Introduction
Neuroendocrine neoplasms (NENs) can originate from
several regions of the body, but are most commonly observed in the
gastrointestinal tract, and, to a lesser extent, in the lungs,
breast, larynx, prostate, bladder, ovaries and cervix. Included
among these, primary NEN of the breast (PNENB) is a rare subtype of
breast cancer, accounting for <1% of all breast tumors (1). Current understanding of this disease
is limited, as researchers have not detected neuroendocrine cells
in normal breast tissue, and it has been suggested that PNENB may
be derived from an early-stage differentiation of breast cancer
tumor cells (2). The definition of
PNENB as well as the diagnostic criteria have been under debate
since this type of cancer was first reported (1,3,4). The
different criteria used for the definition of cases, due to the
overlap in diagnostic features between PNENB and invasive breast
cancer (IBC) with neuroendocrine differentiation (5), have led to a lack of comparability
between studies on the treatment and prognosis of the disease. In
the present article, a case of a patient with PNENB is described
and the current body of literature on PNENB is reviewed.
Case report
A 70-year-old woman was admitted to the Affiliated
Hospital of Inner Mongolia Medical University (Hohhot, China) on
May 22, 2014 with a left breast mass that had been detected >1
month earlier. On examination, a medium-sized (~15×20 mm) mass with
unclear borders was palpable in the upper outer quadrant of the
left breast. Enlarged lymph nodes were also palpable in the left
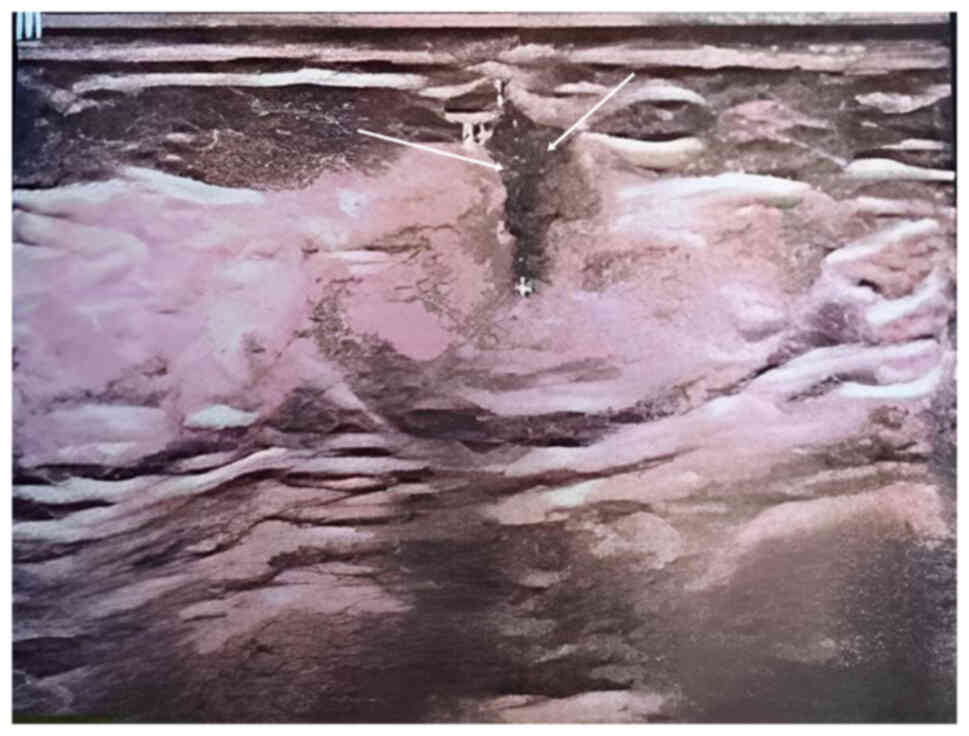
axilla. An ultrasound on May 24, 2014 showed a 15×20-mm irregular
hypoechoic solid mass, identified at 1 o'clock in the left breast,
and two transverse fingers from the nipple, 7.6 mm below the
surface of the skin, with no clear borders. Striated blood flow
signals were observed at the margins of the lesion by color Doppler
flow imaging, which was assessed as Breast Imaging Reporting and
Data System (BI-RADS) category 4b (6) (Fig.
