Introduction
Advanced stage metastatic breast cancer represents
one of the major causes of mortality in women. In 2023 the American
Cancer Society projected the estimated newly diagnosed breast
cancer cases to be at 281,550 with the breast cancer-associated
mortality to be at 43,600 (1).
Clinical triple negative breast cancer (TNBC) is one type of breast
cancer that lacks hormones and growth factor receptor expression
which represents 15–20% of all cases of breast cancer incidence
(2).
The status of receptor expression dictates the
selection of appropriate chemo-endocrine therapy. The treatment
option for TNBC includes the use of anthracycline, platin or
taxane-based cytotoxic chemotherapy. However, these pharmacological
agents frequently exert long-term systemic toxicity and cause
spontaneous or acquired therapy resistance, leading to compromised
therapy response and sub-optimal patient compliance. In addition,
therapy resistance also favors metastatic progression of the
disease due to the emergence of chemo-resistant cancer-initiating
stem cell population (3–5). These aspects of clinical limitations
for conventional chemotherapy emphasize the persisting demand for
the identification of effective nontoxic testable alternatives.
Nutritional herbs constitute the main components of
herbal formulations that are widely used in traditional Chinese
medicine (TCM) for general health and hormonal issues in women. TCM
represents a commonly used therapeutic alternative for
therapy-resistant epithelial organ site cancers. Proven documented
human consumption, low degrees of systemic toxicity and mechanistic
leads for the preclinical efficacy of TCM provides a rationale for
investigating the efficacy of Chinese nutritional herbs used in TCM
as testable alternatives for therapy-resistant breast cancer.
Chinese nutritional herbs have been documented to effectively
target multiple signaling pathways (6,7).
However, the mechanism underlying the preclinical efficacy of
Chinese nutritional herbs is likely to be context-dependent in
cellular models of clinical breast cancer. In hormone receptor
positive Luminal A subtype of breast cancer the growth inhibitory
efficacy of various nutritional herbs such as Cornus
officinalis (CO), Epimedium grandiflorum (EG) and
Lycium barbarum (LB) has been found to be associated with
altered cellular 17β-estradiol metabolism which generates
anti-proliferative metabolites (8–10). By
contrast, in the cellular model for TNBC the anti-proliferative and
pro-apoptotic effects of several nutritional herbs such as
Dipsacus asperoides (DA), CO and Psoaralia
corylifolia (PC) have been found to be associated with
inhibition of the retinoblastoma (RB), RAS, PI3K and AKT signaling
pathways. These pathways are commonly associated with proliferative
cancer cells, and provide growth advantage to primary tumor and to
therapy-resistant cancer stem cells. Additionally, the
pro-apoptotic effects of nutritional herbs have been reported to be
associated with downregulation of anti-apoptotic BCL-2,
upregulation of pro-apoptotic BAX and caspase-regulated apoptotic
signaling pathways (11–14).
Accumulating evidence on effects of nutritional
herbs emphasizes the importance of research into identifying the
common and unique mechanisms of actions mediated by efficacious
nutritional herbs and potential molecular targets. Such an
experimental approach utilizing cellular models of various breast
cancer subtypes can provide evidence for its applicability for
future research directions focused on identifying mechanistic leads
for growth inhibitory efficacy of bioactive agents present in
natural products such as dietary phytochemicals and nutritional
herbs used in TCM. Such research directions may also prioritize
efficacious bioactive agents as potential drug candidates.
The nutritional herbs used in TCM, including
Drynaria fortunei (DF), function as potent anti-oxidative,
anti-angiogenic and immuno-modulatory agents, predominantly through
modulation of several cell signaling pathways (6,7). DF is
a common constituent used in herbal formulations in TCM.
Multi-targeted efficacy of DF, leading to negative growth
regulation, may benefit cancer growth inhibition. However,
anti-cancer mechanisms for the growth inhibitory efficacy of DF on
breast cancer have not been adequately documented. The
multi-functional properties of DF and lack of sufficient evidence
for the effects of DF against breast cancer provide a rationale for
the present study.
The present study is focused on examining the
effects of DF on a cellular model for TNBC to identify mechanistic
pathways and potential molecular targets that may be responsible
for its efficacy.
Materials and methods
Experimental model
Cellular models for clinical TNBC represent valuable
experimental systems for mechanistic investigations that are
focused on assessing the cancer growth inhibitory efficacy of
pharmacological agents. The MDA-MB-231 cell line was originally
isolated from a pleural effusion of a patient with metastatic
breast carcinoma. These carcinoma cells lack the expressions of
estrogen receptor-α (ER-α), progesterone receptor (PR) and
amplified human epidermal growth factor receptor-2 (HER-2),
representing an experimental model for TNBC (15,16).
This cell line was obtained from American Type Culture Collection
(ATCC). The cells were maintained in RPMI culture medium with
L-glutamine and 5% fetal bovine serum (Life Technologies),
following the protocol recommended by the supplier.
Test agent: Herbal formulations in TCM are commonly
prepared as aqueous decoctions in boiling water. These decoctions
are recommended to be consumed by the patients. To simulate patient
consumption, non-fractionated aqueous extract of DF was prepared
according to optimized protocol used in previous publications
(11,12,14).
Briefly, this protocol involves preparation of an aqueous extract
of DF by boiling the herb in deionized water and concentrating the
500 × g supernatant by sequential centrifugations. The stock
solution of the extract was reconstituted in the RPMI culture
medium to obtain a concentration of 1 mg extract/1 ml. For the
experiments stock solution was diluted in the RPMI culture medium
to obtain the concentration range of µg/ml.
