Introduction
Hyalinizing clear cell carcinoma (HCCC) is a rare,
low-grade malignant epithelial tumor predominantly arising in the
salivary glands, with occurrences in the lungs being particularly
uncommon. The 2021 World Health Organization classification of
thoracic tumors first identified HCCC as a distinct pulmonary
epithelial neoplasm, characterized by clear and eosinophilic cells
arranged in trabecular, nest-like or cord-like patterns within a
mucinous and hyalinized stroma. The International Classification of
Diseases for Oncology code for HCCC is 8310/31 (1).
The incidence of pulmonary HCCC is low, with ~20
cases reported worldwide. HCCC typically originates from minor
salivary glands in the tracheobronchial submucosa, leading to
bronchial obstruction, coughing and dyspnea, with hemoptysis being
a less common side effect (2).
Tracheal HCCC is particularly rare, and to the best of our
knowledge, only three cases have been documented in the literature,
all in women aged 46–66 years. Of these cases, two were managed
surgically (2,3), while one was treated with an
endoscopic laser and cryotherapy (4).
The present study reports the case of a 34-year-old
female patient who presented with HCCC of the trachea. The initial
symptoms included a persistent cough and dyspnea, which were
subsequently managed with surgical resection. The present case
highlights the diagnostic and therapeutic challenges posed by this
rare type of tumor and aims to enhance the understanding of its
unique clinical features.
Case report
In February 2021, a 34-year-old woman was referred
to Tianjin Chest Hospital Affiliated to Tianjin University
(Tianjin, China) with a 1-month history of a persistent cough and
intermittent dyspnea, without sputum production, fatigue or chest
