Introduction
Brain radiation necrosis is a severe consequence of
intracranial radiotherapy, which can result in central nervous
system injury and patient mortality (1). The global incidence rate of brain
radiation necrosis has been reported to be 14–15% in patients
following conventional radiotherapy modalities, with high levels
detected after stereotactic radiosurgery treatment (24–68.8%)
(2). Traditionally, glucocorticoids
have been employed as the standard treatment for brain radiation
necrosis (3); however, due to its
complex pathophysiological processes, including
vascular-inflammatory responses, recent studies have proposed the
advantages of bevacizumab in the treatment of brain radiation
necrosis (4,5). However, in patients with brain
metastases from squamous cell carcinoma of the lung, there are few
studies or case reports regarding this treatment, and a lack of
relevant clinical data due to its safety limitations (6,7). The
present study describes the case of a patient who received
bevacizumab after a diagnosis of brain radiation necrosis following
radiotherapy treatment for brain metastasis from squamous cell lung
cancer. The patient was treated with four cycles of bevacizumab
that resulted in the improvement of clinical and imaging
manifestations. The present study discusses the safety of
bevacizumab for the treatment of brain radiation necrosis in
patients with brain metastasis from squamous cell lung cancer, and
further reviews the mechanisms, treatment efficacy and clinical
practice relating to brain radiation necrosis.
Case report
The present case report was approved by the Ethics
Committee of the Affiliated Hospital of North Sichuan Medical
College (approval no. 2024ER253-1; Nanchong, China). The
participant provided written informed consent to participate in the
study, and written informed consent was also obtained from the
individual for the publication of any potentially identifiable
images or data included in this article.
The patient was a 67-year-old man with no history of
food or drug allergies, heart disease, hypertension or diabetes
mellitus who presented to the Affiliated Hospital of North Sichuan
Medical College in July 2019. The patient was diagnosed with
squamous cell carcinoma of the upper lobe of the left lung with
mediastinal lymph node metastasis cT4N2M0 stage IIIB in August
2019, using fiberoptic bronchoscopy, pathological biopsy, computed
tomography, ultrasound and whole-body bone imaging, and other
