Introduction
Based on the latest GLOBOCAN projections, released
by the International Agency for Research on Cancer (IARC), ~20.0
million new cancer cases and 9.7 million cancer deaths were
reported globally in 2022 (1).
Cancer has become a formidable global challenge, significantly
impacting the public health and economy. The cornerstone strategies
for managing cancer encompass surgical procedures, radiotherapy,
chemotherapy, targeted therapy, and breakthrough immunotherapy.
Immune checkpoint inhibitors (ICIs), a prominent type of
immunotherapy, have gained prominence by bolstering immune cell
function and facilitating the eradication of tumor cells (2). Currently, ICIs have shown promising
results in cancer treatment (3).
However, ICIs may also trigger a wide array of immune-related
adverse events (irAEs) in any tissue or organ, with barrier tissues
such as skin, gastrointestinal tract and respiratory epithelium
being the most commonly affected. Additionally, endocrine toxicity
and joint inflammation are relatively common among patients
undergoing therapy with ICIs (4,5). These
irAEs differ from those caused by traditional chemotherapy and
targeted therapy (6,7), and their underlying mechanism remains
elusive. Their clinical manifestations are mostly non-specific,
typically occurring weeks to months after the initiation of
immunotherapy. Although most irAEs can be effectively managed,
there is a risk of severe or even fatal toxicities (8,9).
Therefore, prompt recognition, precise evaluation and timely
intervention are crucial in the management of irAEs (10).
Some microbiome compositions (11), and circulating biomarkers (12) have been identified as potential risk
factors for irAEs. However, the clinical predictive factors for
irAEs risk remain unclear (13). In
the present study, a predictive nomogram-based model to identify
patients with cancer who may develop irAEs following treatment with
ICIs, was developed and evaluated. The model is expected to help
early evaluation of irAEs, thereby optimizing the management of
irAEs, and ultimately improving the quality of life for
patients.
Materials and methods
General information
Data from patients with cancer who received
treatment with ICIs at Xi'an International Medical Center Hospital
(Xi'an, China) from December 2021 to December 2023 retrospectively
collected. The inclusion criteria were as follows: i) male or
female, aged ≥18 years; ii) histologically confirmed solid
malignant tumor; iii) receipted monotherapy with ICIs or
combination therapy with other systemic antitumor agents during
hospitalization; and iv) complete data. Exclusion criteria: i) age
<18 years; ii) non-solid malignant tumor; iii) patients with
severe infection, severe cardiac insufficiency, and severe acute
cardiovascular and cerebrovascular accidents prior to ICIs use; iv)
patients with autoimmune diseases or those who have previously or
currently used immunosuppressants; and v) data is missing, which
affects the judgment of results. Finally, 992 patients were
included, then the patients were divided into the irAEs group
(n=276) and the control group (n=716) based on the occurrence of
irAEs.
Data collection
Patient sex, age, body mass index (BMI), smoking
history, hypertension, diabetes, infection with hepatitis B,
pulmonary disease, primary cancer, metastasis, history of
radiotherapy, type of ICIs and drug combination were collected for
statistical analysis.
Statistical analysis
Statistical analyses were conducted using SPSS 25.0
software (IBM Corp.) with P<0.05 considered to indicate a
statistically significant difference. The number of cases
(percentage) [n (%)] and the chi-square value were used to analyze
differences between the two groups. Data were expressed as the mean
± standard deviation, and non-normally distributed data (for
example, BMI and age) were analyzed using the Mann-Whitney test,
while the type of ICIs and drug combination were analyzed using the
Fisher's exact test. Multivariate analysis was performed by
logistic regression analysis to determine the risk factors for
irAEs. Rv.4.2.2 statistical software (https://www.r-project.org) was used to developed a
nomogram prediction model for irAEs based on the results of
multivariate regression. The original data set was divided into a
training set (n=793) and validation set (n=199). For the prediction
of irAEs in patients with cancer, the Hosmer-Lemeshow (HL) test was
used to evaluate the goodness of fit of the predictive model. In HL
test, P>0.05 indicated that there was a favorable fitting effect
in both data sets. The receiver operating characteristic (ROC)
curve was used to evaluate the performance of the classification
model, and the decision curve analysis (DCA) was used to evaluate
the feasibility of clinical decisions.
Results
Patient characteristics
The characteristics of the patients are summarized
in Table I. The patients included
731 males and 261 females. The mean age was 60.97 (standard
deviation= 11.567). As indicated in Table I, 27.8% of the patients experienced
irAEs (n=276). There was no significant difference in the
occurrence of irAEs between the irAEs group and the Control group
in sex, age, BMI, smoking history, diabetes, infection with
hepatitis B and type of ICIs (P>0.05, Table I). The difference in primary cancer,
metastasis, drug combination, radiotherapy, hypertension, and
pulmonary diseases were statistically significant between the irAEs
group and the Control group (P<0.05, Table I).
 | Table I.Comparison of clinical features of
the 2 groups. |
Table I.
Comparison of clinical features of
the 2 groups.
