Introduction
Lung cancer (LC) is one of the most prevalent
malignant tumors in humans, accounting for ~11.6% of all cancer
cases worldwide, and remains the leading cause of cancer-related
deaths, responsible for nearly 18.4% of total cancer mortality
(1). The 5-year overall survival
rate for LC is <20% (2,3). Based on pathological characteristics,
LC is classified into two subtypes: Small cell lung cancer (SCLC)
and non-small cell lung cancer (NSCLC) (1,4). NSCLC
is particularly concerning due to its high invasiveness, malignancy
and propensity to develop drug resistance, and accounts for ~85% of
all LC cases and remains a leading cause of cancer mortality
globally, contributing to ~125,000 deaths per year in the United
States alone, as projected for 2024 (5). Therefore, there is an urgent need to
identify effective anti-NSCLC therapies with minimal side
effects.
Bergapten, also known as 5-methoxypsoralen, is a
natural furanocoumarin that has garnered increasing attention for
its medicinal potential. It exhibits a wide range of
pharmacological effects, including neuroprotective,
organ-protective, anticancer, anti-inflammatory, antibacterial and
anti-diabetic properties (6).
Network pharmacology, based on systems biology
theory, offers new strategies for investigating the relationship
between drugs and diseases. It integrates systems biology,
multidirectional pharmacobiology, bioinformatics and computer
science. Network pharmacology has shifted biological system
research from the traditional single-drug and single-target model
to a multi-drug and multi-target approach (7). By constructing a
‘component-protein/gene-disease’ network, network pharmacology
enables the high-throughput investigation of molecular regulatory
mechanisms (8). These advantages
make network pharmacology a powerful tool for studying combination
therapies.
Given the urgent need to elucidate effective
anti-NSCLC drugs with minimal side effects, the present study
assessed Bergapten as a promising natural compound with potential
anti-LC properties. The aim of this study was to evaluate the
inhibitory effects of Bergapten on LC cell lines, particularly
through its impact on the PI3K/AKT signaling pathway, which is
crucial for cancer cell survival and proliferation (9). Additionally, the present study
assessed whether Bergapten may promote cellular senescence in LC
cells, potentially limiting tumor progression through its pro-aging
effects.
Materials and methods
Cell culture
NCI-H1975 (cat. no. iCell-h156), NCI-H1299 (cat. no.
iCell-h153) and NCI-H460 (cat. no. iCell-h160) cells were purchased
from iCell Bioscience Inc. and cultured in high-glucose medium
(cat. no. PM00031, Proteintech Group, Inc.) supplemented with 10%
fetal bovine serum (cat. no. F0850; Sigma-Aldrich; Merck KGaA) and
1% penicillin-streptomycin solution (Sigma-Aldrich; Merck KGaA) in
a humidified atmosphere of 5% CO2 at 37°C. Cells were
tested for mycoplasma contamination and all experiments were
performed using cells from passages 3–5. Prior to specific
treatments, cells were starved in serum-free medium for 1 h at
37°C. Treatment with 5-methoxypsoralen and/or SC79 was performed
for 12 h at 37°C.
Reagents
The following reagents were used in the present
study: 5-methoxypsoralen (cat. no. HY-N0370; MedChemExpress); RT
Master Mix for qPCR II (gDNA digester plus; cat. no. HY-K0511A;
MedChemExpress); Protein lysis solution (cat. no. P0013B; Beyotime
Institute of Biotechnology); Cell Counting Kit-8 (CCK-8; cat. no.
C0038; Beyotime Institute of Biotechnology); TRIzol™ (cat. no.
15596018; Invitrogen™; Thermo Fisher Scientific, Inc.); ChamQ
Universal SYBR qPCR Master Mix (cat. no. Q711-02; Vazyme Biotech
Co., Ltd.); fetal bovine serum (cat. no. F0850; Sigma-Aldrich;
Merck KGaA); Phospho-AKT (Ser473) monoclonal antibodies (cat. no.
66444-1-Ig; Proteintech Group, Inc.); AKT monoclonal antibodies
(cat. no. 60203-2-Ig; Proteintech Group, Inc.); PI3K p110 β
polyclonal antibodies (cat. no. 20584-1-AP; Proteintech Group,
Inc.); GAPDH monoclonal antibodies (cat. no. 60004-1-Ig;
Proteintech Group, Inc.); Phospho-PI3K p85 (Tyr458)/p55 (Tyr199)
antibodies (cat. no. 4228; CST Biological Reagents Co., Ltd.); RIPA
Lysis Buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology); BeyoECL Plus (cat. no. P0018S; Beyotime Institute
of Biotechnology); CCK-8 (cat. no. C0037; Beyotime Institute of
Biotechnology); Annexin V-FITC Apoptosis Detection Kit (cat. no.
C1062S; Beyotime Institute of Biotechnology); SC79 (25 mg; cat. no.
SF2730; Beyotime Institute of Biotechnology); and Akt (pS473) +
Total Akt ELISA Kit (cat. no. ab126433; Abcam).
Prediction of 5-methoxypsoralen
target
The Traditional Chinese Medicine Systems
Pharmacology Database and Analysis Platform (TCMSP) database
(https://old.tcmsp-e.com/tcmsp.php)
was searched using the keyword ‘5-methoxypsoralen’ to identify its
targets. Similarly, the SwissTarget database (http://www.swisstargetprediction.ch/)
was queried to obtain additional targets for ‘5-methoxypsoralen’.
The target names of 5-methoxypsoralen were converted into gene
names using the UniProt database (https://www.uniprot.org/). After compiling the
targets, duplicates were merged. Network and target prediction were
conducted using the CytoHubba plug-in for Cytoscape software
(version 3.7.2; Cytoscape Consortium; http://cytoscape.org/).
Prediction of potential targets for
lung cancer
Using ‘lung cancer’ as the keyword, LC-related genes
were retrieved from the GeneCards (https://www.genecards.org/) and DisGeNET (https://disgenet.com/) databases. After merging and
removing duplicates, two independent operators performed the
retrieval and summaries in separate rooms to minimize errors. The
results were then reviewed by a third person for verification.
Obtaining common targets between
5-methoxypsoralen and LC
Using the online tool Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/), the
potential targets of 5-methoxypsoralen and LC were uploaded and the
intersection was taken to identify common 5-methoxypsoralen-LC
targets. A Venn diagram was generated to illustrate the
overlap.
To construct a protein-protein interaction (PPI)
network, the common targets from the Venn diagram were imported
into the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING; http://string-db.org/) database, selecting ‘multiple
proteins’ and setting the species to human. Targets were filtered
with a confidence score of >0.7 and outliers were hidden. The
TSV file was then downloaded and imported into Cytoscape to
construct the PPI network and the ‘5-methoxypsoralen-shared target’
network for anti-LC activity.
Gene ontology (GO) enrichment and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment
To perform enrichment analysis on the common targets
of 5-methoxypsoralen and LC, the bioinformatics toolbar of the
Xiantao academic website was used (https://www.xiantaozi.com/). The functional clustering
option was selected and the common targets were uploaded. GO and
KEGG enrichment functions available in the toolbar was used for the
analysis.
Molecular docking verification
The structure of 5-methoxypsoralen was downloaded
from the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database. The
downloaded file was then opened using PyMOL (v2.2.0; https://www.pymol.org/) and saved in PDB format for
easier docking. The core target structure was obtained from the PDB
database and opened in PyMOL. Water molecules and original ligands
were deleted from the structure. Hydrogen atoms and charges were
added to both the ligands and receptors to create a receptor grid.
Molecular docking was performed using AutoDockVina 1.2.0 (10) and the binding strength between
5-methoxypsoralen and the receptor was evaluated based on the
binding energy (Affinity, kcal/mol). PyMOL was used to visually
display the conformation with the lowest binding energy.
Cell proliferation assay
The experiment consisted of three groups: The blank
group, the experimental group and the negative control group. In
the blank group, cells were cultured under normal conditions
without any additional treatment, serving as a baseline for cell
growth; in the experimental group, cells were treated with
different concentrations of 5-methoxypsoralen; and in the negative
control group, cells were cultured only with culture medium.
