Introduction
Lung cancer is the second most commonly diagnosed
cancer and the leading cause of cancer-related death. In 2020, lung
cancer accounted for ~1.8 million deaths worldwide, with an
estimated 2.2 million new cases diagnosed (1). Non-small cell lung cancer (NSCLC) is
the most prevalent histological type, accounting for ~85% of all
lung cancer cases (2). With the
discovery of immune-related molecules, such as programmed cell
death protein 1 (PD-1), cytotoxic T-lymphocyte protein 4 and other
molecules, the precise treatment of tumors has entered the era of
immunotherapy. Clinical trials have shown that the use of immune
checkpoint inhibitors (ICIs) in combination with chemotherapy is
more effective against NSCLC than chemotherapy alone (3–7).
However, follow-up treatment options remain under investigation for
patients with advanced NSCLC in whom immunotherapy has failed. Some
of these patients receive a new treatment strategy, including
traditional chemotherapy, anti-angiogenic drugs or radiotherapy,
while others choose to continue with immunotherapy, a practice
known as immunotherapy beyond progression (IBP).
Previous studies have demonstrated longer survival
times or continued tumor burden reduction of IBP in patients with
melanoma (8,9) and renal cell carcinoma (10,11).
Limited studies have been reported for patients with NSCLC, and the
clinical outcomes were variable. The KEYNOTE-010 study showed a
good response for patients who received second-course pembrolizumab
(12), but real-world research
demonstrated no significant benefits for continued nivolumab in
advanced NSCLC (13). Therefore,
the merits of IBP after disease progression remain
controversial.
The current study aimed to elucidate the potential
benefits of continuing ICIs after first-line immunotherapy plus
chemotherapy resulted in progression in patients with advanced
NSCLC, and analyzed data by treatment subgroups to indicate the
effects of IBP.
Patients and methods
Patient population
A total of 136 patients with advanced NSCLC who were
hospitalized in Union Hospital Cancer Center (Wuhan, China) between
March 2018 and July 2022, and whose response was assessed as
progressive disease (PD) after receiving first-line immunotherapy
plus chemotherapy, were included. The inclusion criteria were as
follows: i) Pathologically confirmed NSCLC at the time of initial
diagnosis; ii) stage IIIB-IV disease according to the 8th edition
of the American Joint Committee on Cancer staging system (14) or recurrence after surgery; and iii)
received at least two cycles of first-line ICIs in combination with
chemotherapy, and disease status was defined as PD according to the
Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1)
(15). Exclusion criteria were as
follows: i) Sensitized EGFR/ALK/ROS1 alteration; ii) recorded
second primary malignant tumor or condition complicated with
autoimmune diseases; iii) participation in clinical trials; and iv)
a lack of follow-up data.
Patients who received immunotherapy beyond
first-line progression were defined as the IBP group, while those
who was treated with other treatments, such as chemotherapy,
anti-angiogenic therapy or local radiotherapy, were defined as the
non-IBP group. In the present study, further treatment after
first-line progression was selected by the clinician according to
the actual clinical situation, including the economic status and
choice of the patient.
The following clinicopathological data were
collected: Age, sex, histological type, smoking history, sites of
distant metastases and Eastern Cooperative Oncology Group
performance status (ECOG PS) score (16).
Efficacy evaluation
Clinical response to treatment, including complete
response (CR; disappearance of all target lesions), partial
response (PR; at least a 30% decrease in the sum of the diameters
of the target lesions), PD (at least a 20% increase in the sum of
the diameters of the target lesions and an absolute increase of the
sum by at least 5 mm or the appearance of one or more new lesions)
and stable disease (SD; neither sufficient shrinkage to qualify for
PR nor sufficient increase to qualify for PD), was evaluated by
RECIST 1.1 criteria. The objective response rate (ORR) was
calculated as the percentage of patients with the best overall
response of CR and PR (CR + PR). The disease control rate (DCR) was
defined as the percentage of patients with the best overall
response of CR, PR and SD (CR + PR + SD). PFS1 was the interval
from the date of initially receiving first-line treatment to
progression of the disease. PFS2 was the interval from the
second-line treatment to progression or death for any reason. OS
referred to the start of the second-line treatment until death for
any cause or the final follow-up.