|
Characteristics | Immune-related
adverse events group (n=276) | Control group
(n=716) | N=992 |
χ2/Mann-Whitney | P-value |
|---|
| Sex |
|
|
| 0.75 | 0.386 |
|
Female | 78 (28.3%) | 183 (25.6%) | 261 (26.3%) |
|
|
|
Male | 198 (71.7%) | 533 (74.4%) | 731 (73.7%) |
|
|
| Age, years | 60.91±21.906 | 60.99±11.825 | 60.97±11.567 | 97441.5 | 0.735 |
| Body mass index,
kg/m2 | 21.91±2.985 | 21.67±2.011 | 21.74±2.324 | 93678.5 | 0.205 |
| Smoking
history |
|
|
| 0.232 | 0.630 |
|
Yes | 111 (40.2%) | 300 (41.9%) | 411 (41.4%) |
|
|
| No | 165 (59.8%) | 416 (58.1%) | 581 (58.6%) |
|
|
| Diabetes |
|
|
| 2.843 | 0.092 |
|
Yes | 39 (14.1%) | 74 (10.3%) | 113 (11.4%) |
|
|
| No | 237 (85.9%) | 642 (89.7%) | 879 (88.6%) |
|
|
| Infection with
Hepatitis B |
|
|
| 0.952 | 0.329 |
|
Yes | 49 (17.8%) | 109 (15.2%) | 158 (15.9%) |
|
|
| No | 227 (82.2%) | 607 (84.8%) | 834 (84.1%) |
|
|
| Hypertension |
|
|
| 27.354 | <0.001 |
|
Yes | 100 (36.2%) | 145 (20.3%) | 245 (24.7%) |
|
|
| No | 176 (63.8%) | 571 (79.7%) | 747 (75.3%) |
|
|
| Renal
insufficiency |
|
|
| 11.658 | <0.001 |
|
Yes | 21 (7.6%) | 20 (2.8%) | 41 (4.1%) |
|
|
| No | 255 (92.4%) | 696 (97.2%) | 951 (95.9%) |
|
|
| Pulmonary
disease |
|
|
| 4.393 | 0.036 |
|
Yes | 42 (15.3%) | 75 (10.5%) | 117 (11.8%) |
|
|
| No | 233 (84.7%) | 641 (89.5%) | 874 (88.2%) |
|
|
| Radiotherapy |
|
|
| 20.443 | <0.001 |
|
Yes | 51 (18.5%) | 60 (8.4%) | 111 (11.2%) |
|
|
| No | 225 (81.5%) | 656 (91.6%) | 881 (88.8%) |
|
|
| Primary cancer |
|
|
| 14.215 | 0.014 |
| Lung
cancer | 127 (46.0%) | 297 (41.5%) | 424 (42.7%) |
|
|
| Liver
cancer | 48 (17.4%) | 138 (19.3%) | 186 (18.8%) |
|
|
|
Esophagus cancer | 14 (5.1%) | 76 (10.6%) | 90 (9.1%) |
|
|
| Biliary
malignant cancer | 14 (5.1%) | 18 (2.5%) | 32 (3.2%) |
|
|
| Renal
cancer | 7 (2.5%) | 9 (1.3%) | 16 (1.6%) |
|
|
| Other
cancers | 66 (23.9%) | 178 (24.9%) | 244 (24.6%) |
|
|
| Metastasis |
|
|
| 39.633 | <0.001 |
|
Yes | 138 (50.0%) | 206 (28.8%) | 344 (34.7%) |
|
|
| No | 138 (50.0%) | 510 (71.2%) | 648 (65.3%) |
|
|
| Type of ICIs |
|
|
| 5.555 | 0.056 |
|
PD-1 | 244 (88.4%) | 662 (92.5%) | 906 (91.3%) |
|
|
|