Dose response of Drynaria fortunei
(DF)
To determine the effective concentration range, dose
response of DF was determined by measuring cell viability using
Cell Titre Glo assay (Promega Corporation) in accordance with the
protocol provided by the manufacturer. Cell viability was
determined in cells treated with DF at the concentrations of 200,
400, 600, 800 and 1,000 µg/ml at day 7 after seeding, using
Fluoroskan plate reader (Thermo Fisher Scientific Inc.). Cells
maintained in the culture medium without any treatment represented
the control. The data were expressed as the relative luminescent
unit (RLU) and as % inhibition relative to untreated controls.
Anchorage independent (AI) growth
assay
The AI growth formation represents a well-documented
specific and sensitive in vitro surrogate end-point marker
for in vivo tumor formation. This assay was performed
following the optimized protocol (11,12,14).
MDA-MB-231 cell suspension, at a density of 5×105 cells
per ml was prepared in 0.33% agar and treated with DF at the
concentrations of 100, 200, 500, 1,000 and 5,000 µg/ml. Cells
suspended in 0.33% agar without any treatment represented the
control. Non-adherent colonies formed in 0.33% agar at day 21 after
seeding were then counted at 10X magnification. The data were
expressed as AI colony numbers.
Cell cycle progression
Monitoring the cell population at distinct phases of
the cell cycle provides a quantitative measure of cell cycle
progression. The cells were treated with 300, 400 and 800 µg/ml of
DF. Cells maintained in the culture medium without any treatment
represented control Cell cycle analysis was performed according to
the optimized and published protocol (11,12,14).
DNA content was determined using a Becton Dickinson FACSCAN Flow
Cytometer (BD Biosciences) and analyzed using FACS Express software
version 306 (De Novo Software). Distribution of individual cell
population in the G1 (quiescent), S and G2
(proliferative) phases of the cell cycle was determined.
Western blot analysis
Quantitation of cellular proteins by Western blot
analysis represents a commonly used assay. The Western blot assay
was performed according to the optimized and published protocol
(11,12,14).
The cells were treated with 300 and 800 µg/ml of DF. Cells without
any treatment represented control. The cells were harvested and
lysed in radio-immuno precipitation assay (RIPA) buffer containing
protease inhibitors (Sigma-Aldrich), and were centrifuged at 10,000
× g for 15 mins at 4°C. The protein content of the lysates was
determined by the Bradford method and equal quantity of cellular
proteins were separated on 10% sodium dodecyl
sulfate-polyacrylamide gels (SDS-PAGE mini gels (Mini-PROTEAN TGX,
Bio-Rad Laboratories). The gels were directly incubated with
relevant primary and secondary antibodies (Table I). The chemo-luminescent signal was
developed with ECL-plus reagent (Bio-Rad Laboratories), and
detected by autoradiography. The signal intensity of proteins was
quantified using molecular Image GS800 and Quantity One software
(Bio-Rad Laboratories), and was presented as arbitrary scanning
units (ASU).
 | Table I.Antibodies used for western
blotting. |
Table I.
Antibodies used for western
blotting.
| Antibody | Dilution | Cat. no. | Vendor |
|---|
| Primary
antibody |
|
|
|
| Cyclin
E | 1:200 | SC 48420 | SCB |
|
CDK2 | 1:200 | SC 6248 | SCB |
|
E2F1 | 1:100 | SC 137059 | SCB |
| pRB
(Ser 780) | 1:100 | 3590 | CST |
| RB | 1:100 | SC 74562 | SCB |
|
PARP-1 | 1:200 | SC 8007 | SCB |
| Cleaved
PARP-1 | 1:200 | SC 56196 | SCB |
| β-actin
C4 | 1:200 | SC 47778 | SCB |
| Anti-rabbit
secondary antibody |
|
|
|
|
IgG-HRP | 1:1,000 | SC 2337 | SCB |
Caspase assay
This assay represents a quantitative end-point for
the mitochondrial associated apoptotic pathway. Caspase-3/7
activity was measured using Caspase-Glo assay kit (Promega
Corporation), according to the optimized and published protocol
(11,12,14).
Cellular homogenate prepared from the cells treated with DF at 300,
400, 600 and 800 µg/ml, along with that prepared from cells without
any treatment was used to measure the caspase 3/7 activity. The
luminescence was measured using Luminometer (Thermo Fisher
Scientific In.). The data were expressed as relative luminescent
unit (RLU).
Statistical analysis
The experiments for dose response, caspase activity
and pan-caspase inhibitor were conducted in triplicate and data
were presented as mean ± SD. The experiments for AI colony
formation cell cycle progression and RB signaling were conducted in
duplicate and the data were presented as arithmetic means.
Comparison of statistically significant differences among the
common control and multiple treatment groups were analyzed using
analysis of variance (ANOVA) and Dunnett's Multiple Comparison Test
as a post-hoc test using a threshold of α=0.05 using the Microsoft
Excel 2013 XLSTAT-Base software. P<0.05 was considered to
indicate a statistically significant difference.
Results
Dose response of DF
This experiment was conducted to examine the range
of growth inhibitory effects of DF. Treatment of MDA-MB-231 cells
with DF resulted in a concentration dependent reduction in cell
viability. Specifically, DF concentrations of 200, 400, 600, 800
and 1,000 µg/ml resulted in a 6.7, 20.0, 60.0, 80.0 and 93.3%
reduction in cell viability, relative to the untreated control
(Table II). This dose response
experiment identified 625 µg/ml as the 50% proliferation inhibitory
concentration of DF.
 | Table II.Dose response of Drynaria
fortunei. |
Table II.
