Introduction
Tumor metastasis to secondary organs, in addition to
the primary malignant tumor, is a major cause of death. Although
tumor cells can invade any organ in the body, secondary malignant
tumors in the heart are rare, with few cases having been reported
to date. A 35-year single-center autopsy study showed that 61 of
1294 (4.7%) were true heart metastases from solid cancer (1). Approximately 10% of patients with
cancer develop cardiac metastases, which are often associated with
a poor prognosis (2). Lung cancer
is one of the most prevalent malignancies globally and the leading
cause of cancer-related deaths, responsible for 18.0% of all cancer
fatalities (3). A 2020 global
population-based study showed that 20% of lung cancer cases were
squamous cell carcinomas (4). Lung
squamous cell carcinoma is a common type of non-small cell lung
cancer, the survival rates of which have improved due to
advancements in immunotherapy. Tumor immunotherapy is primarily
divided into cellular immunotherapy and immune checkpoint inhibitor
(ICI) therapy. ICIs mainly function by inhibiting the immune
evasion of tumor cells and are widely applied in the treatment of
lung cancer. Current ICIs include PD-1 inhibitors, PD-L1
inhibitors, and CTLA-4 inhibitors. A real-world study has indicated
that among patients receiving immunotherapy combined with
chemotherapy, the median overall survival (OS) for squamous
non-small cell lung cancer (NSCLC) patients is 10.6 months (95% CI,
9.3–11.8); median OS for squamous NSCLC patients undergoing
monotherapy with immunotherapy is 11.3 months (95% CI, 9.8–12.8)
(5). Phase II/III clinical studies
have confirmed the safety and efficacy of PD-L1 inhibitors,
including atezolizumab, pembrolizumab and nivolumab, in the
second-line treatment of advanced lung squamous cell carcinoma
(6–9). In addition to its remarkable efficacy
in the second-line treatment of non-small cell lung cancer (NSCLC),
ICIs also show promising survival benefit data as first-line