PD-L1 | 28 (10.1%) | 51 (18.5%) | 79 (8.0%) |
|
|
| PD-1/
CTLA-4 | 4 (1.4%) | 3 (0.4%) | 7 (0.7%) |
|
|
| Drug
combination |
|
|
| 19.734 | <0.001 |
|
Chemotherapeutics | 166 (60.1%) | 526 (73.5%) | 692 (69.8%) |
|
|
|
Targeted drug | 88 (31.9%) | 158 (22.1%) | 246 (24.8%) |
|
|
|
ICIs | 2 (0.7%) | 0 | 2 (0.2%) |
|
|
|
Monotherapy | 20 (7.2%) | 32 (4.5%) | 52 (5.2%) |
|
|
Risk factors for irAEs in patients
with cancer
Multivariate logistic regression analysis revealed
that hypertension, primary cancer, metastasis, targeted drug
combination and radiotherapy were risk factors for irAEs in
patients with cancer (P<0.05, Table
II).
 | Table II.Risk factors for immune-related
adverse events. |
Table II.
Risk factors for immune-related
adverse events.
|
|
|
|
|
|
| 95% confidence
interval |
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | Coef | S.E. | Wald_Z | P-value | Odds ratio | Lower | Upper |
|---|
| Intercept | −1.620 | 0.139 | −11.647 | <0.001 | 0.198 | 0.151 | 0.26 |
| Hypertension | 0.553 | 0.182 | 3.038 | 0.002 | 1.738 | 1.217 | 2.483 |
| Renal
insufficiency | 0.607 | 0.379 | 1.603 | 0.109 | 1.835 | 0.873 | 3.855 |
| Pulmonary
disease | 0.031 | 0.251 | 0.125 | 0.901 | 1.032 | 0.631 | 1.687 |
| Metastasis | 0.710 | 0.17 | 4.189 | <0.001 | 2.035 | 1.459 | 2.837 |
| Radiotherapy | 0.733 | 0.24 | 3.057 | 0.002 | 2.082 | 1.301 | 3.332 |
| Liver cancer | −0.788 | 0.331 | −2.378 | 0.017 | 0.455 | 0.237 | 0.871 |
| Targeted drug
combination | 1.105 | 0.292 | 3.784 | <0.001 | 3.021 | 1.704 | 5.355 |
Development and validation of a
predictive model for irAEs in patients with cancer
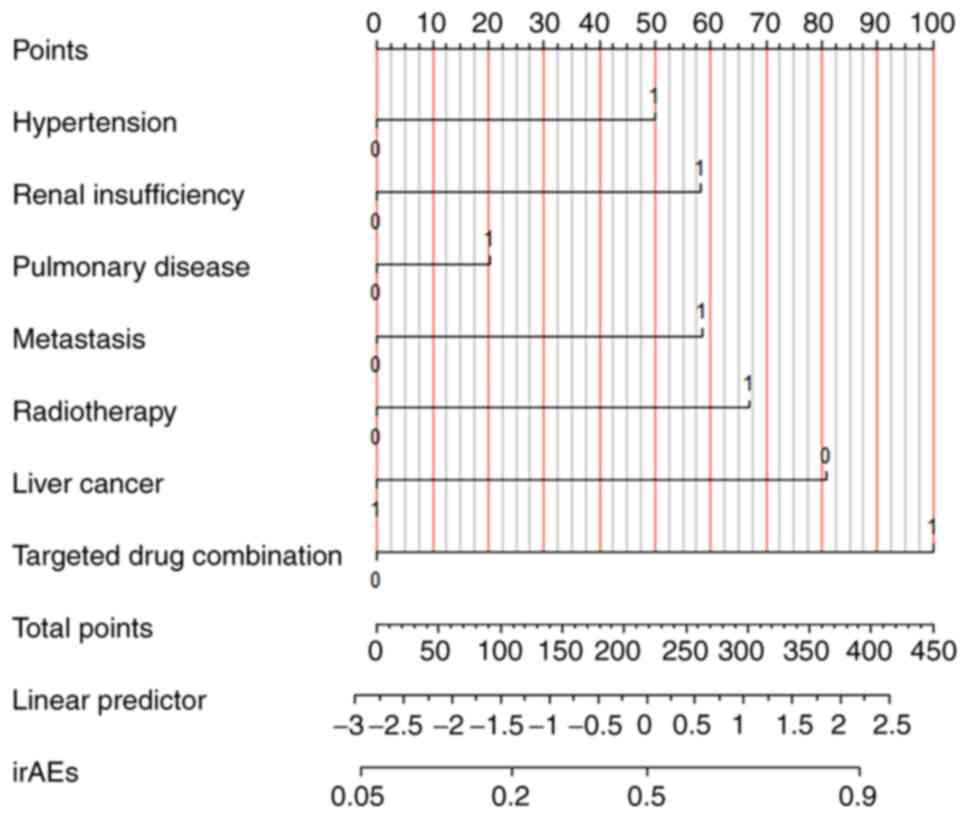
A nomogram prediction model for irAEs was developed
based on the results of multivariate regression (Fig. 1). The original data set was divided
into a training set (n=793) and a validation set (n=199). For the
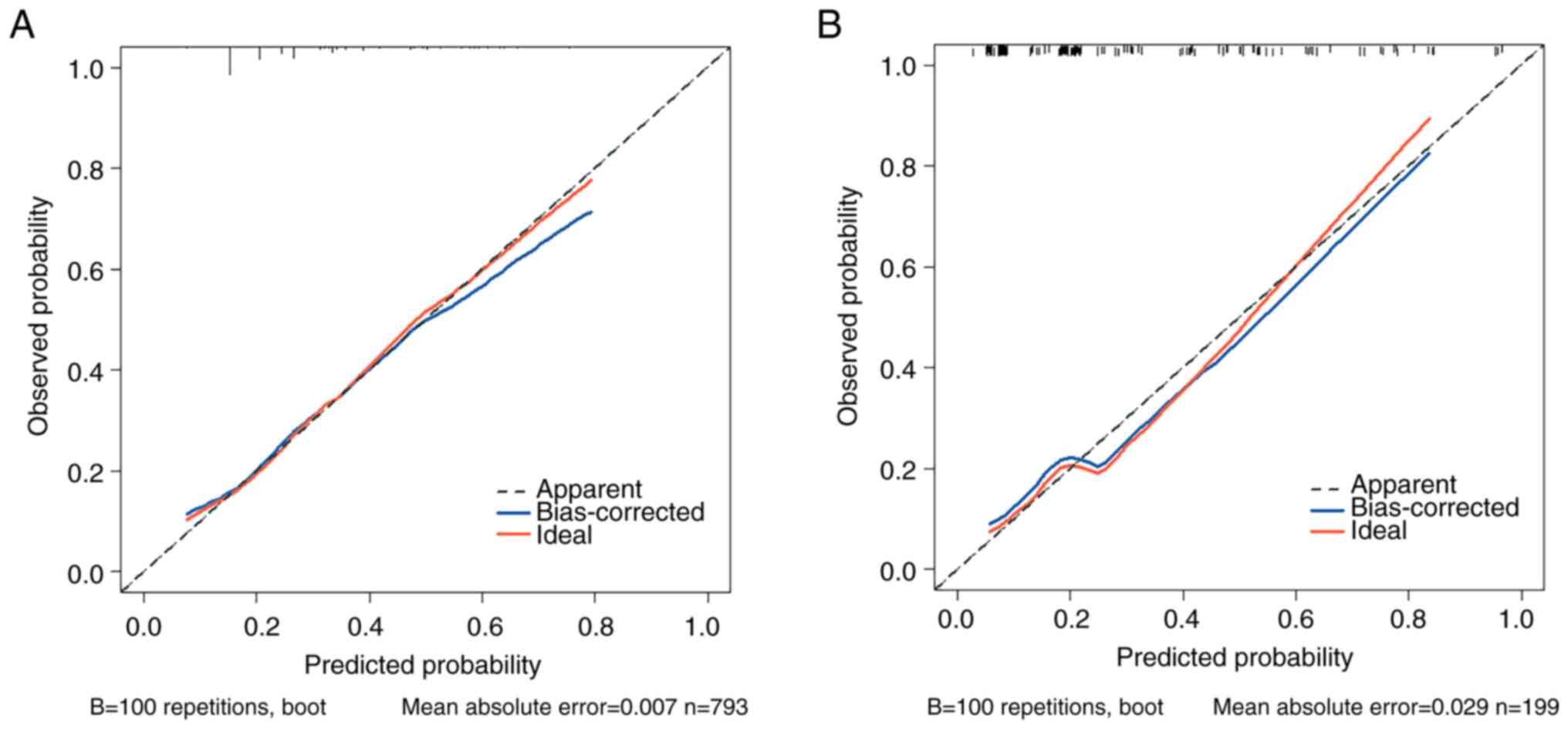
prediction of irAEs in patients with cancer, the HL test
calibration showed that the χ2 was 2.909 (P=0.820) in
the training set and the χ2 was 0.787 (P=0.978) in the
validation set (Fig. 2); the
P>0.05 indicated a favorable fitting effect in both data sets.