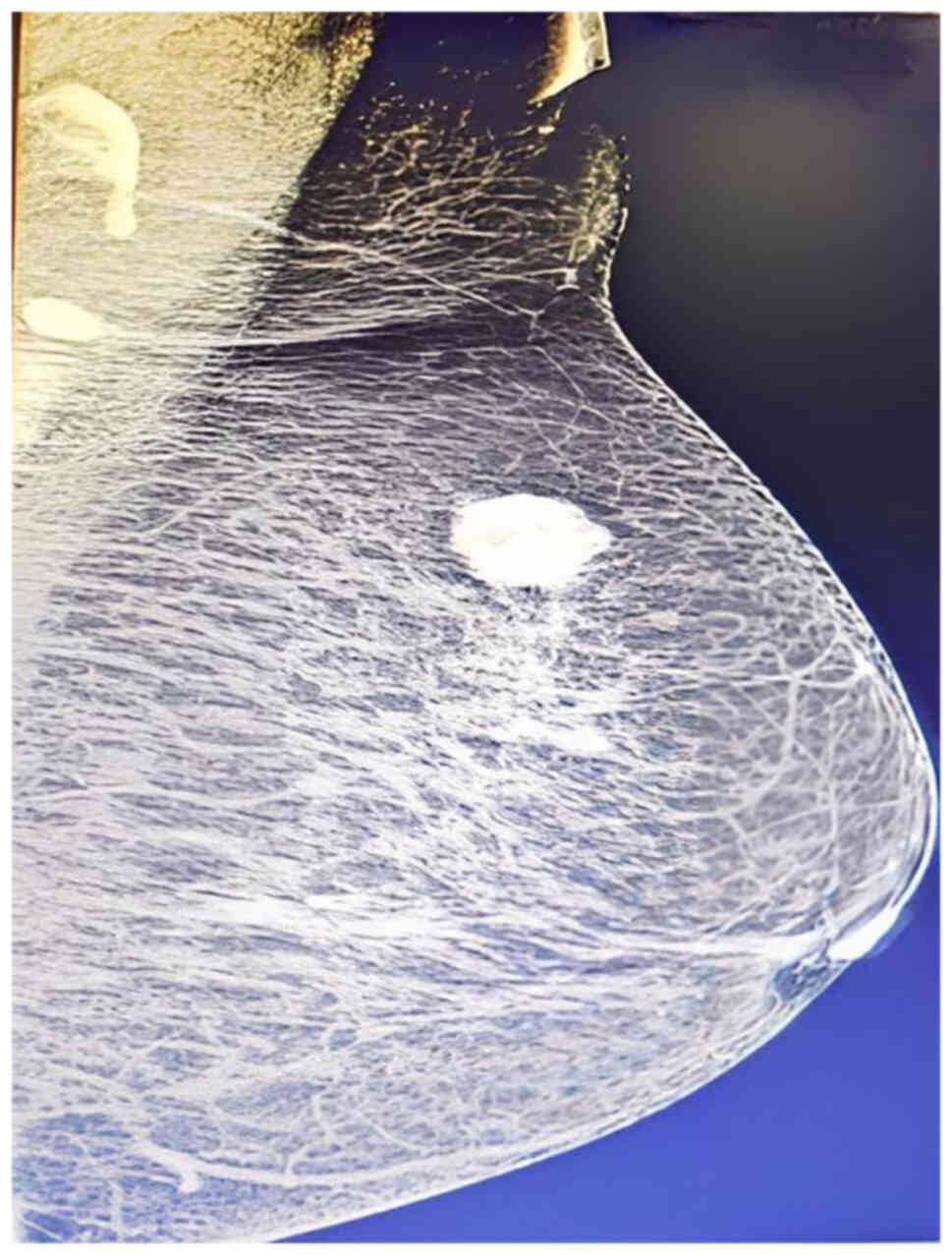
1). A mammography on May 25, 2014 showed a nodular hyperdense
shadow in the upper quadrant of the left outer breast, ~15×20 mm in
size, which was assessed as BI-RADS 4a (Fig. 2). Subsequently, left breast
lumpectomy followed by intraoperative frozen section analysis was
performed. The gross pathological examination revealed a piece of
light-yellow soft tissue of 5×4×3 cm, with a bleeding gray-white
mass inside. The maximum diameter of the mass was 2 cm. Hematoxylin
and eosin staining and immunohistochemical (IHC) staining were
performed to further define the tumor. For hematoxylin and eosin
staining, tissues were fixed with 10% neutral formalin at 24°C for
20 h. Tissue sections were 4 µm thick. Tissues were immersed in
xylene for 5 min, and 75, 85 and 95% gradient ethanol for 1 min.
Tissues were stained with hematoxylin for 6 min at 37°C,
differentiated with 0.5% hydrochloric acid ethanol for 10 sec,
incubated with 0.2% ammonia water for 40 sec and stained with eosin
staining solution for 3 min at 60°C. Staining was observed under a
light microscope. For IHC staining, tissues were fixed with 10%
neutral formalin at 24°C for 20 h and embedded in paraffin. The
tissue sections were 4 µm thick. The tissue sections were blocked
with 15% blocking serum (Fish Serum Blocking Buffer; Thermo Fisher
Scientific, Inc.) for 20 min at 37°C. Tissue sections were
incubated with Cyclin D1 Recombinant Rabbit Monoclonal Antibody
(SP4; dilution, 1:100; cat. no. MA5-14512; Cusabio Technology, LLC)
for 2 h at 37°C, followed by incubation with Goat anti-Human IgG
(H+L) Cross-Adsorbed Secondary Antibody (conjugate, Alexa Fluor™
488; dilution, 1:100; cat. no. A-11013; Cusabio Technology, LLC)
for 2 h at 37°C and sealing with neutral resin. Tissue sections
were observed under a light microscope. A simple left mastectomy
and anterior lymph node biopsy were also performed. The
postoperative pathological results suggested that the mass was a
low-grade neuroendocrine tumor (NET) with negative margins.
