Introduction
Thyroid cancer is the most common carcinoma of the
endocrine glands and accounts for ~1% of all malignancies (1). Papillary thyroid carcinoma (PTC)
represents the most frequently identified histological type (80%)
(1,2). Due to the improvement of modern
imaging methods, the incidence of PTC has increased in the past
15–20 years (predominantly in women). Risk factors for the
development of PTC include ionising radiation, Hashimoto's
thyroiditis or familial adenomatous polyposis. Familial occurrence
is reported to be 4.5%, with a prognosis that is similar to that of
sporadic cases. The tumour may also arise around the thyroglossal
duct or in the ectopic tissue of the thyroid gland. The first
clinical manifestation of PTC is usually a palpable mass in the
thyroid area or cervical lymphadenopathy in cases of metastatic
disease (3). Histopathological
diagnosis is based on the assessment of tumour morphology, nuclear
features of tumour cells (nuclear enlargement and overlapping,
chromatin characteristics, nuclear groove and pseudoinclusion), and
an appropriate immunohistochemical profile, including the markers
of thyroid follicular cells [e.g. thyroid transcription factor 1
(TTF1), paired box gene 8 (PAX-8) and thyroglobulin].
Morphologically, up to 15 variants of PTC (classic, follicular,
cribriform morular, oncocytic, clear cell, spindle cell, tall cell
variants, diffuse sclerosing, columnar, Warthin-like, solid,
hobnail, encapsulated, infiltrative or microinvasive variant) have
been described, with specific prognostic and predictive markers.
The most common histological types include classic PTC,
microcarcinoma and follicular variants of PTC. Mutations of the
BRAFV600E gene are most often detected by molecular
testing (4). The early stage of the
tumour is associated with a favourable prognosis, with a >90%
5-year survival rate. Metastases to the cervical lymph nodes do not
affect prognosis. Local recurrence is reported in 5–20% of cases.
The intermediate risk includes the following histological findings:
Tall cell/hobnail/columnar cell variants, vascular invasion, pN1
with more than five positive lymph nodes and microscopic
extrathyroidal extension to adipose connective tissue. High-risk
cases present with significant extrathyroidal extension (to
muscle), incomplete tumour resection or distant metastasis
(5). Advanced age (≥55 years) at
the time of diagnosis is also a poor prognostic factor. Distant
metastasis (to the lungs, bones and central nervous system)
develops in 10–15% of cases. Breast metastasis of PTC is extremely
rare, particularly in men. To date, to the best of our knowledge,
there have been just two male cases reported worldwide (2,6).
The diagnostic process of thyroid gland cancer
relies on the clinical findings (palpable mass in the thyroid area
or cervical lymphadenopathy in cases of metastatic disease),
ultrasound imaging (a lesion with irregular margins, which is
higher than wider, the presence of microcalcifications and
increased vascularisation), fine-needle aspiration biopsy (FNAB),
and subsequent cytological analysis according to The Bethesda
System for Reporting Thyroid Cytopathology (7).
The treatment of differentiated carcinomas is
surgical, consisting of a total thyroidectomy with subsequent
radioiodine therapy, which serves to eliminate any possible
residual tumour or distant metastasis. At University Hospital
Olomouc (Olomouc, Czech Republic), Olomouc ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up of thyroid cancer
are recommended (8).
Case report
In August 2022, a 63-year-old man was referred to
the Department of Radiology, University Hospital Olomouc from an
external healthcare facility (Hranice, Czech Republic) for a
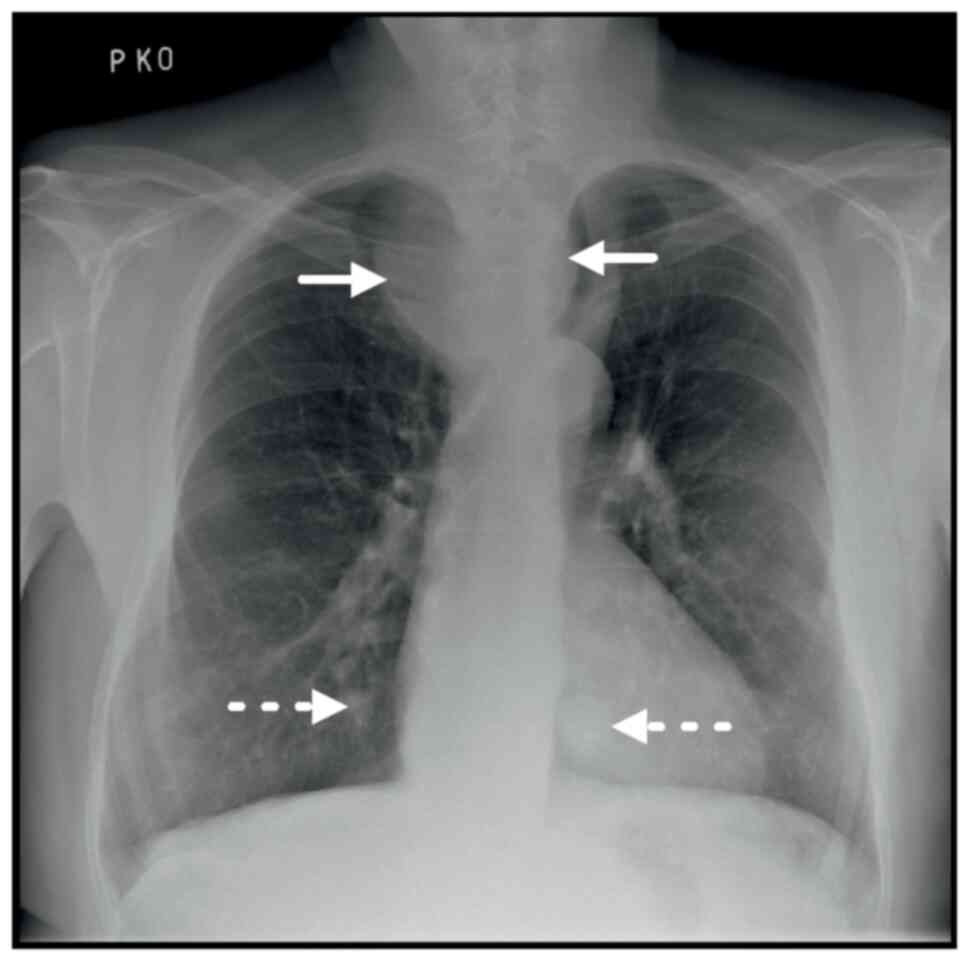
computed tomography (CT) scan of a mediastinal mass described in a
previous chest X-ray (Fig. 1). The
patient's symptoms included dysphonia and intermittent dysphagia.