NCI-H1975, H1299 and H460 cells were seeded at a density of
5×103 cells/well in separate 96-well plates. After cell
attachment, the cells were treated according to their respective
groups for 72 h. Following the removal of the culture medium, CCK-8
solution was added and the cells were incubated for 1 h. DMSO was
then used to dissolve the crystals and absorbance was measured at
490 nm using a microplate reader. The cell survival rate was
calculated as follows: (experimental group-negative control
group)/(blank group-negative control group).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
The experimental groups consisted of a control group
and a 5-methoxypsoralen group. NCI-H1975, H1299 and H460 cells were
individually seeded in 6-well plates at a density of
5×103 cells/well. Once the cells adhered, the determined
concentration of 5-methoxypsoralen from CCK-8 was added and the
cells were treated for 72 h at 37°C in a humidified atmosphere with
5% CO2. Subsequently, TRIzol® reagent was
used to extract the RNA from the cells. The extracted RNA was
reverse-transcribed into cDNA using a commercial RT Master Mix for
qPCR II kit (gDNA digester plus; MedChemExpress). The reverse
transcription conditions were as follows: 25°C for 5 min, 55°C for
15 min and 85°C for 2 min. For quantitative PCR, ChamQ Universal
SYBR qPCR Master Mix (cat. no. Q711-02; Vazyme Biotech Co., Ltd.)
was used, containing SYBR Green I dye as the fluorophore. The
reaction mixture included 1 µg of RNA as template, along with the
provided buffer and dNTPs. The thermocycling protocol was set as
follows: 5°C for 30 sec, then 95°C for 10 sec and 60°C for 30 sec
for 40 cycles. The 2−ΔΔCq method (11) was used to determine the relative
expression of the targeted genes, with Gapdh mRNA serving as
the internal reference. The primers used for RT-qPCR are listed in
Table SI.
AKT phosphorylation assay
The effect of 5-methoxypsoralen was evaluated by
measuring AKT phosphorylation stimulated by SC79 (Akt activator) in
cells. Briefly, NCI-H1975, H1299 and H460 cells 3×103
cells/well were starved for 1 h and 50% of the cells were collected
to measure the basal level of AKT phosphorylation. After cells were
treated with 50 µM 5-methoxypsoralen and/or 4 µg/ml SC79 for 12 h
at 37°C in a humidified atmosphere with 5% CO2, the
remaining 50% of the cells were collected. Phosphorylation of AKT
was measured using an AKT ELISA kit.
Flow cytometry analysis
For cell apoptosis analyses, samples were analyzed
using a BD FACSCanto II flow cytometer (BD Biosciences). Data were
collected and processed using BD FACSDiva software (v8.0.1; BD
Biosciences; http://www.bdbiosciences.com/en-us/products/software/instrument-software/bd-facsdiva-software).
A total of 3×105 NCI-H1975, H1299 and H460 cells were
starved in serum-free medium for 12 h, followed by incubation with
5-methoxypsoralen in fresh serum-containing medium to promote cell
cycle progression for 72 h at 37°C in a humidified atmosphere with
5% CO2. The cells were then collected and fixed with 1
ml ice-cold 70% ethanol and incubated at −20°C for 24 h.
Subsequently, the cells were centrifuged at 380 × g for 5 min at
room temperature. Cell apoptosis was evaluated using the Annexin
V-FITC Apoptosis Detection Kit.
Wound healing assay
A total of 3×105 NCI-H1975, H1299 and
H460 cells were cultured in 6-well tissue culture plates until they
reached 100% confluency at 37°C in a humidified atmosphere with 5%
CO2. Subsequently, they were incubated in starvation
media containing 0.1% FBS for a period of 12 h at 37°C in a
humidified atmosphere with 5% CO2. The monolayer of
cells was then gently scraped using sterile 200 µl pipette tips.
Images were captured using an Olympus CKX53 inverted microscope
(Olympus Corporation) at two migration points, initially and after
24 h of the assay. The gap distances at 0 and 24 h after the wound
were measured, and the migration efficiency was calculated as the
difference between the gap area at 0 and 24 h.
Migration assay
For the migration and invasion assays, NCI-H1975,
H1299 and H460 cells in the logarithmic growth phase were suspended
in serum-free medium and seeded at a density of 3×105
cells/well into the upper chamber of a Transwell insert (8 µm pore
size; Corning Inc.). The lower chamber contained 600 µl culture
medium with 20% fetal bovine serum, serving as a chemoattractant.
Following a 24-h incubation at 37°C in a humidified atmosphere with
5% CO2, non-migrated cells on the upper surface of the
insert were gently removed. Cells that had migrated or invaded to
the lower surface were fixed with 4% paraformaldehyde at room
temperature for 15 min, stained with crystal violet at room
temperature for 5 min and imaged using an Olympus CKX53 inverted
microscope (Olympus Corporation). Cell counts were performed from
at least three random fields per insert to quantify migration and
invasion.
Western blotting
A total of 3×105 NCI-H1975, H1299 and
H460 cells were seeded in 6-well plates and treated with
5-methoxypsoralen for 72 h at 37°C in a humidified atmosphere with
5% CO2. Subsequently, the cells were lysed using the
RIPA lysis kit and the total protein quantification of whole cell
lysates was performed using the BCA protein assay kit (Takara
Biotechnology Co., Ltd.). Equal amounts of 30 µg protein were
separated on a 10–12% SDS-PAGE gel and then electrophoretically
transferred to a PVDF membrane. The membranes were blocked with 5%
non-fat dry milk (cat. no. sc-2324; Santa Cruz Biotechnology, Inc.)
in TBST (Tris-buffered saline with 0.1% Tween-20) for 1 h at room
temperature to prevent non-specific binding. After blocking,
primary antibodies were then applied and incubated overnight at
4°C, including: Phospho-AKT (Ser473; 1:1,000; cat. no. 66444-1-Ig;
Proteintech Group, Inc.), total AKT (1:1,000; cat. no. 60203-2-Ig;
Proteintech Group, Inc.) and GAPDH (1:1,000; cat. no. 60004-1-Ig;
Proteintech Group, Inc.), used as a loading control. The following
secondary antibodies were used at a 1:5,000 dilution: Anti-rabbit
IgG, HRP-conjugated (cat. no. SA00001-2; Proteintech Group, Inc.)
and anti-mouse IgG, HRP-conjugated (cat. no. SA00001-1; Proteintech
Group, Inc.). After a 1-h incubation with the secondary antibodies
at room temperature, the membranes were washed and visualized using
BeyoECL Plus chemiluminescent substrate (cat. no.P0018S; Beyotime
Institute of Biotechnology), and the grayscale values of the
protein bands were analyzed using Image J 1.0 software (https://imagej.net/ij/download.html).
Statistical analysis
Prism software v.9.0 (Dotmatics) was used for
statistical analysis. Values are presented as mean ± standard
deviation. The experiments in the presents study were repeated ≥3
times independently. Comparisons between two groups were performed
using two-tailed Student's t test and comparisons between multiple
groups were performed using one-way ANOVA, followed by the
Bonferroni test. P<0.05 were considered to indicate a
statistically significant difference.
