598 | 0.9908 |
| Demethylases | FTO | 0.3329 | 0.0292 | 0.0730 |
|
| ALKBH5 | 0.2854 | 0.0635 | 0.1271 |
| Reader
proteins | G3BP2 | 0.5410 | 0.0002 | 0.0040 |
|
| PRRC2A | 0.5292 | 0.0003 | 0.0040 |
|
| EIF3A | 0.4764 | 0.0012 | 0.0124 |
|
| YTHDF3 | 0.4291 | 0.0041 | 0.0245 |
|
| G3BP1 | 0.4146 | 0.0057 | 0.0247 |
|
| YTHDF1 | 0.4022 | 0.0075 | 0.0281 |
|
| IGF2BP1 | 0.3453 | 0.0234 | 0.0637 |
|
| IGF2BP3 | 0.2808 | 0.0682 | 0.1279 |
|
|
HNRNPA2B1 | 0.2484 | 0.1082 | 0.1804 |
|
| YTHDC2 | 0.2318 | 0.1347 | 0.2034 |
|
| IGF2BP2 | 0.2234 | 0.1499 | 0.2142 |
|
| YTHDF2 | 0.1746 | 0.2629 | 0.3585 |
|
| HNRNPC | 0.0988 | 0.5283 | 0.6604 |
|
| RBMX | 0.0925 | 0.5554 | 0.6665 |
|
| YTHDC1 | 0.0503 | 0.7489 | 0.8321 |
|
| ELAVL1 | 0.0530 | 0.7359 | 0.8321 |
|
| FMR1 | 0.0018 | 0.9908 | 0.9908 |
Cell culture
HeLa, a human CC cell line, was purchased from
Macgene Biotechnology (https://www.macgene.com/). HeLa cells were routinely
cultured in Dulbecco's modified Eagle's medium (DMEM; Wuhan
Servicebio Technology Co., Ltd.), which was supplemented with 10%
fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology Co.,
Ltd.). Cells were grown at 37°C and 5% CO2 under
humidified conditions and passaged upon reaching 80–90%
confluency.
Cell viability assay
Cell viability was investigated using the Cell
Counting Kit-8 (CCK-8; cat. no. C0038; Beyotime Institute of
Biotechnology) assay. Cells were seeded at a density of
1×104 cells/ml in a 96-well plate at a volume of 100
ml/well. Various concentrations (0–100,000 nM) of DDP (cat. no.
P4394; Sigma-Aldrich; Merck KGaA) were introduced into the culture
medium, with a three-fold gradient to systematically probe the
cytotoxic effects. After a 96-h incubation at 37°C, cell viability
was quantified using the CCK-8 assay and measuring the absorbance,
which was used to calculate the cell survival rate. The subsequent
data were fitted to a dose-response curve to determine the
IC50 of cell proliferation. The equation used to
calculate inhibition (%) was: Inhibition
(%)=[(Ac-As)/(Ac-Ab)]
×100. ‘As’ and ‘Ab’ represent the absorbance
of the experimental wells and the wells with the highest
concentration, respectively. ‘Ac’ represents the
absorbance of the control wells.
DDP-resistant cells construction
HeLa cells were initially treated with 1 µM DDP
which was increased to 2 µM after ~2 months and treatment was
continued at this concentration for another 4 months until
stabilization, resulting in DDP-resistant cells (HeLa/DDP).
Subsequently, HeLa/DDP cells were seeded at a density of
5×105 cells/well into 6-well plates and maintained in
culture medium containing 2 µM cisplatin at 37°C. Next, HeLa/DDP
cells were cultured in the presence of increasing concentrations of
DPP (cat. no. P4394; Sigma-Aldrich; Merck KGaA) to establish the
IC50. The drug sensitivity of the cells were quantified
by determining the IC50 using a cell viability assay.
The resistance index (RI) was calculated as the ratio of the
IC50 of the resistant cells to the IC50 of
the parental cells, which served as a measurement of the relative
resistance. An RI >3 indicated that the resistant cell line was
less sensitive to the drug compared with the parental cell
line.
Western blotting (WB)
WB was used to detect HYOU1 protein levels in three
independent experiments. HeLa and HeLa/DDP cells were harvested and
lysed in Whole Protein Extraction kit (cat. no. WLA019, Wanleibio
Co., Ltd.) for 5 min. The supernatant was centrifuged at 4°C and
10,005 × g for 10 min and the protein concentration was determined
using a bicinchoninic acid kit. Following this, 40 µg of protein
from the supernatant was loaded per lane on a 10% gel and SDS-PAGE
was carried out before the proteins were transferred to a PVDF
membrane. Subsequently, the membrane was blocked with blocking
buffer (cat. no. WLA066; Fast Blocking Western; Wanleibio Co.,
Ltd.) for 1 h at room temperature and then incubated with either
the HYOU1 (cat. no. R383157; 1:500; Chengdu Zen-Bioscience Co.,
Ltd.) or the β-actin (cat. no. WL01372; 1:1,000; Wanleibio Co.,
Ltd.) primary antibody overnight at 4°C. The membranes were then
rinsed with TBST (0.15% Tween20; Wanleibio Co., Ltd.) and incubated
with a secondary antibody (cat. no. WLA023; 1:5,000; Goat
Anti-Rabbit IgG/HRP; Wanleibio Co., Ltd.) for 45 min at 37°C.
Subsequently, the membrane was washed with TBST six times and
visualized using Ultrasensitive ECL Chemiluminescence Kit (cat. no.
WLA006; Wanleibio Co., Ltd.) (32).
The total protein concentration obtained was 2 µg/µl. The intensity
of each band was quantified using Gel-Pro-Analyzer software
(version 4.0; Media Cybernetics, Inc.).
Transfection
All small interfering RNA (siRNA), with a final
concentration of 50 nM, were transiently transfected into HeLa/DDP
cells using Lipofectamine®™ 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 20 min to form transfection complexes
at 37°C. Following a 6-h incubation, the transfection medium was
replaced with fresh growth medium. DDP was added the next day and
the culture was continued for 48 h at 37°C. Transfection efficiency
was semi-quantified using WB. The siRNA sequences (Wanleibio Co.,
Ltd.) used were as follows: HYOU1 sense:
5′-AAGCUGCUGCGUGAGGCUAAUC-3′; anti-sense:
5′-GAUUAAGCCUCACGAGCAGCUU-3′; HYOU1 siRNA-2 sense:
5′-AGCUGGGGAAGAACAUCAAU-3′; anti-sense: 5′-AUUGUUCUUCCCAUCAUCG-3′;
and siRNA negative control (NC) sense: 5′-AUAAACAUCGACUCAAU-3′;
anti-sense: 5′-AUUGAGCUCGAUUGUUAU-3′.