auxiliary examinations. The patient was treated with three cycles
of paclitaxel + cisplatin (paclitaxel 150 mg/kg + cisplatin 75
mg/kg, once every 3 weeks, intravenous drip) and radiotherapy
targeting lung tumor lesions [planning-gross tumor volume (P-GTV):
63.8 Gy/29 Fx; volume-GTV node: 63.8 Gy/29 Fx]. After completion of
radiotherapy, a follow-up examination in January 2020 showed that
the lung lesion was stable and without distant metastasis. The
patient declined further chemotherapy and treatment was suspended.
In March 2020, the patient experienced limb weakness without any
obvious triggers, with no dizziness, headache or other discomfort.
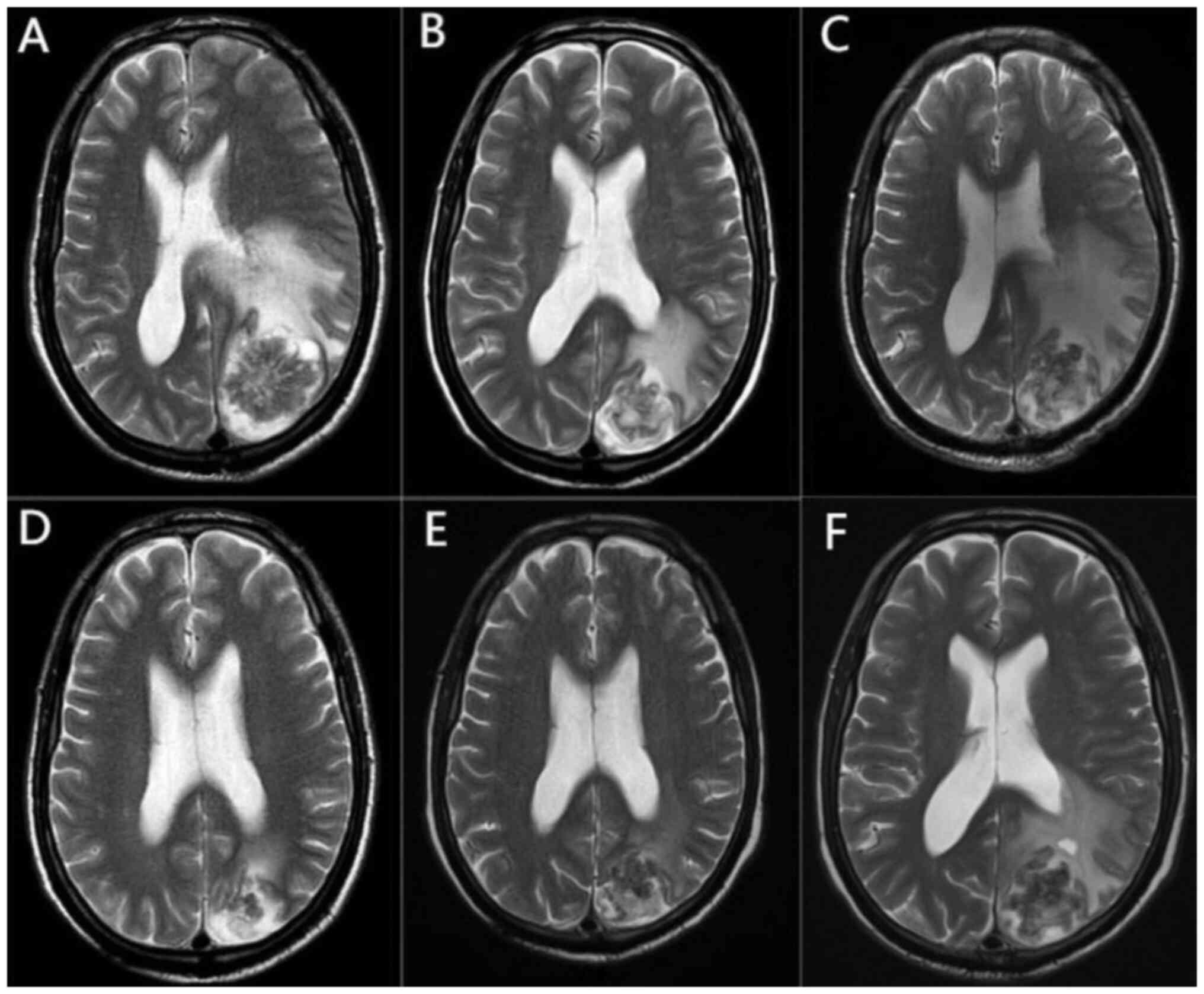
This same month, contrast-enhanced magnetic resonance imaging (MRI)
suggested that a left parieto-occipital lobe mass was accompanied
by obvious peripheral cerebral edema (Fig. 1A), which was considered a brain
metastasis and staged as cT4N2M1b stage IVA. The patient declined
surgery, and local radiation therapy was initiated 10 days after
MRI for brain metastasis with the following dosage and division
pattern: P-GTV: 39 Gy/13 Fx, 3 Gy/Fx, with mannitol (0.25 g mg/kg,
every 6 h, intravenous drip) dehydration to reduce intracranial
pressure and other symptomatic treatments (such as cough
suppressants and expectorants). Because the lung lesion appeared to
be stable and no other distant metastases were found except for in
the brain, three cycles of the original chemotherapy regimen
(paclitaxel 150 mg/kg + cisplatin 75 mg/kg, once every 3 weeks,
intravenous drip) were administered after the completion of
intracranial radiotherapy, and the treatment ended in July
2020.
In August 2020, chest computed tomography with
contrast enhancement showed progression of the lung lesion, whereas
cranial contrast-enhanced MRI showed that the intracranial lesion
had been reduced and the peripheral cerebral edema was reduced
(Fig. 1B). After comprehensive
evaluation, and consultation of the relevant guidelines (8) and the wishes of the patient's family,
second-line docetaxel chemotherapy (75 mg/kg, intravenous drip) was
administered for one cycle. The patient was injected with
polyethylene glycolated recombinant human granulocyte
colony-stimulating factor at the end of chemotherapy and developed
febrile neutropenia after the injection. The treatment was adjusted
to afatinib (40 mg, once a day, oral administration, cycle every 28
days, 14 cycles in total) and the lung mass was markedly
reduced.