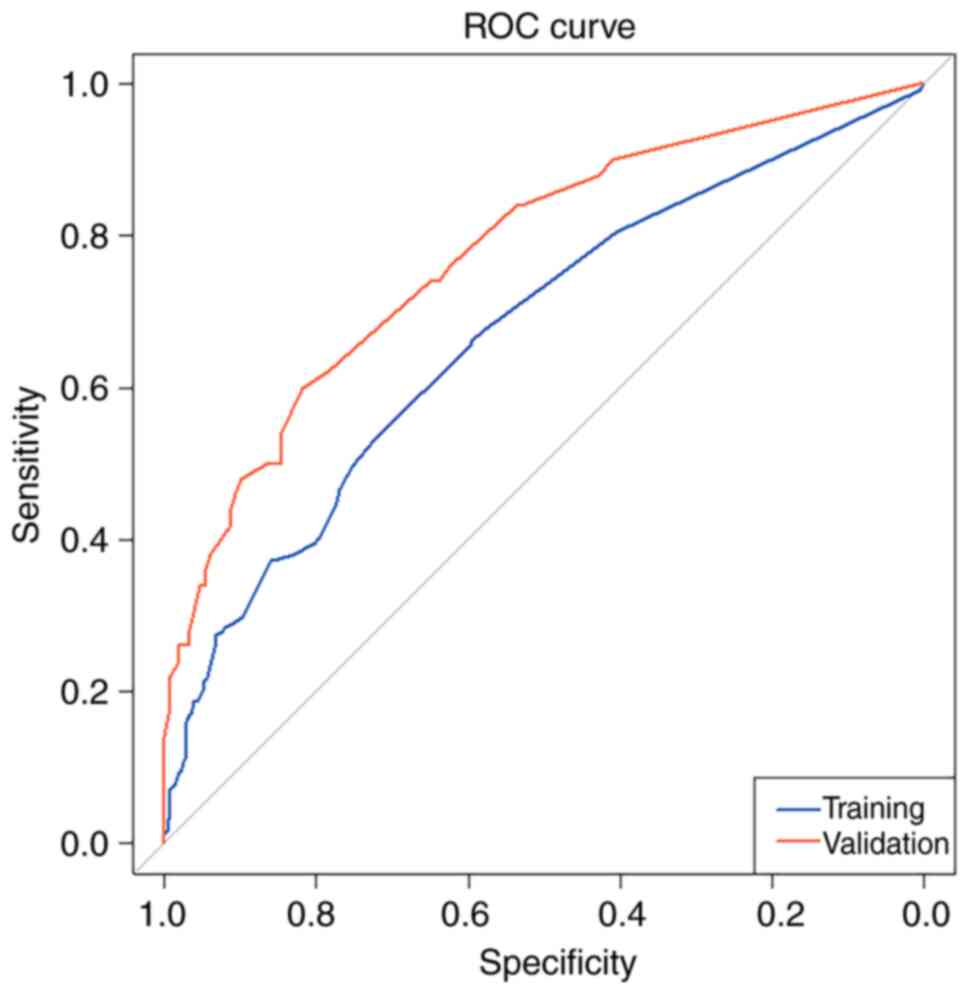
The area under curve (AUC) of ROC curve for the training set data
was 0.672 [95% confidence interval (CI): 0.630, 0.714], while that
for the validation set data was 0.776 (95% CI: 0.700, 0.853), AUC
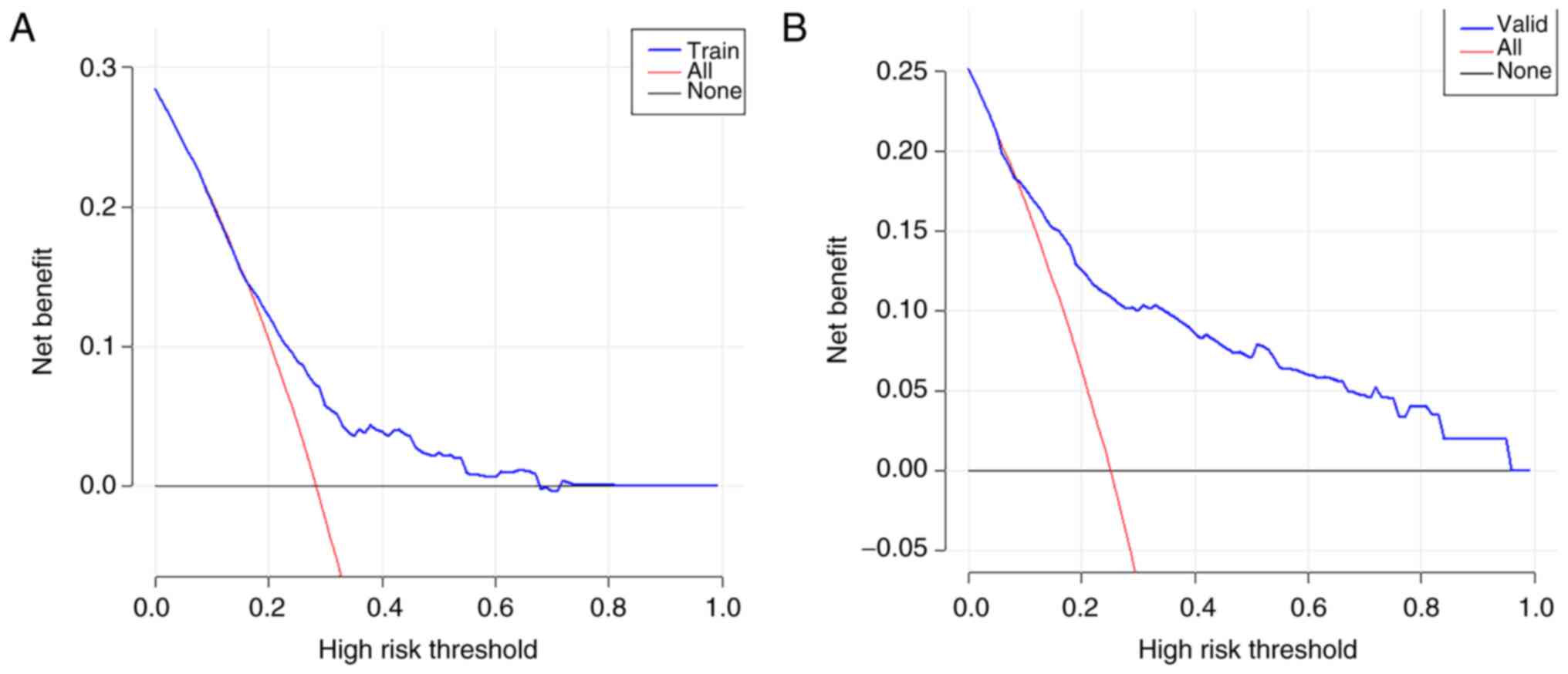
>0.5 showed favorable discrimination of the model (Fig. 3). As revealed in Fig. 4, the DCA showed that the model
performs well and can be used to make beneficial clinical
decisions.
