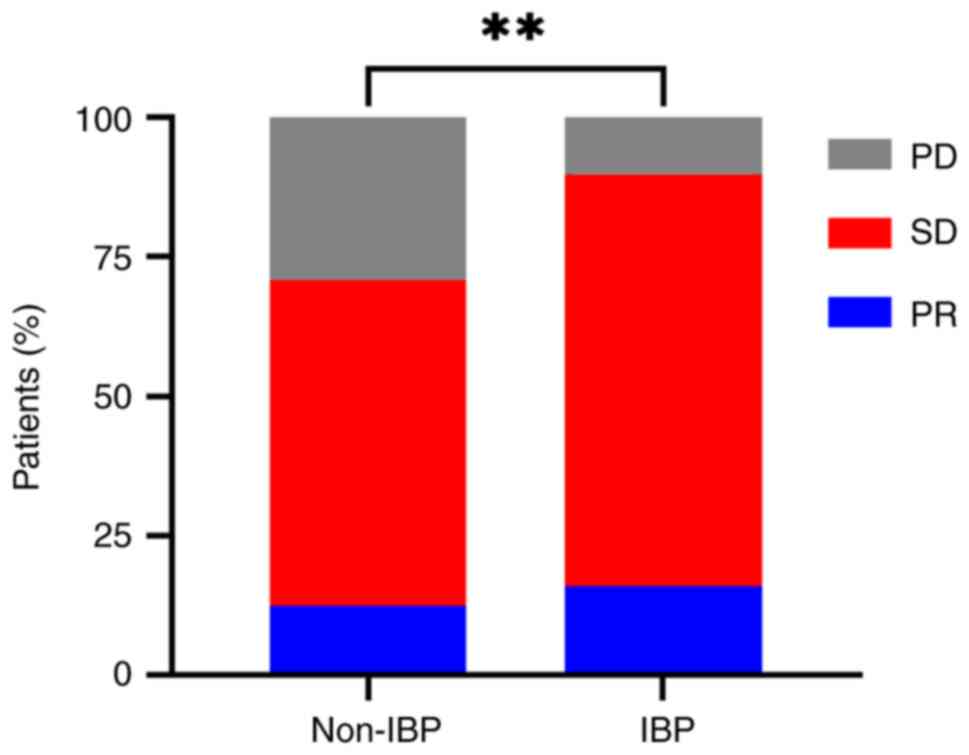
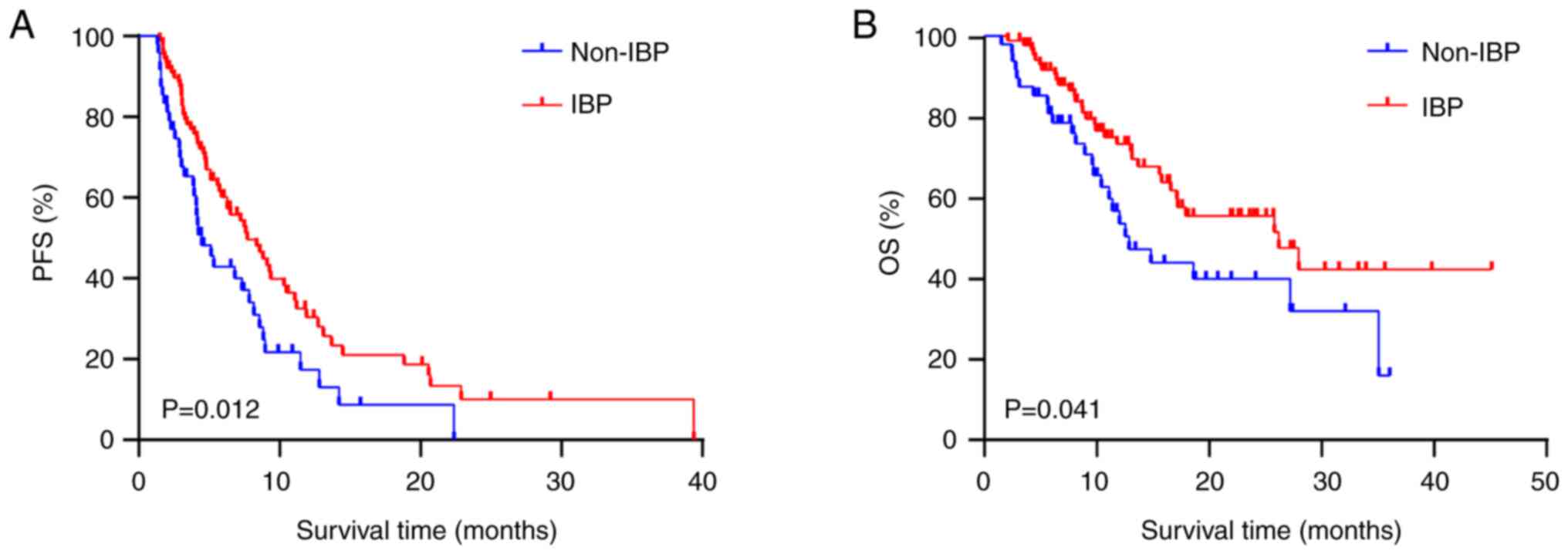
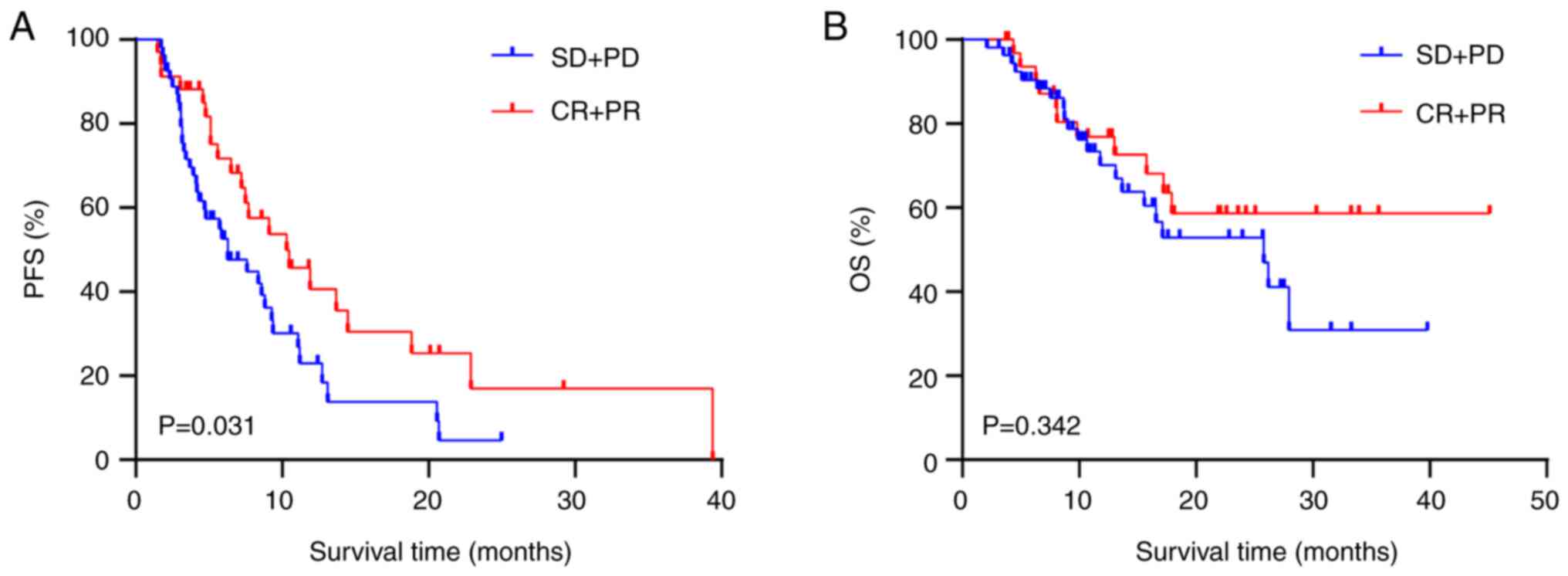
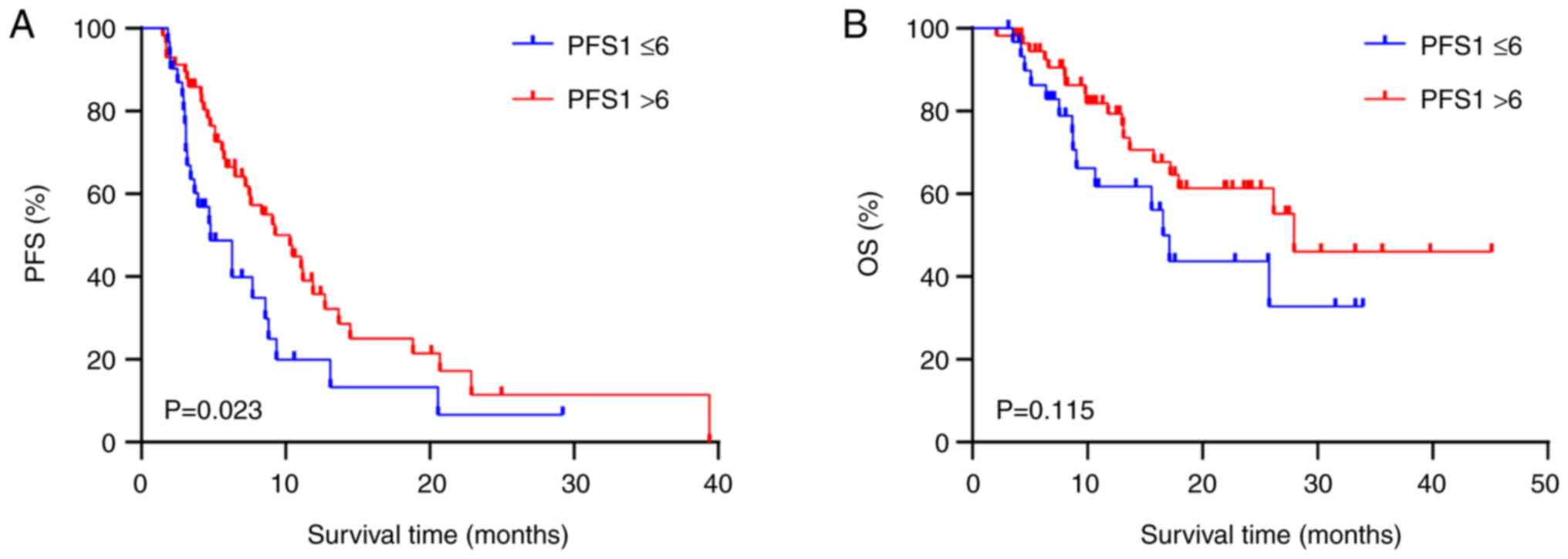
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as first-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R,
Yu Y, Yao W, Chang J, Chen J, et al: Sugemalimab versus placebo, in
combination with platinum-based chemotherapy, as first-line
treatment of metastatic non-small-cell lung cancer (GEMSTONE-302):
Interim and final analyses of a double-blind, randomised, phase 3
clinical trial. Lancet Oncol. 23:220–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Tislelizumab plus chemotherapy
vs chemotherapy alone as first-line treatment for advanced squamous
non-small-cell lung cancer: A phase 3 randomized clinical trial.
JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long GV, Weber JS, Larkin J, Atkinson V,
Grob JJ, Schadendorf D, Dummer R, Robert C, Márquez-Rodas I, McNeil