Statistical analysis
An unpaired student's t-test was used to identify
differentially expressed genes (DEGs) between tumor and normal
samples. OS was defined as the time from the date of initial
surgical resection to the date of mortality or last contact
(censored), which was truncated to 60 months. As the number of
responders and non-responders may not be equal, the ‘surv_cutpoint’
algorithm was used to determine the optimal cut-off to distinguish
between the high and low expression levels of genes. Survival
curves were drawn using the Kaplan-Meier method and statistically
compared using the log-rank test. A univariate Cox regression model
was used to analyze the association between clinical factors and
OS. Hazard ratios (HRs) and 95% confidence intervals (CIs) were
calculated using Cox regression models.
Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analysis was performed using gene set enrichment
analysis (GSEA) from the web-based gene set analysis toolkit
(WebGestalt; http://www.webgestalt.org) (33), with a cut-off value of <0.05 for
the false discovery rate (FDR). The m6A sites of genes
were predicted using a sequence-based RNA adenosine methylation
site predictor (SRAMP) program (http://www.cuilab.cn/sramp/) (34) by inputting the sequences of the
genes. The RMBase version 2.0 platform (http://rna.sysu.edu.cn/rmbase/) (35), a comprehensive resource for RNA
modification data verified using methylated RNA immunoprecipitation
sequencing, m6A-sequence and/or
m6A-crosslinking immunoprecipitation arrays, was used to
validate whether the predicted m6A sites underwent
m6A modification. Subsequently, the interaction
probabilities between predicted m6A site sequence motifs
and the protein sequence of a m6A-associated gene were
retrieved using the RNA-Protein Interaction Prediction (RPISeq)
database (http://pridb.gdcb.iastate.edu/RPISeq/) (36). This database calculated the
interaction probabilities using random forest (RF) and support
vector machine (SVM) methods.
The correlation between gene expression levels and
IC50 values for DDP in the GDSC database was estimated
using Pearson correlation analysis and the ggplot2 package in R
(https://cran.r-project.org/web/packages/ggplot2/index.html)
was used to visualize the results. Comparisons between two groups
were analyzed using the unpaired student's t-test. Comparisons
among multiple groups were analyzed using one-way analysis of
variance (ANOVA) and Tukey's test. P-values were adjusted using the
Benjamini-Hochberg procedure for multiple testing (37) to control for the FDR. FDR <0.05
for multiple testing or P<0.05 was considered to indicate a
statistically significant difference.
Results
HYOU1 is a gene that promotes CC
survival and DDP resistance
Based on CRISPR-Cas9 screening data from CC cell
lines, 699 genes were identified with potential impact on cell
proliferation in CC cell lines, in which the CERES scores were
<-1 in >75% of CC cell lines. Compared with normal samples,
3,309 DEGs were identified in 43 samples with CC derived from the
TCGA-CC1 set (unpaired student's t-test; FDR <0.05
and log2(FC)>0; Fig.
1A). Furthermore, 401 DDP resistance genes were identified in
the non-response group compared with those in the response group
(unpaired student's t-test; P<0.05 and log2(FC)>0;
Fig. 1B). Three genes, including
HYOU1, nonsense-mediated mRNA decay factor (SMG5) and
ankyrin repeat and LEM domain containing 2 (ANKLE2),
were selected as they were significantly upregulated in samples
with CC and in the non-response group when compared with normal
samples and the response group, respectively (Fig. 1C).
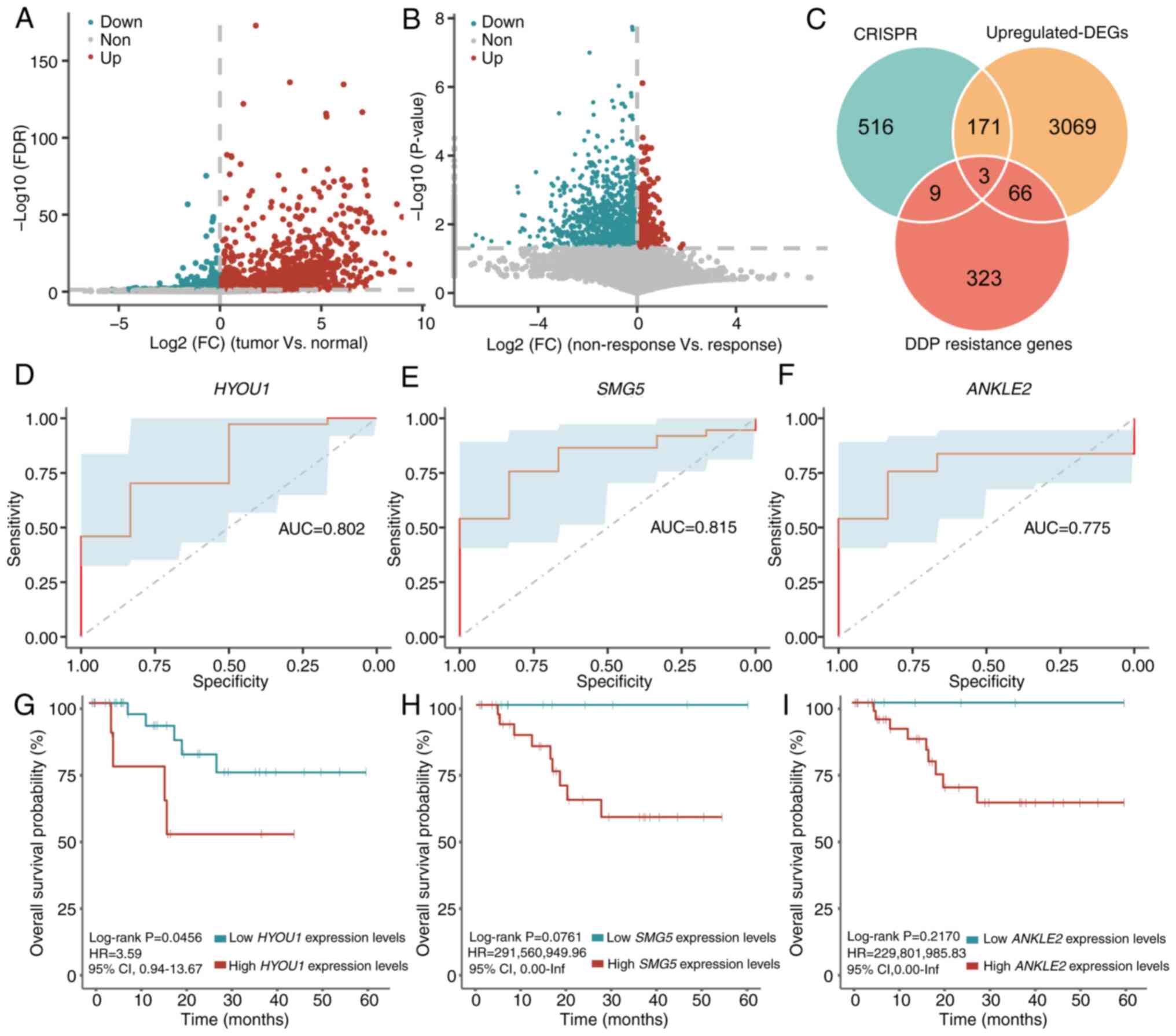 | Figure 1.Identification of key genes in
promoting CC survival and DDP resistance. Volcano plot of
significant DEGs in (A) the tumor group compared with the normal
group, and (B) the non-response group compared with the response
group. (C) Venn diagram of the genes obtained from CRISPR,
CC-associated differential genes and DDP response-associated
differential genes. Receiver operating characteristic curves of (D)
HYOU1, (E) SMG5 and (F) ANKLE2 in
TCGA-CC1 dataset. Kaplan-Meier curve of overall survival
stratified by (G) HYOU1, (H) SMG5 and (I)
ANKLE2 expression in TCGA-CC1 set, respectively.