Statistical analysis
The χ2 test or Fisher's exact test was
used to assess the statistical difference between categorical
variables. PFS time and OS time were analyzed by the Kaplan-Meier
method, and the log-rank test was employed to calculate inter-group
differences. Independent predictors of PFS and OS were determined
by Cox regression models. Multivariate analysis was performed on
variables that were statistically significant in univariate
analysis, and identified independent prognostic factors associated
with OS and PFS. All statistical analyses were performed using SPSS
25.0 (IBM Corp.) and R-4.2.1 (The R Foundation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of patients
The medical records of 136 patients with advanced
NSCLC who experienced PD after first-line chemotherapy combined
with ICIs were retrospectively reviewed. A total of 88 patients
received IBP, while 48 patients received non-IBP treatments
(Table I). Overall, the median age
for all participants was 60 years (range, 25–83 years). 66.9% of
patients (n=91) were aged <65 years and 83.8% were male, while
52.2% were current or former smokers. An ECOG-PS of 0–1 was
recorded in 81.6% of patients (n=111). A total of 84 (61.8%)
patients had adenocarcinoma and 52 (38.2%) had non-adenocarcinoma.
Brain metastases were recorded in 45 (33.1%) patients, bone
metastases in 56 (41.2%) patients and liver metastases in 24
(17.6%) patients. Patient characteristics, including age, sex,
histological type and smoking history, were well-balanced between
IBP and non-IBP groupings.
 | Table I.Baseline characteristics of all
patients. |
Table I.
Baseline characteristics of all
patients.
| Characteristics | All patients, n
(%) | Non-IBP, n (%) | IBP, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.670 |
|
≤65 | 91 (66.9) | 31 (64.6) | 60 (68.2) |
|
|
>65 | 45 (33.1) | 17 (35.4) | 28 (31.8) |
|
| Sex |
|
|
| 0.709 |
|
Male | 114 (83.8) | 41 (85.4) | 73 (83.0) |
|
|
Female | 22 (16.2) | 7 (14.6) | 15 (17.0) |
|
| Histological
type |
|
|
| 0.385 |
|
Adenocarcinoma | 84 (61.8) | 32 (66.7) | 52 (59.1) |
|
|
Non-Adenocarcinoma | 52 (38.2) | 16 (33.3) | 36 (40.9) |
|
| Smoking status |
|
|
| 0.704 |
| Never
smoked | 65 (47.8) | 24 (50.0) | 41 (46.6) |
|
|
Current/former smoker | 71 (52.2) | 24 (50.0) | 47 (53.4) |
|
| ECOG PS |
|
|
| 0.606 |
|
0-1 | 111 (81.6) | 39 (81.3) | 72 (81.8) |
|
| 2 | 25 (18.4) | 9 (18.8) | 16 (18.2) |
|
| Brain
metastases |
|
|
| 0.116 |
| No | 91 (66.9) | 28 (58.3) | 63 (71.6) |
|
|
Yes | 45 (33.1) | 20 (41.7) | 25 (28.4) |
|
| Bone
metastases |
|
|
| 0.932 |
| No | 80 (58.8) | 28 (58.3) | 52 (59.1) |
|
|
Yes | 56 (41.2) | 20 (41.7) | 36 (40.9) |
|
| Liver
metastases |
|
|
| 0.472 |
| No | 112 (82.4) | 38 (79.2) | 74 (84.1) |
|
|
Yes | 24 (17.6) | 10 (20.8) | 14 (15.9) |
|
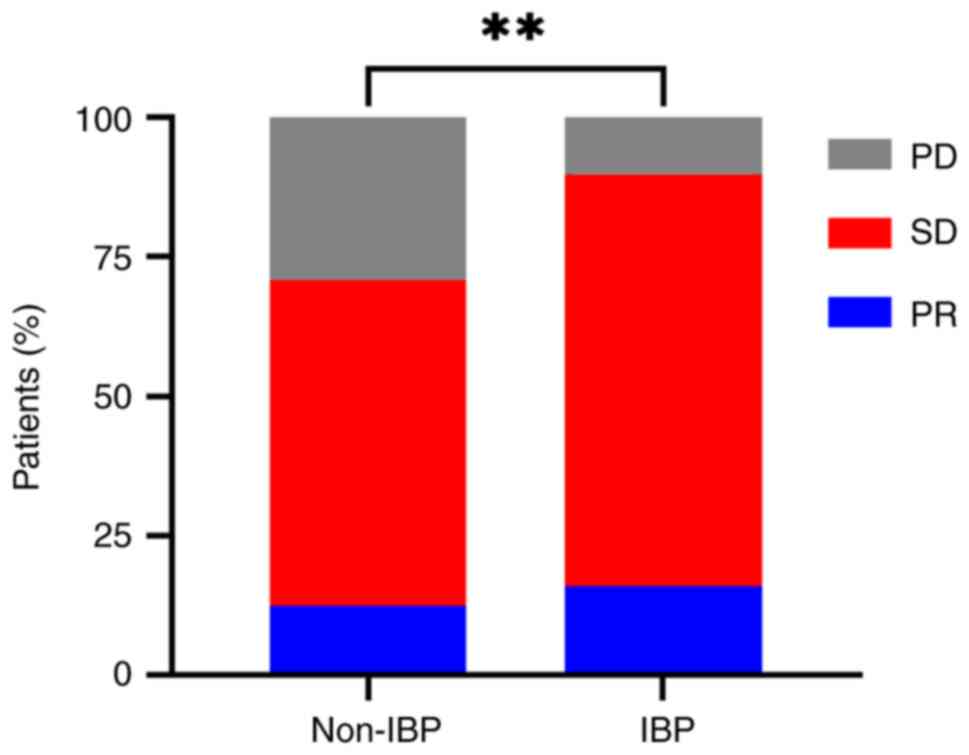
Analysis of treatment response
In order to explore the treatment responses of the
two groups, DCR and ORR were analyzed and compared. The best
response was judged according to RECIST 1.1 (Table II). Responses to first-line
treatment were an ORR of 43.8% and a DCR of 97.9% for the non-IBP
group, and an ORR of 38.6% and a DCR of 92.0% for the IBP group.