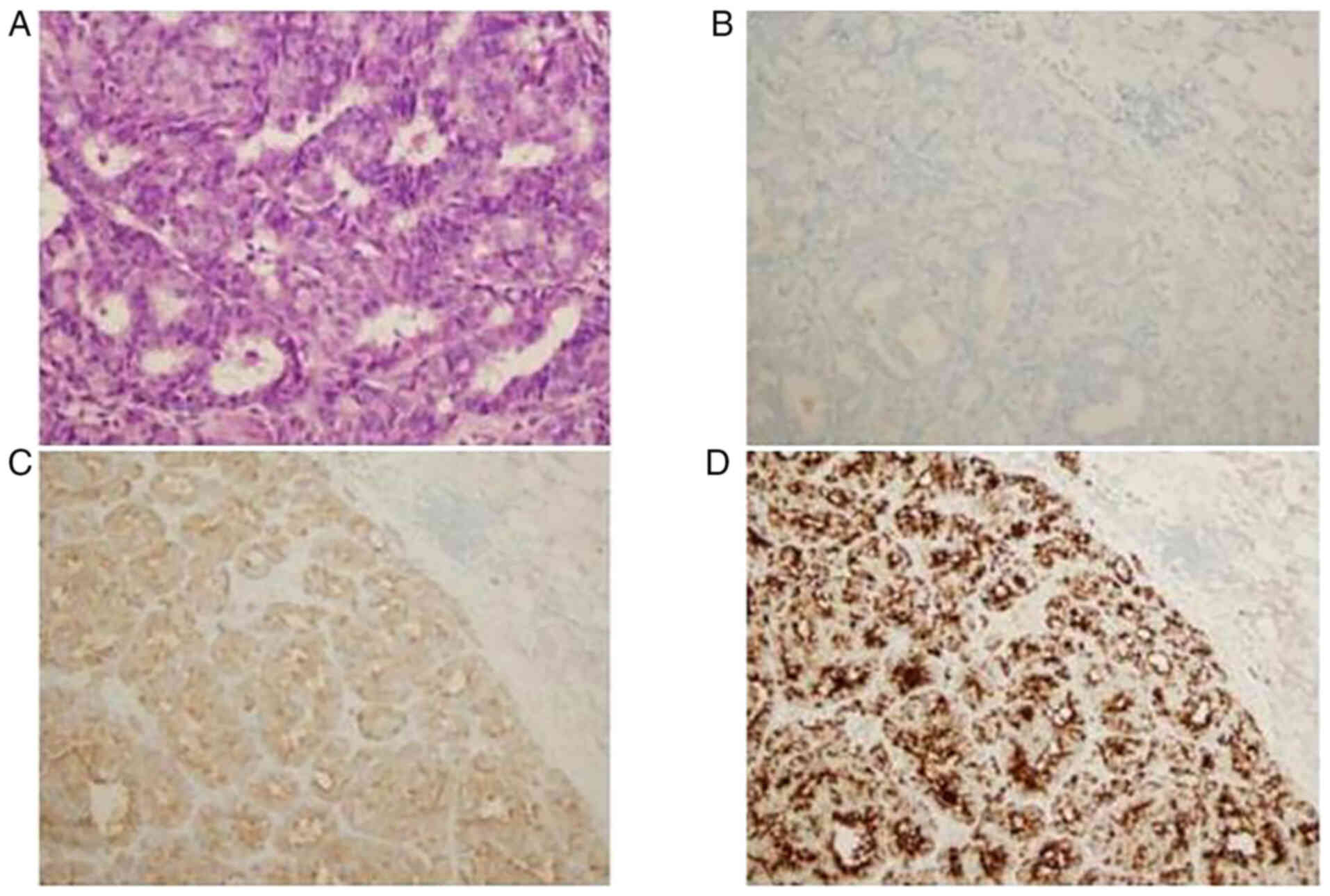
Gross pathological examination confirmed that the
mass had a maximum diameter of ~2 cm. Hematoxylin and eosin
staining showed that it comprised tumor cells that were uniform in
size and rounded, with well-defined nuclei (Fig. 3A) and CD56 negative (Fig. 3B). IHC staining for chromogranin A
(CgA) (Fig. 3C) and synaptophysin
(Syn) (Fig. 3D) was positive, and
detected in >50% of the tissue. Postoperative pathological
diagnosis suggested a low-grade NET of the left breast. IHC
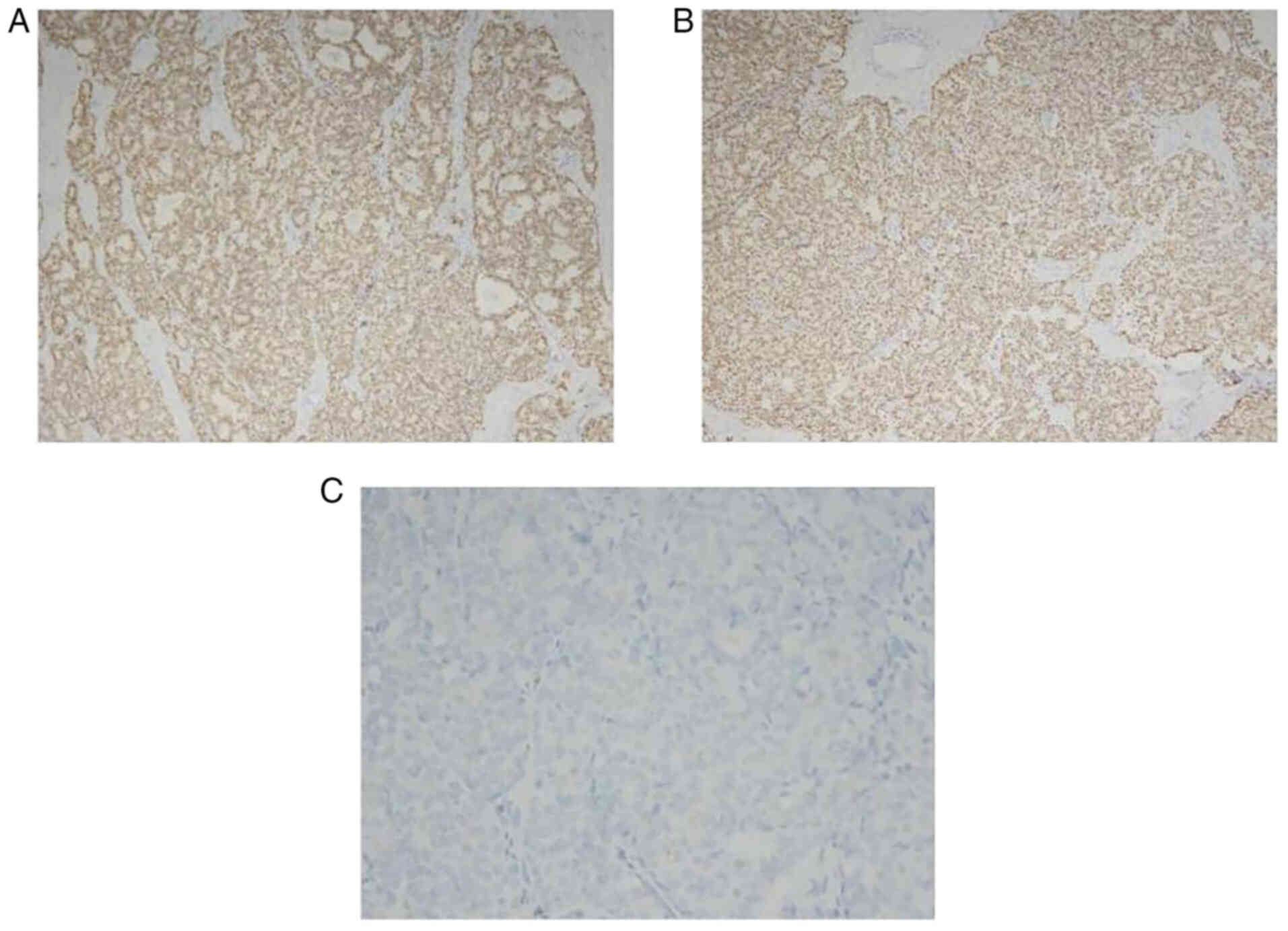
staining results also revealed the presence of estrogen receptors
(ERs, >90%; Fig. 4A),
progesterone receptors (>90%; Fig.