Results
Network pharmacology combined with
molecular docking to explore the mechanism of 5-methoxypsoralen in
the treatment of LC
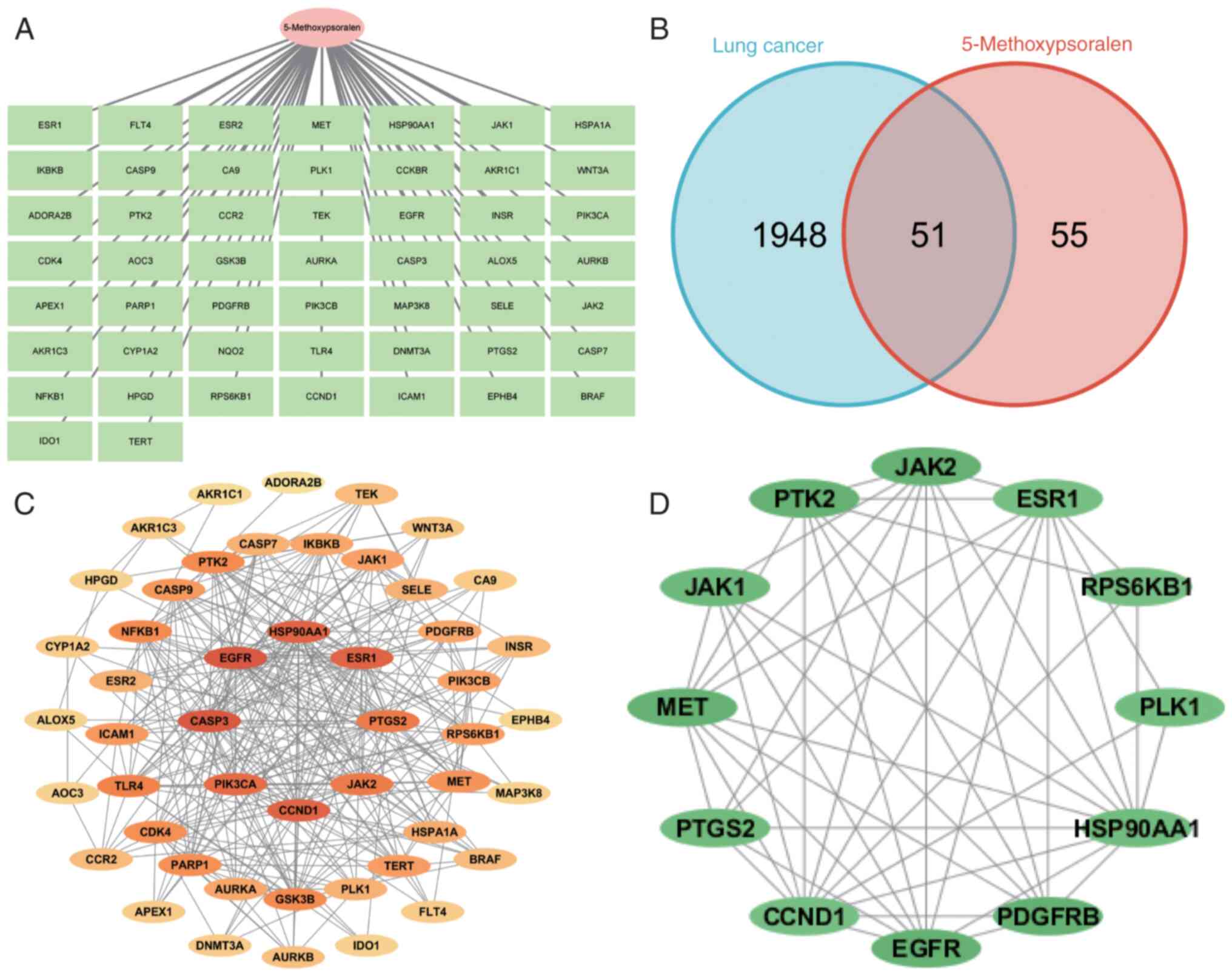
The TCMSP database was used to search for Chinese
medicine containing Bergapten (referred to as ‘bergamot’) by
entering its name in the ‘Herb name’ column. The search yielded
bergamot lactone with Mol ID MOL001945. The target names of
5-methoxypsoralen were converted into gene names using the UniProt
database. The PubChem database provided the Canonical SMILES for
bergapten, which was then entered into the SwissTarget database to
predict its targets. The resulting targets were summarized and
duplicates were removed (Fig.
1A).
To obtain LC-related targets, the GeneCards and
DisGeNET databases were used. After integration and removal of
duplicates, 1,948 target proteins associated with LC were
identified. The Venny 2.1.0 tool was used to map the targets of
5-methoxypsoralen and LC, resulting in a Venn diagram and
identifying 51 shared targets for the treatment of LC with
5-methoxypsoralen (Fig. 1B). These
51 shared targets were further analyzed using the STRING database,
and the results were visualized and adjusted using Cytoscape 3.7.2
software. By hiding single nodes, a PPI network diagram of the
shared targets was created (Fig.
1C). The color intensity of the targets indicates the strength
of protein interactions, with heat shock protein 90 α family class
A member 1 (HSP90AA1), Caspase 3 (CASP3), EGFR, AKT
serine/threonine kinase 1 (AKT1) and prostaglandin-endoperoxide
synthase 2 (PTGS2) nodes exhibiting markedly higher degree values
compared with other nodes, suggesting that these are key targets of
5-methoxypsoralen in its anti-LC activity. The CytoHubba plug-in in
Cytoscape was used to identify the hub genes in the PPI network
diagram (Fig. 1D).
5-methoxypsoralen lactone is involved
in multiple immune-related pathways and biological processes
To assess the biological processes and pathways of
the potential targets, GO and KEGG analyses were performed using
the Xiantao Academic platform. The results of the GO analysis and
KEGG pathway enrichment (P<0.05) are presented in Table I. A total of 51 targets related to
5-methoxypsoralen were identified, which are primarily associated
with the following biological processes: ‘response to peptide’,
‘membrane raft’, ‘membrane microdomain’, ‘protein
serine/threonine/tyrosine kinase activity’ and the ‘PI3K-Akt
signaling pathway’.
 | Table I.Gene Ontology and Kyoto Encyclopedia
of Genes and Genomes analysis of potential targets. |
Table I.
Gene Ontology and Kyoto Encyclopedia
of Genes and Genomes analysis of potential targets.
| Ontology | ID | Description | GeneRatio | BgRatio | P-value | P.adjust |
|---|
| BP | GO:0032355 | Response to
estradiol | 10/51 | 123/18800 |
1.01×10−12 |
2.65×10−9 |
| BP | GO:0018209 | Peptidyl-serine
modification | 13/51 | 338/18800 |
4.2×10−12 |
5.52×10−09 |
| BP | GO:1901653 | Cellular response
to peptide | 13/51 | 361/18800 |
9.6×10−12 |
8.41×10−9 |
| BP | GO:0046777 | Protein
autophosphorylation | 11/51 | 224/18800 |
1.68×10−11 |
1.06×10−8 |
| BP | GO:0033674 | Positive regulation
of kinase activity | 14/51 | 476/18800 |
2.02×10−11 |
1.06×10−8 |
| CC | GO:0045121 | Membrane raft | 9/51 | 326/19594 |
1.44×10−7 |
1.24×10−5 |
| CC | GO:0098857 | Membrane
microdomain | 9/51 | 327/19594 |
1.48×10−7 |
1.24×10−5 |
| CC | GO:0061695 | Transferase
complex, transferring phosphorus-containing groups | 7/51 | 259/19594 |
4.58×10−6 | 0.0003 |
| CC | GO:0005925 | Focal adhesion | 8/51 | 419/19594 |
1.16×10−5 | 0.0005 |
| CC | GO:0030055 | Cell-substrate
junction | 8/51 | 428/19594 |
1.36×10−5 | 0.0005 |
| MF | GO:0004712 | Protein
serine/threonine/tyrosine kinase activity | 20/51 | 446/18410 |
1.23×10−19 |
3.36×10−17 |
| MF | GO:0004713 | Protein tyrosine
kinase activity | 10/51 | 135/18410 |
3.17×10−12 |
4.32×10−10 |
| MF | GO:0004714 | Transmembrane
receptor protein tyrosine kinase activity | 7/51 | 60/18410 |
2.82×10−10 |
2.55×10−8 |
| MF | GO:0019199 | Transmembrane
receptor protein kinase activity | 7/51 | 79/18410 |
2.03×10−9 |
1.38×10−7 |
| MF | GO:0043560 | Insulin receptor
substrate binding | 4/51 | 10/18410 |
1.08×10−8 |
5.89×10−7 |
| KEGG | hsa04151 | PI3K-Akt signaling
pathway | 20/49 | 354/8164 |
2.91×10−15 |
5.65×10−13 |
| KEGG | hsa05212 | Pancreatic
cancer | 11/49 | 76/8164 |
4.72×10−13 |
4.58×10−11 |
| KEGG | hsa05417 | Lipid and
atherosclerosis | 15/49 | 215/8164 |
8.92×10−13 |
5.77×10−11 |
| KEGG | hsa05215 | Prostate
cancer | 11/49 | 97/8164 |
7.5×10−12 |
3.64×10−10 |
| KEGG | hsa05162 | Measles | 12/49 | 139/8164 |
1.98×10−11 |
7.69×10−10 |
Molecular docking and analysis
AutoDockVina was used to perform molecular docking
and evaluate the binding affinity of bergamot lactone to eight key
targets: CASP3, Cyclin D1 (CCND1), estrogen receptor 1 (ESR1),
glycogen synthase kinase-3β (GSK3B), Janus kinase 2 (JAK2), NF-κB,
phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α
(PIK3CA), protein tyrosine kinase 2 (PTK2) and toll-like receptor 4
(TLR4). The ligand used for docking was 5-methoxypsoralen. The
binding energies between 5-methoxypsoralen and the key targets were
all found to be <-5.0 kcal/mol, indicating favorable binding
affinities and suggesting these targets as potential candidates for
5-methoxypsoralen. Notably, the highest docking score (−7.9
kcal/mol) was observed between 5-methoxypsoralen and GSK3B,
implying that GSK3B is a promising target for the anti-LC effects
of 5-methoxypsoralen. The conformation with the lowest binding
energy was visualized using PyMOL v2.2.0 (Fig. 2).