treatments for metastatic NSCLC with high PD-L1 expression (TPS
≥50%) and negative mutation genes. In the KEYNOTE 024 study,
Pembrolizumab alone was significantly more effective than standard
chemotherapy in patients with PD-L1 expression ≥50% of patients in
advanced NSCLC with first-line treatment, with a median OS of 26.3
months vs. 13.4 months (HR: 0.62, 95% CI, 0.48~0.81) (10). Nevertheless, there is insufficient
evidence to demonstrate the efficacy of immunotherapy in the
treatment of cardiac metastases. To the best of our knowledge, the
present case report is the first documented instance of primary
lung cancer with secondary malignancies in the left atrium and left
ventricle. In addition, the response of the case to immunotherapy
is described.
Case report
In November 2023, a 71-year-old male patient
presented to the China-Japan Friendship Hospital (Beijing, China),
with a 2-month history of intermittent cough and chest pain,
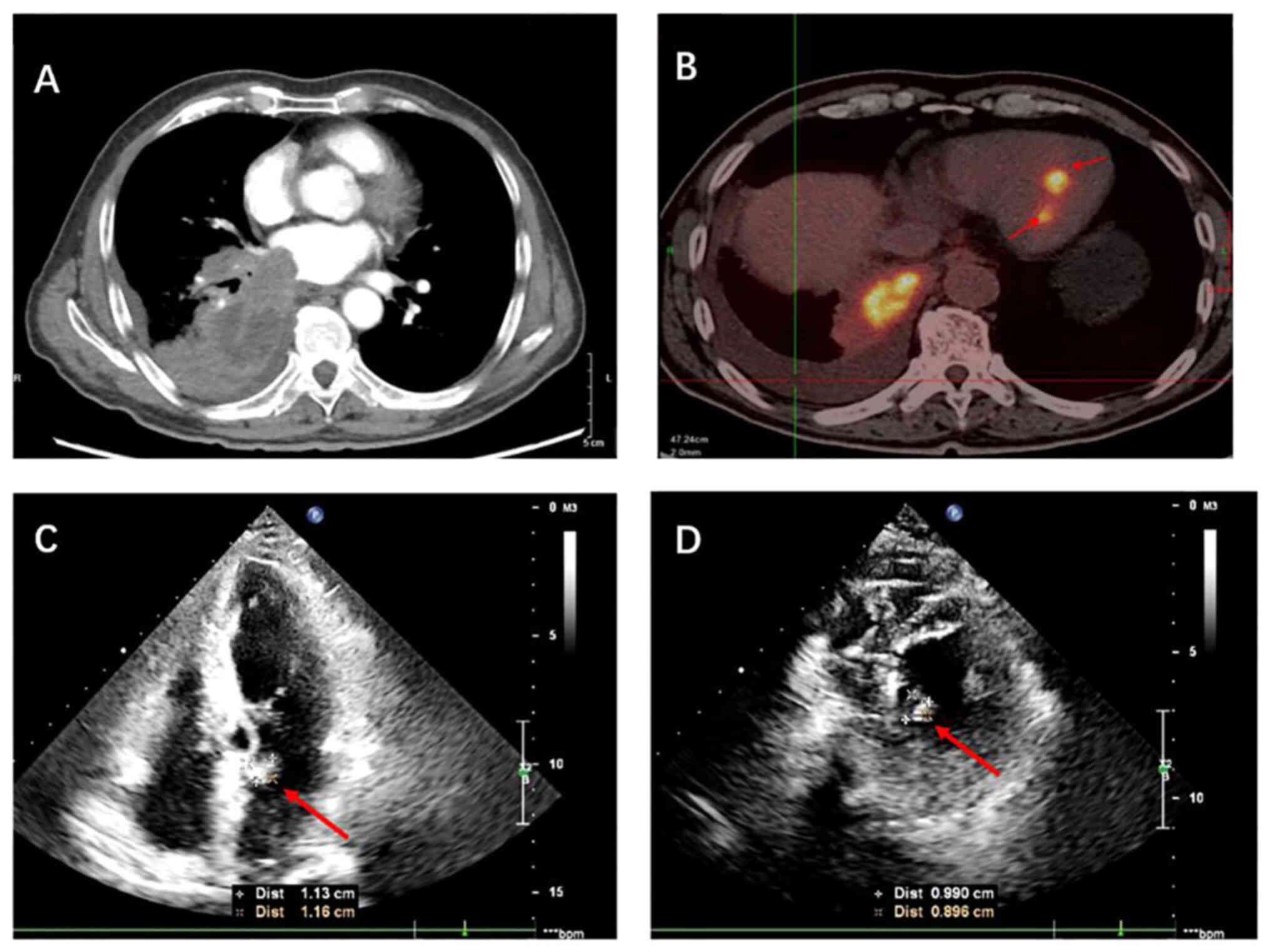
accompanied by fever and hemoptysis. Chest computed tomography (CT)
revealed a perihilar mass in the lower lobe of the right lung,
extending into the pulmonary arteriovenous structures and the left
atrium (Fig. 1A). The patient
underwent fluorodeoxyglucose (FDG) positron emission tomography
(PET)/CT, which identified right lung cancer with multiple lymph
node metastases as well as hypermetabolic foci in the left atrium
and left ventricle of the heart (Fig.
1B). The high FDG uptake revealed by the PET/CT results
confirmed the malignancy of the cardiac tumors, supporting the
diagnosis of metastatic disease. Transthoracic echocardiography
also can show a slightly higher echoic mass attached to the atrial
septum at the base of the proximal anterior mitral valve in the
left atrium, and a slightly higher echo group attached in the
middle of the left ventricular posterior septum (Fig. 1C and D). At this point, the patient
was diagnosed with stage IV NSCLC.