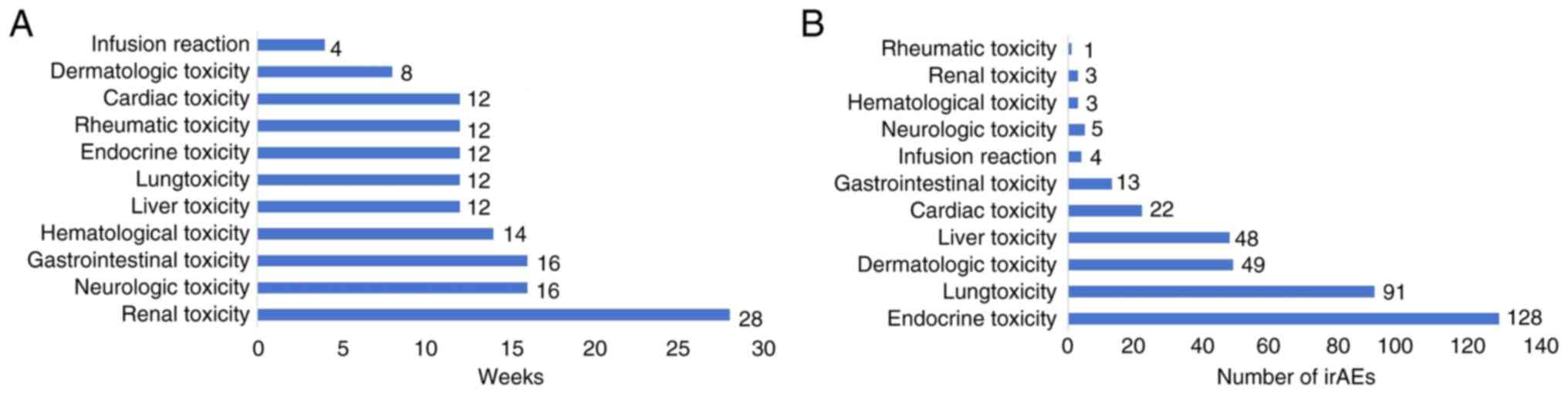
Type of irAEs in patients with
cancer
The incidence of irAEs was 27.8% (276/992),
including endocrine toxicity in 128 cases (12.9%), pulmonary
toxicity in 91 cases (9.2%), dermatologic toxicity in 49 cases
(4.9%), hepatic toxicity in 48 cases (4.8%), cardiac toxicity in 22
cases (2.2%), and gastrointestinal toxicity in 13 cases (1.3%).
Additionally, neurotoxicity, infusion reaction, hematological
toxicity, renal toxicity and rheumatic toxicity were observed
occasionally. The irAEs are categorized based on the Common
Terminology Criteria for Adverse Events, Version 5.0 (CTCAE v5.0)
(https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf#search=%22CTCAE%20v5.0%22).
Notably, among the irAEs exceeding grade 3, lung toxicity (65,
6.5%), liver toxicity (38, 3.8%), dermatologic toxicity (18, 1.8%)
and cardiac toxicity (13, 1.3%) had the highest incidence.
Additionally, there were 31 fatalities (3.1%) related to the irAEs,
mainly including pulmonary toxicity (24, 2.4%), liver toxicity (4,
0.4%), cardiac toxicity (2, 0.2%) and neurologic toxicity (1, 0.1%,
Table III). The irAEs occurred
4–28 weeks after the initiation of ICIs treatment, with a median
time of 16 weeks (Fig. 5A and
B).
 | Table III.Classification and grading of
immune-related adverse events. |
Table III.