In November 2020, the patient reported dizziness and
headache with no obvious cause, and a review of the cranial
contrast-enhanced MRI showed that the area of cerebral edema around
the lesion had considerably increased (Fig. 1C). Taking into account the treatment
history, brain radiation necrosis was considered, and auxiliary
examinations did not reveal other metastases throughout the body.
After mannitol (0.25 g mg/kg, every 6 h, intravenous drip)
dehydration to reduce intracranial pressure and 1 month of
treatment with glucocorticoids (1.0 g, once daily, intravenously
for 3 days, tapering until discontinued), the symptoms were not
significantly relieved, and the efficacy and safety of using
bevacizumab in this patient were discussed. The current lung
lesions were small, did not invade large blood vessels and had no
obvious coagulation abnormalities; therefore, the use of
bevacizumab was considered safe. After consultation with the
patient and their family, bevacizumab was administered at 7.5 mg/kg
once every 3 weeks (intravenous drip) starting in December
2020.
After two cycles of treatment, the headache and
dizziness symptoms had improved, and cranial contrast-enhanced MRI
showed markedly reduced edema around the lesion (Fig. 1D). After four treatment cycles, the
symptoms improved considerably. Cranial contrast-enhanced MRI
showed no significant changes in the intracranial lesion, and the
edema around the lesion was slightly reduced (Fig. 1E), which confirmed the clinical
efficacy of bevacizumab in this patient with brain radiation
necrosis. No significant adverse reactions were observed during
bevacizumab treatment and patient adherence was good. Subsequently,
bevacizumab treatment was discontinued, while afatinib treatment
was continued with regular follow-up. In August 2021, follow-up
cranial MRI showed increased edema around the intracranial lesion
(Fig. 1F) and a diagnosis of
recurrence of radiological cerebral necrosis was considered.
However, due to the lack of clinical symptoms and the care being
unaffordable for the patient, they declined further bevacizumab
treatment. Another follow-up cranial MRI showed that the
intracranial lesion had stabilized without significant changes. The
last follow-up in November 2021 was assessed as stable disease, and
the patient was subsequently lost to follow-up.
Discussion
The classification of radiation-induced brain
necrosis is based on the temporal onset of symptoms, resulting in
three categories: Acute, semi-delayed and late (9). The most prevalent type of
radiation-induced brain necrosis is late necrosis, which is
characterized by the presence of central nervous system injury and
imaging changes 6 months after the completion of radiotherapy. The
incidence of radiation necrosis is considered to be associated with
various factors, including the total radiotherapy dose, the
fractionated dose, a prior history of whole-brain radiotherapy and
the tumor lesion volume. Specifically, a higher total or
fractionated dose, and a larger lesion volume are associated with
an increased incidence of radiation necrosis, which tends to
manifest earlier (10,11). Symptoms of radiation-induced brain
necrosis include vertigo, cephalalgia, mental health conditions,
motor or sensory impairments, amnesia, changes in personality,
cognitive impairment and seizures (12,13),
which greatly reduce the quality of life of patients.
Glucocorticoids are the conventional treatment for brain radiation
necrosis; however, their effectiveness is insufficient to meet
therapeutic requirements, and there is a high occurrence of
negative long-term effects, such as medical hyperadrenocorticism,
infections, diabetes and peptic ulcers (10,14). A
growing body of evidence has suggested that bevacizumab could be
considered a viable therapeutic option for the management of brain
radiation necrosis (15–17).
Previous studies have shown that the development of
brain radiation necrosis is a complex pathophysiological process
caused by the interaction of multiple factors (18–22).
Ionizing radiation induces the production of reactive oxygen
species in glial cells and destroys cellular structures, such as
single-stranded and double-stranded DNA and cell membranes, leading
to cell necrosis or apoptosis, which in turn leads to a series of
inflammatory reactions, disruption of the blood-brain barrier and
the formation of cerebral edema (18,19).