Dose response of Drynaria
fortunei.
| Treatment | Concentration
(µg/ml) | Relative
luminescent unit | Inhibition (%
control) |
|---|
| Control | - | 1.5±0.1 | - |
| DF | 200 | 1.4±0.3 | 6.7 |
|
| 400 | 1.2±0.1 | 20.0 |
|
| 600 | 0.6±0.3 | 60.0 |
|
| 800 | 0.3±0.2 | 80.0 |
|
| 1,000 | 0.1±0.05 | 93.3 |
Effect of DF on AI colony
formation
This experiment was conducted to examine the effect
of DF on anchorage independent (AI) colony formation. This in
vitro end-point biomarker provides the data on the
concentration dependent changes in the AI colony formation in
response to treatment with DF (Table
III). In response to DF concentration of 100, 200, 500, 1,000
and 5,000 µg/ml, the AI colony number was found to decrease with
inhibition rates of 12.5, 25.3, 37.6, 50.5 and 96.1%, relative to
the untreated control. This dose response experiment on AI colony
formation identified 1,000 µg/ml as the 50% inhibitory
concentration of DF.
 | Table III.Effect of DF in anchorage independent
colony formation. |
Table III.
Effect of DF in anchorage independent
colony formation.
| Treatment | Concentration
(µg/ml) | AI colony
number | Inhibition (%
control) |
|---|
| Control | - | 649 | - |
| DF | 100 | 568 | 12.5 |
|
| 200 | 485 | 25.3 |
|
| 500 | 406 | 37.6 |
|
| 1,000 | 321 | 50.5 |
|
| 5,000 | 25 | 96.1 |
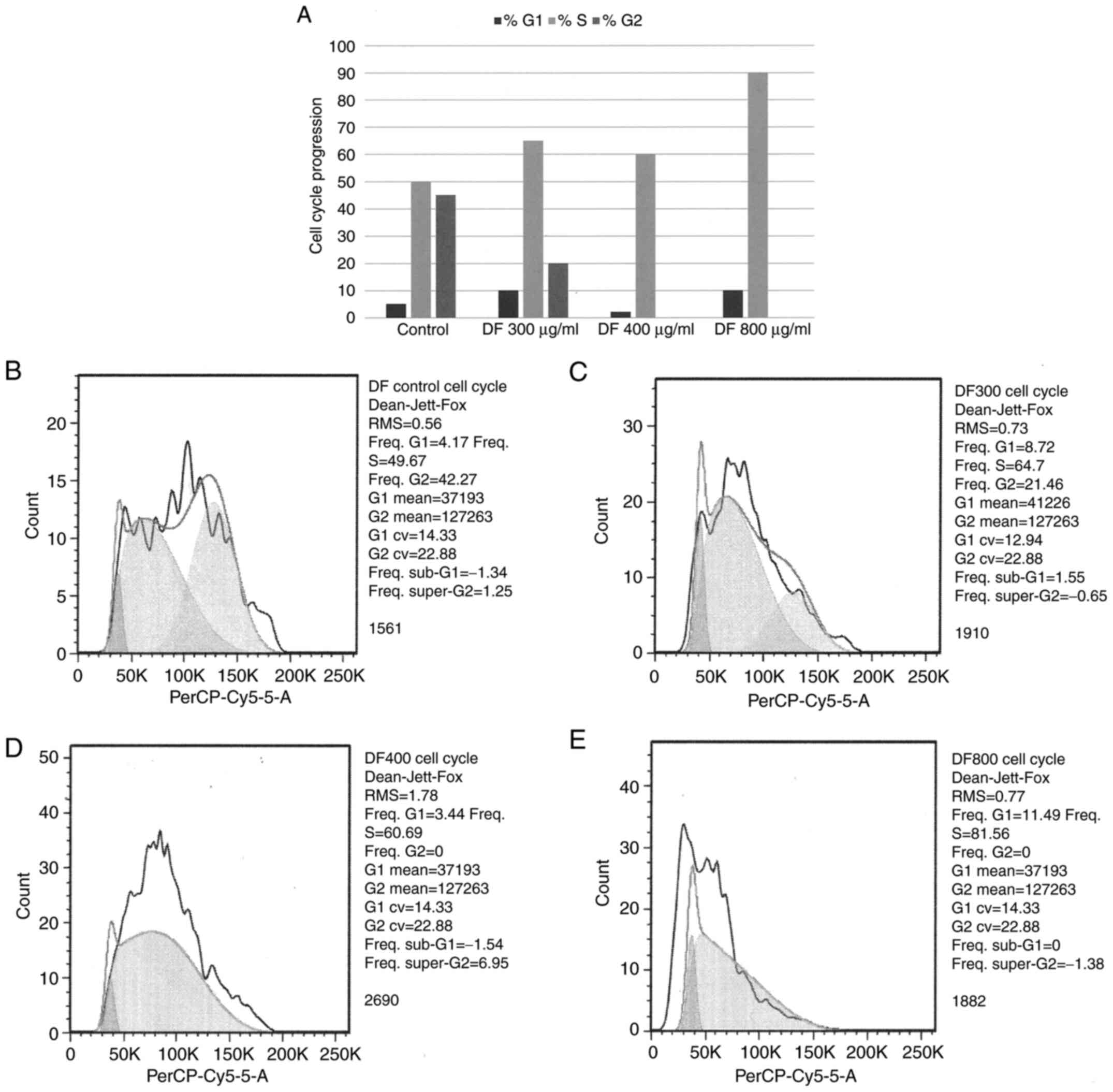
Effect of DF on cell cycle
progression
This experiment was conducted to examine the effect
of DF on the proportion of cells in individual phases of the cell
cycle and therefore, on cell cycle progression. The data presented
showed that treatment with DF resulted in the S-phase arrest of
cells. Additionally, the higher concentrations DF was found to
abrogate the G2-phase of the cell cycle (Fig. 1A). Representative DNA histograms
document the effects of untreated control and of 300, 400 and 800
µg/ml of DF on cell cycle progression. Consistent with the data
presented in Fig. 1A, treatment
with DF resulted in a concentration-dependent S-phase increase of
60.7 and 81.6%, relative to untreated control. In addition, higher
concentration of DF abrogated G2 phase of the cell cycle (Fig. 1B-E).