DDP, cisplatin; AUC, area under the curve; CC, cervical cancer; HR,
hazard ratio; CI, confidence interval; FDR, false discovery rate;
FC, fold change; DEGs, differentially expressed genes; inf,
infinity; TCGA, The Cancer Genome Atlas; CRISPR, clustered
regularly interspaced short palindromic repeats; HYOU1,
hypoxia-upregulated 1 gene; SMG5, nonsense-mediated mRNA decay
factor; ANKLE2, ankyrin repeat and LEM domain containing 2. |
The area under the curve of HYOU1, SMG5 and
ANKLE2 for predicting the response and non-response status
was 0.802, 0.815 and 0.775, respectively (Fig. 1D-F). Finally, for each gene, the
mean expression level was used to stratify patients into high- and
low-expression groups and a survival analysis was performed. The
results showed that there was no significant difference in the OS
between the two groups for the three genes [HYOU1 (high vs.
low expression, 19 vs. 24; log-rank P=0.5412; HR=1.50; 95% CIs,
0.40–5.62), SMG5 (high vs. low expression, 23 vs. 20;
log-rank P=0.5557; HR=1.51; 95% CIs, 0.38–6.07) and ANKLE2
(high vs. low expression, 20 vs. 23; log-rank P=0.6183; HR=1.40;
95% CIs, 0.37–5.22); Fig. S1].
It was hypothesized that the mean value may not be
suitable for distinguishing patients with different responses to
DDP. Therefore, the ‘surv_cutpoint’ algorithm was used to
re-determine the optimal threshold for HYOU1 expression
levels, which was 4.9094. Survival analysis using the
TCGA-CC1 set showed that patients with high HYOU1
expression levels (>4.9094) had a significantly reduced OS
compared with patients with low HYOU1 expression levels
(<4.9094) following DDP treatment (high vs. low expression, 10
vs. 33; log-rank P=0.0456; HR=3.59; 95% CIs, 0.94–13.67; Fig. 1G). Similarly, the ‘surv_cutpoint’
algorithm was used to re-determine the optimal thresholds for
SMG5 and ANKLE2, which were 4.6751 and 3.0310,
respectively. However, high or low SMG5 expression levels
(threshold, 4.6751) and ANKLE2 expression levels (threshold,
3.0310) did not indicate a significantly different OS in the
TCGA-CC1 set [SMG5 (high vs. low expression, 30
vs. 13; log-rank P=0.0761; HR=291,560,949.96; 95% CIs, 0-infinity
(inf); Fig. 1H) and ANKLE2
(high vs. low expression, 35 vs. 8; log-rank P=0.2170;
HR=229,801,985.83; 95% CIs, 0-inf; Fig.
1I)]. Therefore, HYOU1 was selected for follow-up
analyses as a gene associated with the survival of CC cells and DDP
resistance.
Validation of the association of HYOU1
with DDP resistance in independent datasets
In the TCGA-CC2 set, the ‘surv_cutpoint’
algorithm was used to determine the optimal threshold for
HYOU1, which was 4.9094. The 8 patients with high
HYOU1 expression levels (>4.9094) demonstrated a
significantly reduced OS compared with the 32 patients with low
HYOU1 expression levels following DDP treatment (log-rank
P=0.0012; HR=7.09; 95% CIs, 1.81–27.70; Fig. 2A). Using the TCGA-CC3
set, high and low HYOU1 expression levels did not indicate a
significantly different OS in patients that did not receive DDP
treatment (high vs. low expression, 19 vs. 76; log-rank P=0.6254;
HR=1.49; 95% CIs, 0.30–7.38; Fig.
2B). Additionally, according to the GDSC database, the
expression levels of HYOU1 were significantly positively
correlated with the IC50 values of DDP in CC cell lines
(Pearson's correlation analysis; P=0.0384; r=0.58; Fig. 2C).