In particular, radiotherapy-induced endothelial cell damage,
resulting in increased cerebrovascular permeability and
perivascular edema, is one of the main pathogenic mechanisms during
the acute response phase (20). In
this pathophysiological process, vascular endothelial growth factor
(VEGF) and hypoxia-inducible factor-1α (HIF-1α) may serve important
roles. Vascular endothelial cell injury causes impaired tissue
oxygen exchange, and tissue hypoxia further leads to the
upregulation of HIF-1α expression, which promotes the secretion of
VEGF by astrocytes and other cells, which in turn promotes focal
angiogenesis. However, the newly formed blood vessels are highly
permeable and easily damaged, leading to increased local
inflammation and edema, forming a vicious cycle of
hypoxia-edema-hypoxia, ultimately leading to focal brain necrosis
(21,22).
Bevacizumab is a recombinant human monoclonal IgG1
antibody that can competitively inhibit the binding of VEGF to
endothelial cell surface receptors by binding to VEGF, reducing
endothelial cell proliferation and neovascularization, and
decreasing vascular permeability. Bevacizumab has been widely used
in non-small cell lung cancer and other malignant tumors for
targeted antiangiogenic therapy (23). Due to the aforementioned
pathophysiological mechanisms of brain radiation necrosis, the
inhibitory effects of bevacizumab on angiogenesis and its ability
to lower vascular permeability have been suggested as the
mechanisms underlying its palliative effects on brain radiation
necrosis and localized cerebral edema; this was first suggested in
a phase I clinical study in 2007 (24). Since 2007, several case reports and
early clinical studies have reported the use of bevacizumab in
brain radiation necrosis (25–29).
In 2011, Levin et al (30)
published a prospective, placebo-controlled, randomized clinical
study in which 14 patients with symptomatic MRI-supported brain
radiation necrosis were randomized into placebo and bevacizumab
(7.5 mg/kg every 3 weeks) groups. MRI was performed after two
doses, and patients showing effective treatment and tolerance were
re-assessed after two more doses. All patients in the bevacizumab
group experienced varying degrees of relief of subjective symptoms
and imaging signs, whereas there was no significant change in the
placebo group. Furthermore, the safety profile of the bevacizumab
group was favorable (30). In 2018,
Xu et al (15) included 121
cases of brain radiation necrosis in a multicenter randomized
controlled study, where patients were randomly assigned to a
bevacizumab (5 mg/kg every 2 weeks, 4 cycles) and a glucocorticoid
group. Treatment responses were markedly higher in the bevacizumab
group than in the glucocorticoid group (65.5 vs. 31.5%), and
clinical improvement was detected in more patients in the
bevacizumab group than in the glucocorticoid group (62.1 vs. 42.6%)
(15). In addition, several
retrospective studies have provided supportive evidence for the use
of bevacizumab in brain radiation necrosis. In 2015, Sadraei et
al (16) published the results
of a retrospective study in 24 patients with radiation brain
necrosis, showing that all patients treated with bevacizumab
achieved imaging improvements and reduced glucocorticoid use.
Another retrospective study in 2016 supported the same conclusion;
in 14 patients with brain radiation necrosis treated with
bevacizumab, 13 patients showed reduced necrotic brain volumes
(92.86%), and 10 out of 12 symptomatic patients achieved marked
symptomatic improvements with no clinically significant adverse
effects (17). All of the
aforementioned studies provide effective and supportive clinical
evidence for the use of bevacizumab in the treatment of brain
radiation necrosis, but the number of cases reported in clinical
studies to date is generally small, and randomized clinical studies
with larger sample sizes are still needed for further
validation.
Despite the clinical and preclinical evidence, there
is lack of relevant reports on squamous cell carcinoma of the lung.
Bevacizumab treatment has been reported to be associated with a
high risk of severe pulmonary hemorrhage in patients with squamous
cell carcinoma of the lung in a phase II randomized clinical
(30); based on this study, almost
all subsequent clinical studies on bevacizumab for non-small cell
lung cancer have excluded patients with squamous cell carcinoma.