Effect of DF on RB signaling
The RB signaling pathway serves an essential role in
transition of cells in S and G2 phases of the cell cycle wherein
expression status of cyclin E, CDK2, phosphorylated RB (pRB) and
E2F1is critical. This experiment was therefore conducted to examine
the effect of DF on RB signaling activity. Treatment with DF was
found to suppress the expressions of cyclin E, CDK2, pRB and E2F1
levels in a dose-dependent manner (Fig.
2A). The data were also presented as total protein: β-actin
ratio which demonstrated that in response to treatment with DF at
its maximally effective concentration of 800 µg/ml, the expressions
of cyclin E, CDK2 and E2F1 were inhibited by 35.8, 38.7 and 38.5%
(P=0.04) respectively, relative to untreated control (Fig. 2B). The data expressed as pRB: RB
ratio demonstrated that DF at its maximally effective concentration
of 800 µg/ml inhibited this ratio by 74.7% (P=0.01), relative to
the untreated control due to a reduction in pRB levels (Fig. 2C).
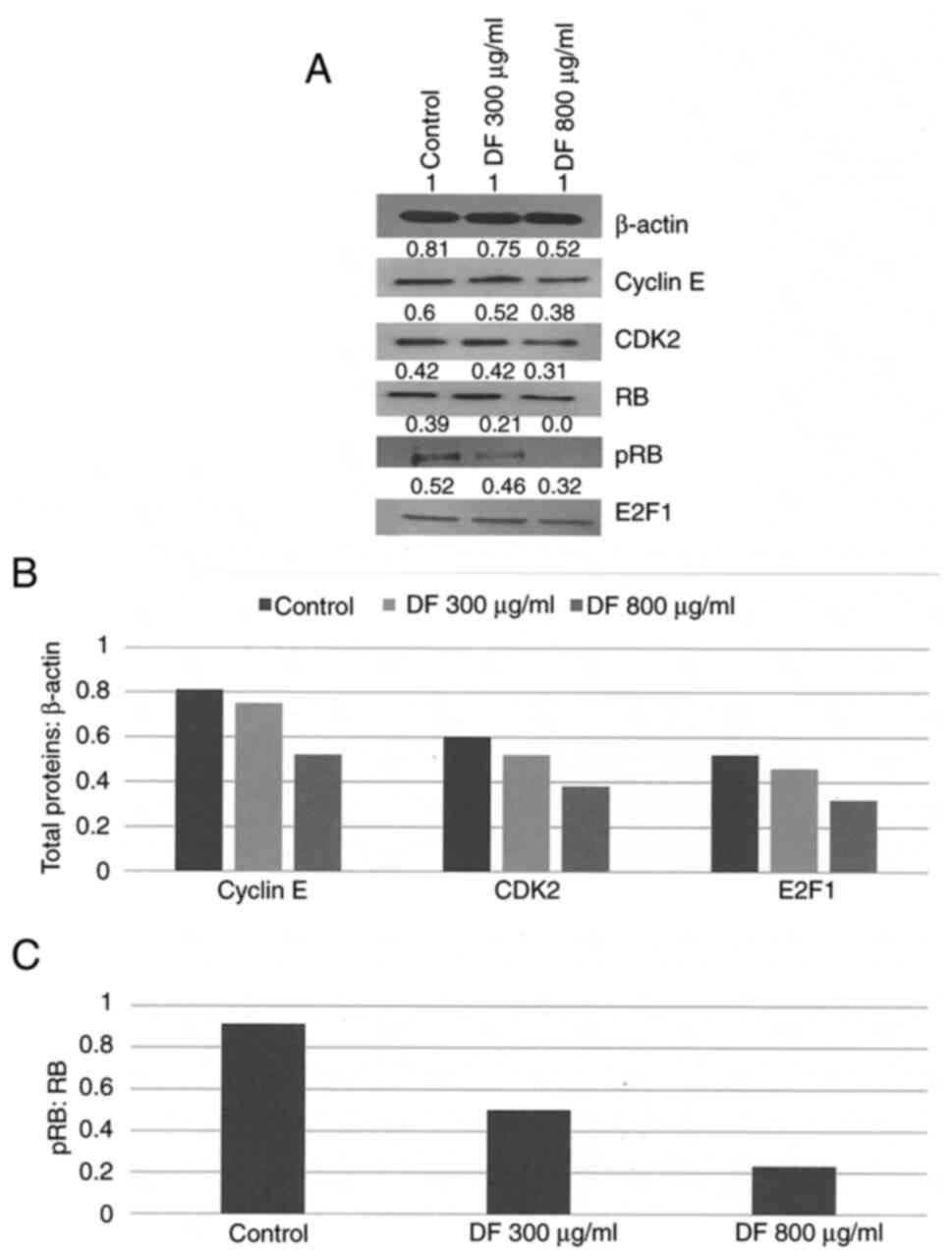 | Figure 2.(A) Effects of DF on RB signaling.