According to the medical history, the patient was also treated for
chronic hypothyroidism and atrial fibrillation that had been
complicated by an embolic stroke in the past. No more clinical data
was available at the time of the CT examination. Laboratory
findings revealed high levels of serum thyroglobulin at 3,847.00
µg/l (normal laboratory reference value, 3.5–77 µg/l), and an
elevation of cancer antigen 15-3 at 78.7 kU/l (normal laboratory
reference value, 0–25 kU/l), with a normal neuron-specific enolase
level of 16.44 µg/l (normal laboratory reference value, 0–30 µg/l).
The level of thyroid-stimulating hormone (TSH) was 0.915 mIU/l
(normal laboratory reference value, 0.55–4.78 mIU/l) and the
antithyroid peroxidase antibody level was 35.6 kU/l (normal
laboratory reference value, 0–60 kU/l).
Based on the indication of the previous chest X-ray
for further evaluation of the mediastinal mass, unenhanced- and
parenchymatous-phase chest CT was performed following the
intravenous administration of an iodine contrast agent (Ultravist
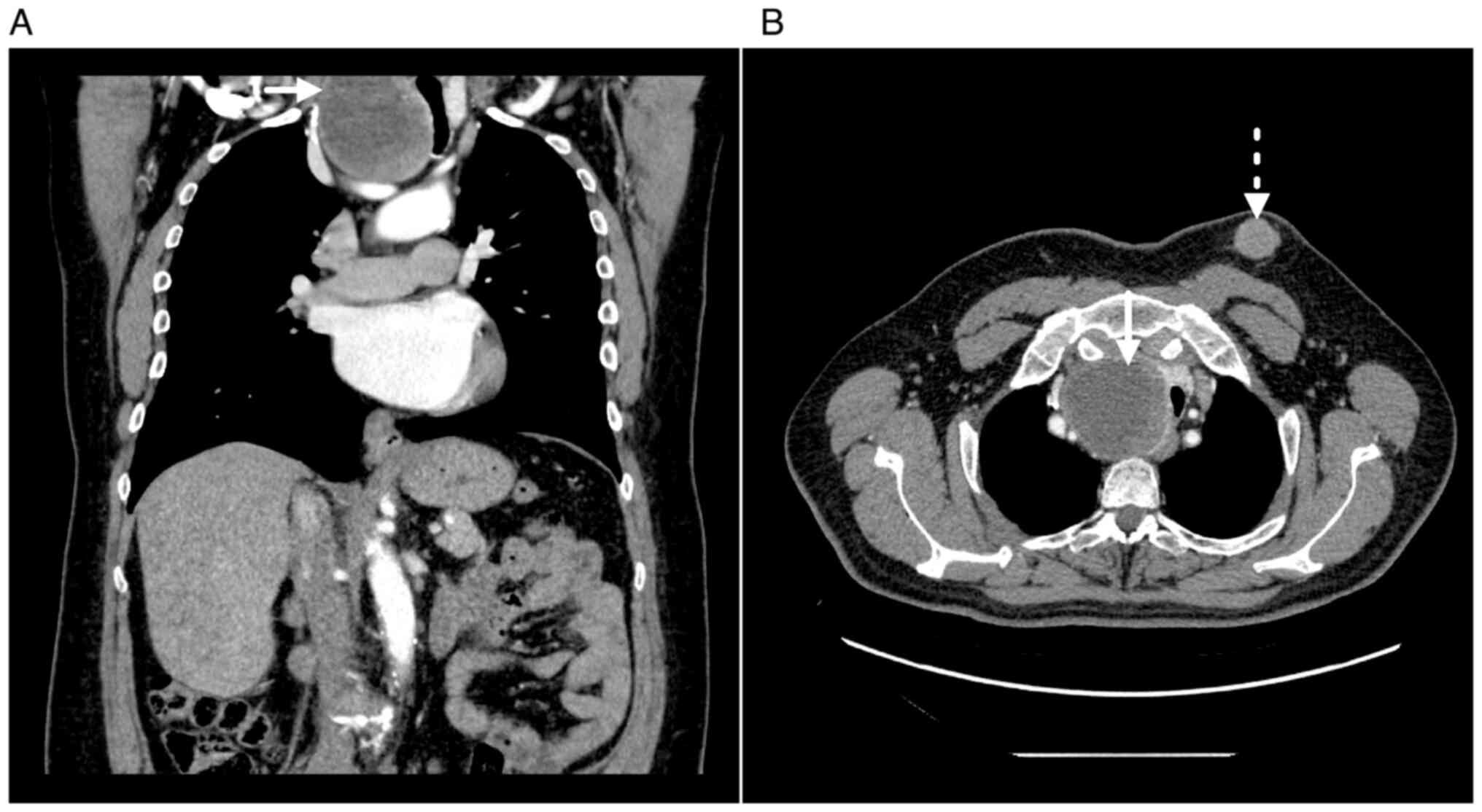
370; Bayer AG). A large hypodense spherical formation was observed
in the upper mediastinum, which was continuous with the right lobe
of the thyroid gland and showed peripheral nodular enhancement