4B) and Ki67 (~10%; data not shown), while IHC staining for
human epidermal growth factor receptor 2 (HER-2) was negative
(Fig. 4C).
Because anterior lymph node metastasis was not
found, the patient did not receive any chemotherapy or other
treatment after surgery. The patient has been followed up for 12
months, with a monthly telephone follow-up, with no axillary lymph
node metastases, and no tumors at other sites have been
detected.
Discussion
PNENB can be distinguished from other types of
breast tumors based on morphological features as well as
neuroendocrine markers. The neuroendocrine differentiation of
breast cancer was first observed in 1963 by Feyrter and Hartmann
(7) in mucinous carcinoma of the
breast. This was followed by the discovery of breast tumors that
were morphologically similar to carcinoid tumors of other organs by
Cubilla and Woodruff (8) in 1977,
who named them primary carcinoid tumors of the breast. In 1985,
Bussolati et al (9) detected
CgA in breast tissue, which provided evidence that some cells in
the breast can exhibit neuroendocrine features. In 2002, Sapino and
Bussolati (10) proposed the first
diagnostic criteria for PNENB. In 2003, the World Health
Organization (WHO) officially recognized PNENB as a distinct type
of breast cancer, and defined PNENB as a tumor of epithelial origin
(11). Following the diagnostic
criteria of Sapino and Bussolati, breast tumors with morphological
features similar to those of NENs originating in the lungs and
gastrointestinal system in which >50% of the tumor cells
expressed neuroendocrine markers were referred to as PNENB, with
CgA and Syn considered as the most sensitive and specific
neuroendocrine markers (11). These
cancers were subdivided into large-cell neuroendocrine carcinomas
(LCNEC), small-cell neuroendocrine carcinomas (SCNEC), and solid
carcinomas based on their morphological features. In 2012, the WHO
Classification of Breast Tumors defined PNENB as a carcinoma with
morphological features similar to those of NEN originating in the
lungs and gastrointestinal system, irrespective of the percentage
of tumor cells expressing neuroendocrine biomarkers. In addition,
it suggested that based on morphology, PNENB can be classified into
three subgroups: Well-differentiated NET of the breast (NETB),
poorly differentiated neuroendocrine carcinomas of the breast
(NECB)/SCNEC, and IBCs with neuroendocrine differentiation,
including solid papillary and mucinous carcinomas (12).
The 5th edition of the WHO Classification of Breast
Tumors, published in 2019, uses the same classification criteria
for PNENB, gastroenterology-pancreas NETs and lung NETs. It also
divides breast tumors in which >90% of tumor cells have
neuroendocrine features into two main categories based on the
degree of differentiation: Well-differentiated NETB and poorly
differentiated NECB, including SCNEC and LCNEC. LCNEC was added to
the classification, and special tissue-type breast cancers,
including solid breast cancer and mucinous carcinoma, were removed
from the PNENB category (13).
Breast tumors in which 10–90% of the cells exhibit neuroendocrine
differentiation are referred to as mixed IBCs or
NETs/neuroendocrine carcinomas (NECs), and tumors in which <10%
of the cells exhibit neuroendocrine differentiation are classified
as either IBC of no special type (IBC-NST) or IBCs of other special
types. The Nottingham grading system can be used to grade PNENB
tumors based on their specific characteristics, independent of the
parameters used in neuroendocrine tumors originating from other
parts of the body (13). It is
noteworthy that although NETB has been classified in the framework
of NETs, it has no defined morphological features, and its
identification among other breast tumors relies on the staining of
neuroendocrine markers, including Syn and CgA, which are also
expressed in non-PNENB tumors. Based on a review of previous
reports, the molecular and genetic characteristics of NETB are not
similar to those of well-differentiated NENs from other sites, but
resemble those of luminal A breast cancer (14). In contrast to NETB, NECB represent a
well-defined entity, showing morphological and clinical analogies
with pulmonary and extra-pulmonary NECs (15).
PNENB occurs predominantly in >60-year-old
postmenopausal women, is very rare in men, and does not differ
markedly from other types of breast cancer in terms of clinical
presentation (16). The majority of
patients present with isolated breast lumps as the primary symptom,
and may also have carcinoid syndrome and clinical manifestations
associated with ectopic hormone secretion, such as paraneoplastic
thrombocytosis and hyperprolactinemia, as well as rare elevations
of α-fetoprotein (17–19). NECB is a high-grade tumor, most
often detected at an advanced stage, and metastasized at the time
of initial diagnosis (20). The
imaging features of PNENB are not specific. Previous studies have
reported that mammography reveals round, ovoid, lobulated or
irregularly shaped masses, most of which have poorly defined
borders with surrounding tissues and burr-free margins, whereas
IBC-NST tends to have an irregular shape with burr-like margins,
and is associated with microcalcifications (21,22).
The majority of cases present on breast ultrasound as an irregular
mass that is hypoechoic or poorly defined, with absent or enhanced
posterior acoustic features (23).