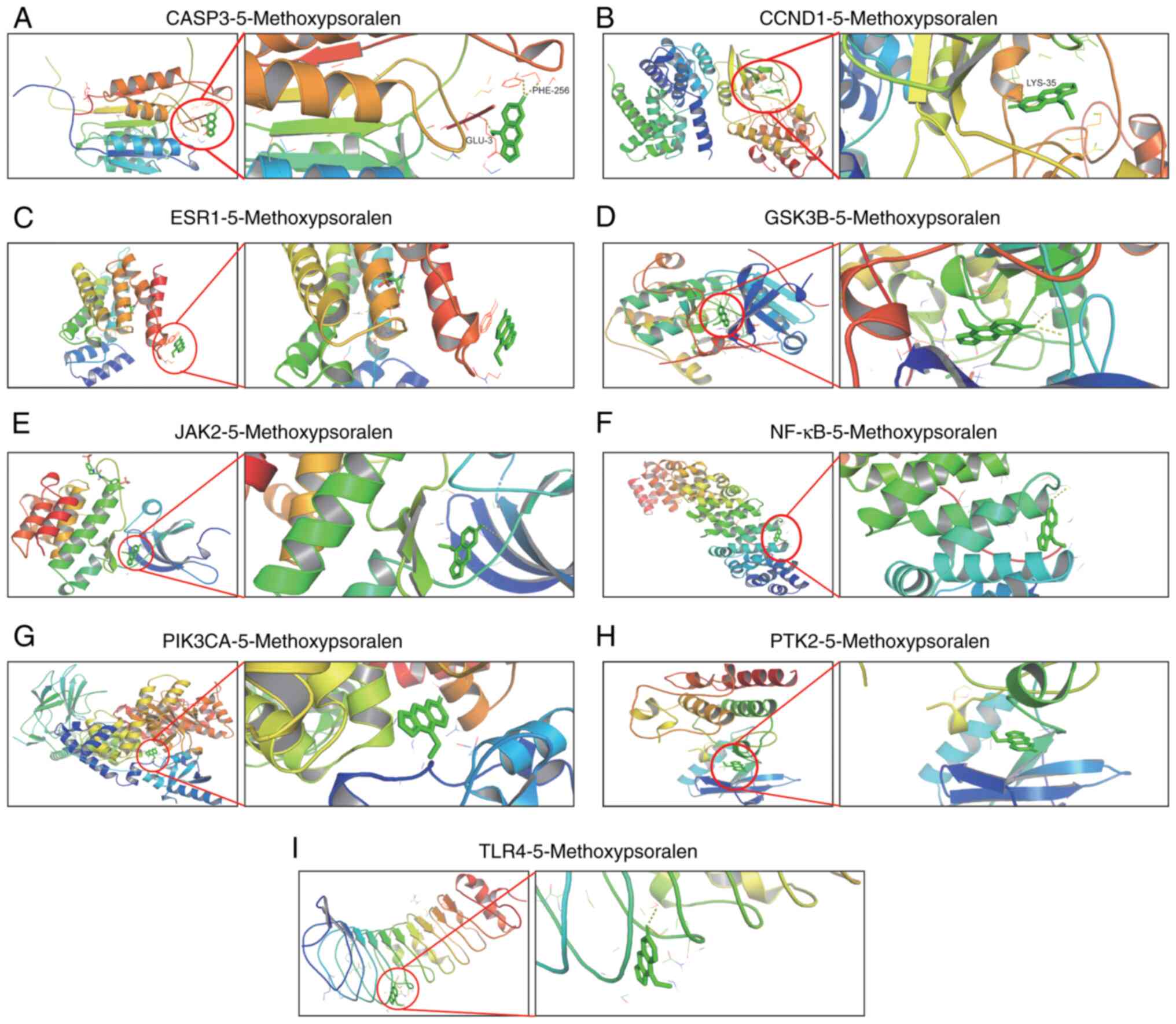 | Figure 2.Molecular model of 5-methoxypsoralen
binding to its predicted protein target. Proteins (A) CASP3, (B)
CCND1, (C) ESR1, (D) GSK3B, (E) JAK2, (F) NF-κB, (G) PIK3CA, (H)
PTK2 and (I) TLR4 were demonstrated to be associated with
5-methoxypsoralen interactions, represented by the blue stick
model. Lines represent residues in the binding site. The light
dashed lines represent hydrogen bonds and the dark dashed lines
demarcate π-π interactions. CASP3, Caspase 3; CCND1, Cyclin D1;
ESR1, estrogen receptor 1; GSK3B, glycogen synthase kinase-3β;
JAK2, Janus kinase 2; PIK3CA, phosphatidylinositol-4,5-bisphosphate
3-kinase, catalytic subunit α; PTK2, protein tyrosine kinase 2;
TLR4, toll-like receptor 4. |
Effects of 5-methoxypsoralen on cell
viability and apoptosis of NCI-H1975, NCI-H1299 and NCI-H460
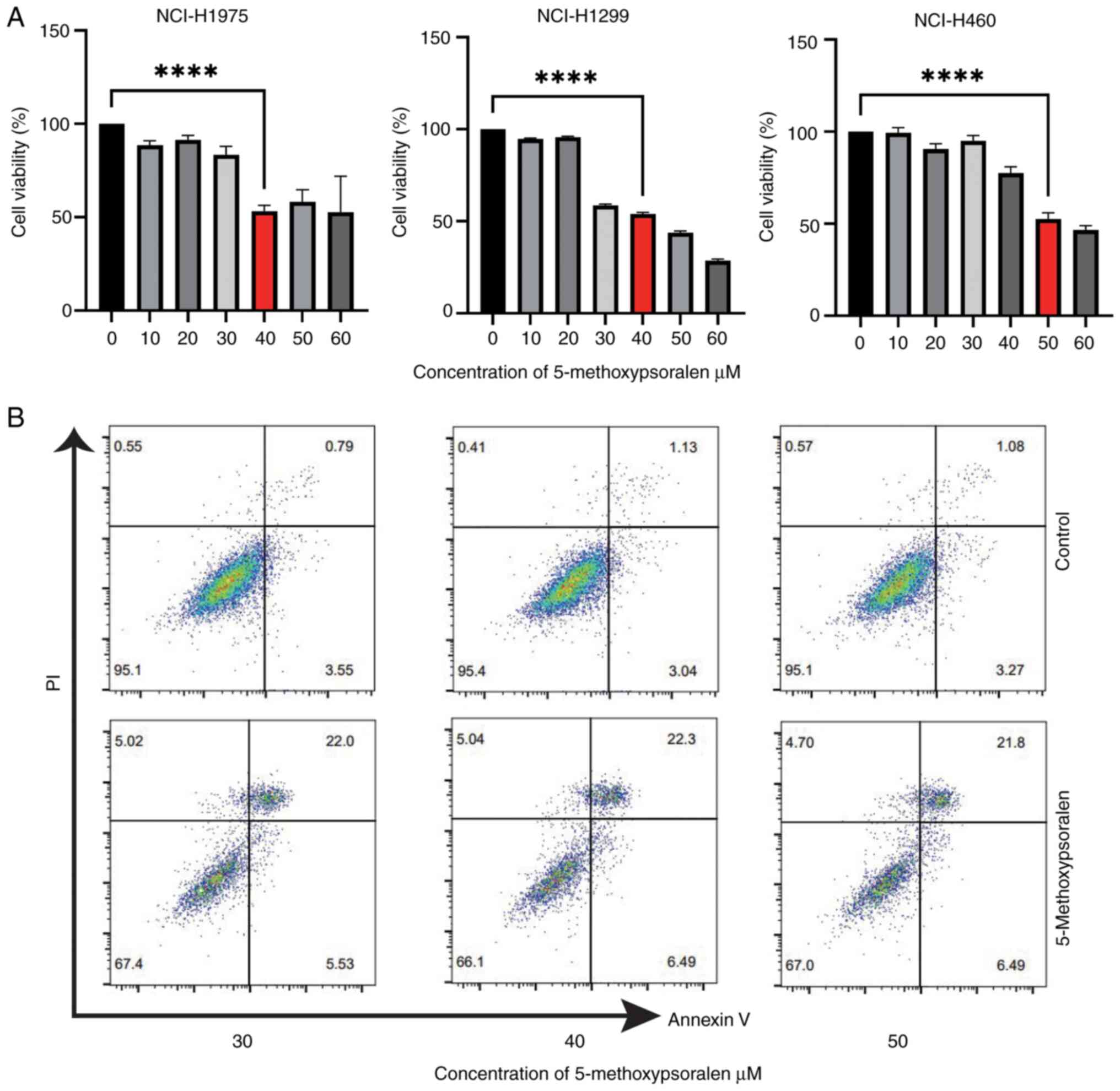
The cytotoxic effect of 5-methoxypsoralen on
NCI-H1975, H1299 and H460 cells was assessed using the CCK-8 assay.