Thus, there is a lack of clinical data on the use of bevacizumab
for brain radiation necrosis in patients with squamous carcinoma of
the lung. In 2017, Remon et al (31) reported the first case of bevacizumab
treatment for brain radiation necrosis in squamous carcinoma of the
lung. In this previous report, a patient with cerebellar metastasis
developed radioencephalic necrosis after stereotactic radiotherapy
and received six cycles of bevacizumab (5 mg/kg every 3 weeks),
achieving a marked improvement in clinical symptoms and imaging
manifestations. This tentatively suggested the efficacy and safety
of bevacizumab for the treatment of brain radiation necrosis in
patients with squamous cell lung cancer. In the present case
report, a patient with brain metastasis from squamous cell lung
cancer exhibited central nervous system symptoms and imaging
changes 8 months after receiving brain radiotherapy, which was
consistent with brain radiation necrosis. After receiving four
cycles of bevacizumab (7.5 mg/kg every 3 weeks), the patient was in
double remission, both regarding clinical symptoms and imaging
manifestations, and there were no adverse reactions detected, such
as pulmonary hemorrhage, further demonstrating the efficacy and
safety of the treatment.
To the best of our knowledge, there are still no
clinical studies on bevacizumab for the treatment of brain
radiation necrosis in patients with squamous cell lung cancer, and
this treatment regimen remains experimental and needs to be
validated by additional clinical studies. Although the present case
report suggested that it may be effective and safe, there is still
a lack of relevant clinical data in this population. Severe
pulmonary hemorrhage remains a serious adverse effect, requiring a
high degree of vigilance when using this drug in patients with
squamous cell lung cancer. Before using bevacizumab in patients
with brain radiation necrosis and squamous cell cancer of the lung,
the risk of pulmonary hemorrhage should be comprehensively
assessed, and factors such as lung tumor invasion of large blood
vessels, coagulation function and the baseline tumor cavity should
be considered (32). Over the
course of treatment, the patient should be continuously and closely
observed for the development of hemoptysis; if this occurs,
bevacizumab should be discontinued and symptomatic treatments
should be immediately administered.
It is worth noting that, because of the difficulty
in regenerating central nervous tissue, the pathological damage
following brain radiation necrosis cannot be reversed, and
recurrence has been observed in a number of studies after
bevacizumab discontinuation (28,33,34).
In the present case, the patient was also observed to have an
imaging manifestation suggestive of recurrence 5 months after
discontinuing bevacizumab. It is generally accepted that, because
bevacizumab acts on neovascularization around the lesion, as long
as the necrotic tissue exists, its peripheral vasculature can
continue to reactively proliferate after bevacizumab
discontinuation, continuing the pathophysiological process of local
hypoxia and edema (35).
Furthermore, when recurrence occurs in patients after stopping
bevacizumab, repeated treatment with bevacizumab seems to be
effective (36). However, the
current case report was unable to observe the efficacy of
bevacizumab for recurrent brain radiation necrosis. Notably, there
is still a lack of clinical data on patients with recurrent
radiation brain necrosis and research remains in the preliminary
stages.
There is no standardized dose of bevacizumab for
treatment of radiation brain necrosis, and most doses used in
clinical studies and case reports are 5–7.5 mg/kg once every 2–3
weeks for ≥2 cycles. The present case used a treatment regimen of
7.5 mg/kg every 3 weeks for four cycles, because the patient wanted
to reduce the frequency of hospital admissions. However,
considering the possible serious adverse effects, such as
hypertension, thromboembolism and hemorrhage (37), lower doses of bevacizumab may be a
superior treatment option. In recent years, several clinical
studies have shown support for low-dose bevacizumab in the
treatment of brain radiation necrosis. A clinical study in 2023
demonstrated that, in 13 patients treated with a low-dose
bevacizumab regimen (400 mg loading dose, then 100 mg every 4
weeks) for brain radiation necrosis, 12 achieved clinical
improvement, all patients achieved imaging improvement and no
clinical adverse effects were observed (38). In addition, a recently published
retrospective study comparing the efficacy and safety of high-dose
(≥5 mg/kg) and low-dose (<5 mg/kg) bevacizumab for the treatment
of brain radiation necrosis showed that clinical and imaging
improvement did not significantly differ between the groups, but
the use of high-dose bevacizumab was associated with a higher
incidence of grade 3 and higher adverse reactions (39). By contrast, two phase II prospective
clinical studies used ultra-low-dose (1 mg/kg every 3 weeks for ≥3
doses) and single low-dose (2.5 mg/kg) treatment regimens of
targeted infusions, and both were clinically efficacious with
favorable safety profiles (40,41).