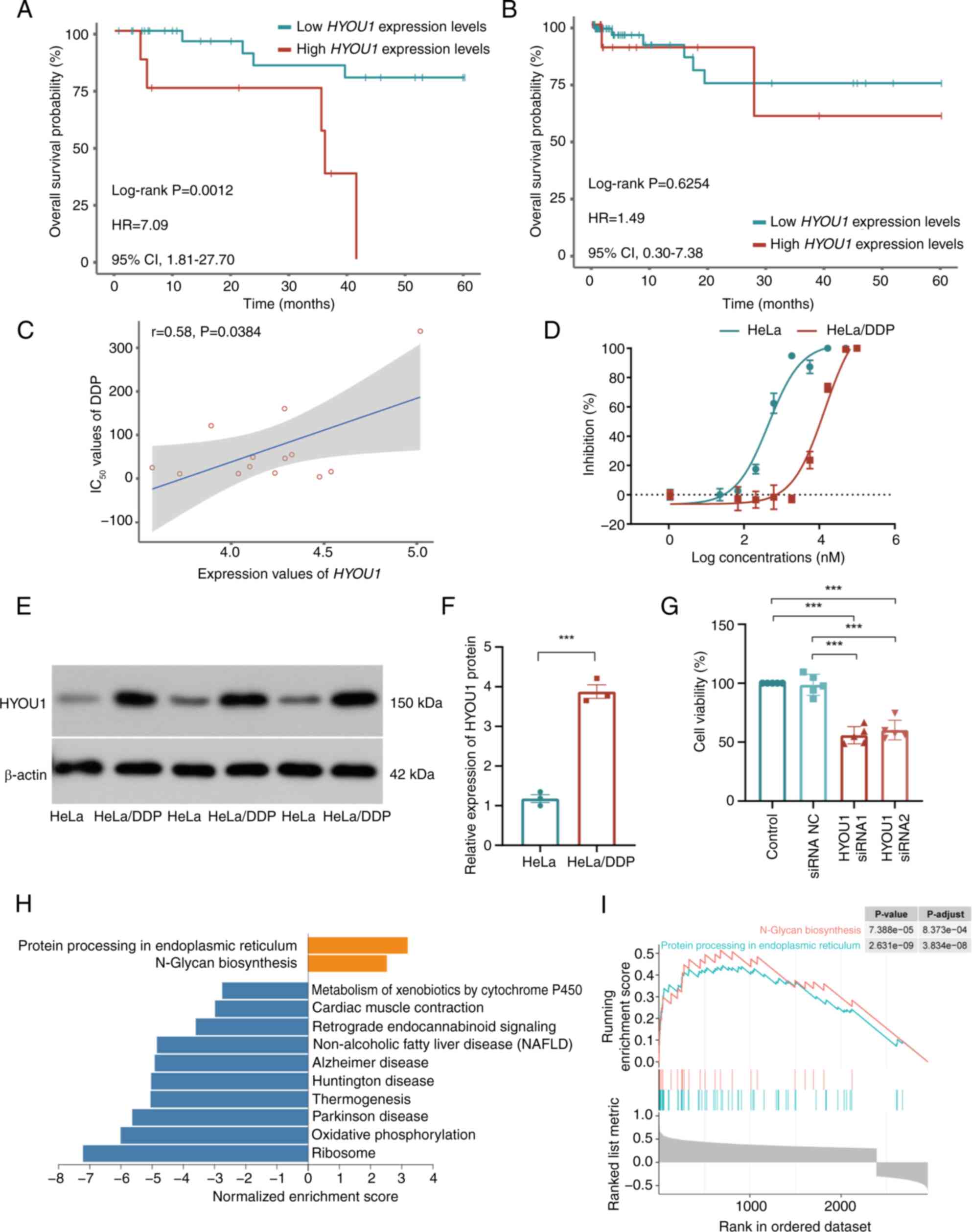 | Figure 2.Validation of HYOU1 in
promoting CC survival and DDP resistance. Kaplan-Meier OS analysis
of patients with high and low expression levels of HYOU1 in
the (A) TCGA-CC2 and (B) TCGA-CC3 datasets.
(C) Point plot of the correlation analysis between the mRNA
expression level values of HYOU1 and IC50 values
of DDP in the Genomics of Drug Sensitivity in Cancer database. (D)
Survival curves of parental HeLa and HeLa/DDP cells that were
subjected to different concentrations of DDP, as measured using the
CCK-8 assay (n=5). (E) Representative western blot showing the
HYOU1 protein expression levels in HeLa and HeLa/DDP cells. (F)
Semi-quantified expression levels of HYOU1 in HeLa and HeLa/DDP
cells (n=3). (G) Proliferation of HeLa/DDP cells treated with DDP
and siRNA (HYOU1 siRNA or siRNA NC) or DDP alone using the CCK-8
assay (n=5), using one-way analysis of variance. (H) Bar plot of
GSEA of HYOU1-associated genes; orange represents the
activation pathway and blue represents the inhibition pathway. (I)
GSEA results for the activation pathways. ***P<0.001. The
statistical difference between two group was analyzed using the
unpaired student's t-test, whereas the statistical difference among
multiple groups was analyzed using one-way analysis of variance and
Tukey's test. CC, cervical cancer; DDP, cisplatin; GSEA, gene set
enrichment analysis; IC50, half-maximal inhibitory
concentration; OS, overall survival; TCGA, The Cancer Genome Atlas;
NC, negative control; siRNA, small interfering RNA; HYOU1,
hypoxia-upregulated 1 gene; HR, hazard ratio; CI, confidence
interval; CCK-8, Cell Counting Kit-8; HeLa/DDP, DDP-resistant HeLa
cells. |
To validate the effect of HYOU1 on the DDP
resistance of CC, HeLa/DDP cells were constructed. The parental
HeLa cells exhibited an IC50 of 1.65 µM. By contrast,
the resistant cells had an IC50 of 15.51 µM,
corresponding to an RI of 9. The IC50 values were
determined using dose-response curves generated from cell viability
assays (Fig. 2D). Using western
blotting, the protein bands revealed an increased HYOU1 expression
level in HeLa/DDP cells across three experiments compared with that
in HeLa cells (Fig. 2E) and the
semi-quantification values in Table
SI further elucidates this. The results showed that the protein
expression of HYOU1 was significantly increased in HeLa/DDP cells
compared with that in parental HeLa cells (unpaired student's
t-test; P=0.0002; Fig. 2F). To
confirm the efficacy of HYOU1 knockdown, knockdown
efficiency was assessed. Using WB analysis, a significant reduction
in protein expression levels of HYOU1 was observed in the knockdown
groups (one-way ANOVA; P<0.001; Fig. S2), indicating the success of
HYOU1 knockdown. Based on this effective knockdown, it was
further revealed that HYOU1 knockdown significantly reduced
the viability of DDP treated cells compared with the control
(one-way ANOVA; P<0.001; Fig.
2G). These results suggest that high HYOU1 expression
levels are associated with resistance to DDP.
To further investigate the function of HYOU1,
2,952 genes that significantly correlated with the expression of
HYOU1 were identified (Pearson correlation analysis; FDR
<0.05; |r|>0.3). These genes were notably enriched in 12
functional pathways (GSEA; FDR <0.05; Fig. 2H). Among these functional pathways,
‘protein processing in endoplasmic reticulum’ and ‘N-glycan
biosynthesis’ were significantly enriched in genes that positively
correlated with HYOU1 and were involved in DDP resistance
(9,13) (Fig.
2I). These results suggest that upregulated expression of
HYOU1 is associated with the accumulation of unfolded
proteins, and may enhance the stress response in the ER and induce
DDP resistance.
m6A modification is
enriched in HYOU1 and increases the stability of the
transcript
Previous preliminary studies report that
m6A modifications are present in almost all types of RNA
molecules in the cell, and regulate the transcriptome to influence
RNA splicing, translation, export, localization and stability
(18–20). To investigate whether the expression
of HYOU1 was regulated by m6A modification, the
online tool SRAMP was used to predict m6A modification
sites on HYOU1. This revealed six HYOU1 sequence
motifs with high confidence (Fig.
3A; Table III).