Responses to second-line treatment were ORRs of 12.5% for the
non-IBP group and 15.9% for the IBP group. There were no
significant differences in treatment response in first-line
treatment between the IBP and non-IBP groups (ORR: P=0.561; DCR:
P=0.313) or for ORR after second-line treatment (P=0.592). However,
the IBP group exhibited a significantly higher DCR than the non-IBP
group after second-line treatment (89.8 vs. 70.8%; P=0.005)
(Fig. 1).
 | Table II.Treatment responses of all
patients. |
Table II.
Treatment responses of all
patients.
| Treatment
response | All patients, n
(%) | Non-IBP, n (%) | IBP, n (%) | P-value |
|---|
| First-line
response |
|
|
|
|
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| PR | 55 (40.4) | 21 (43.8) | 34 (38.6) |
|
| SD | 73 (53.7) | 26 (54.2) | 47 (53.4) |
|
| PD | 8 (5.9) | 1 (2.1) | 7 (8.0) |
|
|
ORR | 55 (40.4) | 21 (43.8) | 34 (38.6) | 0.561 |
|
DCR | 128 (94.1) | 47 (97.9) | 81 (92.0) | 0.313 |
| Second-line
response |
|
|
|
|
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| PR | 20 (14.7) | 6 (12.5) | 14 (15.9) |
|
| SD | 93 (68.4) | 28 (58.3) | 65 (73.9) |
|
| PD | 23 (16.9) | 14 (29.2) | 9 (10.2) |
|
|
ORR | 20 (14.7) | 6 (12.5) | 14 (15.9) | 0.592 |
|
DCR | 113 (83.1) | 34 (70.8) | 79 (89.8) | 0.005 |
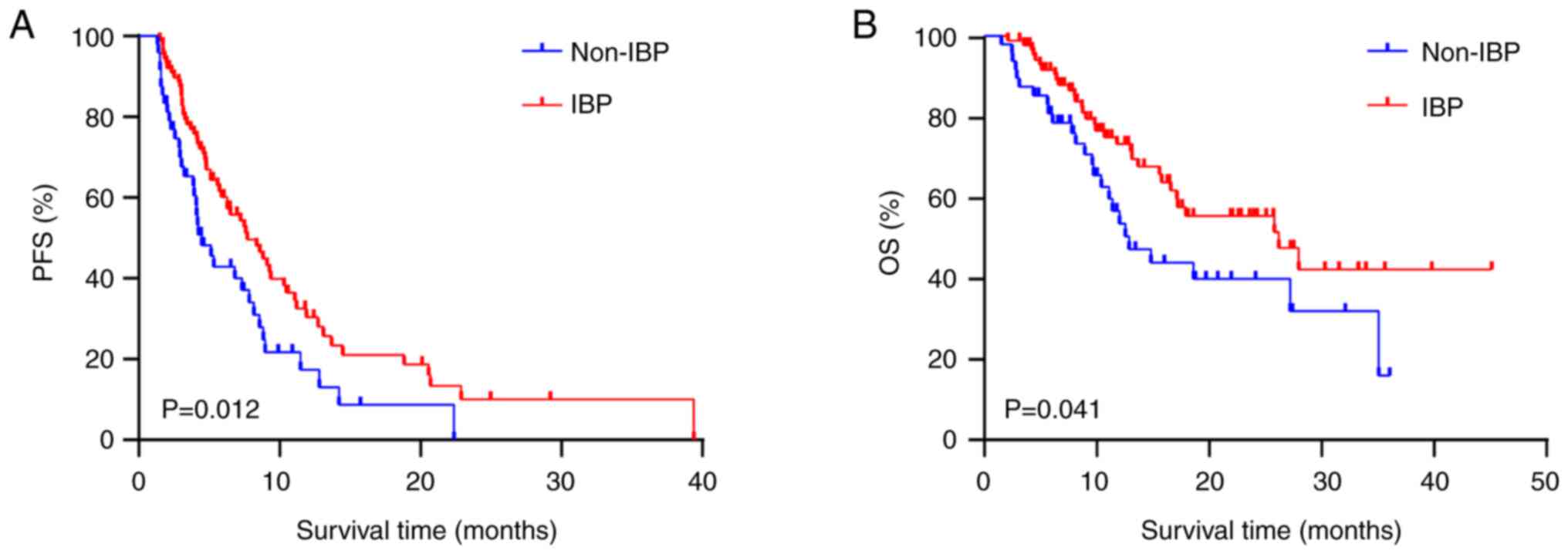
Analysis of survival probability
The median PFS2 time was 7.7 months for the IBP
group and 4.47 months for the non-IBP group. The median OS time was
26.17 months for the IBP group and 12.87 months for the non-IBP
group. Kaplan-Meier plots demonstrated that IBP was significantly
associated with prolonged PFS2 [hazard ratio (HR), 0.597; 95%
confidence interval (CI), 0.379–0.940; P=0.012] and OS (HR, 0.584;
95% CI, 0.334–1.022; P=0.041) times (Fig. 2A and B).