Classification and grading of
immune-related adverse events.
| Classification of
adverse immune events | N=992 (%) | Grade 1–2 | Grade 3–4 | Grade 5 |
|---|
| Endocrine
toxicity | 128 (12.9) | 128 (12.9) | 0 | 0 |
| Lung toxicity | 91 (9.2) | 26 (2.6) | 41 (4.1%) | 24 (2.4%) |
| Dermatologic
toxicity | 49 (4.9) | 31 (3.1) | 18 (1.8%) | 0 |
| Liver toxicity | 48 (4.8) | 10 (1.0) | 34 (3.4%) | 4 (0.4%) |
| Cardiac
toxicity | 22 (2.2) | 9 (0.9) | 11 (1.1%) | 2 (0.2%) |
| Gastrointestinal
toxicity | 13 (1.3) | 9 (0.9) | 4 (0.4%) | 0 |
| Neurologic
toxicity | 5 (0.5) | 4 (0.4) | 0 | 1 (0.1%) |
| Infusion
reaction | 4 (0.4) | 4 (0.4) | 0 | 0 |
| Hematological
toxicity | 3 (0.3) | 1 (0.1) | 2 (0.2%) | 0 |
| Renal toxicity | 3 (0.3) | 3 (0.3) | 0 | 0 |
| Rheumatic
toxicity | 1 (0.1) | 1 (0.1) | 0 | 0 |
Discussion
The development of immunotherapy has resulted in
remarkable progress in the treatment of cancer (3,14).
Targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4),
programmed death protein-1 (PD-1) and programmed death protein
ligand-1 (PD-L1), representative ICIs, have been used for the
treatment of malignant tumors (15,16).
Currently, numerous inhibitors of CTLA-4, PD-1 and PD-L1 have been
registered in China, including nivolumab, pembrolizumab,
tislelizumab, toripalimab, camrelizumab, sintilimab (targeting
PD-1), atezolizumab, durvalumab, sugemalimab (targeting PD-L1), and
cadonilimab (targeting PD-1 and CTLA-4). With the widespread use of
ICIs, the incidence of irAEs has increased, and the use of ICIs in
patients with cancer creates the risk of serious or fatal toxic
reactions (17). Meanwhile, the
early identification, accurate evaluation and timely treatment of
irAEs remain challenging (10).
Therefore, further research is needed to develop more effective
ways to mitigate the risk of irAEs. To address this clinical
problem, a predictive model for irAEs in patients with cancer was
established in the present study.
ICIs have been used for the treatment of various
cancers. In the present study, ICIs were primarily utilized for the
treatment of diverse cancer types, including lung, liver,
esophageal, biliary tract and renal cancer, among other types.
Notably, 65.3% of the patients administered with ICIs in the
present study were in the non-advanced stage, indicating that ICIs
are not only used for the treatment of advanced cancers, but are
also widely used in neoadjuvant, adjuvant and locally advanced
cancer therapy (18,19). The study furthermore showed that
there was no significant difference in the occurrence of irAEs
among different types of ICIs.
Different types of irAEs were observed. In the
present study, 276 patients reported irAEs during treatment with
ICIs, with endocrine toxicity being the most common (n=128, 46.4%)
followed by lung toxicity (n=91, 33.0%), dermatologic toxicity
(n=49, 17.8%) and liver toxicity (n=48, 17.4%). Similarly,
endocrine toxicity is the most common adverse event in patients
undergoing treatment with ICIs (20). The immunological mechanism of
endocrine adverse reactions may be that irAEs are caused by various
pathways such as autoreactive T cells, autoantibodies and
cytokines. The rich blood supply of endocrine glands may increase
their sensitivity to these mechanisms, thus becoming one of the
more commonly affected targets (21). Although the occurrence of endocrine
toxicity is relatively high, its severity is usually limited to
grade 1 or 2 (22). Patient's
symptoms can typically be alleviated by promptly administering
symptomatic treatments, and in most cases, patients are able to
continue therapy with ICIs (23).
The most common fatal irAEs in patients treated with ICIs is lung
toxicity (24), which caused 2.4%
of fatality in the present study. Most irAEs occurred at 8 to 16
weeks after beginning treatment with ICIs. Renal toxicity occurred
later than other adverse reactions, with a median onset time of 28
weeks after beginning treatment with ICIs; infusion reactions
mainly occurred after the first or second ICIs dose. These results
are consistent with previous findings (25). The identification of risk factors
for irAEs is critical for establishing a predictive model. Some
biomarkers have been reported as risk factors of irAEs (10,26).
In the present study, hypertension, primary cancer, metastasis,
targeted drug combination and radiotherapy were identified as risk
factors of irAEs in patients with cancer. However, these factors
should likely have different weights in the predictive model
(27).
Hypertension, one of the risk factors for irAEs, has
attracted considerable attention (28). Previous studies have revealed the
effects of hypertension on the immune system, which is closely
related to the interactions between cytokines and T cells (29). A retrospective study indicated that
the occurrence of irAEs was associated with hypertension in
patients with cancer (30). Through
multifactor analysis, hypertension was confirmed as an independent
risk factor for irAEs (P=0.002).
The primary cancer type is the key factor affecting
irAEs (31). The risk of irAEs in
liver cancer was relatively low in the study. The study also found
that metastasis was a risk factor for irAEs, consistent with
previous findings (32). This
indicates that the primary cancer type and metastatic are crucial
factors in determining the occurrence of irAEs. Therefore, it is
important to tailor an individualized immunotherapy regimen for a
patient's cancer with different characteristics to achieve a
balance between efficacy and safety.
Radiation therapy can stimulate immune mechanisms
and trigger immune responses, especially when combined with ICIs,
thus affecting the safety of immunotherapy (33). Radiation therapy has also been
reported as a risk factor for irAEs (34,35).
Similarly, in this study, radiotherapy was identified as an
independent risk factor for irAEs (P=0.002). In the present study,
subgroup analysis of irAEs of 55 patients with a history of
radiotherapy was conducted. Of these, 34 patients (61.8%) had
undergone radiotherapy in the chest, with an average radiation dose
of 51.976±12.303 Gy, and the median time of irAEs occurrence was 5
months after radiotherapy.
The use of combination therapy regimens may improve
treatment efficacy but amplify irAEs (36,37).
The combination of ICIs and targeted drugs has been shown to
increase the risk of toxicity (38,39).
Similarly, the combination of targeted drugs was identified as an
independent risk factor for irAEs in the present study
(P<0.001). Therefore, when formulating combination immunotherapy
regimens, it is necessary to comprehensively evaluate the risk of
drug combination and closely monitor the adverse reactions during
the treatment.