Park et al (22) suggested
that neuroendocrine differentiation in tumors may influence the
imaging presentation, and that the absence of burr edges on
mammograms and of posterior echo enhancement on ultrasound images
may be indicative of a tumor with neuroendocrine features. When
morphological features of PNENB are not evident, imaging is
necessary to rule out metastatic malignancy in the breast since
≥97% of NECs originate from the gastrointestinal tract or lungs
(24).
The absence of specific features in the routine
examination of PNENB underscores the importance of accurately
diagnosing this condition, as the diagnosis directly influences the
subsequent treatment and prognosis. Diagnosis relies on
morphological features, IHC staining and genetic analyses to
determine the type of cancer and identify therapeutic targets.
Morphologically, NETB consists of dense nests of cells and cellular
trabeculae, and the cells may show spindle, plasma cell-like or
polygonal features, with eosinophilic or thylakoid cytoplasm
separated by a fibrovascular stroma, with little morphological
resemblance to NETs at other sites (14,25).
SCNEC tumors exhibit infiltrative growth, darkly stained nuclei,
inconspicuous nucleoli, high nucleoplasmic ratios, sparse cytoplasm
and ill-defined cytoplasmic boundaries, while LCNEC tumors have
darkly stained nuclei, diverse nuclear morphology and more
cytoplasm than SCNEC tumors (26).
The IHC markers commonly used to identify the presence of
neuroendocrine differentiation are Syn, CgA, neuron-specific
enolase (NSE) and CD56. Syn and CgA are diffusely positive in NECB
and are specific markers, whereas NSE and CD56 are less sensitive
and specific. The transcription factor insulinoma-associated
protein 1 (INSM1) is a relatively novel marker that is detected in
the cytosol and differs from other neuroendocrine markers, which
are detected in the cytoplasm; however, its sensitivity and
specificity are not significantly different from those of Syn and
CgA (27). Zhong et al
(28) investigated the expressed
pattern of ‘second generation’ neuroendocrine markers INSM1,
achaete-scute homolog 1 (ASCL1) and POU class 2 homeobox 3 (POU2F3)
in breast cancers with neuroendocrine morphology. The study found
that INSM1 was more specific than Syn and more sensitive and
specific than CgA, but the positivity rates of ASCL1 (4/35) and
POU2F3 (1/35) were too low to support routine testing of their
expression. Recent studies have demonstrated that syntaxin-1 (STX1)
is a sensitive and specific marker of neuroendocrine cells
(29–31). For example, in a study of NECB in
which STX1 and INSM1 were compared with neuroendocrine markers such
as Syn, CgA and CD56, a sensitivity of 84.7% (50/59) and
specificity of 98.1% were recorded for STX1, suggesting that STX1
has potential as an NE marker (31). However, to the best of our
knowledge, no studies have assessed the expression of these newer
NE markers in the rare PNENB subtype. Juhlin et al (32) suggested that NE markers such as
INSM1, secretagogin, and ISL LIM homeobox 1 are tissue-specific,
which may aid in the detection of the primary tumor site. These new
NE markers may provide novel avenues for research into the clinical
implications of NE differentiation in PNENB. Another study found
that the expression of transcription factors GATA-binding protein 3
(GATA3) and gross cystic disease fluid protein 15 was positive in
tumors originating from the breast and negative in metastatic
tumors, while the expression of caudal-type homeobox 2 and thyroid
transcription factor-1 (TTF-1) was suggestive of metastatic tumors
(33). Notably, TTF-1 also
exhibited strong positivity in high-grade NECB. In addition, the
expression of hormone receptors (HRs) has been shown to contribute
to the diagnosis of PNENB. Specifically, strongly positive HR
expression and negative HER-2 expression is observed in the
majority of PNENBs, which, according to their molecular subtypes,
exhibit tubulointerstitial characteristics (34,35).
However, the proportion of cases of PNENB that are negative for HR
and HER-2 expression is greater than that for invasive ductal
carcinoma (IDC). Several studies have reported ER positivity in NEN
of the lung, pancreas, small intestine and ovary (36–38).
Although ER positivity is not specific, it can be used as a target
for therapeutic agents. For example, Zhang et al (39) found that the tumor in a patient with
poorly differentiated NEC of the breast shrank by 78.87% after 3
months of neoadjuvant endocrine therapy.
PNENB is genetically heterogeneous. It has a
different mutational spectrum from other ER-positive and HER
2-negative IBC-NSTs. In one study, lysine-specific
methyltransferase 2C was found to be the most commonly mutated gene
in PNENB (3/17, 17.6%) and predicted to serve as a driver gene that
may play an important role in the neuroendocrine differentiation of
breast cancer (40). PNENB was also
indicated to have common copy number variants (CNVs) such as 8q,
11q and 17q amplification, with variants in 8q being high-frequency
CNVs and potential targets for tumor therapy (40). Wei et al (40) found that the mutation rates of
members of the PI3K and MAPK signaling pathway in NETB were higher
than those in NECB, suggesting that breast NET and NEC may have
different genetic phenotypes and prognoses, and thus require
different therapeutic strategies. A study by Marchiò et al
(41) showed that the most commonly
mutated genes were GATA3, forkhead box protein A1 (FOXA1), T-box
transcription factor 3 (TBX3) and AT-rich interactive
domain-containing protein 1A in 3/18 cases (17%), and PI3K
catalytic subunit α (PIK3CA), AKT1 and cadherin 1 in 2/18 cases
(11%). In addition, the study indicated that NECB is characterized
by a lower frequency of tumor protein P53 (TP53) and PIK3CA
mutations, and enrichment of FOXA1 and TBX3 mutations compared with
common forms of ductal breast cancer. In another study,
co-mutations in TP53 and RB transcriptional corepressor 1 (RB1)
were found in 6/7 cases of SCNEC (86%), 2/2 cases ambiguous for
small cell vs. large cell morphology (100%) and 2/4 LCNEC cases
(50%). In addition, one case of wild-type TP53/RB1 SCNEC had other
p53 pathway aberrations, specifically amplification of mouse double
minute 2 and 4 homologs, and was found to be RB-negative by IHC
analysis (42). It was suggested
that co-inactivation of TP53 and RB1 may be important for the
pathogenesis of the NEC phenotype in the mammary gland. Vranic
et al (43) detected the
expression of folate receptor 1 (FOLR1), trimethylated Lys-36 of
histone 3 (H3K36me3) and tumor-associated calcium signal transducer
2 (TROP-2) in a subset of NECBs; however, they did not detect any
biomarkers indicating that immune checkpoint inhibitors could be
used, including programmed death ligand 1 expression, tumor
mutational burden and microsatellite instability. However, based on
the detection of TROP-2, FOLR1 and H3K36Me3, they suggested that
antibody-drug conjugates or inhibitors of these proteins may be
potential therapeutic options for the treatment of NECB. Mutations
in the epidermal growth factor receptor (EGFR) gene serve as a
suitable target for most cancers. In one study a rare EGFR
p.Thr790Met (T790M) mutation was found in a patient with poorly
differentiated NEBC, which was suggested to be responsible for the
resistance of the tumor to tyrosine kinase receptor inhibitors
(44).