Treatment with DF resulted in a concentration dependent inhibition
of RB signaling proteins. Data are obtained from two independent
experiments. Representative western blotting is presented. Internal
control is represented by β-actin protein. Signal intensity of
proteins are quantitated from densitometric scans and presented as
arbitrary scanning unit. Protein expression normalized from (B)
β-actin or (C) total RB. (B) Data presented as arithmetic means
from two independent experiments. Total proteins:β-actin ratio for
cyclin E, CDK2 and E2F1. Respective control groups vs. 800 µg/ml DF
for Cyclin E, CDK2 and E2F1, all P=0.04. (C) pRB:RB ratio, data
presented as arithmetic means from two independent experiments.
Control vs. 800 µg/ml DF group, P=0.01. CDK2, cyclin dependent
kinase 2; E2F1, member of the E2F family of transcription factors;
pRB, phosphorylated retinoblastoma protein, RB, retinoblastoma
protein; DF, Drynaria fortunei. |
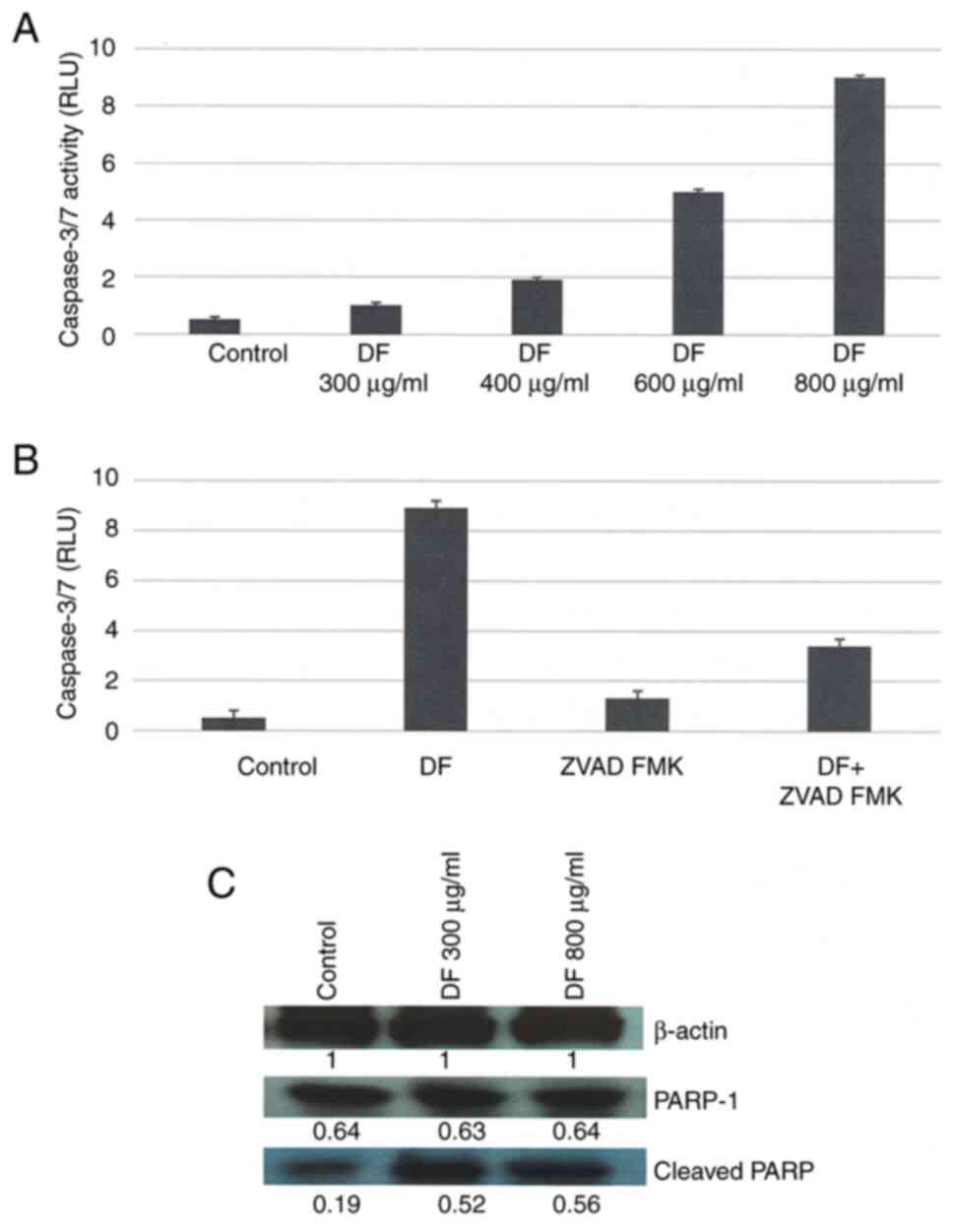
Effect of DF on cellular
apoptosis
Caspase 3/7 activity represents a specific and
sensitive marker for cellular apoptosis. The data presented in
Fig. 3A demonstrated that the
treatment with DF resulted in a concentration-dependent 2X, 4X, 10X
and 17X increase in caspase 3/7 activity, relative to the untreated
control. The experiment conducted to examine the effect of the pan
caspase inhibitor Z-VAD-FMK demonstrated that treatment with DF at
a concentration of 800 µg/ml induced the caspase 3/7 activity
by16.8 X (P=0.01), relative to control. Treatment with DF + 10 µM Z
VAD FMK reduced the caspase 3/7 activity by 61.8% (P=0.04),
relative to DF treated cells (Fig.