Subgroup analyses were conducted, taking patient
characteristics into consideration. IBP produced a higher PFS2 time
(Fig. S1) in most subgroups,
especially for younger patients (P=0.008), males (P=0.009),
patients who had never smoked (P=0.010), those with a histological
type other than adenocarcinoma (P=0.004), patients with ECOG scores
0–1 (P=0.006), and those without brain (P=0.006) or liver (P=0.002)
metastases. Similarly, continuation of ICIs in second-line
treatment produced more favorable OS times for younger patients
(P=0.021), males (P=0.038) and those with ECOG scores 0–1 (P=0.030)
(Fig. S2).
Univariate analysis showed ECOG PS 0–1 (P=0.038),
good response (CR + PR) to initial immunotherapy (P=0.036) and IBP
group (P=0.013) to be associated with prolonged PFS2 time (Table SI). Patients with a
non-adenocarcinoma histological type (P=0.005), ECOG PS 0–1
(P<0.001) and IBP group (P=0.044) were associated with a longer
OS time (Table SII). Multivariate
Cox proportional hazards analyses demonstrated that IBP was an
independent PFS2-related factor (HR, 0.613; 95% CI, 0.403–0.933;
P=0.022; Table SI), but not an
independent factor for OS (HR, 0.613; 95% CI, 0.360–1.045; P=0.072;
Table SII). Moreover, ECOG PS 2
(HR, 1.953; 95% CI, 1.140–3.347; P=0.015; Table SI) and good response (CR + PR) to
initial immunotherapy (HR, 0.579; 95% CI, 0.373–0.901; P=0.015;
Table SI) were independent
PFS2-related factors. Non-adenocarcinoma histological type (HR,
2.339; 95% CI, 1.380–3.964; P=0.002; Table SII) and ECOG PS 2 (HR, 2.897; 95%
CI, 1.616–5.194; P<0.001; Table
SII) were independent OS-related factors.
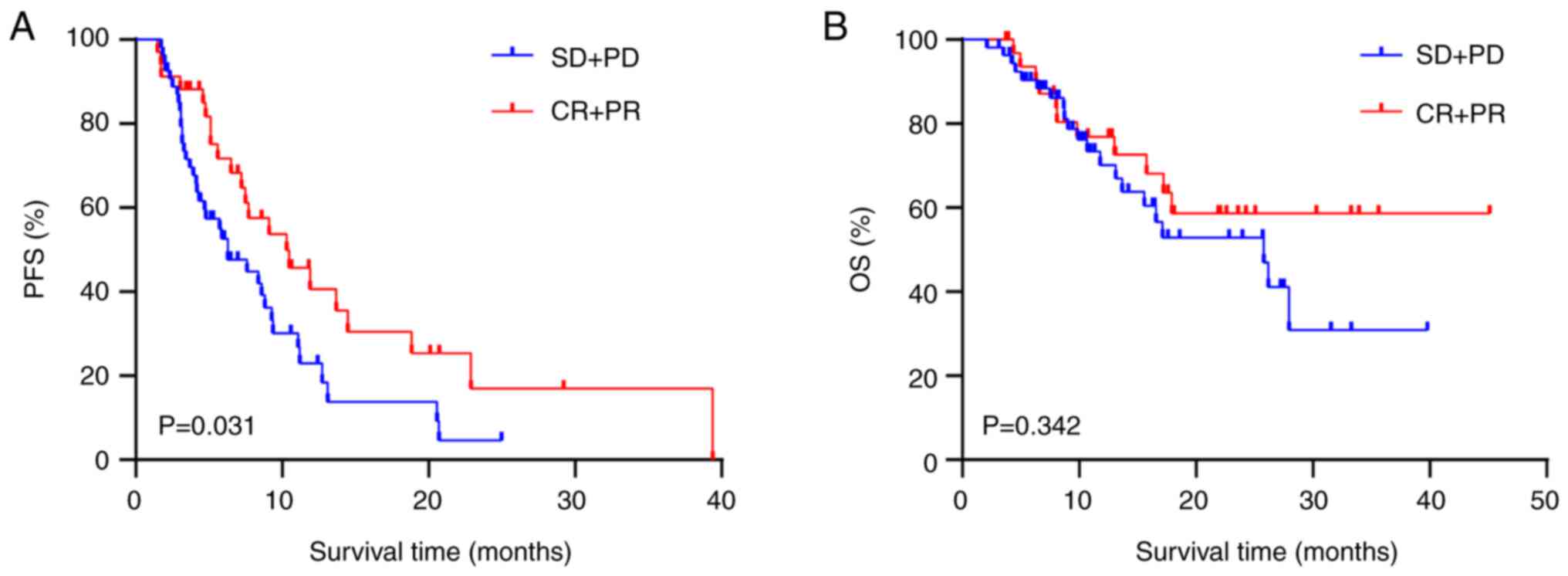
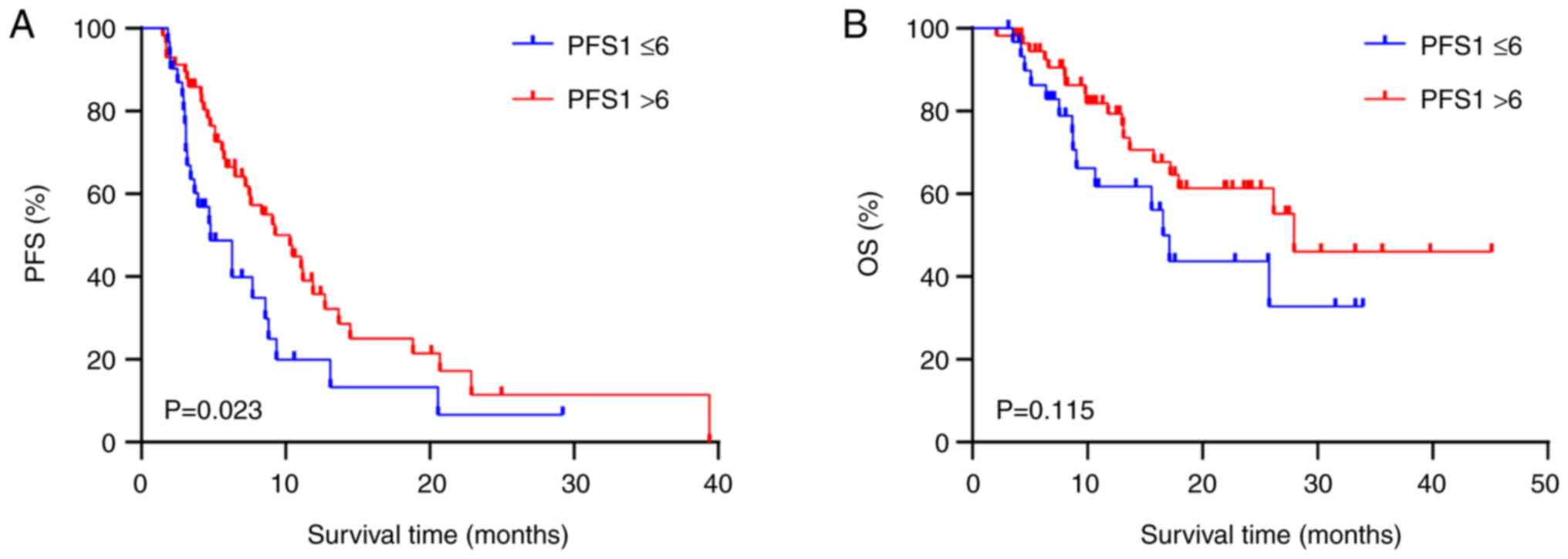
Analysis of IBP efficacy
A subgroup analysis of IBP efficacy was performed
(Table III). Patients whose
initial treatment response was CR + PR showed a prolonged PFS2 time
compared with those whose treatment response was SD + PD after
prior ICIs (mPFS2: 10.3 vs. 6.27 months; HR, 0.574; 95% CI,
0.346–0.952; P=0.031; Fig. 3A).