Due to the rarity of PNENB, no standard treatment
guideline currently exists. Surgery is the first-line treatment
option, as it is for other IBC-NSTs (16,45).
Other treatment modalities include adjuvant radiotherapy,
chemotherapy, endocrine therapy, targeted therapy and neoadjuvant
chemotherapy (16,46). NECB has been typically treated with
chemotherapy in previous cases in the relevant literature due to
its high histological grade and metastasis at the time of
detection. Adjuvant chemotherapy can be used in patients at a high
risk of recurrence and neoadjuvant chemotherapy can be used in
patients with preoperative downstaging or locally advanced disease
before surgery (16,45,47).
Platinum-based drugs and etoposide chemotherapeutic agents are the
most widely used chemotherapeutic agents for small cell lung cancer
and extrapulmonary poorly differentiated LCNEC or SCNEC, while
combinations of anthracycline- and paclitaxel-containing
chemotherapeutic agents are generally used for other types of PNENB
(48). Somatostatin (SS) therapy is
mediated through the SS receptor (SSTR) on the surface of tumor
cells. SSTR2A, SSTR2B, SSTR3 and SSTR5 have been found to be
expressed in breast NET and NEC (49,50),
of which SSTR2A and SSTR5 exhibit a high affinity for growth
inhibitor analogs. Positron emission tomography using
gallium-68-labelled SS analogs has potential for use in the
diagnosis of PNENB, while SS analogs combined with a β-emitting
lutetium-177 radioisotope are now approved for use in the treatment
of NET (50), and may also serve as
a therapeutic option in the management of PNENB.
To date, the prognosis of PNENB is unclear, which
may be attributed to the different diagnostic criteria used for the
inclusion of cases in different studies and the small sample sizes.
However, most studies have shown that PNENB has a poorer prognosis
than NST-IDC (51,52). Tumor staging and histological
grading remain the primary prognostic factors. An analysis of PNENB
using data from the Surveillance, Epidemiology, and End Results
database indicated that age, marital status, the place of
registration, surgery, American Joint Committee on Cancer staging
and breast subtype are independent prognostic factors (53). Although race was found to be
significantly associated with PNENB in univariate Cox and
Kaplan-Meier analyses, no significant association was observed in
multivariate Cox analysis, which may be attributed to the
elimination of spurious associations when confounding variables
were accounted for in the multivariate analysis (53). The analysis also showed that,
compared with non-surgical treatment, surgical intervention for
excision of the primary tumor or for non-cancer-related purposes
was effective in improving prognosis. It was suggested that in the
latter scenario, the surgery may improve the prognosis by improving
the quality of life of the patient (53).
In conclusion, PNENBs are a rare, heterogeneous
subtype of breast tumors, which are yet to be fully understood.
Although previous studies have identified several driver genes and
mutated genes with high mutation rates, the exact diagnosis
continues to rely on the examination of traditional neuroendocrine
markers, namely Syn and CgA. Moreover, there are no standard
treatment guidelines for this disease, with surgery being the
first-line approach, as it is for other IBC-NSTs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HJ collected the clinical, imaging and pathological
data of the patient, and wrote the manuscript. ML conceived and
designed the study, and revised the manuscript. HJ and ML confirm
the authenticity of all the raw data. Both authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent for publication was obtained from
the patient. All identifying information has been removed or
anonymized to ensure confidentiality.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosen LE and Gattuso P: Neuroendocrine
tumors of the breast. Arch Pathol Lab Med. 141:1577–1581. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rakha E and Tan PH: Head to head: Do
neuroendocrine tumours in the breast truly exist? Histopathology.
81:2–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lebeau A and Denkert C: Updated WHO
classification of tumors of the breast: The most important changes.