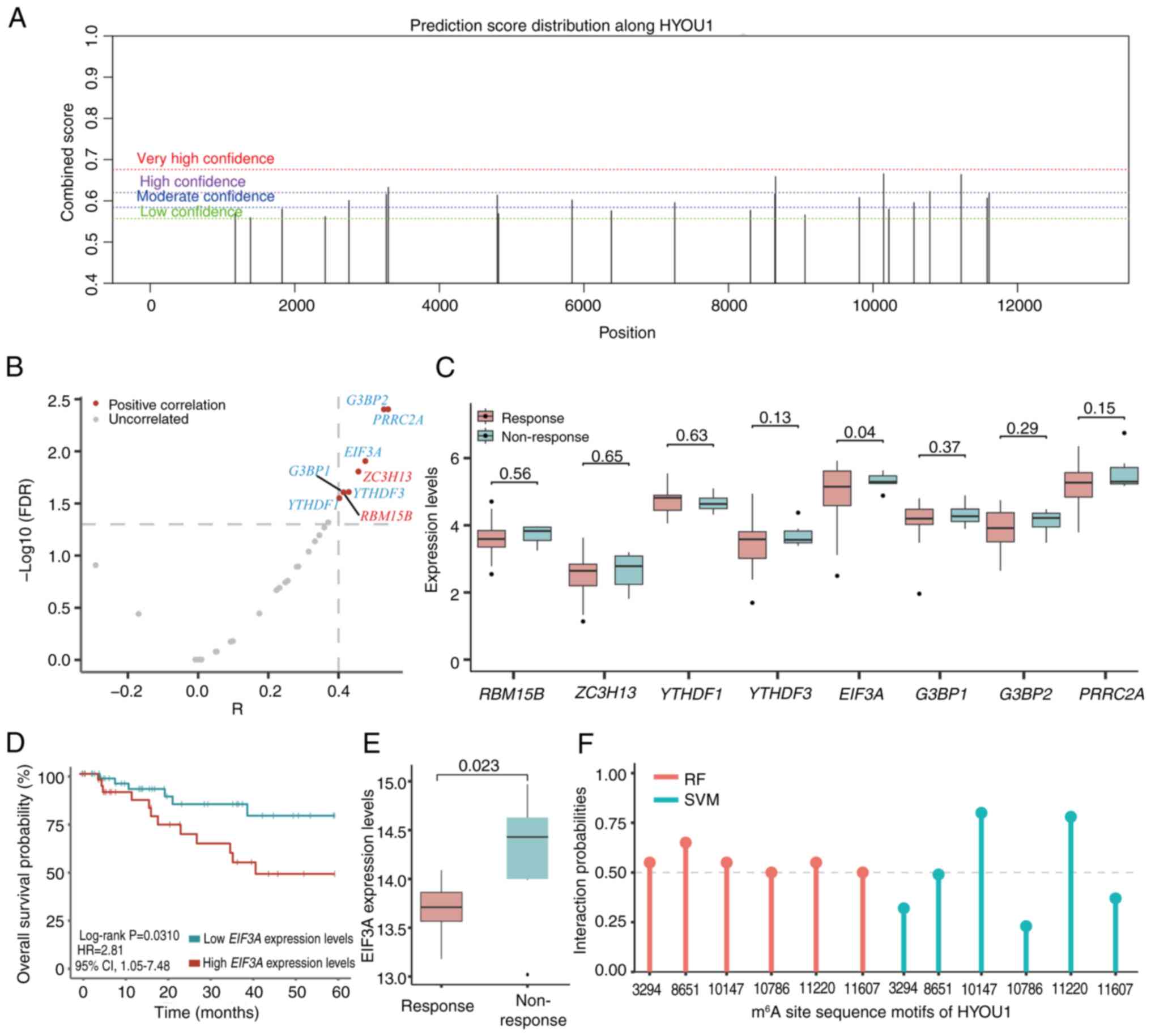 | Figure 3.Analysis of predicted HYOU1
m6A sites and m6A-associated genes. (A)
m6A sites of HYOU1 were predicted using the
sequence-based RNA adenosine methylation site predictor program
(https://www.cuilab.cn/sramp). (B)
Volcano plot of m6A-associated genes that significantly
correlated with the expression of HYOU1. Red,
methyltransferases and blue, reader proteins. (C) Boxplot of
m6A-associated gene expression levels in the
non-responsive and responsive groups. (D) Kaplan-Meier curves of
the overall survival stratified by the EIF3A expression
levels of patients from the TCGA-CC1 or
TCGA-CC2 datasets. (E) Boxplot of the EIF3A
expression levels in non-responsive and responsive groups in the
GSE56363 dataset (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE56363).
(F) Lollipop chart of the interaction probabilities of EIF3A with
the six m6A site sequence motifs according to RPISeq
predictions. TCGA, The Cancer Genome Atlas, RF, random forest; SVM,
support vector machine; HYOU1, hypoxia-upregulated 1 gene;
m6A, N6-methyladenosine; EIF3A, eukaryotic
translation initiation factor 3 subunit A; CC, cervical cancer; HR,
hazard ratio; CI, confidence interval; R, Pearson correlation
coefficient; FDR, false discovery rate. |
 | Table III.Hypoxia-upregulated 1 gene sequence
motifs with high confidence. |
Table III.
Hypoxia-upregulated 1 gene sequence
motifs with high confidence.
|
|
|
|
|
|
|
| RMBase version
2.0a |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Position | Sequence context
(5′-3′) | Score (binary) | Score (KNN) | Score
(spectrum) | Score
(combined) | Decision | Motif
scoreb | Support
Numc |
|---|
| 3,294 |
UGGGAAAACUGGAAGACAUGGAAC | 0.71 | 0.56 | 0.53 | 0.63 | m6A site
(high confidence) | 294.79 | 8 |
|
|
UUUCAAAAUGUAUUCUAAGGA |
|
|
|
|
|
|
|
| 8,651 |
CCUUUGUCCCAUAGACUUCAGGAC | 0.72 | 0.74 | 0.57 | 0.66 | m6A site
(high confidence) | - | - |
|
|
UUGACACUCCGAGACCUGGAG |
|
|
|
|
|
|
|
| 10,147 |
UCCGUCUCAAAAAAAAAAAAGGAC | 0.76 | 0.80 | 0.52 | 0.67 | m6A site
(high confidence) | - | - |
|
|
UAUUCAAGGGGUUUGUUCAGU |
|
|
|
|
|
|
|
| 10,786 |
UUCAGAACCUGAGAAAGUAGAGAC | 0.72 | 0.70 | 0.48 | 0.62 | m6A site
(high confidence) | - | - |
|
|
UGGUGAGUUGGAGCAACCAUG |
|
|
|
|
|
|
|
| 11,220 |
CACUCCAGCCUGGGCAACAGAGAC | 0.70 | 0.77 | 0.61 | 0.66 | m6A site
(high confidence) | - | - |
|
|
UCUGUCUCAAAAAACAGAGUA |
|
|
|
|
|
|
|
| 11,607 |
AGCGGCCUUUGAAGAACGACGAAC | 0.69 | 0.56 | 0.53 | 0.62 | m6A site
(high confidence) | - | - |
|
|
UAUAACCCCCACCUCUGUUUU |
|
|
|
|
|
|
|
The correlation between m6A-associated
genes and the expression of HYOU1 using the
TCGA-CC1 set was analyzed and eight
m6A-associated genes were found that significantly
correlated with the expression of HYOU1 (Pearson's
correlation analysis; FDR <0.05; |r|>0.4; Fig. 3B; Table
II). Among these genes, the expression of EIF3A was
significantly upregulated in the non-response group compared with
that of the response group (unpaired student's t-test; P=0.0399;
FC=1.07; Fig. 3C). Furthermore, the
‘surv_cutpoint’ algorithm was used to determine the optimal
thresholds for EIF3A, which was 5.2442. Survival analysis
indicated that patients with high EIF3A expression levels
(>5.2442) had a significantly reduced OS compared with patients
with low EIF3A expression levels (<5.2442) following DDP
treatment using TCGA-CC data integrated with TCGA-CC1
and TCGA-CC2 sets (high vs. low expression, 35 vs. 48;
log-rank P=0.0310; HR=2.81; 95% CIs, 1.05–7.48; Fig. 3D). In an independent dataset of
patients with CC (GSE56363), the expression of EIF3A was
significantly increased in the non-response group compared with the
response group (unpaired student's t-test; P=0.0228; FC=1.04;
Fig. 3E).