Patients with a PFS1 >6 months had a higher PFS2 time than
patients with a PFS1 ≤6 months (mPSF2: 10.3 vs. 4.8 months; HR,
0.555; 95% CI, 0.311–0.911; P=0.023; Fig. 4A). However, no difference in OS time
was observed between the CR + PR group and the SD + PD group for
first-line treatment (P=0.342; Fig.
3B) or between the PFS1 >6 months group and the PFS1 ≤6
months group (P=0.115; Fig.
4B).
 | Table III.Clinical characteristics of
immunotherapy beyond progression group (n=88). |
Table III.
Clinical characteristics of
immunotherapy beyond progression group (n=88).
| Clinical
characteristics | Patients, n
(%) |
|---|
| First-line
response |
|
| CR +
PR | 34 (38.6) |
| SD +
PD | 54 (61.4) |
| PFS1 time,
months |
|
| ≤6 | 31 (35.2) |
|
>6 | 57 (64.8) |
| Second-line
treatment |
|
|
Monotherapy | 13 (14.8) |
|
Combination therapy | 75 (85.2) |
| Immunotherapy
options |
|
| Use the
same ICI | 10 (11.4) |
| Change
the ICI | 78 (88.6) |
Second-line immunotherapy treatment strategies were
analyzed for their impact on efficacy. The patients treated with
three agents [chemo (chemotherapy) + immunotherapy (IO) +
anti-angiogenic therapy (anti-angio); n=28] had prolonged PFS2
times (mPSF2: 9.1 vs. 7.6 months; HR, 0.821; 95% CI, 0.466–1.445;
P=0.481; Fig. S3A) compared with
those treated with two agents (chemo + IO; n=40), although the
difference was not statistically significant. OS times were
comparable between the two groups (mOS: 27.97 vs. 26.17 months; HR,
0.917; 95% CI, 0.412–2.042; P=0.831; Fig. S3B).
Discussion
Immunotherapy is the usual treatment choice for
NSCLC without driver gene mutations. ICIs relieve the inhibition of
immune cells and enhance antitumor activity (17). However, patients may show individual
response patterns to ICIs, including late treatment response and
pseudo-progression (18), meaning
that the optimal treatment duration remains uncertain. Much
controversy surrounds the continuation of immunotherapy following
progression after first-line immunochemotherapy and the potential
patient benefits.
Previous studies have shown that IBP is effective
for advanced NSCLC. Ricciuti et al (19) reported that discontinuation of
immunotherapy at first progression was associated with shorter
survival times. Another study also reported that the IBP group
experienced longer OS times compared with the non-IBP group
(20). Conversely, Metro et
al (21) explored the outcomes
of chemotherapy or pembrolizumab beyond first-line pembrolizumab in
patients with advanced NSCLC and programmed cell death 1 ligand 1
(PD-L1) ≥50%, and found no significant difference in
post-progression survival time.
IBP was initially explored in lung cancer, but these
patients received first-line or multiline antitumor therapy prior
to IBP or ICI monotherapy rather than immunotherapy plus
chemotherapy prior to IBP (13,19,20).
Few studies have explored whether IBP in patients with NSCLC after
first-line immunotherapy combined with chemotherapy is beneficial
(22). In the present study, IBP
achieved superior PFS2 (P=0.012) and OS (P=0.041) times after the
progression of first-line immunotherapy combined with chemotherapy
for the current cohort of patients, which is in line with previous
studies (19,20). However, retrospective studies
reported no statistical difference in PFS2 and OS times between the
IBP group and the non-IBP group (13,22).
The inconsistent results may be due to the small sample sizes and
retrospective nature of the studies. A superior DCR was also
identified (P=0.005) in the present study, similar to that found in
a previous retrospective study (23). Moreover, IBP was found to be an
independent prognostic factor for PFS time but not for OS time. In
addition, patients with longer PFS times (PFS1 >6 months) or a
favorable treatment response (CR + PR) to first-line treatment
showed improved PFS2 times, which was consistent with previous
findings (22,24). Therefore, for patients with a better
response to prior treatment (PFS1 >6 months or CR + PR), the
present results suggest a potential benefit from continued
immunotherapy after first-line immunotherapy plus chemotherapy
progression. The limited impact on OS time may be associated with
an insufficient follow-up time and the limited sample size in the
current study. Although not all patients could be followed up for a
total of 40–50 months due to being included at different time
periods, further follow-up along with an expanded data sample
analysis is required in order to better clarify the clinical
benefits of IBP.
After first-line chemoimmunotherapy, the combination
of immunotherapy with anti-angiogenic drugs is a viable option.
Anti-angiogenic drugs can normalize abnormal tumor vasculature and
regulate the tumor immune microenvironment (25–27).
Co-administration of anti-angiogenic drugs may thus improve the
efficacy of immunotherapy (28).