Der Pathologe. 42:270–280. 2021.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pareja F, Vahdatinia M, Marchio C, Lee
SSK, Da Cruz Paula A, Derakhshan F, da Silva EM, Selenica P, Dopeso
H, et al: Neuroendocrine tumours of the breast: A genomic
comparison with mucinous breast cancers and neuroendocrine tumours
of other anatomic sites. J Clin Pathol. 75:10–17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salemis NS: Primary neuroendocrine
carcinoma of the breast: A rare presentation and review of the
literature. Intractable Rare Dis Res. 9:233–246. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ
and Dogan BE: BI-RADS® fifth edition: A summary of
changes. Diagn Interv Imaging. 98:179–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feyrter F and Hartmann G: On the carcinoid
growth form of the carcinoma mammae, especially the carcinoma
solidum (gelatinosum) mammae. Frankf Z Pathol. 73:24–39. 1963.(In
German). PubMed/NCBI
|
|
8
|
Cubilla AL and Woodruff JM: Primary
carcinoid tumor of the breast: A report of eight patients. Am J
Surg Pathol. 1:283–292. 1977. View Article : Google Scholar
|
|
9
|
Bussolati G, Gugliotta P, Sapino A, Eusebi
V and Lloyd RV: Chromogranin-reactive endocrine cells in
argyrophilic carcinomas (“carcinoids”) and normal tissue of the
breast. Am J Pathol. 120:186–192. 1985.PubMed/NCBI
|
|
10
|
Sapino A and Bussolati G: Is detection of
endocrine cells in breast adenocarcinoma of diagnostic and clinical
significance? Histopathology. 40:211–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John E, Fattaneh N, Tavassoli A and
Devilee A: Pathology and genetics of tumors of the breast and
female genital organs. Iarc; 2003
|
|
12
|
Eymard H, Pittella J and Alfredo JAB: The
new WHO classification of breast tumors. J Brasileiro de Patologia
e Medicina Laboratorial. 48:406–407. 2012.
|
|
13
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 WHO classification of tumors of the breast. Histopathology.
77:181–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uccella S, Finzi G, Sessa F and La Rosa S:
On the endless dilemma of neuroendocrine neoplasms of the breast: A
journey through concepts and entities. Endocr Pathol. 31:321–329.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
La Rosa S and Uccella S: Classification of
neuroendocrine neoplasms: Lights and shadows. Rev Endocr Metab
Disord. 22:527–538. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Dai S, Xu J, Liu L, Yu J and Sun T:
Primary neuroendocrine tumor of the breast: Current understanding
and future perspectives. Front Oncol. 12:8484852022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kherbek H, Skef J, Kherbek S, Abdulrahman
SA, Alshehabi Z and Zahlouk N: Primary neuroendocrine carcinoma of
the breast with paraneoplastic thrombocytosis; a rare case report.
Ann Med Surg (Lond). 77:1036642022.PubMed/NCBI
|
|
18
|
Wang J, Wang X, Du W, Guo Y, Yang X, Pan J
and Yin L: Primary neuroendocrine carcinoma of the breast with
markedly elevated alpha-fetoprotein: A case report. Transl Cancer
Res. 10:2503–2508. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, He L, Lv W and Wang N:
Neuroendocrine carcinoma of the breast with hyperprolactinemia:
Report of two cases and a minireview. Int J Clin Exp Pathol.
13:1457–1462. 2020.PubMed/NCBI
|
|
20
|
Lavigne M, Menet E, Tille JC, Lae M,
Fuhrmann L, Bonneau C, Deniziaut G, Melaabi S, Ng CCK, Marchiò C,
et al: Comprehensive clinical and molecular analyses of
neuroendocrine carcinomas of the breast. Modern Pathol. 31:68–82.
2018. View Article : Google Scholar
|
|
21
|
Kayadibi Y, Erginoz E, Cavus GH, Kurt SA,
Ozturk T and Velidedeoglu M: Primary neuroendocrine carcinomas of
the breast and neuroendocrine differentiated breast cancers:
Relationship between histopathological and radiological features.
Eur J Radiol. 147:1101482022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park YM, Wu Y, Wei W and Yang WT: Primary
neuroendocrine carcinoma of the breast: Clinical, imaging, and
histologic features. Am J Roentgenol. 203:W221–W230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang ED, Kim MK, Kim JS and Whang IY:
Primary neuroendocrine tumor of the breast: Imaging features.
Korean J Radiol. 14:395–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hörsch D, Schmid KW, Anlauf M, Darwiche K,
Denecke T, Baum RP, Spitzweg C, Grohé C, Presselt N, Stremmel C, et
al: Neuroendocrine tumors of the bronchopulmonary system (typical
and atypical carcinoid tumors): Current strategies in diagnosis and
treatment. Conclusions of an expert meeting February 2011 in
Weimar, Germany. Oncol Res Treat. 37:266–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pareja F and D'Alfonso TM: Neuroendocrine
neoplasms of the breast: A review focused on the updated World
Health Organization (WHO) morphologic classification. Br J.
26:1160–1167. 2020.PubMed/NCBI
|
|
26
|
Adegbola T, Connolly CE and Mortimer G:
Small cell neuroendocrine carcinoma of the breast: A report of
three cases and review of the literature. J Clin Pathol.
58:775–778. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy M, Buehler DG, Zhang R, Schwalbe ML,
Baus RM, Salamat MS, Lloyd RV and Rosenbaum JN: Expression of
insulinoma-associated protein 1 (INSM1) and orthopedia homeobox
(OTP) in tumors with neuroendocrine differentiation at rare sites.
Endocr Pathol. 30:35–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong E, Pareja F, Hanna MG, Jungbluth AA,
Rekhtman N and Brogi E: Expression of novel neuroendocrine markers
in breast carcinomas: A study of INSM1, ASCL1, and POU2F3. Human
Pathol. 127:102–111. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zombori T, Turkevi-Nagy S, Sejben A,
Juhász-Nagy G, Cserni G, Furák J, Tiszlavicz L, Krenács L and
Kővári B: The panel of syntaxin 1 and insulinoma-associated protein
1 outperforms classic neuroendocrine markers in pulmonary
neuroendocrine neoplasms. APMIS. 129:186–194. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kővári B, Turkevi-Nagy S, Báthori Á,
Fekete Z and Krenács L: Syntaxin 1: A novel robust immunophenotypic
marker of neuroendocrine tumors. Int J Mol Sci. 21:12132020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Turkevi-Nagy S, Báthori Á, Böcz J, Krenács
L, Cserni G and Kővári B: Syntaxin-1 and insulinoma-associated
protein 1 expression in breast neoplasms with neuroendocrine
features. Pathol Oncol Res. 27:16100392021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Juhlin CC, Zedenius J and Höög A: Clinical
routine application of the second-generation neuroendocrine markers
ISL1, INSM1, and secretagogin in neuroendocrine neoplasia: Staining
outcomes and potential clues for determining tumor origin. Endocr
Pathol. 31:401–410. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohanty SK, Kim SA, DeLair DF, Bose S,
Laury AR, Chopra S, Mertens RB and Dhall D: Comparison of
metastatic neuroendocrine neoplasms to the breast and primary
invasive mammary carcinomas with neuroendocrine differentiation.