The patient exhibited no heart-related symptoms, and
an electrocardiogram (ECG) revealed a normal sinus rhythm. A
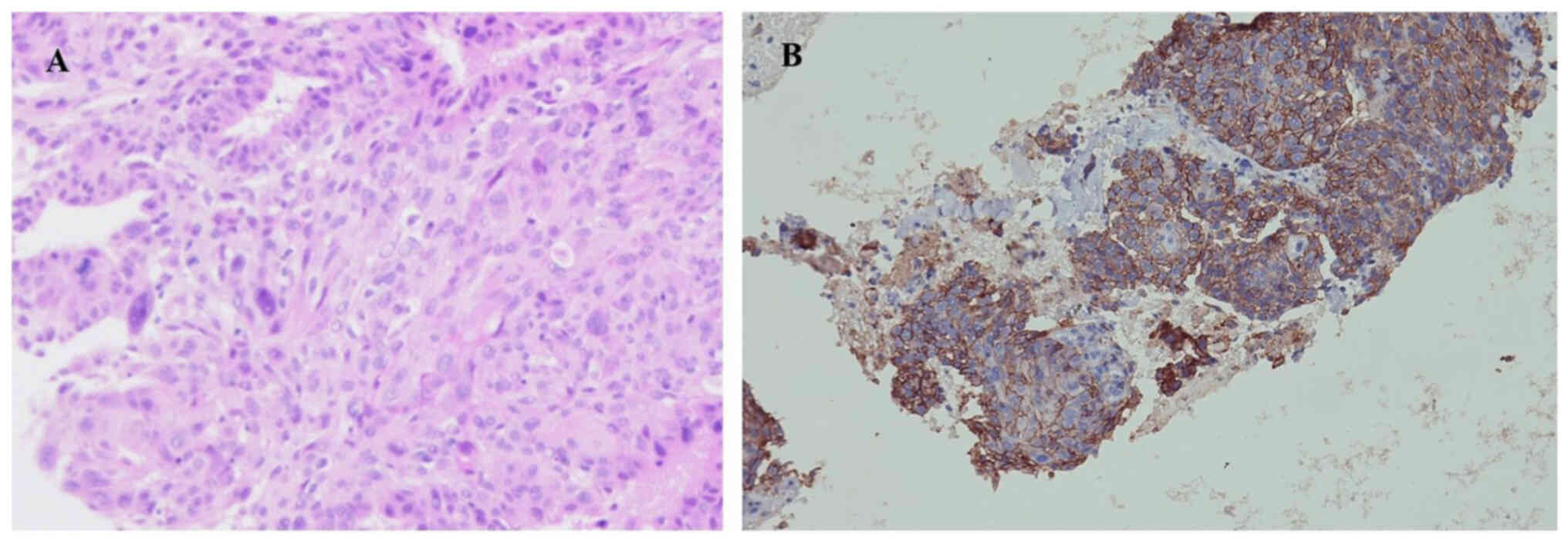
subsequent biopsy via fiberoptic bronchoscopy confirmed lung
squamous cell carcinoma (Fig. 2A);
morphology and immunohistochemistry were consistent with squamous
cell carcinoma, and necrosis was visible in the focal area. Due to
the low incidence of genetic mutations in lung squamous cell
carcinoma, current guidelines, including European Society for
Medical Oncology (11), the
American Society of Clinical Oncology (ASCO) (12) and the National Comprehensive Cancer
Network (NCCN) (13), do not
recommend routine genetic testing for all cases, classifying it as
a level II recommendation. Moreover, the patient had financial
limitations. Therefore, molecular testing was not performed.
Immunohistochemistry revealed that the tumor exhibited a high level
of programmed cell death-ligand 1 (PD-L1) expression, with a tumor
proportion score (TPS) of ~80% (Fig.
2B). According to the ASCO (12), NCCN (13), and the Chinese Society of Clinical
Oncology (CSCO) (14) guidelines,
immunotherapy alone is recommended for patients with lung cancer
who have a PD-L1 TPS of ≥50% and a performance status of 0 or 1,
which indicates the patient is capable of normal movement or
experiences fatigue during strenuous exercise and physical
exertion. The patient has a performance status of 0. The CTONG1901
study demonstrated that sintilimab is effective and well-tolerated
in patients with advanced NSCLC, regardless of PD-L1 expression
levels, and exhibits similar efficacy and safety to pembrolizumab
(15). Additionally, considering
the economic burden on patients, sintilimab is more appropriate for
this patient. Therefore, sintilimab (200 mg every 3 weeks) was
administered to the patient as the selected immunotherapy.