3B). The experiment conducted to examine the effect of DF on
the expression of cleaved poly(ADP-ribose) polymerase-1 (PARP-1)
demonstrated that treatment with 800 µg/ml DF increased the
expression of cleaved PARP by 1.9X (P=0.01), relative to control
(Fig. 3C).
Discussion
TNBC is considered to be an aggressive subtype of
breast cancer, with an incidence of 15–20% and disparity towards
the African American ethnic population (2). TNBC lacks ER-α, PR and HER-2
expression which is noted to readily acquire resistance to a range
of long-term conventional cytotoxic chemotherapy such as
anthracyclines (including doxorubicin), platins (such as
carboplatin) and taxols (such as paclitaxel). Treatment options
using cytotoxic conventional chemotherapeutic are based on
documented preclinical efficacy of these agents in TNBC models.
TNBC has also been known to harbor a therapy-resistant
cancer-initiating stem cell population, which proliferates
following chemotherapy (5). The
TNBC subtype is notable for tumor heterogeneity predominantly due
to extensive cellular plasticity and phenotypic diversity. Amongst
the multiple subtypes of TNBC the M TNBC subtype has documented the
presence of putative chemo-resistant cancer-initiating stem cells.
The MDA-MB-231 cell line model for TNBC belongs to the M TNBC
subtype (17). Established cellular
models for the M TNBC subtypes include cell lines derived from
clinical breast carcinoma such as MDA-MB-157, BT 549, SUM 159PT and
MDA-MB-231. These cell lines have been documented to exhibit
hyper-proliferation in vitro and tumorigenesis in
vivo, and thereby, provide valuable experimental approaches for
cell line based and tumor transplant-based investigations on growth
inhibitory efficacy of nutritional herbs. Published evidence on the
MDA-MB-231 model has been documented to exhibit growth inhibitory
efficacy of several mechanistically distinct nutritional herbs used
in TCM (11–14).
Experiments in the present study were designed based
on the MDA-MB-231 model for clinical TNBC to examine effects of DF,
a nutritional herb that represents a common component of herbal
formulations used in TCM.
Inhibitory effects of DF on triple negative
MDA-MB-231 cells were found in the present study, as evidenced by
the effective reduction of cell viability in adherent culture and
reduction in the number of AI colonies formed in a non-adherent
culture. AI colony formation represents an in vitro marker
for in vivo tumor formation. Collectively, these data
provide evidence of the susceptibility for growth inhibition
mediated by DF in the present experimental model and for the
effective reduction in cancer risk. The present preclinical
evidence provides a proof of concept for possible benefit of DF for
prevention of clinical breast cancer.
The RB signaling represents one of the major tumor
suppressor pathways responsible for the regulation of cellular
proliferation, differentiation and apoptosis. The tumor suppressive
function of RB has been documented to be compromised in cases of
clinical TNBC and is associated with accelerated cell cycle
progression due to the inactivation of negative growth regulatory
proteins and the inhibition of cellular apoptosis (18). RB signaling targets G1 - S phase
transition through the cyclin D1-CDK 4/6- pRB-E2F axis where p16
INK4 functions as a major CDK inhibitor. Additionally,
RB signaling targets the S and/or G2/M phases of the cell cycle
through the cyclin E-CDK2- pRB-E2F axis where p21
CIP1/waf1 and p27 KIP1 function as the major
CDK inhibitors. In the RB signaling cascade expression of E2F
family of transcription factors represents a critical event
preceding expression of RB target genes (19,20).
In the present study DF at the concentration of 800 µg/ml
represented the maximally effective dose that could inhibit the
expression of cyclin E, CDK2 and E2F1 and RB phosphorylation.
The growth inhibitory efficacy of nutritional herbs
CO, DA, PC in the present TNBC model has been documented to
associate with inhibition of RB signaling through the cyclin
D1-CDK4/6-pRB axis (11,12). Additionally, treatment with DA also
resulted in inhibition of RAS, PI3K and AKT signaling which have
also been reported to be constitutively activated in
hyper-proliferative cancer cells (14). By contrast, growth inhibition by DF
in the present study was associated with the inhibition of Cyclin
E, CDK2, E2F1 expression and RB phosphorylation. Therefore,
individual nutritional herbs may affect the expression of distinct
proteins involved in the RB signaling pathway. Collectively the
data on nutritional herbs suggest that distinct mechanisms of
action and distinct molecular targets may be responsible for their
respective inhibitory efficacy.
It is conceivable that constitutive bioactive agents
present in nutritional herbs may target several signaling pathways
for effective growth inhibition (7). The effects of nutritional herbs on
cellular growth appear to be context dependent. Extract prepared
from the rhizomes of DF containing flavonoids has been documented
to exert proliferation promoting effects in estrogen responsive
breast cancer cells and in osteoblastic cells through the
osteopontin-related pathways possibly regulated by the functional
estrogen receptor (21,22). DF has also been reported to promote
proliferation of human umbilical vascular endothelial cells through
VEGF and MMP related pathways to induce angiogenesis which was
demonstrated through the chicken chorio-allentoic membrane assay
(23). Network pharmacology and
transcriptomic analysis for the effects of DF has demonstrated the
targeting efficacy of multiple relevant genes such as TP53, AKT1,
immune function related STAT1, STAT 3, IL6 and IL10, and estrogen
receptor genes ESR1 and ESR2 coding for ER-α and ER-β, respectively
(23–25). Proliferative inhibitory effects of
DF found in the present ER negative TNBC model suggest that the
distinct proliferation-modulating effects of DF may be related to
ER function.