Mod Pathol. 29:788–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ang D, Ballard M, Beadling C, Warrick A,
Schilling A, O'Gara R, Pukay M, Neff TL, West RB, Corless CL and
Troxell ML: Novel mutations in neuroendocrine carcinoma of the
breast: Possible therapeutic targets. Appl Immunohistochem Mol
Morphol. 23:97–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martinez EO, Jorns JM, Kong AL, Kijak J,
Lee WY, Huang CC and Cortina CS: Primary breast neuroendocrine
tumors: An analysis of the national cancer database. Ann Surg
Oncol. 29:6339–6346. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castellanos MR, Fanous E, Thaker R, Flory
MJ, Seetharamu N, Dhar M, Starr A and Strange TJ: Expression
patterns and clinical significance of estrogen receptor in
non-small cell lung cancer. Pathol Res Pract. 241:1542982023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohanty SK, Kim SA, DeLair DF, Bose S,
Laury AR, Chopra S, Mertens RB and Dhall D: Comparison of
metastatic neuroendocrine neoplasms to the breast and primary
invasive mammary carcinomas with neuroendocrine differentiation.
Mod Pathol. 29:788–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perry KD, Reynolds C, Rosen DG, Edgerton
ME, T Albarracin C, Gilcrease MZ, Sahin AA, Abraham SC and Wu Y:
Metastatic neuroendocrine tumour in the breast: A potential mimic
of in-situ and invasive mammary carcinoma. Histopathology.
59:619–630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Liu C, Zheng C, Ren Q, Wang Q,
Gao X, He Y, Wu J, Chen G, Li X and Ma Z: Efficacy of neoadjuvant
endocrine therapy in patients with poorly differentiated
neuroendocrine carcinoma of the breast: A case report. Medicine
(Baltimore). 99:e226522020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei Y, Ke X, Yu J, Jing Q, Bu H, Zeng X
and Wei B: Clinical and genomic analyses of neuroendocrine
neoplasms of the breast. Mod Pathol. 35:495–505. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchiò C, Geyer FC, Ng CK, Piscuoglio S,
De Filippo MR, Cupo M, Schultheis AM, Lim RS, Burke KA,
Guerini-Rocco E, et al: The genetic landscape of breast carcinomas
with neuroendocrine differentiation. J Pathol. 241:405–419. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bean GR, Najjar S, Shin SJ, Hosfield EM,
Caswell-Jin JL, Urisman A, Jones KD, Chen YY and Krings G: Genetic
and immunohistochemical profiling of small cell and large cell
neuroendocrine carcinomas of the breast. Mod Pathol. 35:1349–1361.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vranic S, Palazzo J, Sanati S, Florento E,
Contreras E, Xiu J, Swensen J and Gatalica Z: Potential novel
therapy targets in neuroendocrine carcinomas of the breast. Clin
Breast Cancer. 19:131–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sagan OA, Rothstein A, Jambunathan B,
Hadziahmetovic M, Antoniolli A and Rashid MH: Case report:
Neuroendocrine breast carcinoma with a germline EGFR T790M
mutation. Front Oncol. 13:11768682023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Acar A, Tolan HK, Ozbagriacik M and
Ezberci F: Primary neuroendocrine carcinoma of the breast: A 5-year
experiences. Ann Ital Chir. 91:23–26. 2020.PubMed/NCBI
|
|
46
|
Irelli A, Sirufo MM, Morelli L, D'Ugo C,
Ginaldi L and De Martinis M: Neuroendocrine Cancer of the Breast: A
Rare Entity. J Clin Med. 9:14522020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gallo M, Campione S, Di Vito V, Fortunati
N, Lo Calzo F, Messina E, Ruggeri RM, Faggiano A and Colao AAL:
Primary neuroendocrine neoplasms of the breast: Still open issues.
Front Endocrinol (Lausanne). 11:6102302021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frame MT, Gohal J, Mader K and Goodman J:
Primary small cell carcinoma of the breast: An approach to medical
and surgical management. Cureus. 15:e479812023.PubMed/NCBI
|
|
49
|
Kontogeorgos G, Thodou E and Choreftaki T:
Investigation of somatostatin receptor profile of neuroendocrine
carcinomas of the breast. Pathol Res Pract. 216:1530662020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Harris PE and Zhernosekov K: The evolution
of PRRT for the treatment of neuroendocrine tumors; what comes
next? Front Endocrinol (Lausanne). 13:9418322022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang J, Wei B, Albarracin CT, Hu J,
Abraham SC and Wu Y: Invasive neuroendocrine carcinoma of the
breast: A population-based study from the surveillance,
epidemiology and end results (SEER) database. BMC Cancer.
14:1472014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang L, Roy M, Lin H, Shen Y, Albarracin
C, Huo L, Chen H, Wei B, Bedrosian I, Bu H and Wu Y: Validation of
prognostic significance of the proposed uniform classification
framework in neuroendocrine neoplasms of the breast. Breast Cancer
Res Treat. 186:403–415. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xia L, Lai J, Liu X, Kong F, Qiu S, Hu H,
Zhu S and Cao J: Epidemiological and survival outcomes of
neuroendocrine carcinoma of the breast: A SEER data analysis.
Transl Cancer Res. 12:1951–1962. 2023. View Article : Google Scholar : PubMed/NCBI
|