Sequence docking prediction analyses with the RPISeq
database confirmed, with high probabilities and confidence, that
the EIF3A reader may bind with the six m6A site motifs
of HYOU1 (interaction probabilities >0.5; Table IV; Fig.
3F), including the ‘3294’, ‘8651’, ‘10147’, ‘10786’, ‘11220’
and ‘11607’ sites. Furthermore, searching for the HYOU1 gene
on the RMBase version 2.0 platform revealed that the m6A
site (‘3294’) of HYOU1, which exhibited a high probability
of binding with EIF3A, was modified by m6A modification
(Table III).
 | Table IV.Probability of binding based on
predictions using the RNA-protein interaction prediction database
(http://pridb.gdcb.iastate.edu/RPISeq/). |
Table IV.
Probability of binding based on
predictions using the RNA-protein interaction prediction database
(http://pridb.gdcb.iastate.edu/RPISeq/).
| HYOU1
sequence motif position | Method of
interaction probabilities | EIF3A reader |
|---|
| 3,294 |
RF | 0.55 |
|
| SVM | 0.32 |
| 8,651 |
RF | 0.65 |
|
| SVM | 0.49 |
| 10,147 |
RF | 0.55 |
|
| SVM | 0.80 |
| 10,786 |
RF | 0.50 |
|
| SVM | 0.23 |
| 11,220 |
RF | 0.55 |
|
| SVM | 0.78 |
| 11,607 |
RF | 0.50 |
|
| SVM | 0.37 |
Discussion
Resistance to DDP-based chemotherapy is the leading
cause of mortality for patients with CC. By integrating
multidimensional publicly available data of CC, the present study
identified HYOU1 as an important gene, the overexpression of
which was associated with DDP resistance in patients with CC. The
association between high HYOU1 expression levels and DDP
resistance was revealed using data from 53 patients with CC and
cell lines. Mechanistic analyses suggested that EIF3A
overexpression might be associated with HYOU1 depending on
the m6A modification and was associated with DDP
resistance.
HYOU1 belongs to the heat shock protein 70
family and is expressed in numerous cell types, such as epithelial
cells, neuronal cells and cardiomyocytes (38,39).
It is induced by various types of stress, such as hypoxia, ER
stress, ischemia and glucose deprivation (40). Previous studies reveal that
HYOU1 is upregulated in various tumors (such as ovarian
cancer and breast cancer) and is involved in tumorigenesis and
tumor growth (41,42). The study by Liu and Wang (43) demonstrates that HYOU1 is
upregulated in CC cell lines. In addition, the study by Zhou et
al (44) indicates the
expression of HYOU1 in the tissues of nasopharyngeal
carcinoma, which is associated with poor prognosis. Additionally,
HYOU1 is associated with the expansion and metastatic
activity of epithelial ovarian tumor cell lines (41). However, the association of
HYOU1 with DDP resistance has not yet been investigated. The
present study was the first to demonstrate that HYOU1 was
associated with DDP resistance in patients with CC. An independent
cohort of patients with CC was used to indicate that high
HYOU1 expression levels were associated with poor prognosis
only in the patients that received DDP treatment. Additionally,
pharmacogenomic data indicated that high HYOU1 expression
levels were associated with high IC50 values of DDP.
However, the correlation was not strong, which may be due to the
small sample size and should be further validated in a large-scale
dataset. In addition, the present study demonstrated that high
HYOU1 expression levels were associated with resistance to
DDP using WB experiments and knockdown experiments of HYOU1
in HeLa/DDP cells.
The m6A modification serves an important
role in regulating RNA stability and participates in biological
activities (such as response to stress and RNA stability) and
clinical outcomes in patients with cancer (45,46).
The present study found that m6A modifications were
enriched within HYOU1 and that HYOU1 expression
levels were significantly associated with the m6A
reader, EIF3A. Analysis of TCGA-CC data showed that
EIF3A was significantly associated with DDP resistance and
poor survival in patients treated with DDP. Sequence docking
indicated that EIF3A had docking activity with the
m6A site sequence motifs of HYOU1. EIF3A is the
largest subunit of EIF3, which is an important factor in
translation initiation. EIF3A can bind with the 5′-untranslated
region to promote the translation of cap-independent mRNAs
(47). Expression of EIF3A
may influence cancer cell proliferation as this malignant phenotype
can be reversed by knocking down EIF3A in cancer cells
(48). Previously, the study by Su
et al (49), using ribosome
profiling with HEK293T upon CRISPR-Cas9-induced
methyltransferase-like protein 16 (METTL16; a methyltransferase)
knockdown (GSE156796), reports that METTL16 directly interacts with
EIF3A/B, thereby promoting the translation of >4,000 mRNA
transcripts. The analysis of the data (49) reveals that METTL16 knockdown
suppresses the translation efficiency of HYOU1
(log2(FC)=−1.21), suggesting that the dysregulation of HYOU1
might be dependent on the m6A modification. The study by
Xu et al (50) demonstrates
that variation in EIF3A contributes to platinum-based
chemotherapy resistance in patients with lung cancer. To the best
of our knowledge, the role of EIF3A in the DDP resistance of
patients with CC has not been studied before. In the present study,
it was demonstrated that EIF3A may promote DDP resistance in
CC by inducing HYOU1 overexpression depending on the
m6A modification.
However, there were limitations in the present
study. Firstly, the associations of HYOU1 with DDP resistance needs
to be validated using a larger number of patients with CC in future
studies. Secondly, the underlying regulatory mechanism was only
preliminarily investigated and it was found that EIF3A may
promote DDP resistance in CC by inducing HYOU1
overexpression depending on the m6A modification.