The pro-apoptotic effects of nutritional herbs CO.
DA, PC are evidenced by increases in the Sub G0 (apoptotic) phase
of the cell cycle, inhibited expression of anti-apoptotic BCL-2
protein, upregulated expression of pro-apoptotic BAX protein and
increased caspase 3/7 activity (11–14).
In the present study treatment with the maximally effective dose of
DF at 800 µg/ml is associated with an increase in caspase 3/7
activity and its reduction by the pan-caspase inhibitor Z-VAD-FMK.
Apoptotic cells are known to exhibit cleavage of the full-length
PARP-1 protein to the truncated PARP-1. Treatment with DF resulted
in increased PARP-1 cleavage. Therefore, the pro-apoptotic effects
of DF are highly likely to be supported by modulation of additional
apoptosis-related markers.
Collectively, the data on the effects of DF on RB
signaling, induction of caspase 3/7 activity, its inhibition by the
pan caspase inhibitor Z-VAD-FMK and increased cleavage of PARP-1
presented in the present study substantiated the mechanistic leads
for the growth inhibitory efficacy of DF. Furthermore, the present
experimental approach facilitates future research on cellular
models for M TNBC using in vitro experiments on cell lines
and in vivo tumor transplant experiments focused on
identifying additional nutritional herbs and putative molecular
targets for their growth inhibitory efficacy.
In conclusion, investigations into the growth
inhibitory efficacy of nutritional herbs on the various models of
TNBC including the present model for TNBC has identified
mechanistic pathways mediating anti-proliferative and pro-apoptotic
processes (11–14). Therefore, it is conceivable that
multiple bioactive agents in the non-fractionated aqueous extracts
may be effective in an interactive manner, affecting cancer cell
survival specific PI3K, AKT and m TOR pathways, and through their
effects on MAPK, MEK, ERK signaling pathways (6,7).
The experimental approach using the present TNBC
model provided several scientifically robust rationales for future
investigations. Major bioactive agents present in DF include
flavones, anthocyanins, terpenes, polyphenolic acids and lignans
(22,24). These agents may be responsible for
growth inhibition, either individually or in combination. Reliable
cancer stem cell models may represent valuable experimental
approaches for examining cancer stem cell-targeting efficacy of
bioactive agents present in natural products (26,27).
Cancer stem cell specific telomerase activity (28), epigenetic modulation (29), and stem cell plasticity through
epithelial-mesenchymal transition (30) represent valuable therapeutic
targets. Pharmacological agents or natural products that target
these processes may also represent potential novel drug candidates
(31–33).
Acknowledgements
Not applicable.
Funding
Support for the present research was provided by the
philanthropic contribution to the American Foundation for Chinese
Medicine from The Sophie Stenbeck Family Foundation.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contribution
NTT designed the experimental protocols, analyzed
and interpreted the primary data, and prepared the manuscript. HBN
conducted the experiments and participated in the preparation of
the manuscript. GYCW initiated research projects on preventive
efficacy of Chinese nutritional herbs on breast cancer. The efforts
of GYCW on translating clinical aspects of herbal medicine into
mechanism-based evidence for preclinical efficacy has provided a
scientifically robust rationale for current research directions.
GYCW also recommended the nutritional herb for the present study,
analyzed and interpreted the data and participated in the
preparation of the manuscript. NTT and HBN confirm the authenticity
of all the raw data. All the authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Nitin T. Telang, ORCID ID:
0000-0002-9059-8995.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdfJune
7–2024
|
|
2
|
Lin NU, Vanderplas A, Hughes ME, Theriault
RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP and Weeks JC:
Clinicopathologic features, patterns of recurrence, and survival
among women with triple-negative breast cancer in the National
Comprehensive Cancer Network. Cancer. 118:5463–5472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Velloso FJ, Bianco FJR, Farias JO, Torres
NE, Ferruzo PY, Anschau V, Jessus-Farreira HC, Chng TH, Sogayar MC,
Zerbini LF and Correa RG: The crossroads of breast cancer
progression: Insights into the modulation of major signaling
pathways. Onco Targets Ther. 10:5491–5524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Won KI and Spruck C: Triple-negative
breast cancer therapy: Current and future perspectives (Review).
Int J Oncol. 57:1245–1261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gradishar WJ, Moran MS and Abraham J: NCCN
Clinical Practice Guidelines in Oncology: Breast Cancer Version 4.