following the administration of the iodine contrast agent. The mass
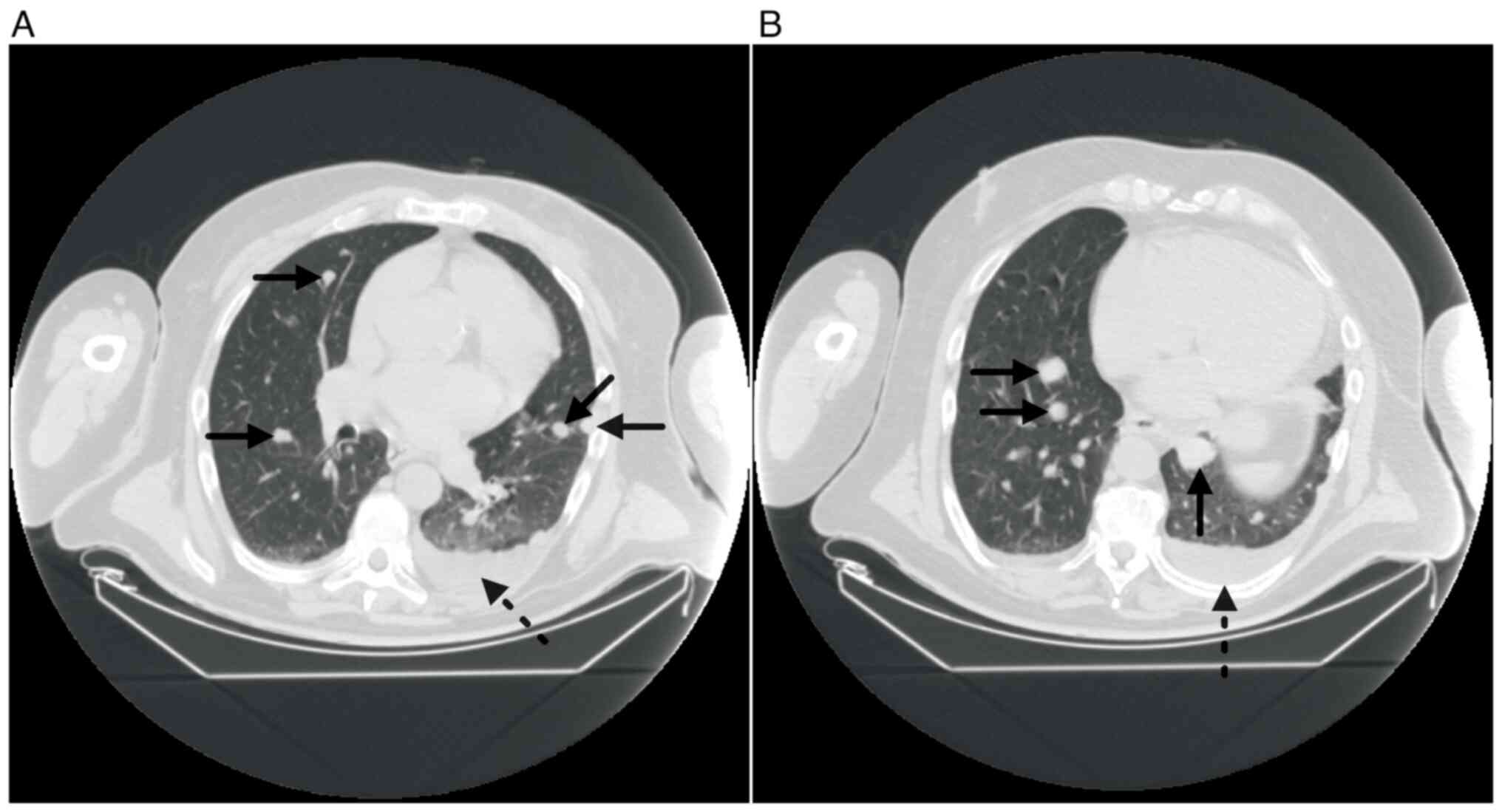
had compressed and dislocated the trachea to the left (Fig. 2A). Furthermore, the lung parenchyma
contained small non-specific nodulations bilaterally, and a
follow-up examination in 3–6 months was therefore recommended
according to the Fleischner Society if the patient was not already
under follow-up elsewhere (9). The
samples of the suspicious mediastinal mass subsequently obtained by
FNAB were not considered representative for the purposes of
histological examination due to the extended necrosis of the
tumour, therefore cytology images were not obtained.
In the patient's left breast, an irregular lobulated
subcutaneous formation measuring 40×30×50 mm was observed. The
breast lesion was homogenously enhanced after the administration of
the contrast medium, presenting with smooth margins and fat
stranding in the surrounding adipose tissue, without axillary
lymphadenopathy at the time of examination. The lesion was
relatively distant from the breast gland, it did not exhibit
typical male breast cancer (MBC) localisation
(retroareolar/periareolar location) and it did not contain spicula;
therefore, on the basis of the imaging methods, the lesion was not
considered to have originated in the breast (Fig. 2B).