Prolonged OS times compared with those for chemotherapy or
chemotherapy plus anti-angiogenic inhibitor have been shown
(29). The present study analyzed
triplet therapy compared with doublet therapy after the progression
of first-line treatment. No significant differences were found, but
the addition of anti-angiogenic therapy in second-line treatment
remains a viable option. The relative merits of ICI combinations or
monotherapy in second-line treatment could not be evaluated during
the present work due to limited sample sizes. However, combination
therapy tends to exhibit superior efficacy (24,30).
Furthermore, switching the administration of anti-PD-1 and
anti-PD-L1 antibodies as ICI rechallenge may be a treatment option
(31), but such an analysis was
outside the scope of the current study.
There are several limitations to the current study.
Firstly, it was a single-center retrospective study with a small
sample size and therefore selection bias could not be avoided.
Secondly, given the complexity and practicality of clinical
practice, RECIST were used rather than immune-related response
criteria, immune RECIST (iRECIST) or immune-related RECIST
(32–34). Pseudo-progression and delayed
response may not be fully evaluated with RECIST, and more accurate
criteria such as iRECIST are expected to be used in the future.
Thirdly, no data was available regarding PD-L1 expression and tumor
mutational burden before first-line and second-line treatment.
PD-L1 expression may be useful to evaluate the use of IBP when
there is failure of the first-line treatment. In addition, more
analyses are required to evaluate PFS and OS differences based on
the initial PD-L1 expression levels. Lastly, immune-related adverse
events (irAEs) between the two groups were not compared, as the
majority of patients lacked data regarding irAEs, although
continuation of immunotherapy is not considered to significantly
increase the incidence of grade3 or grade4 irAEs (19). Immunotherapy was tolerated well by
most of the current patients and no discontinuation due to severe
irAEs was recorded in the present study.
Overall, in the present study, the clinical benefits
of continued immunotherapy after progression with first-line
chemotherapy combined with immunotherapy are indicated. However,
large prospective clinical studies are required to confirm these
findings.
In conclusion, in the present study, patients with
advanced NSCLC benefited from continuing ICI treatment after
failure of prior chemotherapy plus immunotherapy. IBP should be
therefore be considered for patients with advanced NSCLC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 8207112731).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XG, YH, KZ and LL conceived and designed this study.
IDM, FG, YX participated in data collection and data curation
(organising and maintaining data). XG, YH, JC and LZ analyzed the
data. LL provided the administrative support. XG and YH drafted the
manuscript and all authors reviewed it. KZ and LL confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Ethics
Committee of the Tongji Medical College, Huazhong University of
Science and Technology (approval no. S139). The requirement for
informed consent for participation was waived due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as first-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R,
Yu Y, Yao W, Chang J, Chen J, et al: Sugemalimab versus placebo, in
combination with platinum-based chemotherapy, as first-line
treatment of metastatic non-small-cell lung cancer (GEMSTONE-302):
Interim and final analyses of a double-blind, randomised, phase 3
clinical trial. Lancet Oncol. 23:220–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Tislelizumab plus chemotherapy
vs chemotherapy alone as first-line treatment for advanced squamous
non-small-cell lung cancer: A phase 3 randomized clinical trial.
JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long GV, Weber JS, Larkin J, Atkinson V,
Grob JJ, Schadendorf D, Dummer R, Robert C, Márquez-Rodas I, McNeil
C, et al: Nivolumab for patients with advanced melanoma treated
beyond progression: Analysis of 2 phase 3 clinical trials. JAMA
Oncol. 3:1511–1519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beaver JA, Hazarika M, Mulkey F, Mushti S,
Chen H, He K, Sridhara R, Goldberg KB, Chuk MK, Chi DC, et al:
Patients with melanoma treated with an anti-PD-1 antibody beyond
RECIST progression: A US food and drug administration pooled
analysis. Lancet Oncol. 19:229–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
George S, Motzer RJ, Hammers HJ, Redman
BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM and Rini
BI: Safety and efficacy of nivolumab in patients with metastatic
renal cell carcinoma treated beyond progression: A subgroup
analysis of a randomized clinical trial. JAMA Oncol. 2:1179–1186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Motzer RJ, Sharma P, Wagstaff
J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski
PG, et al: Treatment beyond progression in patients with advanced
renal cell carcinoma treated with nivolumab in CheckMate 025. Eur
Urol. 72:368–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herbst RS, Garon EB, Kim DW, Cho BC,
Perez-Gracia JL, Han JY, Arvis CD, Majem M, Forster MD, Monnet I,
et al: Long-term outcomes and retreatment among patients with
previously treated, programmed death-ligand 1-positive, advanced
non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol.