Treatment with 40 µM 5-methoxypsoralen for 72 h significantly
reduced cell viability in NCI-H1975 and H1299 cells compared with
untreated control cells (Fig. 3A).
Similarly, treatment with 50 µM 5-methoxypsoralen for 72 h
significantly inhibited cell viability in NCI-H460 cells relative
to the untreated control group. Therefore, these concentrations
were selected for further experiments. Additionally, the effect of
5-methoxypsoralen on apoptosis in LC cells was evaluated (Fig. 3B) and the results indicated that
5-methoxypsoralen treatment promoted apoptosis in all three types
of LC cell. In conclusion, these findings suggest that
5-methoxypsoralen exhibits significant cytotoxic effects on
NCI-H1975, NCI-H1299 and NCI-H460 cells.
Molecular docking target protein
verification
CASP3 is a classic indicator of apoptosis and CCND1
is a key driver of the malignant transformation of SCLC (12). Preclinical data support that ESR1
can stimulate NSCLC cell growth (13). GSK3B positive expression in LC is
associated with more advanced tumor stages and worse overall
survival (14). Inhibition of JAK2
signaling can enhance radiotherapy in lung cancer models (15). Furthermore, although NF-κB has key
physiological functions in normal cells (especially immune cells),
specific inhibition of NF-κB in LC cells is crucial for alleviating
inflammation and preventing LC. Blocking NF-κB promotes apoptosis
in LC cells (16). High expression
of PIK3CA is also associated with NSCLC in patients with a history
of smoking (17). PTK2 is
considered a novel therapeutic target for overcoming acquired
EGFR-TKI resistance in NSCLC (18).
Compared with non-LC tissues, TLR4 levels are higher in LC tissues
and TLR4 helps LC cells evade the immune system by releasing
immunosuppressive cytokines and enhancing resistance to
pro-apoptotic factors (19).
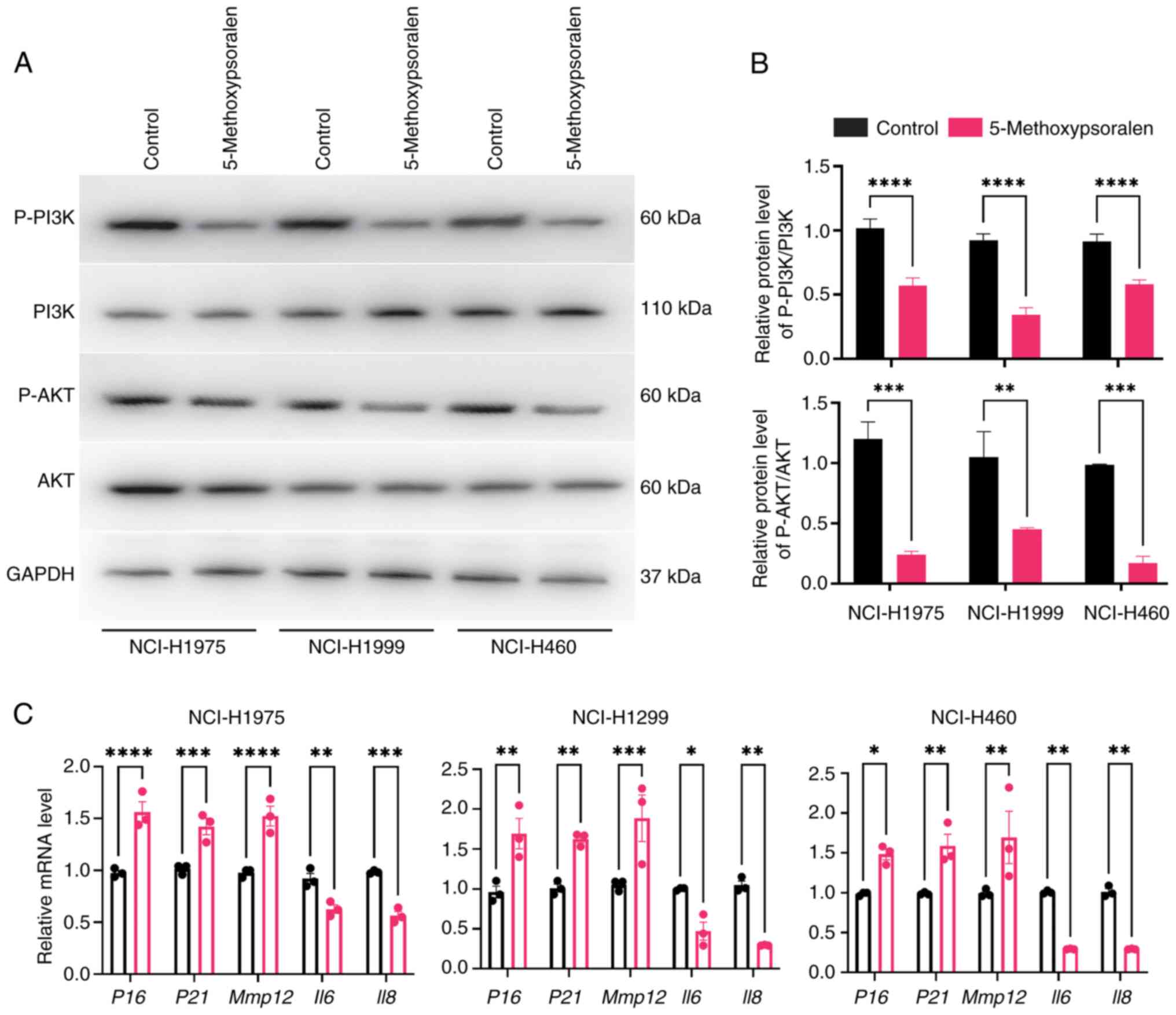
In the present study, 50 µM 5-methoxypsoralen was
used to evaluate the molecular docking results. The RT-qPCR
experiments demonstrated that 5-methoxypsoralen significantly
upregulated the expression of CASP3 in the three LC cell
lines and significantly inhibited the expression of CCND1, ESR1,
GSK3B, JAK2, NFΚB, PIK3CA, PTK2 and TLR4, in comparison
with controls (Fig. S1). These
findings not only confirm the reliability of the molecular docking
results but also suggest that 5-methoxypsoralen may have the
potential to promote apoptosis, inhibit proliferation, and reduce
immune escape in lung cancer cells.