discomfort. The physical examination was unremarkable, showing a
normal thoracic structure with clear breath sounds bilaterally.
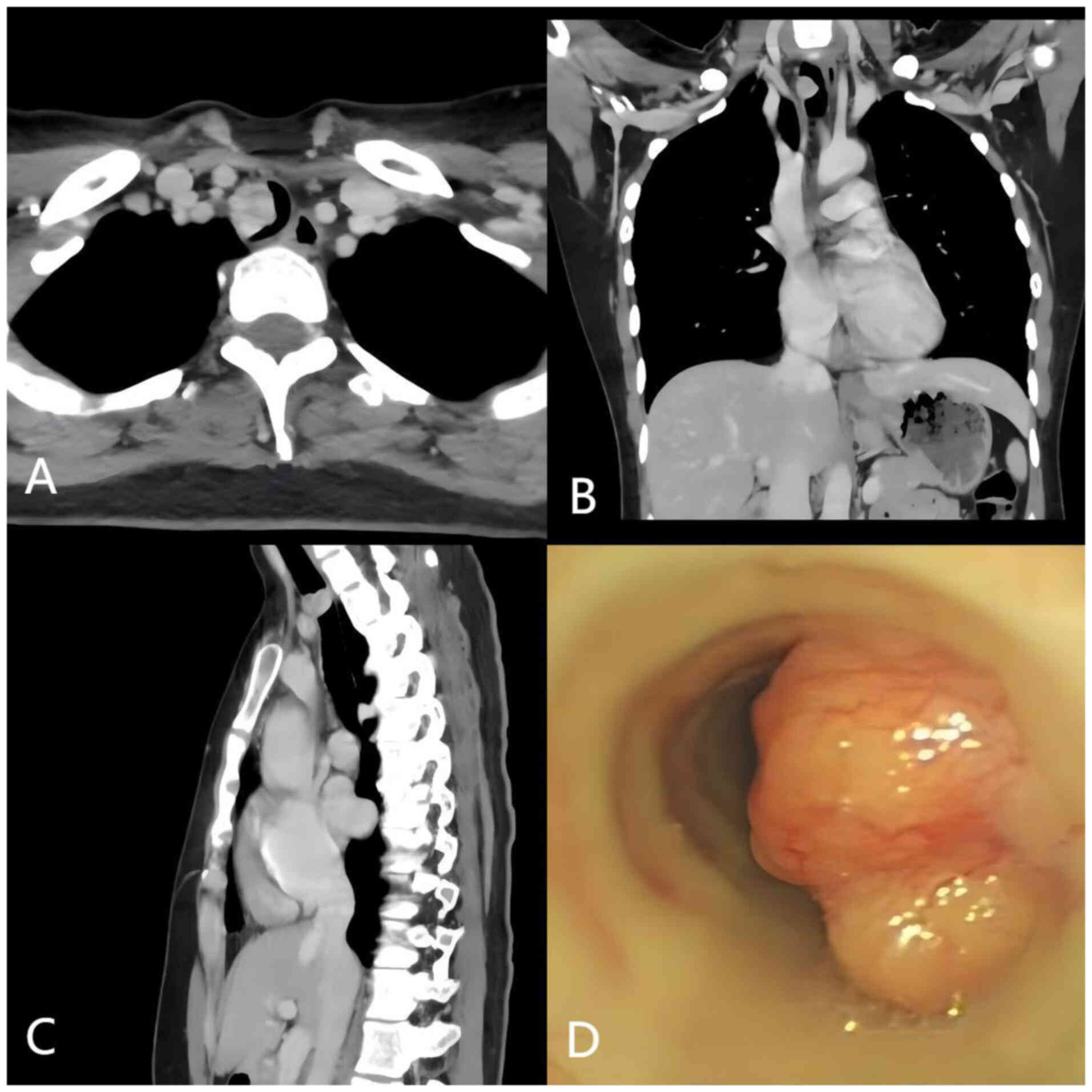
Enhanced chest computed tomography (CT) scan results
showed a hypervascular nodule, measuring 2.3×2.0 cm, on the
posterior wall of the trachea at the level of the thoracic inlet,
with no evidence of metastasis to the regional lymph nodes. The
lesion occupied approximately two-thirds of the tracheal lumen and
was classified as cT2N0M0 based on the 8th edition of the TNM
staging system established by the Union for International Cancer
Control and the American Joint Committee on Cancer (5). The patient's lactate dehydrogenase
(LDH) level was 186 U/l, which was within the normal range (120–250
U/l). Although elevated LDH levels can be associated with tumor
presence, the patient's LDH value remained normal, suggesting that
LDH may not always be indicative of malignancy in such cases
(6). Routine blood count and
biochemical analyses were also within normal ranges. The patient
reported no personal or family history of tumor-related genetic
disorders. Evidence of right-sided tracheal wall invasion raised
concerns for possible transmural extension. Subsequent bronchoscopy
results showed a broad-based, lobulated mass within the trachea,
seven cartilage rings below the glottis, causing significant
luminal obstruction (Fig. 1).
A preoperative biopsy suggested poorly
differentiated squamous cell carcinoma based on histological and
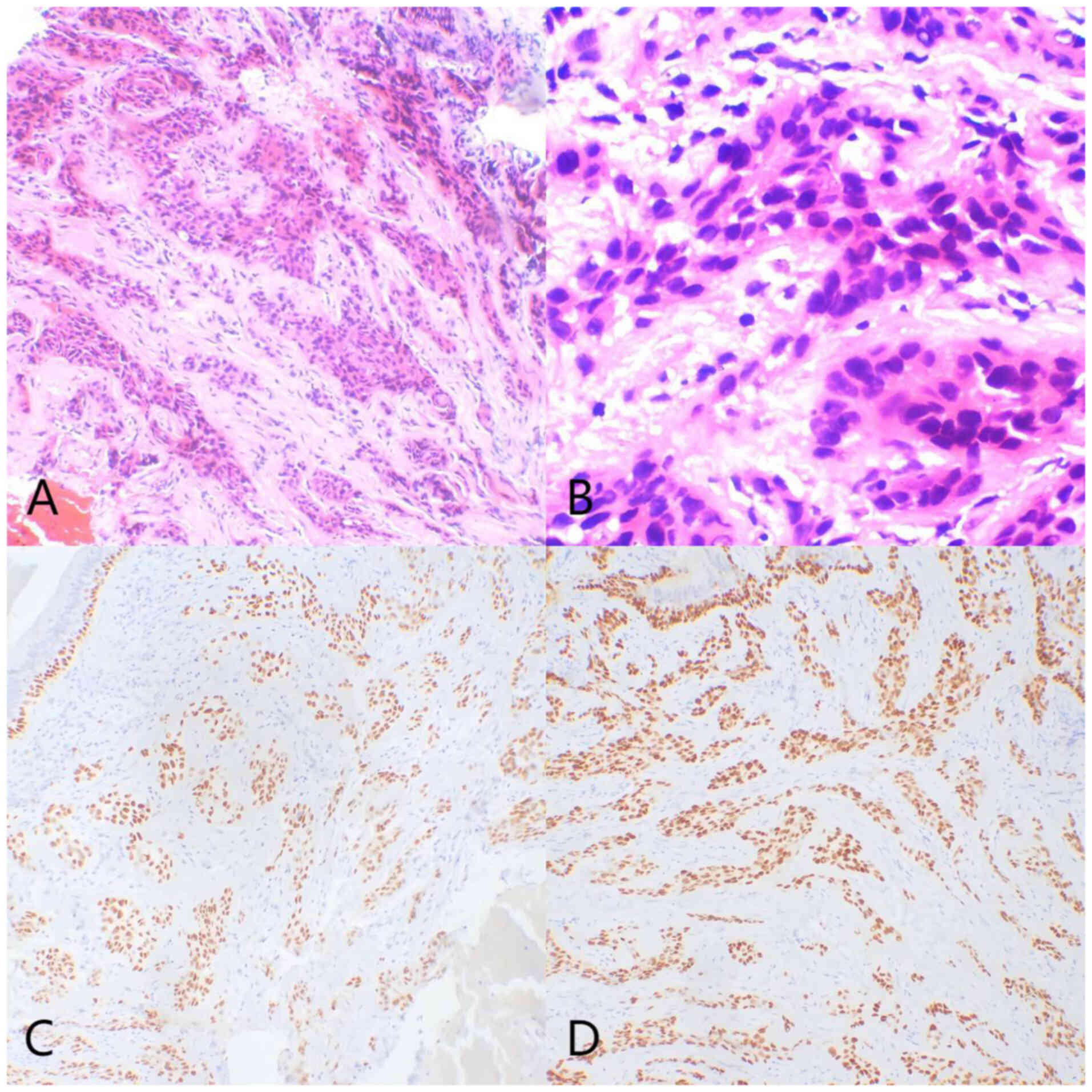
immunohistochemical findings. Histologically, small round tumor
cells were observed infiltrating the submucosa, arranged in
trabecular or nest-like patterns within fibrous connective tissue.
Tumor cells exhibited mild nuclear atypia, uniform size and