38:1580–1590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enomoto T, Tamiya A, Matsumoto K, Adachi
Y, Azuma K, Inagaki Y, Kouno S, Taniguchi Y, Saijo N, Okishio K and
Atagi S: Nivolumab treatment beyond progressive disease in advanced
non-small cell lung cancer. Clin Transl Oncol. 23:582–590. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wankhede D: Evaluation of eighth AJCC TNM
sage for lung cancer NSCLC: A meta-analysis. Ann Surg Oncol.
28:142–147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer E, Therasse P, Bogaerls J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang S, Qin C, Hu H, Liu T, He Y, Guo H,
Yan H, Zhang J, Tang S and Zhou H: Immune checkpoint inhibitors in
non-small cell lung cancer: Progress, challenges, and prospects.
Cells. 11:3202022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borcoman E, Kanjanapan Y, Champiat S, Kato
S, Servois V, Kurzrock R, Goel S, Bedard P and Le Tourneau C: Novel
patterns of response under immunotherapy. Ann Oncol. 30:385–396.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricciuti B, Genova C, Bassanelli M, De
Giglio A, Brambilla M, Metro G, Baglivo S, Dal Bello MG, Ceribelli
A, Grossi F and Chiari R: Safety and efficacy of nivolumab in
patients with advanced non-small-cell lung cancer treated beyond
progression. Clin Lung Cancer. 20:178–185.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stinchcombe TE, Miksad RA, Gossai A,
Griffith SD and Torres AZ: Real-world outcomes for advanced
non-small cell lung cancer patients treated with a PD-L1 inhibitor
beyond progression. Clin Lung Cancer. 21:389–394.e3. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Metro G, Addeo A, Signorelli D, Gili A,
Economopoulou P, Roila F, Banna G, De Toma A, Rey Cobo J, Camerini
A, et al: Outcomes from salvage chemotherapy or pembrolizumab
beyond progression with or without local ablative therapies for
advanced non-small cell lung cancers with PD-L1 ≥50% who progress
on first-line immunotherapy: Real-world data from a European
cohort. J Thorac Dis. 11:4972–4981. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu M, Hao Y, Zeng X, Si J and Song Z:
Immune checkpoint inhibitors beyond first-line progression with
prior immunotherapy in patients with advanced non-small cell lung
cancer. J Thorac Dis. 15:1648–1657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge X, Zhang Z, Zhang S, Yuan F, Zhang F,
Yan X, Han X, Ma J, Wang L, Tao H, et al: Immunotherapy beyond
progression in patients with advanced non-small cell lung cancer.
Transl Lung Cancer Res. 9:2391–2400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian T, Yu M, Yu Y, Wang K, Tian P, Luo Z,
Ding Z, Wang Y, Gong Y, Zhu J, et al: Immune checkpoint inhibitor
(ICI)-based treatment beyond progression with prior immunotherapy
in patients with stage IV non-small cell lung cancer: A
retrospective study. Transl Lung Cancer Res. 11:1027–1037. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bourhis M, Palle J, Galy-Fauroux I and
Terme M: Direct and indirect modulation of T cells by VEGF-A
counteracted by anti-angiogenic treatment. Front Immunol.
12:6168372021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kazerounian S and Lawler J: Integration of
pro- and anti-angiogenic signals by endothelial cells. J Cell
Commun Signal. 12:171–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang H and Wang M: Prospect of
immunotherapy combined with anti-angiogenic agents in patients with
advanced non-small cell lung cancer. Cancer Manag Res.
11:7707–7719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reckamp KL, Redman MW, Dragnev KH,
Minichiello K, Villaruz LC, Faller B, Al Baghdadi T, Hines S,
Everhart L, Highleyman L, et al: Phase II randomized study of
ramucirumab and pembrolizumab versus standard of care in advanced
non-small-cell lung cancer previously treated with
immunotherapy-lung-MAP S1800A. J Clin Oncol. 40:2295–2306. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu X, Chu X, Wu Y, Zhou J, Zhao J, Zhou F,
Han C and Su C: Favorable clinical outcomes of checkpoint
inhibitor-based combinations after progression with immunotherapy
in advanced non-small cell lung cancer. Cancer Drug Resist.
4:728–739. 2021.PubMed/NCBI
|
|
31
|
Kitagawa S, Hakozaki T, Kitadai R and
Hosomi Y: Switching administration of anti-PD-1 and anti-PD-L1
antibodies as immune checkpoint inhibitor rechallenge in
individuals with advanced non-small cell lung cancer: Case series
and literature review. Thorac Cancer. 11:1927–1933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai R, Li W, Du N and Cui J: Challenges of
evaluating immunotherapy efficacy in solid tumors. Chin J Cancer
Res. 31:853–861. 2019. View Article : Google Scholar : PubMed/NCBI
|