C, et al: Nivolumab for patients with advanced melanoma treated
beyond progression: Analysis of 2 phase 3 clinical trials. JAMA
Oncol. 3:1511–1519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beaver JA, Hazarika M, Mulkey F, Mushti S,
Chen H, He K, Sridhara R, Goldberg KB, Chuk MK, Chi DC, et al:
Patients with melanoma treated with an anti-PD-1 antibody beyond
RECIST progression: A US food and drug administration pooled
analysis. Lancet Oncol. 19:229–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
George S, Motzer RJ, Hammers HJ, Redman
BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM and Rini
BI: Safety and efficacy of nivolumab in patients with metastatic
renal cell carcinoma treated beyond progression: A subgroup
analysis of a randomized clinical trial. JAMA Oncol. 2:1179–1186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Motzer RJ, Sharma P, Wagstaff
J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski
PG, et al: Treatment beyond progression in patients with advanced
renal cell carcinoma treated with nivolumab in CheckMate 025. Eur
Urol. 72:368–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herbst RS, Garon EB, Kim DW, Cho BC,
Perez-Gracia JL, Han JY, Arvis CD, Majem M, Forster MD, Monnet I,
et al: Long-term outcomes and retreatment among patients with
previously treated, programmed death-ligand 1-positive, advanced
non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol.
38:1580–1590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enomoto T, Tamiya A, Matsumoto K, Adachi
Y, Azuma K, Inagaki Y, Kouno S, Taniguchi Y, Saijo N, Okishio K and
Atagi S: Nivolumab treatment beyond progressive disease in advanced
non-small cell lung cancer. Clin Transl Oncol. 23:582–590. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wankhede D: Evaluation of eighth AJCC TNM
sage for lung cancer NSCLC: A meta-analysis. Ann Surg Oncol.