occasional clear cytoplasm, with no significant keratinization or
mitotic figures observed (Fig. 2A and
B). Immunohistochemical staining revealed diffuse strong
positivity for p40 and p63 (Fig. 2C and
D), while markers such as CD56, TTF-1, Napsin A, and
Synaptophysin were negative, supporting squamous differentiation.
CEA showed partial positivity, while Ki-67 demonstrated a low
proliferative index (<10%), and weak positivity for p53 was
noted. Based on these findings and the biopsy limitations, an
initial diagnosis suggesting poorly differentiated squamous cell
carcinoma was made, with final confirmation deferred to the
surgical resection specimen. In February 2021, the patient
underwent a median sternotomy with resection of the tracheal tumor
and an end-to-end tracheal anastomosis, all under general
anesthesia. The tumor measured ~2×1.5×1 cm and was firm in
consistency. Surgical dissection achieved a 1-cm margin on both the
superior and inferior edges of the tumor, which was resected using
electrocauterization. The primary technical challenges included the
proximity of the tumor to critical vascular structures, such as the
brachiocephalic artery and common carotid artery, necessitating
meticulous dissection to avoid injury to these vessels and the
recurrent laryngeal nerve. Additionally, the occupation of the
tumor in the tracheal lumen complicated intraoperative airway
management. A staged distal tracheal intubation technique was
employed to maintain airway patency and oxygenation during tumor
resection and tracheal reconstruction. Continuous suturing ensured
a watertight anastomosis, minimizing the risk of postoperative
airway stenosis or fistula formation. Care was taken to preserve
the recurrent laryngeal nerve, preventing postoperative vocal cord
dysfunction.