In summary, a low-dose regimen may be a better choice than the
standard bevacizumab dose, but to the best of our knowledge, there
are no prospective studies comparing different regimens, and more
evidence is needed on optimal dosages and cycle durations.
In conclusion, the clinical efficacy of bevacizumab
as a potentially recommended drug for the treatment of brain
radiation necrosis has been recognized, but most current clinical
evidence has limitations. For example, the safety of its use in
patients with squamous cell lung cancer, the tendency to relapse
after drug discontinuation, and the optimal dosage and duration of
its use still need to be further discussed with a higher level of
clinical evidence. In clinical practice, the treatment of radiation
brain necrosis remains challenging, particularly in patients with
squamous cell lung cancer. Although the present case report
suggested that bevacizumab may offer therapeutic potential for this
condition, further evidence is needed to confirm its safety. When
bevacizumab use is warranted, an experimental approach is
recommended, carefully assessing the bleeding risk of the patient,
and tailoring the dosage and duration of therapy to the needs of
the individual. Close monitoring for adverse effects throughout
treatment is also essential.
Acknowledgements
Not applicable.
Funding
Financial support was received for the research, authorship
and/or publication of this article. This work was supported by the
Sichuan Science and Technology Program (grant no. 2022NSFSC1554),
the Research Project of North Sichuan Medical College (grant no.
CBY20-QA-Z11) and the Doctoral Research Startup Fund Project of the
Affiliated Hospital of North Sichuan Medical College (grant no.
2019-248).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
KG and BZ conceptualized the study and wrote the
original manuscript. BZ conducted a relevant survey of the
background to this study and provided grant support. BZ and HC
searched the literature and obtained case-related data. KG, SM and
DM analyzed data and relevant literature. BZ and DM reviewed and
edited the final draft. DM was responsible for managing this
research project. KG and BZ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of The Declaration of Helsinki and was approved by the
Ethics Committee of Affiliated Hospital of North Sichuan Medical
College (approval no. 2024ER253-1; 2024-04-03).
Patient consent for publication
Written informed consent was provided by the patient
to obtain clinical data and information, as well as for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chin LS, Ma L and DiBiase S: Radiation
necrosis following gamma knife surgery: a case-controlled
comparison of treatment parameters and long-term clinical follow
up. J Neurosurg. 94:899–904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ali FS, Arevalo O, Zorofchian S, Patrizz
A, Riascos R, Tandon N, Blanco A, Ballester LY and Esquenazi Y:
Cerebral radiation necrosis: Incidence, pathogenesis, diagnostic
challenges, and future opportunities. Curr Oncol Rep. 21:662019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kotsarini C, Griffiths PD, Wilkinson ID
and Hoggard N: A systematic review of the literature on the effects
of dexamethasone on the brain from in vivo human-based studies:
Implications for physiological brain imaging of patients with
intracranial tumors. Neurosurgery. 67:1799–815; discussion 1815.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Ren H and Fu J: Treatment of
radiation-induced brain necrosis. Oxid Med Cell Longev.
2021:47935172021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao G, Khan M, Zhao Z, Arooj S, Yan M and
Li X: Bevacizumab treatment of radiation-induced brain necrosis: A
systematic review. Front Oncol. 11:5934492021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS: Toxicities of antiangiogenic
therapy in non-small-cell lung cancer. Clin Lung Cancer. 8 (Suppl
1):S23–S30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanna N, Johnson D, Temin S, Baker S Jr,
Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl
NB, et al: Systemic therapy for stage IV non-small-cell lung
cancer: American society of clinical oncology clinical practice
guideline update. J Clin Oncol. 35:3484–3515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martino A, Krainik A, Pasteris C, Hoffmann
D, Chabardes S, Berger F, Le Bas JF, Cantin S, Attye A and Grand S:
Neurological imaging of brain damages after radiotherapy and/or
chimiotherapy. J Neuroradiol. 41:52–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le Rhun E, Dhermain F, Vogin G, Reyns N
and Metellus P: Radionecrosis after stereotactic radiotherapy for
brain metastases. Expert Rev Neurother. 16:903–914. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matuschek C, Bölke E, Nawatny J, Hoffmann
TK, Peiper M, Orth K, Gerber PA, Rusnak E, Lammering G and Budach
W: Bevacizumab as a treatment option for radiation-induced cerebral
necrosis. Strahlenther Onkol. 187:135–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung MC, Chan AS, Law SC, Chan JH and
Tse VK: Impact of radionecrosis on cognitive dysfunction in
patients after radiotherapy for nasopharyngeal carcinoma. Cancer.