The patient had a history of good health, with no
known hypertension, coronary heart disease or cardiomyopathy. A
comprehensive assessment was performed, including detailed
inquiries about cardiac-associated symptoms and a physical
examination. The pre-treatment evaluations also included blood
tests to assess cardiac enzyme levels, electrocardiography and
echocardiography. However, cardiac MRI was not performed due to the
financial limitations of the patient. The patient reported no
discomfort, and all cardiac examination results were normal. After
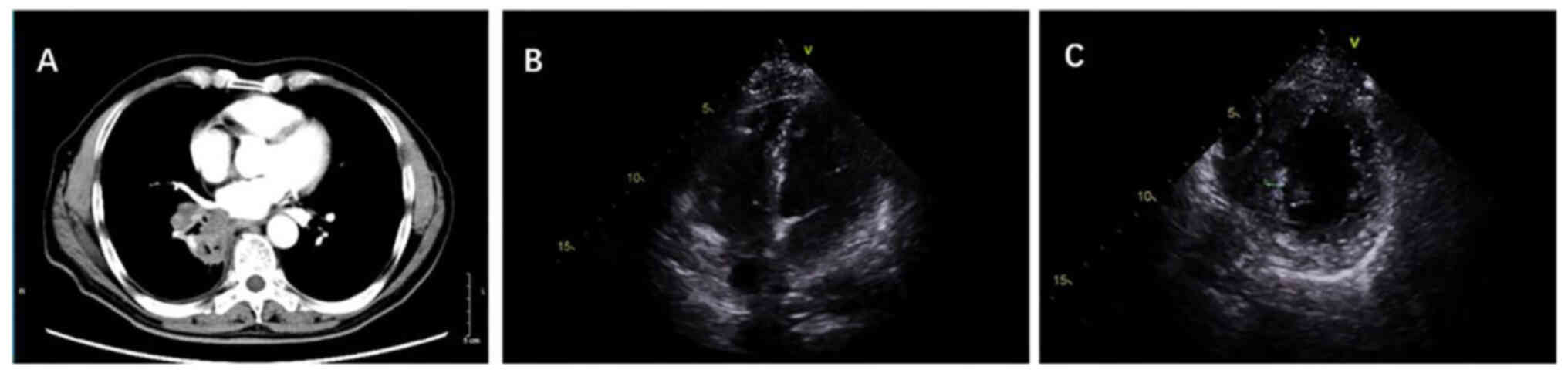
two cycles of immunotherapy, echocardiography showed the
disappearance of the left atrial metastasis and a slight reduction
in the left ventricular metastasis (Fig. 3). Furthermore, the lung mass
demonstrated a marked response, with a clear reduction in size.
At this time, the patient was found to have elevated
alanine transaminase (ALT) and aspartate transaminase (AST) levels,
which were ~3-fold the upper limit of normal. This raised the
suspicion of immune-mediated hepatitis (IMH), rated as grade 2
according to the Common Terminology Criteria for Adverse Events
(CTCAE), version 5.0 (16),
prompting a switch to paclitaxel plus cisplatin chemotherapy.
Subsequently, following protection of the liver with glutathione
and magnesium isoglycyrrhizinate, and without the administration of
additional treatments such as glucocorticoids, the ALT and AST
levels were normalized. Considering the long history of alcohol use
by the patient and the exclusion of other chronic liver diseases,
it is hypothesized that the transient liver injury may have been
medication-induced, occurring in the context of alcoholic liver
disease. Following the exclusion of organic liver damage, liver
function was regularly monitored and remained normal during the
subsequent two cycles of chemotherapy. Given the favorable response
to immunotherapy, the treatment was resumed and cycles 3–8 were
completed. Throughout the treatment, the liver function remained
stable within normal limits, and ultrasound and CT examinations
showed no abnormalities.