5-methoxypsoralen inhibits the growth
of LC cells in vitro
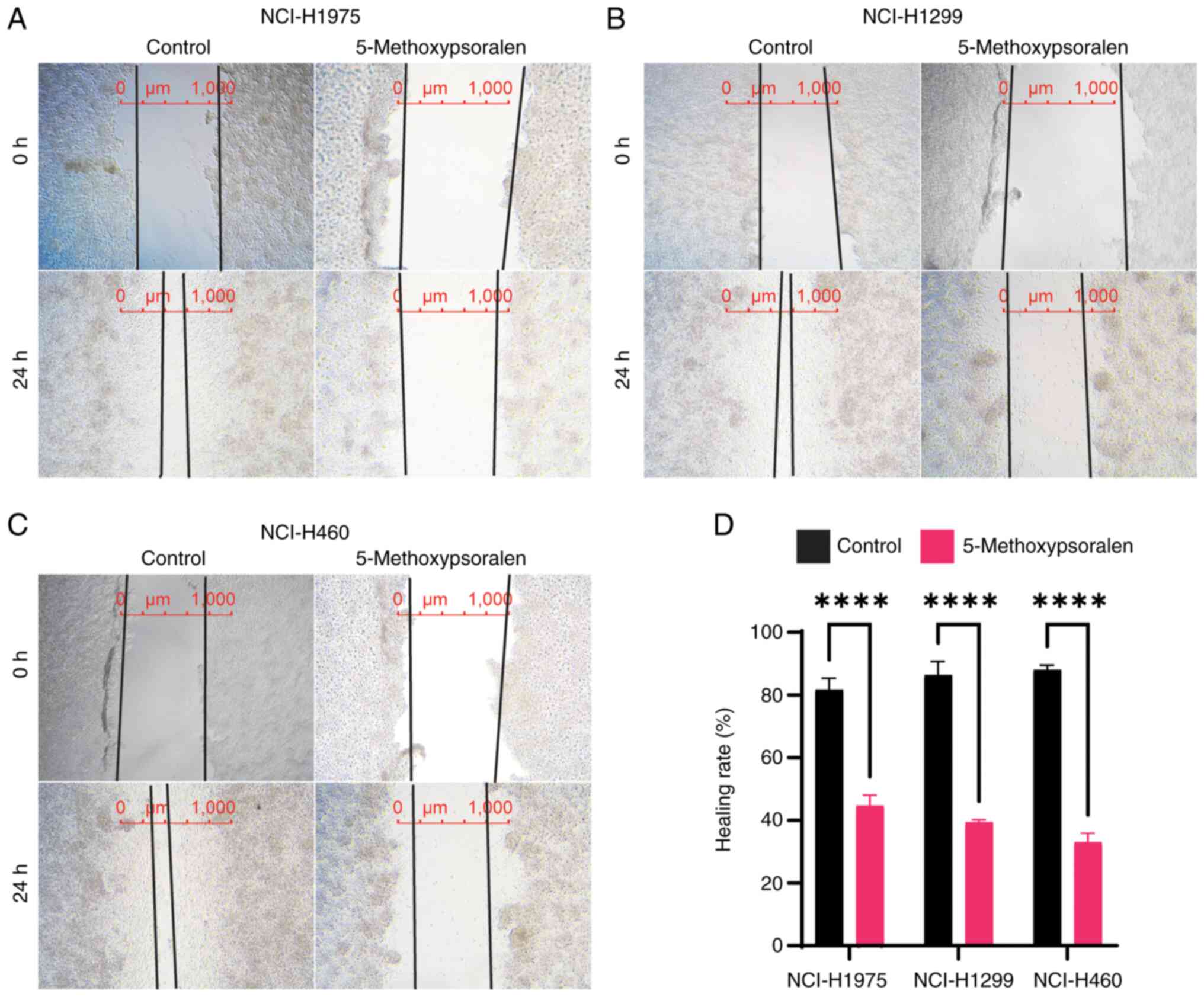
A wound healing assay was performed to measure cell
migration, where the distance between the edges of three cell
monolayers (wound width) was measured after 24 h of treatment with
5-methoxypsoralen. The results indicated that 5-methoxypsoralen at
concentrations of 40 or 50 µM significantly reduced the migration
of LC cells, in comparison with controls (Fig. 4). Additionally, the migration of LC
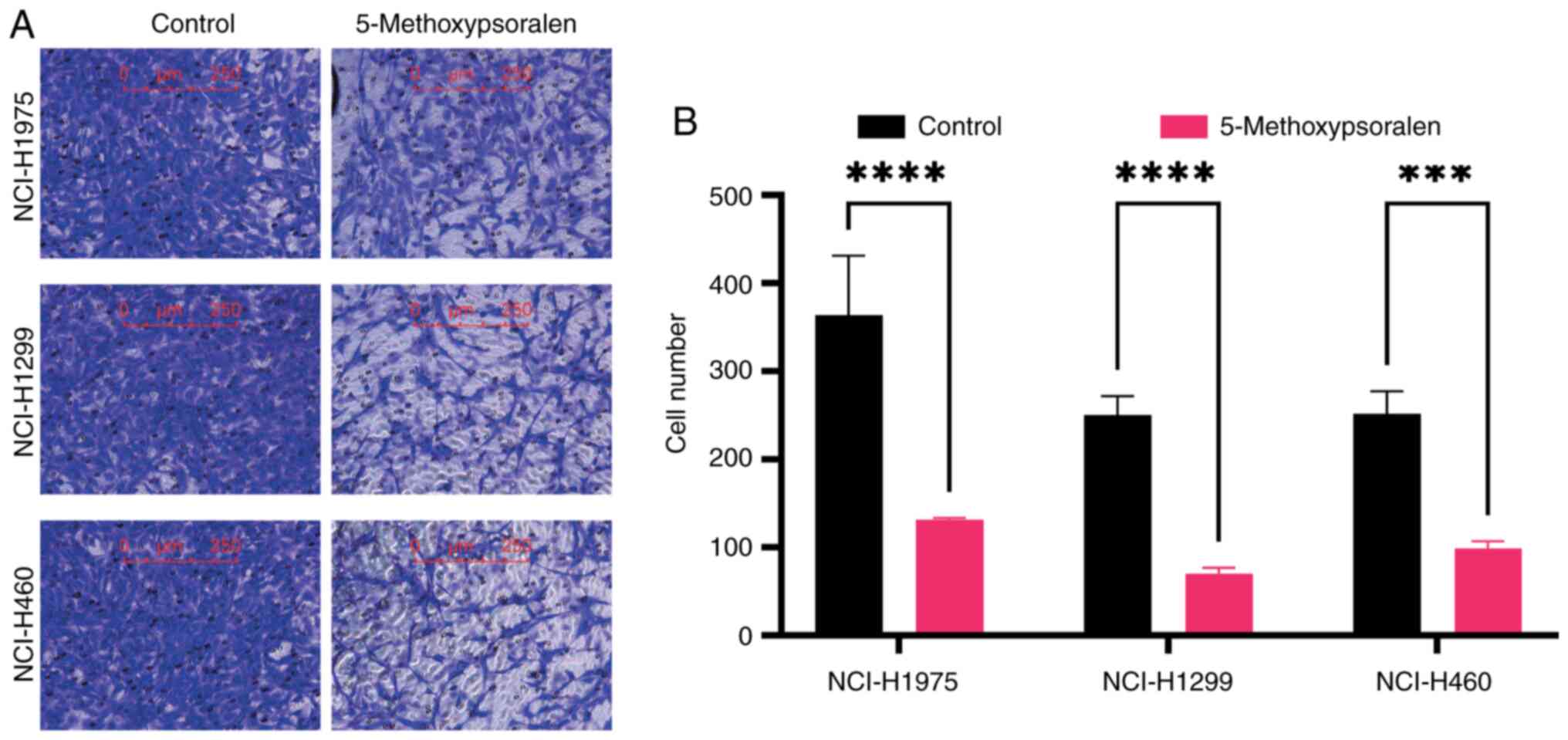
cells was evaluated using a Transwell assay. The findings
demonstrated that 5-methoxypsoralen at concentrations of 40 or 50
µM significantly inhibited the migration ability of LC cells
compared with controls, particularly in NCI-H1299 cells (Fig. 5).
5-methoxypsoralen inhibits the
PI3K/AKT pathway in lung cancer cells and promotes cellular
senescence
The KEGG results indicated that 5-methoxypsoralen
may impact the PI3K/AKT pathway in LC, which was subsequently
evaluated in vitro. The results demonstrated a significant
reduction in the expression of P-PI3K and P-AKT in the
5-methoxypsoralen group compared with the control group. However,
no significant differences were observed in the expression of total
AKT and PI3K between the groups (Fig.
6A and B).
During cellular senescence, several changes occur in
DNA, proteins, secreted factors and cell morphology. This includes
the secretion of a substantial amount of pro-inflammatory factors
(20). RT-qPCR results revealed
that treatment with 5-methoxypsoralen significantly activated the
aging markers P16 and P21, in comparison with the
control group. Additionally, 5-methoxypsoralen treatment also
influenced senescence-associated secretory phenotype (SASP), with
increased expression of MMP12 and decreased levels of
IL6 and IL8 (Fig.
6C).
AKT activator SC79 attenuates the
negative effects of 5-methoxypsoralen on LC cells
To further assess whether the regulatory effect of
5-methoxypsoralen on cells depends on the PI3K/AKT pathway, SC79,
an AKT activator, for verification. SC79 is known for its high
safety profile and ability to activate multiple phosphorylation
sites of AKT. Even after SC79 is removed, sustained increases in
Akt phosphorylation levels can be observed both in cell cultures
and in vivo (21).
In the present study, AKT activity significantly
increased in all three cell types following treatment with SC79,
compared with the 5-methoxypsoralen group (Fig. 7A). Subsequently, the effect of SC79
on cell apoptosis was evaluated. The results revealed that SC79
treatment, after 5-methoxypsoralen exposure, significantly reduced
the mRNA levels of apoptotic markers CASP3, 7 and 9
in comparison with the 5-methoxypsoralen group (Fig. 7B), suggesting that AKT activation
inhibits cell apoptosis and that 5-methoxypsoralen exerts its
pro-apoptotic effect through AKT.