97:2019–2026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XS, Ying HM, He XY, Zhou ZR, Wu YR
and Hu CS: Treatment of cerebral radiation necrosis with nerve
growth factor: A prospective, randomized, controlled phase II
study. Radiother Oncol. 120:69–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang CL, Liu L, Li Z and Buttgereit F:
The novel strategy of glucocorticoid drug development via targeting
nongenomic mechanisms. Steroids. 102:27–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Y, Rong X, Hu W, Huang X, Li Y, Zheng
D, Cai Z, Zuo Z and Tang Y: Bevacizumab monotherapy reduces
radiation-induced brain necrosis in nasopharyngeal carcinoma
patients: A randomized controlled trial. Int J Radiat Oncol Biol
Phys. 101:1087–1095. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sadraei NH, Dahiya S, Chao ST, Murphy ES,
Osei-Boateng K, Xie H, Suh JH, Peereboom DM, Stevens GH and
Ahluwalia MS: Treatment of cerebral radiation necrosis with
bevacizumab: The cleveland clinic experience. Am J Clin Oncol.
38:304–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang H, Yuan X, Zheng Y, Li X, Chang JY,
Wang J, Wang X, Yuan Z and Wang P: A study on the evaluation method
and recent clinical efficacy of bevacizumab on the treatment of
radiation cerebral necrosis. Sci Rep. 6:243642016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rahmathulla G, Marko NF and Weil RJ:
Cerebral radiation necrosis: A review of the pathobiology,
diagnosis and management considerations. J Clin Neurosci.
20:485–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Remler MP, Marcussen WH and Tiller-Borsich
J: The late effects of radiation on the blood brain barrier. Int J
Radiat Oncol Biol Phys. 12:1965–1969. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levin VA, Edwards MS and Byrd A:
Quantitative observations of the acute effects of X-irradiation on
brain capillary permeability: Part I. Int J Radiat Oncol Biol Phys.
5:1627–1631. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nonoguchi N, Miyatake SI, Fukumoto M,
Furuse M, Hiramatsu R, Kawabata S, Kuroiwa T, Tsuji M, Fukumoto M
and Ono K: The distribution of vascular endothelial growth
factor-producing cells in clinical radiation necrosis of the brain:
pathological consideration of their potential roles. J Neurooncol.
105:423–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoritsune E, Furuse M, Kuwabara H, Miyata
T, Nonoguchi N, Kawabata S, Hayasaki H, Kuroiwa T, Ono K, Shibayama
Y and Miyatake S: Inflammation as well as angiogenesis may
participate in the pathophysiology of brain radiation necrosis. J
Radiat Res. 55:803–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia J, Hurwitz HI, Sandler AB, Miles D,
Coleman RL, Deurloo R and Chinot OL: Bevacizumab
(Avastin®) in cancer treatment: A review of 15 years of
clinical experience and future outlook. Cancer Treat Rev.
86:1020172020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez J, Kumar AJ, Conrad CA and Levin
VA: Effect of bevacizumab on radiation necrosis of the brain. Int J
Radiat Oncol Biol Phys. 67:323–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torcuator R, Zuniga R, Mohan YS, Rock J,
Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M and
Mikkelsen T: Initial experience with bevacizumab treatment for
biopsy confirmed cerebral radiation necrosis. J Neurooncol.