Following the release of the CT findings, the
referring physician communicated with the radiologist regarding
information provided by the external healthcare facility in
Hranice, Czech Republic, where an initial biopsy had been performed
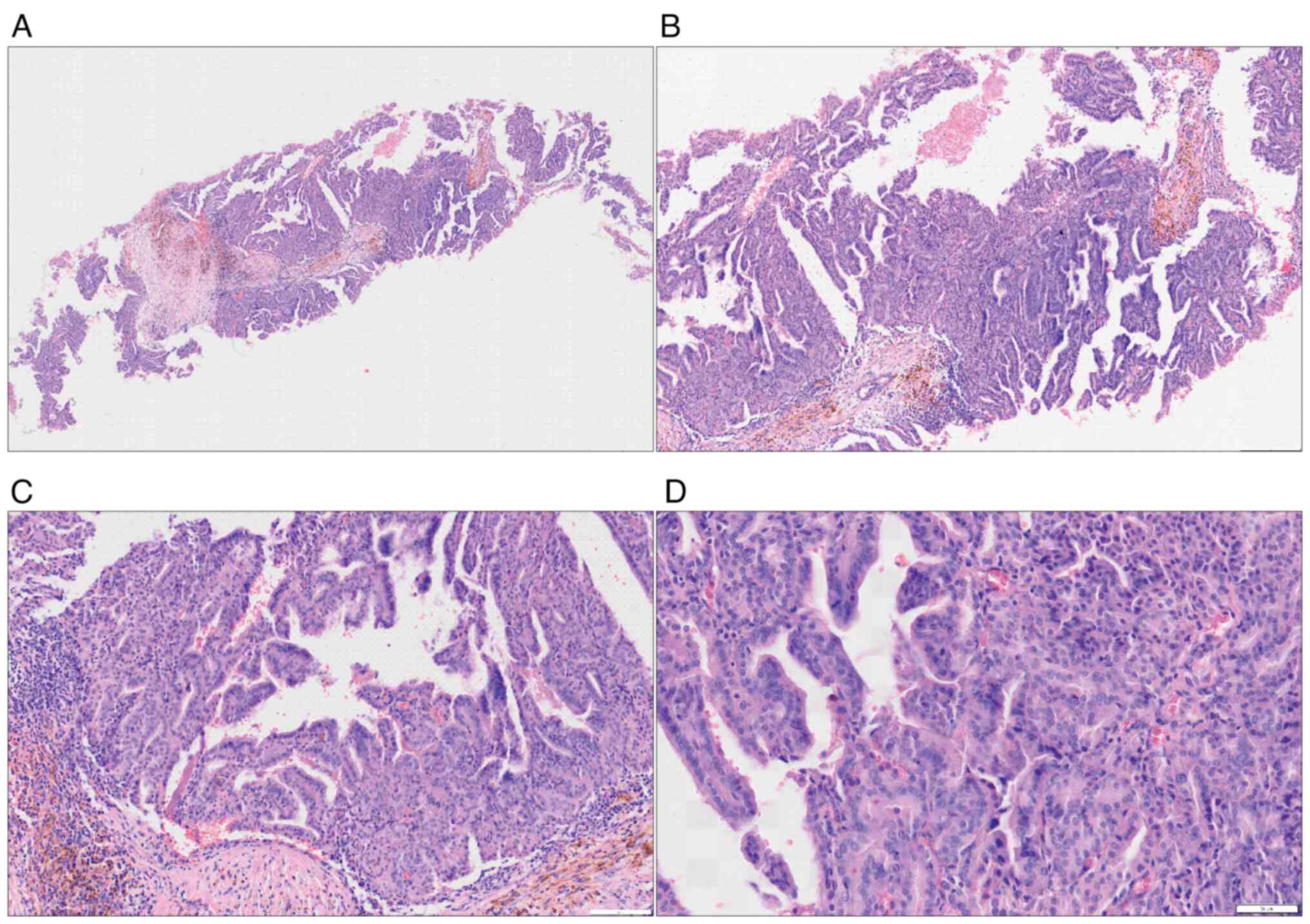
on the patient in June 2022. The histopathological analysis from
that biopsy confirmed a diagnosis of invasive breast cancer,
specifically identified as no special type (NST), which also
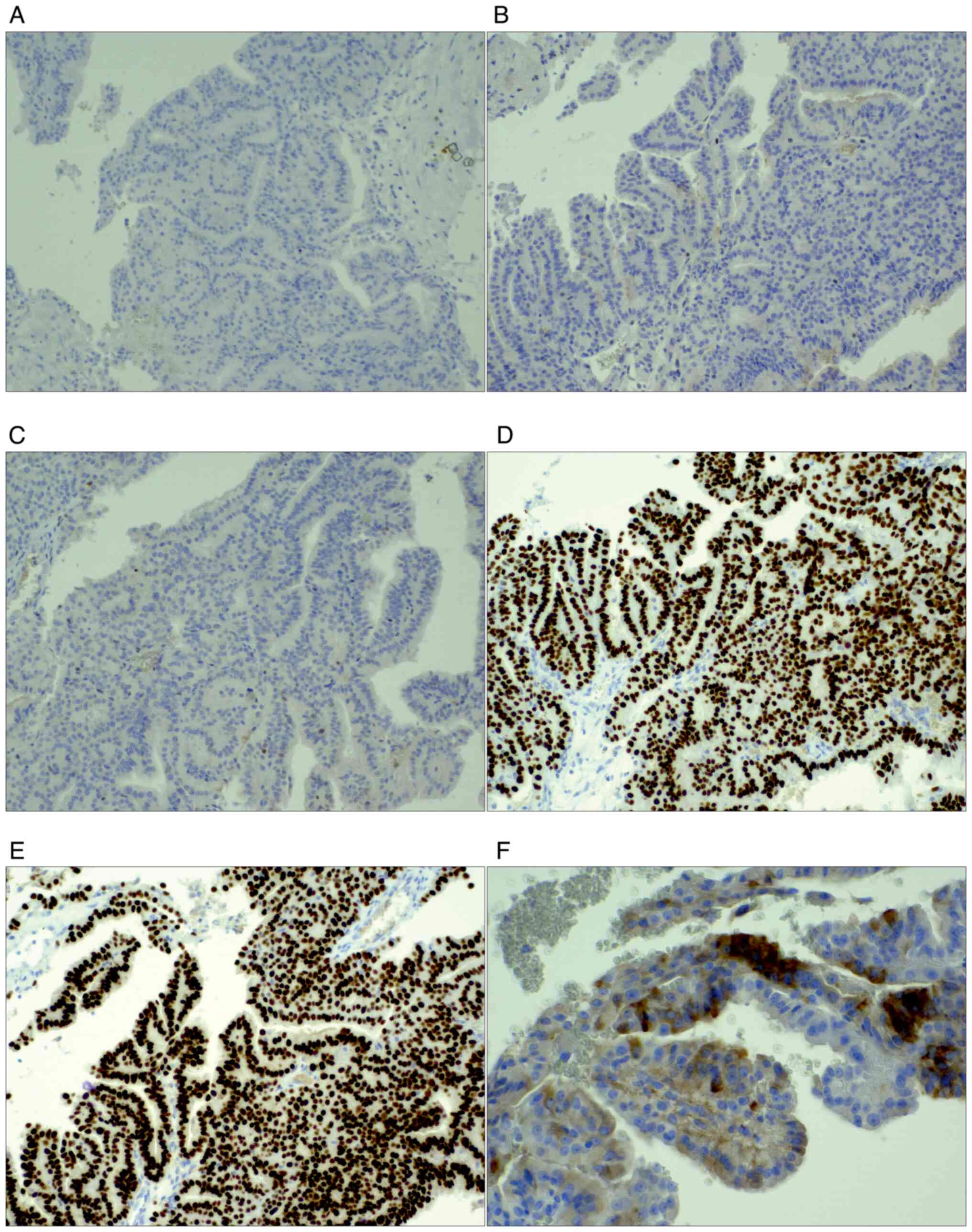
exhibited focal papillary features (Fig. 3). Immunohistochemically, the cancer
cells lacked the expression of oestrogen receptor (ER; 0%) and GATA
binding protein 3 (GATA3) (Fig. 4).