2022.Available from:. www.nccn.org
|
|
6
|
Ye L, Jia Y, Ji KE, Saunders AJ, Xue K, Ji
J, Mason MD and Jiang WG: Traditional Chinese medicine in
prevention and treatment of breast cancer and metastasis. Oncol
Lett. 10:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Z, Zhang Q, Yu L, Zhu J, Cao Y and
Gao X: The signaling pathways and targets of traditional Chinese
medicine and natural medicine in triple-negative breast cancer. J
Ethnopharmacol. 264:1132492021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Telang NT, Li G, Sepkovic DW, Bradlow HL
and Wong GYC: Anti-proliferative effects of Chinese herb Cornus
officinalis in a cell culture model for estrogen receptor positive
clinical breast cancer. Mol Med Rep. 5:22–28. 2012.PubMed/NCBI
|
|
9
|
Telang N, Li G, Katdare M, Sepkovic D,
Bradlow L and Wong G: Inhibitory effects of Chinese nutritional
herbs in isogenic breast carcinoma cells with modulated estrogen
receptor function. Oncol Lett. 12:3949–3957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Telang NT, Li G, Katdare M, Sepkovic DW,
Bradlow HL and Wong GYC: The nutritional herb Epimedium
grandiflorum inhibits the growth in a model for the Luminal A
molecular subtype of breast cancer. Oncol Lett. 13:2477–2482. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Telang NT, Nair HB and Wong GYC: Growth
inhibitory efficacy of Cornus officinalis in a cell culture model
for triple-negative breast cancer. Oncol Lett. 17:5261–5266.
2019.PubMed/NCBI
|
|
12
|
Telang NT, Nair HB and Wong GYC: Growth
inhibitory efficacy of the nutritional herb Psoralia corylifolia in
a model of triple-negative breast cancer. Int J Funct Nutr.
2:82021. View Article : Google Scholar
|
|
13
|
Telang NT, Nair HB and Wong GYC: Growth
inhibitory efficacy of Chinese herbs in a cellular model for
triple-negative breast cancer. Pharmaceuticals (Basel).
14:13182021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Telang N, Nair HB and Wong GYC:
Anti-proliferative and pro-apoptotic effects of Dipsacus asperoides
in a cellular model for triple-negative breast cancer. Arch Breast
Cancer. 9:66–75. 2022. View Article : Google Scholar
|
|
15
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark I, Bayani N, Cope JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subik K, Lee JF, Baxret I, Strzepak T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG and
Tang P: The expression pattwerns of ER, PR, HER-2, CK 5/6, EGFR, Ki
67 and AR by immunohistochemical analysis in breast cancer cell
lines. Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
17
|
Lehmann BD, Colaprico A, Silva TC, Chen J,
An H, Ban Y, Huang H, Wang L, James JL, Balko JM, et al:
Multi-omics analysis identifies therapeutic vulnerabilities in
triple-negative breast cancer subtypes. Nat Commun. 12:62762021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burkhart DL and Sage J: Cellular
mechanisms of tumor suppression by the retinoblastoma gene. Nat Rev
Cancer. 8:671–682. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HZ, Tsai SY and Leone G: Emerging
roles of E2Fs in cancer: An exit from cell cycle control. Nat Rev
Cancer. 9:785–797. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang EJ, Lee WJ, Cho SH and Choi SW:
Proliferative effects of flavan-3-olsand propelargonidins from
rhizomes of Drynaria fortunei on MCF-7 and osteoblastic cells. Arch
Pharmacol Res. 26:620–630. 2003. View Article : Google Scholar
|
|
22
|
Wong KC, Pang WY, Wang XL, Mok SK, Lai WP,
Chow HK, Leung PC, Yao SX and Wong MS: Drynaria fortunei-derived
total flavonoid fraction and isolated compounds exert
oestrogen-like protective effects in bone. Br J Nutr. 110:475–485.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang ST, Chang CC, Pang JH, Huang HS,
Chou SC, Kao MC and You HL: Drynaria fortunei promoted angiogenesis
associated with modified MMP-2/TMP-2 balance and activation of VEGF
ligand/receptors expression. Front Pharmacol. 9:9792018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiao X, Lin XH, Liang YH, Dong J, Guo DA
and Ye M: Comprehensive chemical analysis of the rhizomes of
Drynaria fortune by orthogonal pre-separation and liquid
chromatography mass spectrometry. Planta Med. 80:330–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie L, Zhao S, Zhang X, Huang W, Qiao L,
Zhan D, Ma C, Gong W, Dang H and Lu H: Wenshengyang recipe treats
infertility through hormonal regulation and inflammatory responses
revealed by transcriptome analysis and network pharmacology. Front
Pharmacol. 13:9175442022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Telang NT: Stem cell models for breast and
colon cancer: Experimental approach for drug discovery. Int J Mol
Sci. 23:92232022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Telang NT: Natural products as drug
candidates for breast cancer (Review). Oncol Lett. 26:3492023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hannen R and Bartsch JW: Essential roles
of telomerase reverse transcriptase hTERT in cancer stemness and
metastasis. FEBS Lett. 592:2023–2031. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar VE, Nambiar R, De Souza C, Nguyen A,
Chien J and Lam KS: Targeting epigenetic modifiers of tumor
plasticity and cancer stem cell behavior. Cells. 11:14032022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gooding AJ and Scheiman WP:
Epithelial-mesenchymal transition programs and cancer stem cell
phenotypes Mediators of breast cancer therapy resistance. Mol
Cancer Res. 18:1257–1270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganeshan K and Xu B: Telomerase inhibitors
from natural products and their anticancer potential. Int J Mol
Sci. 19:132017. View Article : Google Scholar
|
|
32
|
Liskova A, Kubatka P, Samec M, Zubor P,
Mlyneck M, Bielik T, Samuel SM, Zulli A, Kwon TK and Busselberg D:
Dietary phytochemicals targeting cancer stem cells. Molecules.
24:8992019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin W: Role of JAK/STAT 3 signaling in the
regulation of metastasis, the transition of cancer stem cells and
chemo-resistance of cancer by epithelial-mesenchymal transition.
Cells. 9:2172020. View Article : Google Scholar : PubMed/NCBI
|