Multivariate logistic regression analysis revealed
that hypertension, primary cancer, metastasis, targeted drug
combination and radiotherapy were risk factors for irAEs in the
present study (P<0.05). To facilitate the identification of
patients with cancer at high risk for irAEs, a nomogram-based
predictive model was developed based on relevant risk factors. The
HL test calibration showed that the χ2 was 2.909
(P=0.820) in the training set and the χ2 was 0.787
(P=0.978) in the validation set; P>0.05 indicated a favorable
fitting effect in both data sets. The AUC of ROC curve for the
training set data was 0.672 (95% CI: 0.630, 0.714), while that for
the validation set data was 0.776 (95% CI: 0.700, 0.853); AUC
>0.5 showed favorable discrimination of the model. DCA was shown
in Fig. 4, which indicated that the
model performed well and was feasible for making beneficial
clinical decisions. The predictive nomogram-based model established
in the present study has favorable predictive value for predicting
irAEs risk in patients with cancer. A recent study on
immune-associated pneumonia in patients with cancer demonstrated
the efficacy of nomogram-based models for predicting irAE risk
(40). Another study on
postoperative infection complications in patients with gastric
cancer also demonstrated the efficacy of the nomogram-based model
(41). Therefore, the developed
model can effectively identify high-risk patients with irAEs,
thereby facilitating early identification of irAEs, and improving
the prognosis of patients.
The present study had certain limitations. First, it
was a retrospective study, and potential confounding factors could
not be ruled out. Second, newly emerged irAEs-related biomarkers
(for example, cytokines and chemokines) were not considered when
developing the predictive model. These biomarkers require further
investigation. Finally, compared with external validation, the
internal validation applied in the present study may be less
robust.
In conclusion, the results of the present study
indicate that hypertension, primary cancer, metastasis, targeted
drug combination and radiotherapy are significant risk factors for
irAEs following treatment with ICIs in patients with cancer. The
predictive model is highly effective and can accurately identify
high-risk groups for irAEs, laying a solid foundation for
individualized immunotherapy strategies. This model is expected to
enable early evaluation of irAEs, thereby optimizing the management
strategies of irAEs, and ultimately significantly improving the
quality of life for patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hospital project of Xi'an
International Medical Center Hospital (grant no. 2021QN009).
Availability of data and materials
The data generated in the present study are not
publicly available due to patient privacy purposes but may be
requested from the corresponding author.
Authors' contributions
YT, JS, LX and TS designed the study. YT, JS, LW and
YZ collected, analyzed and interpreted the data. YT and TS wrote
the original draft of the manuscript. JS and YZ reviewed the
manuscript. JS and YT confirm the authenticity of all the raw data.
All authors contributed to the article, and read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Ethics Committee of Xi'an International Medical Center
Hospital (approval no. 202110; approval date: 2021.11.8; Xi'an,
China). This was a retrospective study; therefore, the written
informed consent was exempted from all participants included in the
current study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med.
8:328rv3242016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robert C: A decade of immune-checkpoint
inhibitors in cancer therapy. Nat Commun. 11:38012020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramos-Casals M and Siso-Almirall A:
Immune-Related adverse events of immune checkpoint inhibitors. Ann
Intern Med. 177:ITC17–ITC32. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin Q, Wu L, Han L, Zheng X, Tong R, Li L,
Bai L and Bian Y: Immune-related adverse events of immune
checkpoint inhibitors: A review. Front Immunol. 14:11679752023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Champiat S, Lambotte O, Barreau E, Belkhir
R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M,
Durrbach A, et al: Management of immune checkpoint blockade
dysimmune toxicities: A collaborative position paper. Ann Oncol.
27:559–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dougan M, Luoma AM, Dougan SK and
Wucherpfennig KW: Understanding and treating the inflammatory
adverse events of cancer immunotherapy. Cell. 184:1575–1588. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara Y, Horita N, Adib E, Zhou S,
Nassar AH, Asad ZUA, Cortellini A and Naqash AR: Treatment-related
adverse events, including fatal toxicities, in patients with solid
tumours receiving neoadjuvant and adjuvant immune checkpoint
blockade: A systematic review and meta-analysis of randomised
controlled trials. Lancet Oncol. 25:62–75. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhang X, Li W, Du Y, Hu W and
Zhao J: Biomarkers and risk factors for the early prediction of
immune-related adverse events: A review. Hum Vaccin Immunother.
18:20188942022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCulloch JA, Davar D, Rodrigues RR,
Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM,
Fernandes MR, et al: Intestinal microbiota signatures of clinical
response and immune-related adverse events in melanoma patients
treated with anti-PD-1. Nat Med. 28:545–556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smithy JW, Faleck DM and Postow MA: Facts
and hopes in prediction, diagnosis, and treatment of immune-related
adverse events. Clin Cancer Res. 28:1250–1257. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponvilawan B, Khan AW, Subramanian J and
Bansal D: Non-invasive predictive biomarkers for immune-related
adverse events due to immune checkpoint inhibitors. Cancers
(Basel). 16:12252024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan L, Li Y, Chen JY, Zheng YF and Xu XM:
Immune checkpoint modulators in cancer immunotherapy: Recent
advances and combination rationales. Cancer Lett. 456:23–28. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makuku R, Khalili N, Razi S,
Keshavarz-Fathi M and Rezaei N: Current and future perspectives of
PD-1/PDL-1 blockade in cancer immunotherapy. J Immunol Res.
2021:66614062021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russano M, Cortellini A, Giusti R, Russo
A, Zoratto F, Rastelli F, Gelibter A, Chiari R, Nigro O, De Tursi
M, et al: Clinical outcomes of NSCLC patients experiencing early
immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors
leading to treatment discontinuation. Cancer Immunol Immunother.