94:63–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boothe D, Young R, Yamada Y, Prager A,
Chan T and Beal K: Bevacizumab as a treatment for radiation
necrosis of brain metastases post stereotactic radiosurgery. Neuro
Oncol. 15:1257–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng X, Zhao R, Wu S, Shen G, Ding L, Sun
B and Wang J: Efficacy of repeated low-dose bevacizumab treatment
with long-dosing interval for radiation-induced brain necrosis: A
case report. Cancer Biol Ther. 18:63–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alessandretti M, Buzaid AC, Brandão R and
Brandão EP: Low-dose bevacizumab is effective in radiation-induced
necrosis. Case Rep Oncol. 6:598–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wang
E, Zhang N and Dai J: Reversal of cerebral radiation necrosis with
bevacizumab treatment in 17 Chinese patients. Eur J Med Res.
17:252012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel
JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR and Jackson
EF: Randomized double-blind placebo-controlled trial of bevacizumab
therapy for radiation necrosis of the central nervous system. Int J
Radiat Oncol Biol Phys. 79:1487–1495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Remon J, Le Péchoux C, Caramella C,
Dhermain F, Louvel G, Soria JC and Besse B: Brain Radionecrosis
Treated with bevacizumab in a Patient with Resected squamous cell
carcinoma of the Lung. J Thorac Oncol. 12:e1–e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sandler AB, Schiller JH, Gray R, Dimery I,
Brahmer J, Samant M, Wang LI and Johnson DH: Retrospective
evaluation of the clinical and radiographic risk factors associated
with severe pulmonary hemorrhage in first-line advanced,
unresectable non-small-cell lung cancer treated with carboplatin
and paclitaxel plus bevacizumab. J Clin Oncol. 27:1405–1412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Huang X, Jiang J, Hu W, Hu J, Cai J,
Rong X, Cheng J, Xu Y, Wu R, et al: Clinical variables for
prediction of the therapeutic effects of bevacizumab monotherapy in
nasopharyngeal carcinoma patients with radiation-induced brain
necrosis. Int J Radiat Oncol Biol Phys. 100:621–629. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furuse M, Nonoguchi N, Kawabata S,
Yoritsune E, Takahashi M, Inomata T, Kuroiwa T and Miyatake S:
Bevacizumab treatment for symptomatic radiation necrosis diagnosed
by amino acid PET. Jpn J Clin Oncol. 43:337–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang H, Shi S, Yuan Z and Chang JY:
Bevacizumab treatment for radiation brain necrosis: Mechanism,
efficacy and issues. Mol Cancer. 18:212019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Furuse M, Kawabata S, Kuroiwa T and
Miyatake SI: Repeated treatments with bevacizumab for recurrent
radiation necrosis in patients with malignant brain tumors: A
report of 2 cases. J Neurooncol. 102:471–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taugourdeau-Raymond S, Rouby F, Default A
and Jean-Pastor MJ; French network of pharmacovigilance centers, :
Bevacizumab-induced serious side-effects: A review of the French
pharmacovigilance database. Eur J Clin Pharmacol. 68:1103–1107.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tijtgat J, Calliauw E, Dirven I, Vounckx
M, Kamel R, Vanbinst AM, Everaert H, Seynaeve L, Van Den Berge D,
Duerinck J and Neyns B: Low-dose bevacizumab for the treatment of
focal radiation necrosis of the brain (fRNB): A single-center case
series. Cancers (Basel). 15:25602023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao M, Wang X, Wang X, Niu G, Liu X, Zhao
S, Wang Y, Yu H, Huo S, Su H, et al: Can low-dose intravenous
bevacizumab be as effective as high-dose bevacizumab for cerebral
radiation necrosis? Cancer Sci. 115:589–599. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhuang H, Zhuang H, Shi S and Wang Y:
Ultra-low-dose bevacizumab for cerebral radiation necrosis: A
prospective Phase II clinical study. Onco Targets Ther.
12:8447–8453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dashti SR, Kadner RJ, Folley BS, Sheehan
JP, Han DY, Kryscio RJ, Carter MB, Shields LBE, Plato BM, La Rocca
RV, et al: Single low-dose targeted bevacizumab infusion in adult
patients with steroid-refractory radiation necrosis of the brain: A
phase II open-label prospective clinical trial. J Neurosurg.
137:1676–1686. 2022. View Article : Google Scholar : PubMed/NCBI
|