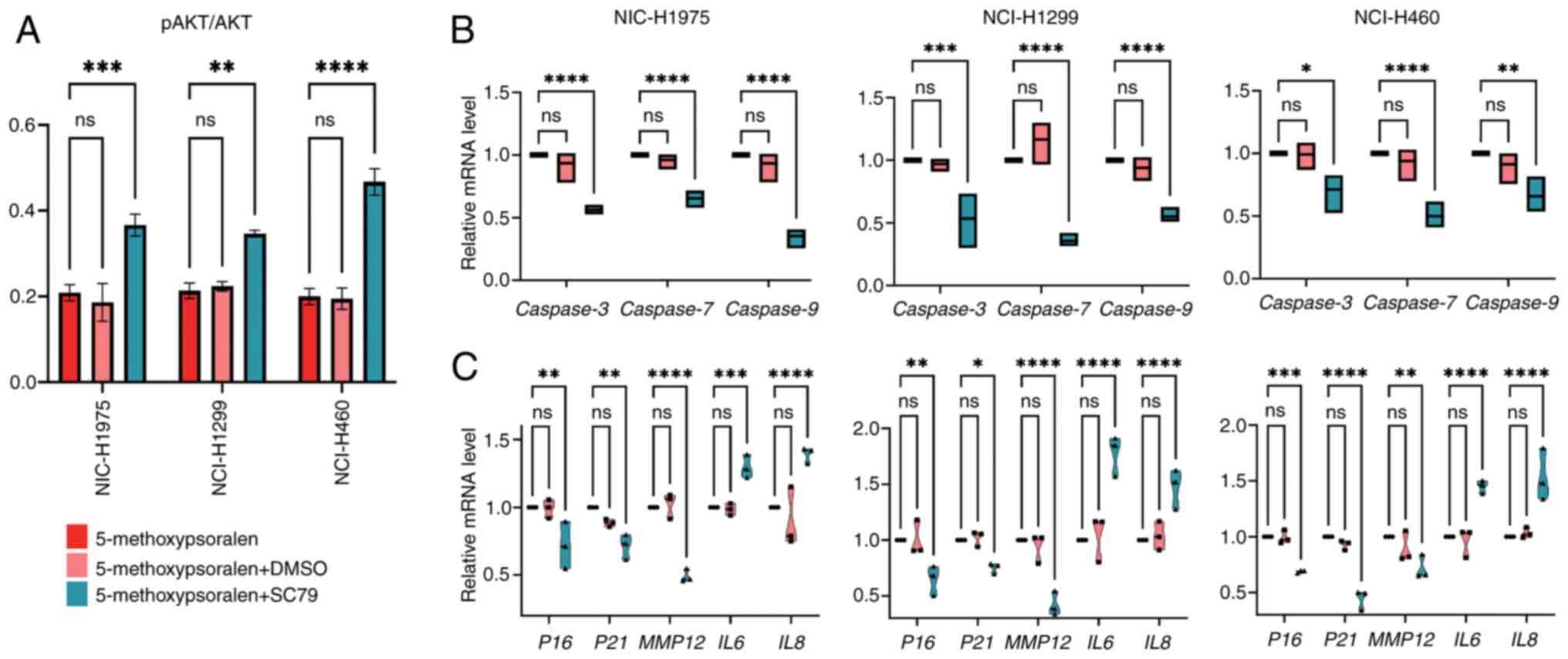 | Figure 7.SC79 (4 µg/ml) antagonizes the
negative effects of 5-methoxypsoralen on non-small cell lung
cancer. (A) ELISA was used to detect the AKT activation levels of
three cell lines after treatment with 5-methoxypsoralen,
5-methoxypsoralen + DMSO and 5-methoxypsoralen + SC79. (B) RT-qPCR
analysis determined the Caspase3, 7 and 9 mRNA levels
after treatment with 5-methoxypsoralen, 5-methoxypsoralen + DMSO
and 5-methoxypsoralen + C79. (C) RT-qPCR analysis determined the
P16, P21, MMP12, IL6 and IL8 mRNA levels after
treatment with 5-methoxypsoralen, 5-methoxypsoralen + DMSO and
5-methoxypsoralen + SC79. n=3. *P<0.05; **P<0.01;
***P<0.001; ****P<0.0001. RT-qPCR, reverse
transcription-quantitative PCR; MMP12, matrix metallopeptidase 12;
ns, no significant difference. |
Additionally, the impact of SC79 on SASP was
assessed. Following combined treatment with SC79 and
5-methoxypsoralen, the mRNA levels of P16, P21 and
MMP12 decreased, whilst the mRNA levels of IL6 and
IL8 increased in comparison with the 5-methoxypsoralen group
(Fig. 7C). These findings suggest
that the ability of 5-methoxypsoralen to promote tumor cell
senescence and inhibit inflammation is also dependent on AKT
activity.
Discussion
The present study assessed the anticancer effects of
5-methoxypsoralen on the human LC cell lines, NCI-H1975, H1299 and
H460. The results demonstrated that 5-methoxypsoralen effectively
inhibited the viability of these cell types, attributed to its
ability to induce apoptosis and senescence, as well as its
inhibition of the PI3K/AKT signaling pathway. Network pharmacology
analysis revealed that 5-methoxypsoralen may exert its anti-LC
effects primarily by targeting HSP90AA1, CASP3, EGFR, AKT1, GSK3B
and PTGS2. Molecular docking results showed strong binding between
Bergapten (5-methoxypsoralen) and these targets, with the highest
binding affinity observed for GSK3B. This suggests that
5-methoxypsoralen exerts its anti-LC effects by modulating GSK3B
and its associated pathways.
The KEGG enrichment analysis results revealed that
5-methoxypsoralen may have a therapeutic effect on LC through the
PI3K-AKT signaling pathway (Table
I). The PI3K-AKT signaling pathway is known to be involved in
cell growth, survival and metabolism (22). Previous studies have demonstrated
that this pathway interacts with DNA damage response, which is a
key factor in aging (23). The
activation of PI3K cascade leads to the activation of downstream
survival molecules, such as AKT (24). The PI3K/AKT pathway serves a crucial
role in regulating cell growth, motility and survival during the
progression and metastasis of cancer; it is frequently activated in
cancer cells. Furthermore, activation of the AKT signaling pathway
results in resistance to apoptosis, including the inactivation of
anti-apoptotic genes and pro-apoptotic factors (25).
There is increasing evidence linking cellular
senescence and inflammation. Cellular senescence is a natural and
unavoidable process in organisms, resulting in permanent cell cycle
arrest (26,27). Whilst aging is associated with
several diseases, senescent cells can also have a positive role. In
cancer, aging acts as an effective barrier against tumorigenesis
(28). A characteristic of
senescent cells is the elevated activity of lysosomal
β-galactosidase. Senescent cells secrete pro-inflammatory factors
such as IL-6, TNF-α and matrix metallopeptidase 12 (MMP12), known
as the SASP, which can induce physiological changes in the
surrounding environment, including inflammation, tumor formation
and growth arrest. Studies have demonstrated that certain SASP
factors, like IL-1β, can induce senescence in normal cells, whilst
IL-6 can further accelerate the senescence process in senescent
cells. The loss of IL-6 can disrupt the inflammation-related SASP
network and the aging paracrine pathway (29). There has been a rise in studies
focusing on the senescence of LC cells. In vitro, A549 cells
irradiated with a single high dose exhibit SASP (30). Moreover, knockdown of MMP12 inhibits
the growth and migration of lung adenocarcinoma cells (31).
The present study aimed to evaluate the effects of
5-methoxypsoralen on senescence and SASP in LC cell lines. The
results from RT-qPCR analysis indicated that 5-methoxypsoralen can
induce senescence in LC cells. Whilst both IL6 and
IL8 are part of SASP, they serve different roles in LC. IL-6
is known to promote NSCLC metastasis by upregulating T-cell
immunoglobulin domain and mucin domain 4 (TIM-4) (32), whereas reducing IL-8 secretion
inhibits LC metastasis to the brain (33). Notably, 5-methoxypsoralen was
reported to inhibit the levels of IL6 and IL8 in LC
cell lines, suggesting that it not only promotes senescence in LC
cells but also inhibits chronic inflammation in the cancer
micro-environment, thereby limiting tumor progression. Moreover,
the present study demonstrated that the regulation of
5-methoxypsoralen on LC cell lines was significantly impeded after
the use of the AKT activator SC79, which also suggests that
5-methoxypsoralen does exert its anticancer effect in vitro
through PI3K/AKT.
The case report by Hashimoto et al (34) highlights the clinical challenges
posed by metastatic lung adenocarcinoma, including the
aggressiveness and resilience of metastatic cells. Understanding
how Bergapten and similar compounds affect metastatic lesions could
provide critical insights into their broader therapeutic potential,
particularly in preventing or treating metastasis in patients with
LC.
To fully understand the therapeutic potential of
Bergapten, it is critical to bridge the gap between in vitro
concentrations and achievable in vivo plasma concentrations
in humans. Whilst the in vitro results of the present study
are promising, demonstrating significant effects on cancer cell
viability, apoptosis and pathway modulation, the concentrations
used may not be directly translatable to clinical settings without
considering factors like bioavailability, delivery methods and
potential toxicity. Therefore, further pharmacokinetic studies and
in vivo experiments are needed to determine the clinical
feasibility of using Bergapten as an anticancer agent and to
optimize its delivery to achieve therapeutic concentrations at the
target site.