Further m6A RNA immunoprecipitation experiments in
EIF3A-transfected and knockout cells are needed to validate the
findings.
In conclusion, HYOU1 was identified as a key
gene associated with DDP resistance in CC. HYOU1 expression
levels may serve as an indicator for assessing the suitability of
DDP treatment as a therapeutic strategy. Mechanistically,
EIF3A may induce HYOU1 overexpression depending on
the m6A modifications in CC cells and may be a candidate
to target for the treatment of patients with CC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Outstanding Youth
Foundation of Heilongjiang Province of China (grant no.
YQ2023H002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RW, JD, MZ, ZW, SW, SL and LQ contributed to the
conception and design of the present study. Material preparation,
data collection and analysis were performed by RW, JD and MZ. ZW
and SW prepared Fig. 1, Fig. 2, Fig.
3. LQ and SL confirm the authenticity of all the raw data. The
first draft of the manuscript was written by LQ and SL and all
authors commented on previous versions of the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CC
|
cervical cancer
|
|
DDP
|
cisplatin
|
|
HYOU1
|
hypoxia-upregulated 1 gene
|
|
ER
|
endoplasmic reticulum
|
|
m6A
|
N6-methyladenosine
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
TCGA
|
The Cancer Genome Atlas
|
|
OS
|
overall survival
|
|
GDSC
|
Genomics of Drug Sensitivity in
Cancer
|
|
HRs
|
hazard ratios
|
|
CIs
|
confidence intervals
|
|
GSEA
|
gene set enrichment analysis
|
|
WebGestalt
|
web-based gene set analysis
toolkit
|
|
FDR
|
false discovery rates
|
|
SMG5
|
nonsense-mediated mRNA decay
factor
|
|
SRAMP
|
sequence-based RNA adenosine
methylation site predictor
|
|
WB
|
western blotting
|
|
RI
|
resistance index
|
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorusso D, Petrelli F, Coinu A,
Raspagliesi F and Barni S: A systematic review comparing cisplatin
and carboplatin plus paclitaxel-based chemotherapy for recurrent or
metastatic cervical cancer. Gynecol Oncol. 133:117–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kishimoto S, Kawazoe Y, Ikeno M, Saitoh M,
Nakano Y, Nishi Y, Fukushima S and Takeuchi Y: Role of Na+,
K+-ATPase alpha1 subunit in the intracellular accumulation of
cisplatin. Cancer Chemother Pharmacol. 57:84–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bai ZL, Wang YY, Zhe H, He JL and Hai P:
ERCC1 mRNA levels can predict the response to cisplatin-based
concurrent chemoradiotherapy of locally advanced cervical squamous
cell carcinoma. Radiat Oncol. 7:2212012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Fraser M, Abedini MR, Bai T and
Tsang BK: Regulation of apoptosis-inducing factor-mediated,
cisplatin-induced apoptosis by Akt. Br J Cancer. 98:803–808. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashrafizadeh M, Zarrabi A, Hushmandi K,
Kalantari M, Mohammadinejad R, Javaheri T and Sethi G: Association
of the epithelial-mesenchymal transition (EMT) with cisplatin
resistance. Int J Mol Sci. 21:40022020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Avril T, Vauleon E and Chevet E:
Endoplasmic reticulum stress signaling and chemotherapy resistance
in solid cancers. Oncogenesis. 6:e3732017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Visioli F, Wang Y, Alam GN, Ning Y, Rados
PV, Nör JE and Polverini PJ: Glucose-regulated protein 78 (Grp78)
confers chemoresistance to tumor endothelial cells under acidic
stress. PLoS One. 9:e1010532014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu R, Warri A, Jin L, Zwart A, Riggins RB,
Fang HB and Clarke R: NF-kappaB signaling is required for XBP1
(unspliced and spliced)-mediated effects on antiestrogen
responsiveness and cell fate decisions in breast cancer. Mol Cell
Biol. 35:379–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Mercier M, Lefranc F, Mijatovic T,
Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R and
Mathieu V: Evidence of galectin-1 involvement in glioma
chemoresistance. Toxicol Appl Pharmacol. 229:172–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rao S, Oyang L, Liang J, Yi P, Han Y, Luo
X, Xia L, Lin J, Tan S, Hu J, et al: Biological function of HYOU1
in tumors and other diseases. Onco Targets Ther. 14:1727–1735.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q,
Jin Z, Yang C and Chen Y: The role of m6A modification in the
biological functions and diseases. Signal Transduct Target Ther.
6:742021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu Y, Dominissini D, Rechavi G and He C:
Gene expression regulation mediated through reversible m(6)A RNA
methylation. Nat Rev Genet. 15:293–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knuckles P, Lence T, Haussmann IU, Jacob
D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D, et
al: Zc3h13/Flacc is required for adenosine methylation by bridging
the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery
component Wtap/Fl(2)d. Genes Dev. 32:415–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pendleton KE, Chen B, Liu K, Hunter OV,
Xie Y, Tu BP and Conrad NK: The U6 snRNA m(6)A Methyltransferase
METTL16 regulates SAM synthetase intron retention. Cell.
169:824–835. e142017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendel M, Chen KM, Homolka D, Gos P,
Pandey RR, McCarthy AA and Pillai RS: Methylation of structured rna
by the m(6)A writer METTL16 Is essential for mouse embryonic
development. Mol Cell. 71:986–1000. e112018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC,
Shi H, Cui X, Su R, Klungland A, et al: Differential m(6)A,
m(6)A(m), and m(1)A demethylation mediated by FTO in the cell
nucleus and cytoplasm. Mol Cell. 71:973–985. e52018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mauer J, Luo X, Blanjoie A, Jiao X,
Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q,
et al: Reversible methylation of m(6)A(m) in the 5′ cap controls
mRNA stability. Nature. 541:371–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su Y, Wang B, Huang J, Huang M and Lin T:
YTHDC1 positively regulates PTEN expression and plays a critical
role in cisplatin resistance of bladder cancer. Cell Prolif.
56:e134042023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu S, Yun J, Tang W, Familiari G,
Relucenti M, Wu J, Li X, Chen H and Chen R: Therapeutic m(6)A
eraser ALKBH5 mRNA-Loaded exosome-liposome hybrid nanoparticles
inhibit progression of colorectal cancer in preclinical tumor
models. ACS Nano. 17:11838–11854. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu Y, Wan A, Lin Z, Lu X and Wan G: N
(6)-Methyladenosine modification: A novel pharmacological target
for anti-cancer drug development. Acta Pharm Sin B. 8:833–843.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi L, Li Y, Qin Y, Shi G, Li T, Wang J,
Chen L, Gu Y, Zhao W and Guo Z: An individualised signature for
predicting response with concordant survival benefit for lung
adenocarcinoma patients receiving platinum-based chemotherapy. Br J
Cancer. 115:1513–1519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Behan FM, Iorio F, Picco G, Gonçalves E,
Beaver CM, Migliardi G, Santos R, Rao Y, Sassi F, Pinnelli M, et
al: Prioritization of cancer therapeutic targets using CRISPR-Cas9
screens. Nature. 568:511–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyers RM, Bryan JG, McFarland JM, Weir
BA, Sizemore AE, Xu H, Dharia NV, Montgomery PG, Cowley GS, Pantel
S, et al: Computational correction of copy number effect improves
specificity of CRISPR-Cas9 essentiality screens in cancer cells.