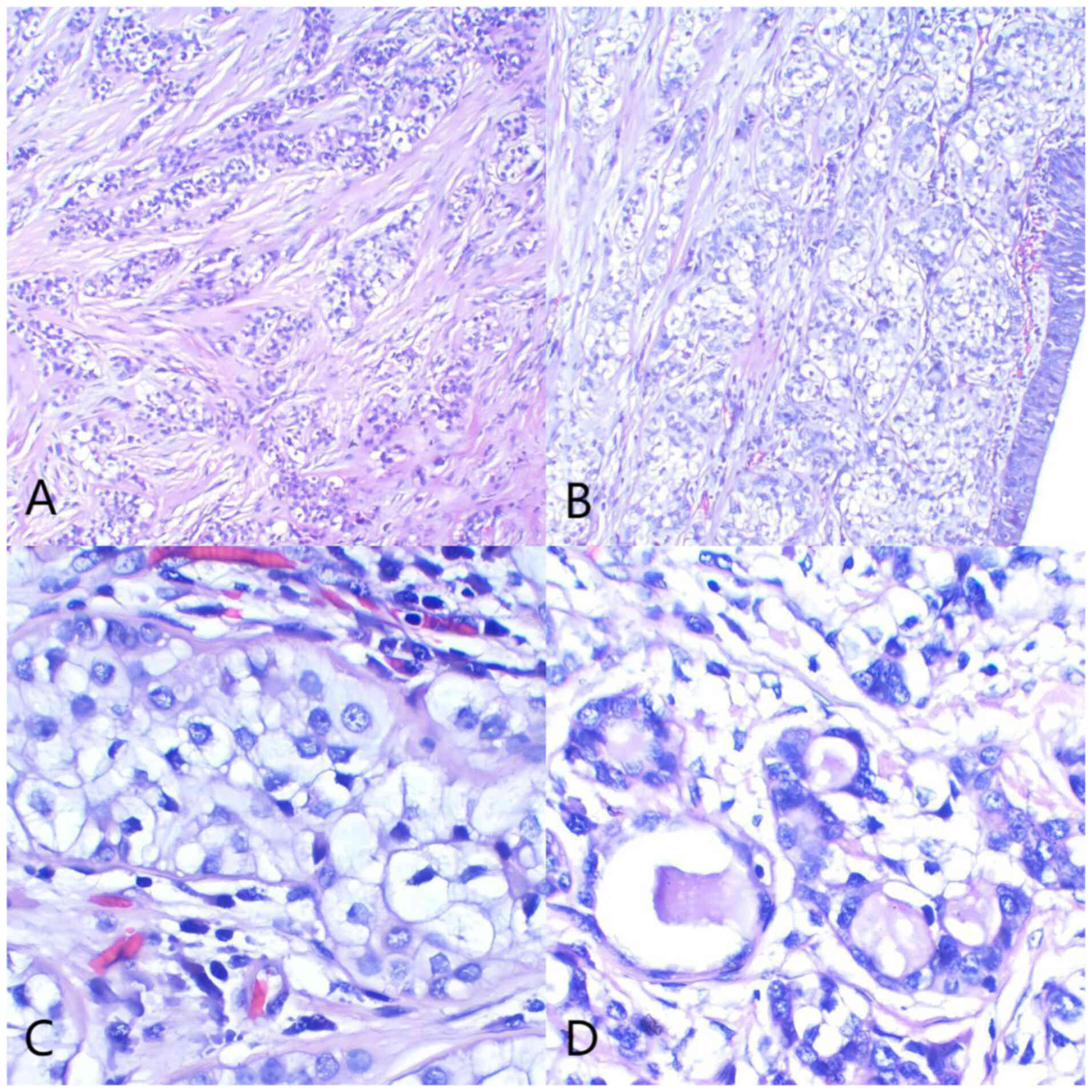
The pathological examination showed a 4.2-cm segment
of resected trachea with a proximal and distal circumference of 2.5
and 2.0 cm, respectively. A polypoid, gray-yellow mass measuring
1.7×1.2×1.2 cm protruded into the lumen. Histologically, the tumor
was in the submucosa and consisted of cells arranged in trabecular,
cord-like and nest-like patterns within a hyalinized stroma. The
tumor cells were round to oval and uniform in size, with
well-defined cell membranes, mild atypia, clear to eosinophilic
cytoplasm, regular nuclear membranes, inconspicuous nucleoli and
rare mitotic figures. A number of cells formed glandular structures
with mucin secretion, and no keratinization was observed. Mucinous
degeneration was noted in areas of the stroma. Tumor tissues were
fixed in 10% neutral buffered formalin at room temperature for 24
h, embedded in paraffin, and sectioned at a thickness of 4 µm.