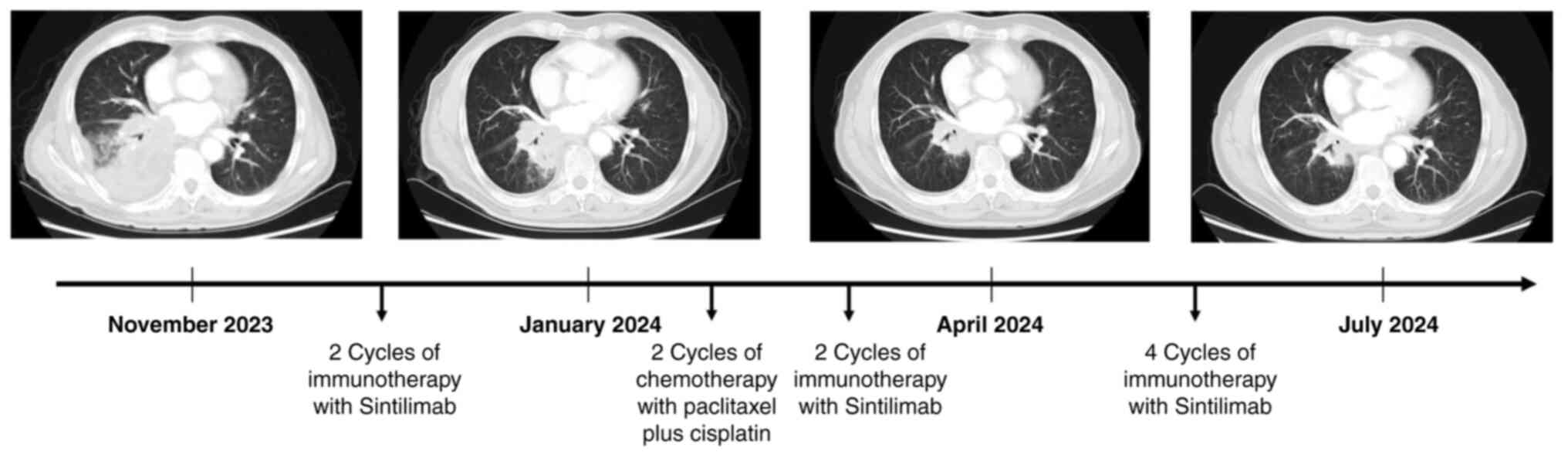
As of July 2024, the patient had completed 8 cycles
of immunotherapy and 2 rounds of chemotherapy. The chest CT scan
showed a steady reduction in the pulmonary lesion (Fig. 4), while the metastatic focus in the
left ventricle remained unchanged. The patient was comprehensively
reassessed every 3 months, which involved examination with CT scans
of the chest and abdomen, ECG and echocardiograms. Furthermore, a
brain MRI was conducted every 6 months. To date, the patient has
reported no discomfort, and no evidence of ischemic infarction has
been detected in other organs. Immunotherapy continues to provide
clear clinical benefits.
Discussion
Nearly all primary tumors are able to metastasize to
the heart. Frequent sources of cardiac metastases include malignant
melanoma, lymphoma and lung cancer, and the most common route of
metastasis to the heart is lymphatic spread to the epicardium and
pericardium (17). However, other
mechanisms, including direct extension and hematogenous
dissemination, can also occur (18). Metastasis involving the endocardium
and heart chambers is rare (19),
and often undetectable before autopsy. To the best of our
knowledge, there have been no previous reports of simultaneous
metastasis to both the left ventricle and left atrium. Lung cancer
most commonly metastasizes to the brain, bones, lymph nodes and
liver (20), with cardiac invasion
being rarely reported (21).
In the present case, tumor cells from the lung had
invaded the left atrium directly through the pulmonary veins and
through hematogenous metastasis. This is the most direct and
challenging method of metastasis. Given the pumping function of the
left heart, it is not easy to contemplate that tumor cells would be
able to enter the left atrium through the pulmonary veins in the
midst of turbulent blood flow. Furthermore, if tumor cells manage
to enter the left atrium, they would then need to pass through the
mitral valve into the left ventricle and successfully implant in
the endocardium. Following two rounds of immunotherapy using
sintilimab, the primary lung tumor was markedly reduced in size,
and the tumor tissue that had spread to the pulmonary veins and
left atrium had completely disappeared. This outcome indirectly
demonstrates that the cardiac metastasis occurred via hematogenous
spread. Consequently, following active treatment of the primary
mass, the cardiac metastases responded well to therapy.
In the present case, the secondary cardiac tumors
rapidly diminished or disappeared due to high responsiveness to
immunotherapy. However, this response carries a risk of
hematogenous metastasis due to the likelihood that tumor cells will
enter the bloodstream. Tumors in the left ventricle are
particularly prone to spreading through the bloodstream to various
organs, including critical ones such as the brain and kidneys,
which are vulnerable to tumor embolism. While examination results
did not show any evidence of ischemic infarction caused by tumor
embolism in the systemic organs of the present case, this risk
requires close monitoring in future treatments.