The index of proliferated Ki-67, a nuclear marker indicating tumour
cell proliferation, was 8%.
However, the findings of a potential thyroid gland
tumour, significantly elevated serum thyroglobulin levels (3,847.00
µg/l) and atypical imaging attributes of the mammary lesion were
challenging for the multi-specialty board. Given the increasing
doubts about the tumour's mammary origin, a second
histopathological reading of the breast lesion was requested. The
Department of Clinical and Molecular Pathology, University Hospital
Olomouc, performed the second histopathological reading. Tissue
samples 4-mm thick were fixed in 10% neutral buffered formalin for
24 h at room temperature, before being embedded in paraffin within
24 h. The formalin-fixed and paraffin embedded tissue slides were
with an automated stainer using hematoxylin solution and
counterstained with eosin Y solution at 65°C for 10 min. Slides
were assessed under an Olympus BX46 light microscope (Olympus
Corporation) with magnification ×100-400. The tissue blocks were
stored at room temperature before immunohistochemical examination.
The standardised protocol for immunohistochemistry, including the
incubation of sections with primary and secondary antibodies for
automated use (Ventana BenchMark ULTRA; Roche Diagnostics), was
performed on 4-µm thick formalin-fixed paraffin-embedded tissue
samples. An Olympus BX46 light microscope (Olympus Corporation) was
used for their analysis.
The results received from the Department of Clinical
and Molecular Pathology, University Hospital Olomouc, revealed
positive expression of TTF1, PAX-8 and thyroglobulin, and the
absence of gross cystic disease fluid protein 15 expression in the
tumour cells (Fig. 4). The levels
of TSH, anti-thyroid peroxidase antibodies and thyroglobulin
antibodies were not elevated in the tissue sample.
Based on the aforementioned findings, the lesion in
the left breast was reassessed as a metastasis of a PTC. In
November 2022, the patient underwent a total thyroidectomy using
the cervical approach, a partial sternotomy and a left-sided breast
metastasectomy, with a good postoperative clinical course.
The surgically resected specimen of the left breast
measured 90×90×50 mm and contained a lesion measuring 40×35×58 mm.
The lesion was characterised by distinct margins, a reddish-pink
colour and a soft consistency, with the presence of a focal
haemorrhage, and was completely removed. Histologically, the
findings were consistent with the core cut biopsy, showing
metastasis of a focally necrotising PTC. The tumour cells did not
reach the resection margins of the breast tissue.
Postoperative examination of the thyroid gland
revealed that the left lobe measured 53×28×25 mm. The resection
slices showed a homogenous brown appearance of the thyroid tissue,
histologically consistent with normal thyroid gland tissue without
a tumour. The right lobe of the thyroid gland measured 105×70×70
mm. The lobe was almost entirely infiltrated by a tumour of a
beige-pink colour, with fairly distinct margins and measuring
68×65×85 mm. The tumour was extensively necrotic, with a centrally
degraded cavity consisting of soft, brownish necrotic masses. In
certain areas, the tumour nearly reached the thyroid gland capsule,
but it did not infiltrate it macroscopically. Microscopically, the
tumour was described as being composed of thyroid gland tissue,
with structures of necrotising papillary carcinoma, with a focal
(up to 20%) solid type of growth. Structures of an anaplastic
thyroid carcinoma were not found. Blood vessel invasion and
extracapsular invasion to the surrounding thyroid tissue were
detected, while invasion beyond the thyroid gland was not.