The present study on the effect of Bergapten on
NSCLC has advantages and limitations. Network pharmacology was used
to predict the key targets and pathways involved in the anticancer
effect of Bergapten, which was further assessed in vitro.
The strong binding affinity of Bergapten to key proteins was
confirmed by molecular docking, which enhanced the understanding of
its mechanism of action. However, the results of the present study
are based on in vitro assays and cannot fully replicate the
complexity of organisms. Therefore, in vivo validation is
required. In addition, the present study mainly targeted the
PI3K/AKT pathway and did not assess other related pathways in the
progression of NSCLC. For instance, mitochondrial deoxyguanosine
kinase has been reported to markedly influence apoptosis and
autophagy in lung adenocarcinoma cells (35), offering insights into the potential
mechanisms through which Bergapten might exert its effects on cell
viability and survival in NSCLC. The interplay between
mitochondrial function and the PI3K/AKT pathway could further
elucidate the mechanistic basis for the anticancer activity of
Bergapten. In addition, the present study did not address the
long-term safety and potential side effects of Bergapten;
therefore, its clinical application needs further study.
In conclusion, the present study used network
pharmacology and molecular docking technology to predict the
potential anti-LC target of 5-methoxypsoralen and its mechanism of
action. The combined experimental findings further support the
notion that 5-methoxypsoralen may promote LC cell senescence and
exert its pharmacological effects in treating LC by inhibiting the
PI3K/AKT signaling pathway, regulating SASP and the
micro-environment, and modulating the expression of
P16/P21 aging-related genes.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the Chongqing Science and
Technology Bureau (grant no. cstc2019jcyj-msxmX0190).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YC conceived and designed the study with input from
QX. YF performed the majority of the experiments and analyzed the
data. HZ and PW assisted with data analysis and interpretation. YX
conducted the cell viability and migration assays. YC and QX
supervised the project. YC wrote the manuscript with contributions
from all authors. All authors read and approved the final version
of the manuscript. YC and QX confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pakzad R, Mohammadian-Hafshejani A,
Ghoncheh M, Pakzad I and Salehiniya H: The incidence and mortality
of lung cancer and their relationship to development in Asia.
Transl Lung Cancer Res. 4:763–774. 2015.PubMed/NCBI
|
|
3
|
Amini A, Byers LA, Welsh JW and Komaki RU:
Progress in the management of limited-stage small cell lung cancer.
Cancer. 120:790–798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Ao X, Yu W, Zhang Y and Wang J:
Biogenesis, functions, and clinical implications of circular RNAs
in non-small cell lung cancer. Mol Ther Nucleic Acids. 27:50–72.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Cancer Society: Cancer Facts
& Figures 2024. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.htmlOctober
22–2024
|
|
6
|
Liang Y, Xie L, Liu K, Cao Y, Dai X, Wang
X, Lu J, Zhang X and Li X: Bergapten: A review of its pharmacology,
pharmacokinetics, and toxicity. Phytother Res. 35:6131–6147. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo TT, Lu Y, Yan SK, Xiao X, Rong XL and
Guo J: Network pharmacology in research of chinese medicine
formula: Methodology, application and prospective. Chin J Integr
Med. 26:72–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Chen B, Chen S, Lin M, Chen Y, Jin
S, Chen W and Zhang Y: Applications of network pharmacology in
traditional Chinese medicine research. Evid Based Complement
Alternat Med. 2020:16469052020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng Y, Wang Y, Zhou C, Mei W and Zeng C:
PI3K/Akt/mTOR pathway and its role in cancer therapeutics: Are we
making headway? Front Oncol. 12:8191282022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eberhardt J, Santos-Martins D, Tillack AF
and Forli S: AutoDock Vina 1.2.0: New docking methods, expanded
force field, and python bindings. J Chem Inf Model. 61:3891–3898.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gautschi O, Ratschiller D, Gugger M,
Betticher DC and Heighway J: Cyclin D1 in non-small cell lung
cancer: A key driver of malignant transformation. Lung Cancer.
55:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siegfried JM, Hershberger PA and Stabile
LP: Estrogen receptor signaling in lung cancer. Semin Oncol.
36:524–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alves M, Borges DP, Kimberly A, Martins
Neto F, Oliveira AC, de Sousa JC, Nogueira CD, Carneiro BA and
Tavora F: Glycogen synthase kinase-3 beta expression correlates
with worse overall survival in non-small cell lung cancer-A
clinicopathological series. Front Oncol. 11:6210502021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Moretti L, Giacalone NJ, Schleicher
S, Speirs CK, Carbone DP and Lu B: Inhibition of JAK2 signaling by
TG101209 enhances radiotherapy in lung cancer models. J Thorac
Oncol. 6:699–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Li Z, Bai L and Lin Y: NF-kappaB
in lung cancer, a carcinogenesis mediator and a prevention and
therapy target. Front Biosci (Landmark Ed). 16:1172–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang Y, Li J, Li J and Che G:
Clinical significance of PIK3CA gene in non-small-cell lung cancer:
A systematic review and meta-analysis. Biomed Res Int.
2020:36082412020.PubMed/NCBI
|
|
18
|
Tong X, Tanino R, Sun R, Tsubata Y,
Okimoto T, Takechi M and Isobe T: Protein tyrosine kinase 2: A
novel therapeutic target to overcome acquired EGFR-TKI resistance
in non-small cell lung cancer. Respir Res. 20:2702019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoden B, DeRubeis D, Martinez-Moczygemba
M, Ramos KS and Zhang D: Understanding the role of Toll-like
receptors in lung cancer immunity and immunotherapy. Front Immunol.
13:10334832022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Payea MJ, Anerillas C, Tharakan R and
Gorospe M: Translational control during cellular senescence. Mol
Cell Biol. 41:e00512–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jo H, Mondal S, Tan D, Nagata E, Takizawa
S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, et al:
Small molecule-induced cytosolic activation of protein kinase Akt
rescues ischemia-elicited neuronal death. Proc Natl Acad Sci USA.
109:10581–10586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maidarti M, Anderson RA and Telfer EE:
Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the
oocyte: Implications for primordial follicle activation, oocyte
quality and ageing. Cells. 9:2002020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oh SJ, Erb HHH, Hobisch A, Santer FR and
Culig Z: Sorafenib decreases proliferation and induces apoptosis of
prostate cancer cells by inhibition of the androgen receptor and
Akt signaling pathways. Endocr Relat Cancer. 19:305–319. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kowald A, Passos JF and Kirkwood TBL: On
the evolution of cellular senescence. Aging Cell. 19:e132702020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiley CD and Campisi J: The metabolic
roots of senescence: Mechanisms and opportunities for intervention.
Nat Metab. 3:1290–1301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calcinotto A, Kohli J, Zagato E,
Pellegrini L, Demaria M and Alimonti A: Cellular senescence: Aging,
cancer, and injury. Physiol Rev. 99:1047–1078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salama R, Sadaie M, Hoare M and Narita M:
Cellular senescence and its effector programs. Genes Dev.
28:99–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tesei A, Arienti C, Bossi G, Santi S, De
Santis I, Bevilacqua A, Zanoni M, Pignatta S, Cortesi M, Zamagni A,
et al: TP53 drives abscopal effect by secretion of
senescence-associated molecular signals in non-small cell lung
cancer. J Exp Clin Cancer Res. 40:892021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv FZ, Wang JL, Wu Y, Chen HF and Shen XY:
Knockdown of MMP12 inhibits the growth and invasion of lung
adenocarcinoma cells. Int J Immunopathol Pharmacol. 28:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C, Yang L, Xu H, Zheng S, Wang Z, Wang
S, Yang Y, Zhang S, Feng X, Sun N, et al: Systematic analysis of
IL-6 as a predictive biomarker and desensitizer of immunotherapy
responses in patients with non-small cell lung cancer. BMC Med.
20:1872022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Zheng H, Xiong J, Huang Y, Li H, Jin
H, Ai S, Wang Y, Su T, Sun G, et al: miR-596-3p suppresses brain
metastasis of non-small cell lung cancer by modulating YAP1 and
IL-8. Cell Death Dis. 13:6992022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hashimoto K, Nishimura S and Akagi M: Lung
adenocarcinoma presenting as a soft tissue metastasis to the
shoulder: A case report. Medicina (Kaunas). 57:1812021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu C, Qin Q and Cong H: Research progress
on the relationship between mitochondrial deoxyguanosine kinase and
apoptosis and autophagy in lung adenocarcinoma cells. Cancer
Insight. 1:53–62. 2022. View Article : Google Scholar
|