Research indicates that immune-related adverse
events (irAEs) might be a positive prognostic marker in patients
with lung cancer and may potentially improve treatment outcomes
(22). However, the decision to
resume immune checkpoint inhibitor (ICI) therapy after the
resolution of irAEs is clinically challenging, due to uncertainties
concerning the safety and benefits of such retreatment. According
to current guidelines (23,24), ICI therapy should be paused for
patients with a 2-grade elevation in transaminase levels (3-5-fold
the normal value) until levels return to grade 1 or baseline based
on the CTCAE. For IMH of grade ≥4, the permanent discontinuation of
ICI therapy is recommended. However, it has been suggested that for
patients with grade 3 or 4 immune hepatitis, appropriate
interventions such as glucocorticoids may enable the resumption of
ICI therapy to be considered once liver function improves to grade
≤1, depending on the overall condition, risk assessment and
judgment of the patient by the physician (25). A clinical study (26) found that among patients irAE with no
prior treatment response, those who resumed immunotherapy
experienced longer progression-free survival and overall survival
times than those whose immunotherapy was discontinued, indicating
potential benefits from retreatment. In the present case, the
patient showed a positive response after two cycles of
immunotherapy, indicating that immunotherapy was a suitable
treatment. Therefore, after the careful monitoring and follow-up of
liver function, the decision was made to restart immunotherapy to
provide continued survival benefits. It is recommended that the
resumption of ICIs should be guided by the clinical background and
specific requirements of the patient.
Currently, the primary approach to managing
secondary cardiac tumors is effectively treating the tumor itself.
ICIs have been demonstrated to be an effective therapy for lung
cancer and are recommended as the first treatment for lung squamous
cell carcinoma in ASCO, NCCN and CSCO (12–14).
However, clinical data to support the safety and efficacy of
first-line ICIs in patients with cardiac malignancies are lacking,
and the treatment risk is also unknown.
Although the present patient presented with IMH, no
serious symptoms of toxicity or adverse effects, including
heart-associated issues, were observed. Given that immunotherapy
operates through mechanisms distinct from the direct cytotoxic
effects of traditional chemotherapy, it is crucial to monitor and
manage adverse effects on organs other than those targeted by the
therapy as well as to assess its effectiveness.
In conclusion, immunotherapy shows promise in the
treatment of cardiac metastases secondary to malignant tumors, as
demonstrated by the positive response in the present case of lung
squamous cell carcinoma. Despite the rarity of such cases and the
limited evidence from clinical trials, the present case highlights
the potential for immunotherapy to offer clear therapeutic benefits
and improve the quality of life of the patient. The findings may
serve as a valuable reference to guide the treatment approach in
similar cases.
Acknowledgements
Not applicable.
Funding
This study was supported by Capital's Funds for Health
Improvement and Research (grant no. 2022-2-4065), National
High-Level Hospital Clinical Research Funding (grant no.
2022-NHLHCRF-LX-02-0111).
Availability of data and materials
The data generated in the present may be requested
from the corresponding author.
Authors' contributions
YS and YZ participated in study design, and wrote
the original manuscript draft. YY and HL obtained medical images
and analyzed patient data. SL analyzed pathological images and made
the diagnosis. HC was involved in drafting the manuscript, revising
it critically for important intellectual content, data analysis and
gave final approval of the version to be published. ZL and RL were
involved in data collection, drafting the manuscript and confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication of the case details and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nova-Camacho LM, Gomez-Dorronsoro M,
Guarch R, Cordoba A, Cevallos MI and Panizo-Santos A: Cardiac
metastasis from solid cancers: A 35-year single-center autopsy
study. Arch Pathol Lab Med. 147:177–184. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts WC: Primary and secondary
neoplasms of the heart. Am J Cardiol. 80:671–682. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Vaccarella S, Morgan E, Li M,
Etxeberria J, Chokunonga E, Manraj SS, Kamate B, Omonisi A and Bray
F: Global variations in lung cancer incidence by histological
subtype in 2020: A population-based study. Lancet Oncol.