Following surgical treatment, radioiodine therapy
was initiated to eliminate possible tumour residues or metastases,
and the patient received a single dose of 8.6 GBq radioiodine-131
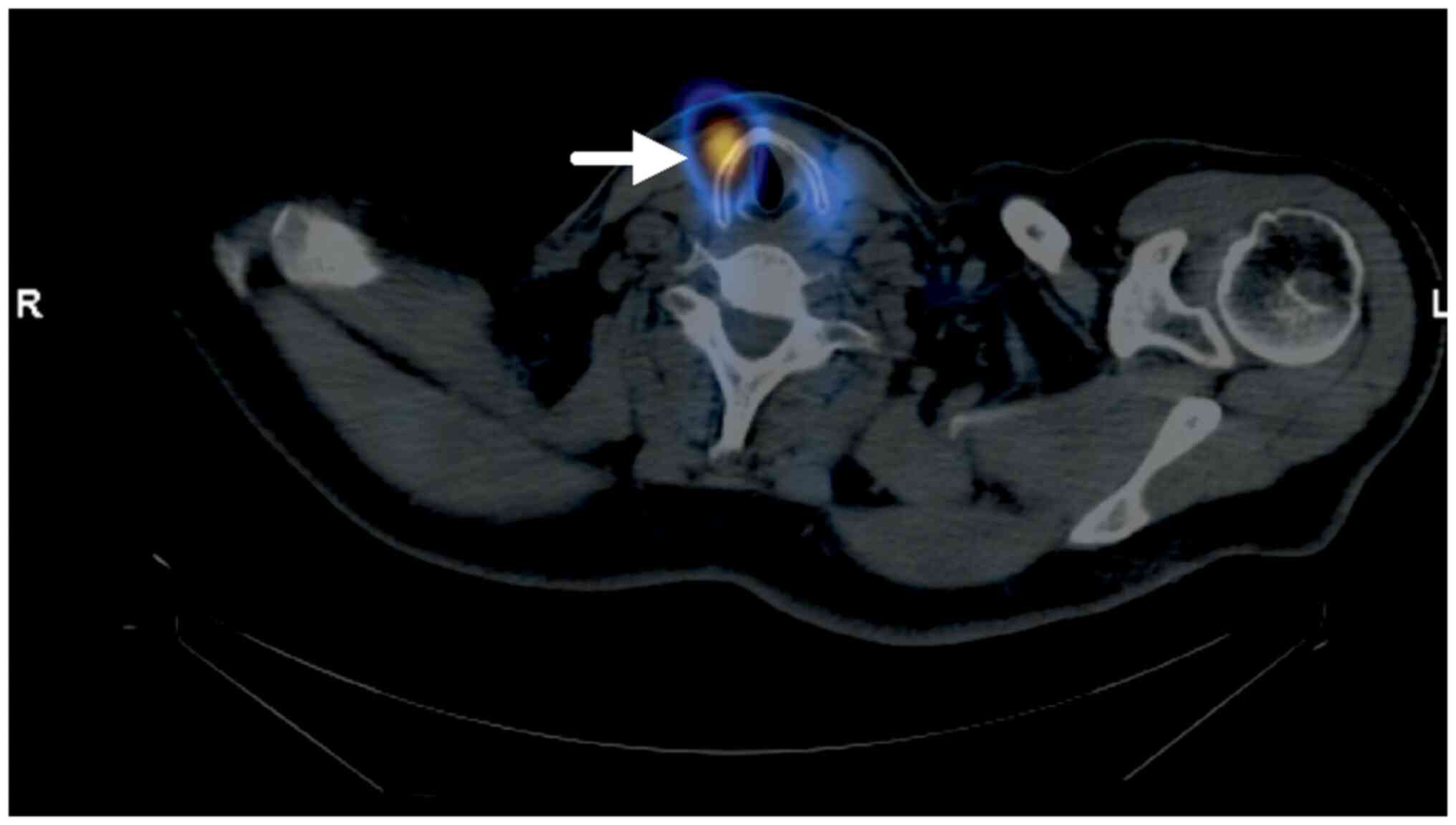
via oral administration. In January 2023, whole-body scintigraphy
with radioiodine-131 was subsequently performed, with findings of
bilateral paratracheal tumour residues (Fig. 5). In addition, the CT scan revealed
an increase in the quantity and diameter of lung parenchyma
nodulations (Fig. 6). Therefore,
the lung parenchyma lesions were considered to be metastases. In
May 2023, oral lenvatinib (protein kinase inhibitor) therapy was
initiated at a dose of 24 mg/day. After 7 days, lenvatinib therapy
was discontinued due to deterioration in the patient's health and
the patient was admitted to an external healthcare facility. Since
August 2023, no new information about the patient's condition had
been received, and the patient died during the hospitalisation.
Follow-up tools vary according to the histological type, initial
treatment, initial risk of persistent/recurrent disease and
response to treatment (8). In the
present study, physical examination, laboratory examination (serum
level of TSH, thyroglobulin and thyroglobulin antibody) and
ultrasound examination of the neck every 6–12 months would have
been recommended according to the ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up of thyroid cancer (8).
Discussion
To the best of our knowledge, only two reported
cases of metastatic PTC of the male breast are reported in the
literature (2,6). In both males, the solitary lesions had
presented as a primary manifestation of PTC located in the left
breast, as was the case in the present study. An initial
pathological misdiagnosis of MBC was also present in both
referenced cases.
The incidence of metastasis from differentiated
thyroid cancer to the breast is 1–2%. In the case of women
generally, the metastasis is typically located in the upper outer
quadrant (2). In the present case,
the mass was distributed in the upper inner quadrant, fairly
distant from the nipple, which is not typical for a primary
malignancy arising from the breast in the male population.
MBC accounts for <1% of all breast cancer cases.
The risk of developing cancer increases with age; men are often
diagnosed later than women, which leads to a worse prognosis as a
result of the more advanced disease stage and comorbidities
(10,11). The most common type of MBC is
invasive carcinoma of NST, and when compared with breast cancer in
women, a lobular type of cancer is very rare in men (12). Clinical presentation typically
includes a painless, periareolar, eccentric mass (12,13),
although bloody discharge from the nipple may be present (13). Mammography images of MBC show a very
dense lobulated mass, often with spicules, skin thickening or
nipple retraction, and microcalcifications are seen sporadically.