28:142–147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer E, Therasse P, Bogaerls J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang S, Qin C, Hu H, Liu T, He Y, Guo H,
Yan H, Zhang J, Tang S and Zhou H: Immune checkpoint inhibitors in
non-small cell lung cancer: Progress, challenges, and prospects.
Cells. 11:3202022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borcoman E, Kanjanapan Y, Champiat S, Kato
S, Servois V, Kurzrock R, Goel S, Bedard P and Le Tourneau C: Novel
patterns of response under immunotherapy. Ann Oncol. 30:385–396.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ricciuti B, Genova C, Bassanelli M, De
Giglio A, Brambilla M, Metro G, Baglivo S, Dal Bello MG, Ceribelli
A, Grossi F and Chiari R: Safety and efficacy of nivolumab in
patients with advanced non-small-cell lung cancer treated beyond
progression. Clin Lung Cancer. 20:178–185.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stinchcombe TE, Miksad RA, Gossai A,
Griffith SD and Torres AZ: Real-world outcomes for advanced
non-small cell lung cancer patients treated with a PD-L1 inhibitor
beyond progression. Clin Lung Cancer. 21:389–394.e3. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Metro G, Addeo A, Signorelli D, Gili A,
Economopoulou P, Roila F, Banna G, De Toma A, Rey Cobo J, Camerini
A, et al: Outcomes from salvage chemotherapy or pembrolizumab
beyond progression with or without local ablative therapies for
advanced non-small cell lung cancers with PD-L1 ≥50% who progress
on first-line immunotherapy: Real-world data from a European
cohort. J Thorac Dis. 11:4972–4981. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu M, Hao Y, Zeng X, Si J and Song Z:
Immune checkpoint inhibitors beyond first-line progression with
prior immunotherapy in patients with advanced non-small cell lung
cancer. J Thorac Dis. 15:1648–1657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge X, Zhang Z, Zhang S, Yuan F, Zhang F,
Yan X, Han X, Ma J, Wang L, Tao H, et al: Immunotherapy beyond
progression in patients with advanced non-small cell lung cancer.
Transl Lung Cancer Res. 9:2391–2400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian T, Yu M, Yu Y, Wang K, Tian P, Luo Z,
Ding Z, Wang Y, Gong Y, Zhu J, et al: Immune checkpoint inhibitor
(ICI)-based treatment beyond progression with prior immunotherapy
in patients with stage IV non-small cell lung cancer: A
retrospective study. Transl Lung Cancer Res. 11:1027–1037. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bourhis M, Palle J, Galy-Fauroux I and
Terme M: Direct and indirect modulation of T cells by VEGF-A
counteracted by anti-angiogenic treatment. Front Immunol.
12:6168372021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kazerounian S and Lawler J: Integration of
pro- and anti-angiogenic signals by endothelial cells. J Cell
Commun Signal. 12:171–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang H and Wang M: Prospect of
immunotherapy combined with anti-angiogenic agents in patients with
advanced non-small cell lung cancer. Cancer Manag Res.
11:7707–7719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reckamp KL, Redman MW, Dragnev KH,
Minichiello K, Villaruz LC, Faller B, Al Baghdadi T, Hines S,
Everhart L, Highleyman L, et al: Phase II randomized study of
ramucirumab and pembrolizumab versus standard of care in advanced
non-small-cell lung cancer previously treated with
immunotherapy-lung-MAP S1800A. J Clin Oncol. 40:2295–2306. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu X, Chu X, Wu Y, Zhou J, Zhao J, Zhou F,
Han C and Su C: Favorable clinical outcomes of checkpoint
inhibitor-based combinations after progression with immunotherapy
in advanced non-small cell lung cancer. Cancer Drug Resist.
4:728–739. 2021.PubMed/NCBI
|
|
31
|
Kitagawa S, Hakozaki T, Kitadai R and
Hosomi Y: Switching administration of anti-PD-1 and anti-PD-L1
antibodies as immune checkpoint inhibitor rechallenge in
individuals with advanced non-small cell lung cancer: Case series
and literature review. Thorac Cancer. 11:1927–1933. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai R, Li W, Du N and Cui J: Challenges of
evaluating immunotherapy efficacy in solid tumors. Chin J Cancer
Res. 31:853–861. 2019. View Article : Google Scholar : PubMed/NCBI
|