24:1206–1218. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waterhouse D, Lam J, Betts KA, Yin L, Gao
S, Yuan Y, Hartman J, Rao S, Lubinga S and Stenehjem D: Real-world
outcomes of immunotherapy-based regimens in first-line advanced
non-small cell lung cancer. Lung Cancer. 156:41–49. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung Cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-Positive
Non-Small-Cell Lung Cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park K, Vansteenkiste J, Lee KH,
Pentheroudakis G, Zhou C, Prabhash K, Seto T, Voon PJ, Tan DSW,
Yang JCH, et al: Pan-Asian adapted ESMO Clinical Practice
Guidelines for the management of patients with locally-advanced
unresectable non-small-cell lung cancer: A KSMO-ESMO initiative
endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol.
31:191–201. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaiyesimi IA, Leighl NB, Ismaila N, Alluri
K, Florez N, Gadgeel S, Masters G, Schenk EL, Schneider BJ, Sequist
L, et al: Therapy for stage IV non-small cell lung cancer without
driver alterations: ASCO living guideline. version 2023.3. Clin
Oncol. 42:e23–e43. 2024.PubMed/NCBI
|
|
13
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-Small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oncology Society of Chinese Medical
Association and Chinese Medical Association Publishing House, .
Chinese Medical Association guideline for clinical diagnosis and
treatment of lung cancer (2023 edition). Zhonghua Yi Xue Za Zhi.
103:2037–2074. 2023.(In Chinese). PubMed/NCBI
|
|
15
|
Maggie Liu SY, Huang J, Deng JY, Xu CR,
Yan HH, Yang MY, Li YS, Ke EE, Zheng MY, Wang Z, et al: PD-L1
expression guidance on sintilimab versus pembrolizumab with or
without platinum-doublet chemotherapy in untreated patients with
advanced non-small cell lung cancer (CTONG1901): A phase 2,
randomized, controlled trial. Sci Bull (Beijing). 69:535–543. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 5. US Department of Health and Human
Services, National Institutes of Health, National Cancer Institute,
. 2017.Available from. https://ctep.cancer.gov/search/search.asp?zoom_query=CTCAE&Action=Go%3EOctober
10–2024
|
|
17
|
Maleszewski JJ and Anavekar NS: Neoplastic
pericardial disease. Cardiol Clin. 35:589–600. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dragoescu EA and Liu L: Pericardial fluid
cytology: An analysis of 128 specim-ens over a 6-year period.
Cancer Cytopathol. 121:242–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Butany J, Leong SW, Carmichael K and
Komeda M: A 30-year analysis of cardiac neoplasms at autopsy. Can J
Cardiol. 21:675–680. 2005.PubMed/NCBI
|
|
20
|
Xie S, Wu Z, Qi Y, Wu B and Zhu X: The
metastasizing mechanisms of lung cancer: Recent advances and
therapeutic challenges. Biomed Pharmacother. 138:1114502021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu FY, Zhou Q, Yang JJ, Zhong WZ, Chen
ZH, Deng W, He YY, Chen HJ, Zeng Z, Ke EE, et al: Distribution and
prognosis of uncommon metastases from non-small cell lung cancer.
BMC Cancer. 16:1492016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Ma W, Wu D, Lyu M, Zheng Q, Wang
T, Zhou J and Liu C: Prognostic relevance of immune-related adverse
events in lung cancer patients undergoing immune checkpoint
inhibitor therapy: A systematic review and meta-analysis. Transl
Lung Cancer Res. 13:1559–1584. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thompson JA, Schneider BJ, Brahmer J,
Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M,
Dunnington D, et al: NCCN Guidelines Insights: Management of
Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc
Netw. 18:230–241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brahmer JR, Lacchetti C and Thompson JA:
Management of Immune-Related Adverse Events in Patients Treated
With Immune Checkpoint Inhibitor Therapy: American Society of
Clinical Oncology Clinical Practice Guideline Summary. J Oncol
Pract. 14:247–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K; ESMO Guidelines Committee, :
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29
(Suppl 4):iv264–iv266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A,
Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF,
Long NM, et al: Safety and Efficacy of Re-treating with
immunotherapy after immune-related adverse events in patients with
NSCLC. Cancer Immunol Res. 6:1093–1099. 2018. View Article : Google Scholar : PubMed/NCBI
|