Ultrasound examination evaluates tumour size, skin invasion or
infiltration to the muscles of the chest wall and regional lymph
node involvement. The MBC itself presents as a significantly
hypoechoic lesion, often lobulated, with or without acoustic
shadowing and visible vascularisation on sonography (14). The positive expression of ER,
progesterone receptor and B-cell lymphoma 2 (an apoptosis
suppressor gene) (15), and the
lack of expression of human epidermal growth factor receptor 2
(16), are often observed in
MBC.
The location of the breast lesion in the male
patient of the present study was not typical for MBC
(peri/retroareolar location). The mass had smooth margins without
signs of invasive extension to the surrounding structures of the
chest wall, which is not characteristic for MBC; therefore, on the
basis of the imaging methods, the lesion was suspected of
originating in a location other than the mammary gland.
During histopathological examination, especially
when dealing with core-cut biopsy samples, the differential
diagnosis can pose certain challenges. Despite the well-defined
histological pattern of thyroid cancer in a surgical specimen, the
examination of a core-cut biopsy can be limited, so that not all
the specific morphological features are identified on a small
tumour area. The presence of a solid growth pattern without typical
nuclear features can be misleading, so focused immunohistochemical
analysis is necessary. The standardized protocol for
immunohistochemistry in the present study, including the incubation
of sections with primary and secondary antibodies, is provided in
Table I. Nuclear positivity of
GATA3 expression indicated the mammary origin of the tumour in this
case. The lack of ER expression could be explained by the
alteration of gene regulation. However, increased GATA3 and PAX-8
expression was also observed in the thyroid cancer. The subsequent
immunohistochemical determination of positive expression of TTF1
and thyroglobulin, the specific markers of thyroid differentiation,
confirmed the considered diagnosis of metastatic thyroid
cancer.
 | Table I.Immunohistochemistry antibodies. |
Table I.
Immunohistochemistry antibodies.
| Primary antibody | Supplier | Clone | Catalogue number | Dilution | Incubation
temperature, °C | Incubation time,
min | Secondary
antibody | Supplier | Catalogue number | Dilution | Incubation
temperature, °C | Incubation time,
min |
|---|
| GATA3 | Cell | L50-823 | 390M-14 | 1:200 | 36 | 32 | OptiView | Ventana/ | 760-700 | RTU | 36 | 8+8 |
|
| Marque; |
|
|
|
|
| DAB | Roche |
|
|
|
|
|
| Millipor |
|
|
|
|
| detection |
|
|
|
|
|
|
| Sigma |
|
|
|
|
| kit |
|
|
|
|
|
| ER | Ventana; | SP1 | 790-4325 | RTU | 36 | 16 | UltraView | Ventana/ | 760-500 | RTU | 36 | 8 |
|
| Roche |
|
|
|
|
| Universal | Roche |
|
|
|
|
|
| Diagnostics |
|
|
|
|
| DAB |
|
|
|
|
|
|
|
|
|
|
|
|
| detection |
|
|
|
|
|
|
|
|
|
|
|
|
| kit |
|
|
|
|
|
| TTF1 | Biocare | SPT 24 | ACR3126C | 1:100 | 36 | 32 | UltraView | Ventana/ | 760-500 | RTU | 36 | 8 |
|
| Medical |
|
|
|
|
| Universal | Roche |
|
|
|
|
|
| LLC |
|
|
|
|
| DAB |
|
|
|
|
|
|
|
|
|
|
|
|
| detection |
|
|
|
|
|
|
|
|
|
|
|
|
| kit |
|
|
|
|
|
| Thyroglobulin | Dako; Agilent | DAK-Tg6 | M0781 | 1:200 | 36 | 32 | UltraView
Universal | Ventana/Roche | 760-500 | RTU | 36 | 8 |
|
| Technologies,
Inc. |
|
|
|
|
| DAB detection |
|
|
|
|
|
|
|
|
|
|
|
|
| kit |
|
|
|
|
|
| PAX-8 | Abcam | SP348 | ab227707 | 1:100 | 36 | 32 | UltraView | Ventana/ | 760-500 | RTU | 36 | 8 |
|
|
|
|
|
|
|
| Universal | Roche |
|
|
|
|
|
|
|
|
|
|
|
| DAB |
|
|
|
|
|
|
|
|
|
|
|
|
| detection |
|
|
|
|
|
|
|
|
|
|
|
|
| kit |
|
|
|
|
|
In terms of differential diagnosis, it is necessary
to consider the tall cell variant of papillary breast carcinoma,
previously termed breast tumour resembling the tall cell variant of
PTC, which has been frequently described in the literature
(17–20). The possibility of a second primary
tumour must also be considered within the differential diagnosis
(21,22), as well as tumour-to-tumour
metastasis (23,24), although this is rare.
In conclusion, PTC metastasis to the male breast
tissue is extremely rare worldwide, and it may cause diagnostic
doubts or incorrect diagnosis followed by an unsuitable medical
therapeutic approach. In instances where imaging methods reveal a
soft-tissue lesion within the male breast that fails to meet the
established criteria for typical MBC manifestations, it is
imperative to conduct further assessment and rule out alternative
neoplastic origins. Multi-specialty collaboration considering the
results of imaging methods, laboratory findings, the biopsy method
applied and histopathological analysis involving an appropriate
panel of the immunohistochemical profile is pivotal to establishing
the definitive and correct diagnosis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Internal Grant of the Palacky
University (grant no. IGA_LF_2024_022), the Ministry of Health of
the Czech Republic-Conceptual Development of Research Organization
(grant no. FNOl, 00098892), and the Ministry of Education, Youth
and Sports of the Czech Republic - Conceptual Development of
Research Organization (grant no. UPOL, 61989592).
Availability of data and materials
The data generated in the present study are included
in the figures and table of this article.
Authors' contribution
VM and LV conceived and designed the study. VM, LV,
EM analysed and confirmed the imaging method examination results.
DS and MU analysed and confirmed the pathological data. KV provided
the surgical aspect to the study. KV, MU and EM revised the
manuscript before submission.