Nat Genet. 49:1779–1784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang W, Soares J, Greninger P, Edelman EJ,
Lightfoot H, Forbes S, Bindal N, Beare D, Smith JA, Thompson IR, et
al: Genomics of drug sensitivity in cancer (GDSC): A resource for
therapeutic biomarker discovery in cancer cells. Nucleic Acids Res.
41((Database issue)): D955–D961. 2013.PubMed/NCBI
|
|
28
|
Li Y, Xiao J, Bai J, Tian Y, Qu Y, Chen X,
Wang Q, Li X, Zhang Y and Xu J: Molecular characterization and
clinical relevance of m(6)A regulators across 33 cancer types. Mol
Cancer. 18:1372019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Harada BT and He C: Regulation of
gene expression by N(6)-methyladenosine in cancer. Trends Cell
Biol. 29:487–499. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang H, Weng H and Chen J: m(6)A
modification in coding and non-coding RNAs: Roles and therapeutic
implications in cancer. Cancer Cell. 37:270–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nombela P, Miguel-Lopez B and Blanco S:
The role of m(6)A, m(5)C and Ψ RNA modifications in cancer: Novel
therapeutic opportunities. Mol Cancer. 20:182021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qiu F, Liu Q, Xia Y, Jin H, Lin Y and Zhao
X: Circ_0000658 knockdown inhibits epithelial-mesenchymal
transition in bladder cancer via miR-498-induced HMGA2
downregulation. J Exp Clin Cancer Res. 41:222022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao Y, Wang J, Jaehnig EJ, Shi Z and
Zhang B: WebGestalt 2019: Gene set analysis toolkit with revamped
UIs and APIs. Nucleic Acids Res. 47:W199–W205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zeng P, Li YH, Zhang Z and Cui Q:
SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based
on sequence-derived features. Nucleic Acids Res. 44:e912016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xuan JJ, Sun WJ, Lin PH, Zhou KR, Liu S,
Zheng LL, Qu LH and Yang JH: RMBase v2.0: Deciphering the map of
RNA modifications from epitranscriptome sequencing data. Nucleic
Acids Res. 46((D1)): D327–D334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi Y, Zhao Y, Huang Y and Wang D: A brief
review of RNA-protein interaction database resources. Noncoding
RNA. 3:62017.PubMed/NCBI
|
|
37
|
Hochberg Y and Benjamini Y: More powerful
procedures for multiple significance testing. Stat Med. 9:811–818.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsukamoto Y, Kuwabara K, Hirota S, Ikeda
J, Stern D, Yanagi H, Matsumoto M, Ogawa S and Kitamura Y: 150-kD
oxygen-regulated protein is expressed in human atherosclerotic
plaques and allows mononuclear phagocytes to withstand cellular
stress on exposure to hypoxia and modified low density lipoprotein.
J Clin Invest. 98:1930–1941. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giffin L, Yan F, Major MB and Damania B:
Modulation of Kaposi's sarcoma-associated herpesvirus interleukin-6
function by hypoxia-upregulated protein 1. J Virol. 88:9429–9441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuwabara K, Matsumoto M, Ikeda J, Hori O,
Ogawa S, Maeda Y, Kitagawa K, Imuta N, Kinoshita T and Stern DM:
Purification and characterization of a novel stress protein, the
150-kDa oxygen-regulated protein (ORP150), from cultured rat
astrocytes and its expression in ischemic mouse brain. J Biol Chem.
271:5025–5032. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Zhang NX, Ye HY, Song PP, Chang W,
Chen L, Wang Z, Zhang L and Wang NN: HYOU1 promotes cell growth and
metastasis via activating PI3K/AKT signaling in epithelial ovarian
cancer and predicts poor prognosis. Eur Rev Med Pharmacol Sci.
23:4126–4135. 2019.PubMed/NCBI
|
|
42
|
Stojadinovic A, Hooke JA, Shriver CD,
Nissan A, Kovatich AJ, Kao TC, Ponniah S, Peoples GE and Moroni M:
HYOU1/Orp150 expression in breast cancer. Med Sci Monit.
13:BR231–BR239. 2007.PubMed/NCBI
|
|
43
|
Liu J and Wang Y: Long non-coding RNA
KCNQ1OT1 facilitates the progression of cervical cancer and tumor
growth through modulating miR-296-5p/HYOU1 axis. Bioengineered.
12:8753–8767. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou Y, Liao Q, Li X, Wang H, Wei F, Chen
J, Yang J, Zeng Z, Guo X, Chen P, et al: HYOU1, regulated by
LPLUNC1, is up-regulated in nasopharyngeal carcinoma and associated
with poor prognosis. J Cancer. 7:367–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Desrosiers R, Friderici K and Rottman F:
Identification of methylated nucleosides in messenger RNA from
Novikoff hepatoma cells. Proc Natl Acad Sci USA. 71:3971–3975.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saletore Y, Meyer K, Korlach J, Vilfan ID,
Jaffrey S and Mason CE: The birth of the Epitranscriptome:
Deciphering the function of RNA modifications. Genome Biol.
13:1752012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Meyer KD, Patil DP, Zhou J, Zinoviev A,
Skabkin MA, Elemento O, Pestova TV, Qian SB and Jaffrey SR: 5′ UTR
m(6)A promotes cap-independent translation. Cell. 163:999–1010.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dong Z, Liu LH, Han B, Pincheira R and
Zhang JT: Role of eIF3 p170 in controlling synthesis of
ribonucleotide reductase M2 and cell growth. Oncogene.
23:3790–3801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Su R, Dong L, Li Y, Gao M, He PC, Liu W,
Wei J, Zhao Z, Gao L, Han L, et al: METTL16 exerts an
m(6)A-independent function to facilitate translation and
tumorigenesis. Nat Cell Biol. 24:205–216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu X, Han L, Yang H, Duan L, Zhou B, Zhao
Y, Qu J, Ma R, Zhou H and Liu Z: The A/G allele of eIF3a rs3740556
predicts platinum-based chemotherapy resistance in lung cancer
patients. Lung Cancer. 79:65–72. 2013. View Article : Google Scholar : PubMed/NCBI
|

