71:865–874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Felip E, Altorki N, Zhou C, Csoszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M,
Lee HC, Yopp AC, Zhou J, Wang L, Wen X, et al: Atezolizumab plus
bevacizumab versus active surveillance in patients with resected or
ablated high-risk hepatocellular carcinoma (IMbrave050): A
randomised, open-label, multicentre, phase 3 trial. Lancet.
402:1835–1847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khoja L, Day D, Chen TWW, Siu LL and
Hansen AR: Tumour- and class-specific patterns of immune-related
adverse events of immune checkpoint inhibitors: A systematic
review. Ann Oncol. 28:2377–2385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wright JJ, Powers AC and Johnson DB:
Endocrine toxicities of immune checkpoint inhibitors. Nat Rev
Endocrinol. 17:389–399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elshafie O, Khalil AB, Salman B, Atabani A
and Al-Sayegh H: Immune checkpoint inhibitors-induced
endocrinopathies: Assessment, management and monitoring in a
comprehensive cancer centre. Endocrinol Diabetes Metab.
7:e005052024. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panagiotou E, Ntouraki S, Vathiotis IA,
Livanou ME, Trimis A, Evangelou G, Charpidou A, Syrigos K and Peppa
M: Endocrine immune-related adverse events are independent
predictors of survival in patients with lung cancer. Cancers
(Basel). 16:17642024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sears CR, Peikert T, Possick JD, Naidoo J,
Nishino M, Patel SP, Camus P, Gaga M, Garon EB, Gould MK, et al:
Knowledge gaps and research priorities in immune checkpoint
inhibitor-related pneumonitis. An official American thoracic
society research statement. Am J Respir Crit Care Med. 200:e31–e43.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bracamonte-Baran W and Kim ST: The current
and future of biomarkers of immune related adverse events. Rheum
Dis Clin North Am. 50:201–227. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu HR, Zhu PF, Deng YY, Chen ZL and Yang
L: Predictive value of NLR and PLR for immune-related adverse
events: A systematic review and meta-analysis. Clin Transl Oncol.
26:1106–1116. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chennamadhavuni A, Abushahin L, Jin N,
Presley CJ and Manne A: Risk factors and biomarkers for
immune-related adverse events: A practical guide to identifying
high-risk patients and rechallenging immune checkpoint inhibitors.
Front Immunol. 13:7796912022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh MV, Chapleau MW, Harwani SC and
Abboud FM: The immune system and hypertension. Immunol Res.
59:243–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao W, Liu W, Chang R, Yang M, Xin K, Liu
J, Wang Y, Ren M, Xie J and Yang Y: Safety and clinical efficacy of
immune checkpoint inhibitors in advanced gastric cancer in the real
world. J Cancer Res Clin Oncol. 150:1802024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Remolina-Bonilla YA, Jimenez-Franco B, Lam
ET and Bourlon MT: Immune-related adverse events involving multiple
organ sites in a patient treated with nivolumab plus ipilimumab.
Oncology (Williston Park). 34:171–174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vokes EE, Ready N, Felip E, Horn L, Burgio
MA, Antonia SJ, Aren Frontera O, Gettinger S, Holgado E, Spigel D,
et al: Nivolumab versus docetaxel in previously treated advanced
non-small-cell lung cancer (CheckMate 017 and CheckMate 057):
3-year update and outcomes in patients with liver metastases. Ann
Oncol. 29:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bardoscia L, Pasinetti N, Triggiani L,
Cozzi S and Sardaro A: Biological bases of immune-related adverse
events and potential crosslinks with immunogenic effects of
radiation. Front Pharmacol. 12:7468532021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaverdian N, Beattie J, Thor M, Offin M,
Shepherd AF, Gelblum DY, Wu AJ, Simone CB II, Hellmann MD, Chaft
JE, et al: Safety of thoracic radiotherapy in patients with prior
immune-related adverse events from immune checkpoint inhibitors.
Ann Oncol. 31:1719–1724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu X, Wang J, Zhang T, Zhou Z, Deng L,
Wang X, Wang W, Liu W, Tang W, Wang Z, et al: Comprehensive
pneumonitis profile of thoracic radiotherapy followed by immune
checkpoint inhibitor and risk factors for radiation recall
pneumonitis in lung cancer. Front Immunol. 13:9187872022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoos A: Development of immuno-oncology
drugs-from CTLA4 to PD1 to the next generations. Nat Rev Drug
Discov. 15:235–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reynolds KL, Arora S, Elayavilli RK, Louv
WC, Schaller TH, Khandelwal A, Rothenberg M, Khozin S, Guidon AC,
Dougan M, et al: Immune-related adverse events associated with
immune checkpoint inhibitors: A call to action for collecting and
sharing clinical trial and real-world data. J Immunother Cancer.
9:e0028962021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn MJ, Sun JM, Lee SH, Ahn JS and Park K:
EGFR TKI combination with immunotherapy in non-small cell lung
cancer. Expert Opin Drug Saf. 16:465–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spigel DR, Reynolds C, Waterhouse D, Garon
EB, Chandler J, Babu S, Thurmes P, Spira A, Jotte R, Zhu J, et al:
Phase 1/2 study of the safety and tolerability of nivolumab plus
crizotinib for the first-line treatment of anaplastic lymphoma
kinase translocation-positive advanced non-small cell lung cancer
(CheckMate 370). J Thorac Oncol. 13:682–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Lv F, Wang Y and Du Z: Establishment
and validation of nomogram for predicting immuno checkpoint
inhibitor related pneumonia. BMC Pulm Med. 22:3312022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong Z, Liu G, Tu L, Su X and Yu Y:
Establishment of a prediction model of postoperative infection
complications in patients with gastric cancer and its impact on
prognosis. J Gastrointest Oncol. 14:1250–1258. 2023. View Article : Google Scholar : PubMed/NCBI
|