Ethics approval and consent to
participate
The patient provided written informed consent for
the examination, including consent to use documentation anonymously
for scientific and statistical purposes.
Patient consent for publication
The patient provided written informed consent for
the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTC
|
papillary thyroid carcinoma
|
|
MBC
|
male breast cancer
|
|
FNAB
|
fine-needle aspiration biopsy
|
|
CT
|
computed tomography
|
|
ER
|
oestrogen receptor
|
|
PAX-8
|
paired box gene 8
|
|
NST
|
no special type
|
|
TSH
|
thyroid-stimulating hormone
|
|
TTF1
|
thyroid transcription factor 1
|
References
|
1
|
Giuffrida R, Adamo L, Iannolo G, Vicari L,
Giuffrida D, Eramo A, Gulisano M, Memeo L and Conticello C:
Resistance of papillary thyroid cancer stem cells to chemotherapy.
Oncol Lett. 12:687–691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parasuraman L, Kane SV, Pai PS and
Shanghvi K: Isolated metastasis in male breast from differentiated
thyroid carcinoma - oncological curiosity. A case report and review
of literature. Indian J Surg Oncol. 7:91–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shukla N, Osazuwa-Peters N and Megwalu UC:
Association between age and nodal metastasis in papillary thyroid
carcinoma. Otolaryngol Head Neck Surg. 165:43–49. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Podolski A, Castelluci E and Halmos B:
Precision medicine: BRAF mutations in thyroid cancer. Precis Cancer
Med. 2:292019. View Article : Google Scholar
|
|
5
|
Lloyd RV, Buehler D and Khanafshar E:
Papillary thyroid carcinoma variants. Head Neck Pathol. 5:51–56.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Worapongpaiboon R and Vongsaisuwon M:
Breast metastasis of papillary thyroid carcinoma. BMJ Case Rep.
15:e2510812022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali SZ, Baloch ZW, Cochand-Priollet B,
Schmitt FC, Vielh P and VanderLaan PA: The 2023 Bethesda system for
reporting thyroid cytopathology. Thyroid. 33:1039–1044.
2023.PubMed/NCBI
|
|
8
|
Filetti S, Durante C, Hartl D, Leboulleux
S, Locati LD, Newbold K, Papotti MG and Berruti A; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Thyroid cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 30:1856–1833. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nair A, Devaraj A, Callister MEJ and
Bladwin DR: The Fleischner society 2017 and British thoracic
society 2015 guidelines for managing pulmonary nodules: Keep calm
and carry on. Thorax. 73:806–812. 2018. View Article : Google Scholar
|
|
10
|
Miao H, Verkooijen HM, Chia KS, Bouchardy
C, Pukkala E, Larønningen S, Mellemkjær L, Czene K and Hartman M:
Incidence and outcome of male breast cancer: An international
population-based study. J Clin Oncol. 29:4381–4386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lautrup MD, Thorup SS, Jensen V, Bokmand
S, Haugaard K, Hoejris I, Jylling AB, Joernsgaard H, Lelkaitis G,
Oldenburg MH, et al: Male breast cancer: A nation-wide
population-based comparison with female breast cancer. Acta Oncol.
57:613–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doyle S, Steel J and Porter G: Imaging
male breast cancer. Clin Radiol. 66:1079–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khalkhali I and Cho J: Male breast cancer
imaging. Breast J. 21:217–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gadam S, Heller SL, Babb JS and Gao Y:
Male breast cancer risk assessment and screening recommendations in
high-risk men who undergo genetic counseling and multigene panel
testing. Clin Breast Cancer. 21:e74–e79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muir D, Kanthan R and Kanthan SC: Male
versus female breast cancers. A population-based comparative
immunohistochemical analysis. Arch Pathol Lab Med. 127:36–41. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bloom KJ, Govil H, Gattuso P, Reddy V and
Francescatti D: Status of HER-2 in male and female breast
carcinoma. Am J Surg. 182:389–392. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Colella R, Guerriero A, Giansanti M,
Sidoni A and Bellezza G: An additional case of breast tumor
resembling the tall cell variant of papillary thyroid carcinoma.
Int J Surg Pathol. 23:217–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tosi AL, Ragazzi M, Asioli S, Del Vecchio
M, Cavalieri M, Eusebi LH and Foschini MP: Breast tumor resembling
the tall cell variant of papillary thyroid carcinoma: Report of 4
cases with evidence of malignant potential. Int J Surg Pathol.
15:14–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eusebi V, Damiani S, Ellis IO, Azzopardi
JG and Rosai J: Breast tumor resembling the tall cell variant of
papillary thyroid carcinoma: Report of 5 cases. Am J Surg Pathol.
27:1114–1118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pitino A, Squillaci S, Spairani C, Rassu
PC and Cosimi MF: Tall cell variant of papillary breast carcinoma:
An additional case with review of the literature. Pathologica.
109:162–167. 2017.PubMed/NCBI
|
|
21
|
Zhong J, Lei J, Jiang K, Li Z, Gong R and
Zhu J: Synchronous papillary thyroid carcinoma and breast ductal
carcinoma: A rare case report and literature review. Medicine
(Baltimore). 96:e61142017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong H, Chen J and Tang SC: Synchronous
papillary thyroid carcinoma and breast ductal carcinoma. J Int Med
Res. 48:3000605209487102020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raveendrannair AK, Mathews A, Varghese BT
and Jayasree K: Papillary carcinoma thyroid serving as recipient
tumor to carcinoma breast: A rare example of tumor-to-tumor
metastasis. Indian J Pathol Microbiol. 62:122–124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiziltan G, Bozdogan N and Ozaslan C:
Breast cancer metastasis into thyroid papillary carcinoma: A case
report. Breast J. 27:547–549. 2021. View Article : Google Scholar : PubMed/NCBI
|