Sections were stained with hematoxylin and eosin at room
temperature (hematoxylin for 5 min and eosin for 2 min). The
histological features were observed under a Leica light microscope
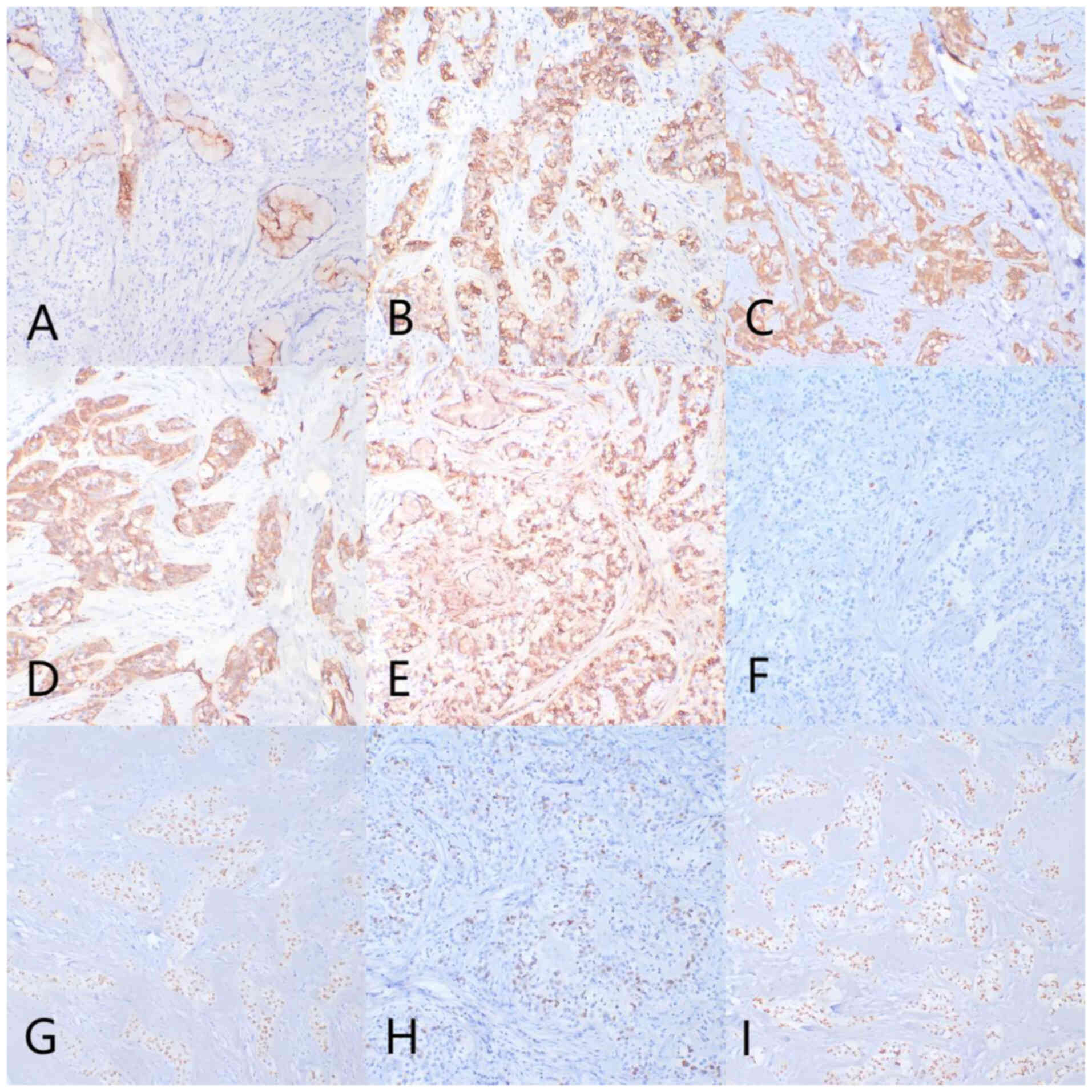
(DM3000) with magnifications of ×100, ×200 and ×400 (Fig. 3). Immunohistochemical analysis
demonstrated specific marker profiles as follows: Diffuse strong
positivity for carcinoembryonic antigen (CEA), cytokeratin (CK)7,
CK5/6, pan-CK and epithelial membrane antigen, diffuse positivity
for p40 and p63, and focal positivity for p53. Tissue sections were
blocked with 5% normal goat serum (Beyotime Institute of
Biotechnology) at room temperature for 30 min. Primary antibodies,
including CEA (dilution 1:200; cat. no. ZM-0096; ZSGB-Bio), CK7
(dilution 1:200; cat. no. ab92742; Abcam), CK5/6 (dilution 1:200;
cat. no. ab52635; Abcam), pan-CK (dilution 1:300; cat. no. ZM-0069;
ZSGB-Bio), EMA (dilution 1:200; cat. no. ab124964; Abcam), p40
(dilution 1:200; cat. no. ab235897; Abcam), p63 (dilution 1:200;
cat. no. ab735; Abcam), Ki-67 (dilution 1:200; cat. no. ab16667;
Abcam) and p53 (dilution 1:200; cat. no. ab26; Abcam), were
incubated at 4°C overnight. Secondary antibody (HRP-conjugated
anti-rabbit IgG; dilution 1:500; cat. no. 111-035-003; Jackson
ImmunoResearch) was incubated at room temperature for 30 min.
Chromogen detection was performed using the DAB kit (ZSGB-Bio)
following the manufacturer's protocol. The Ki-67 proliferative
index was low, at <5% (Fig.
4).
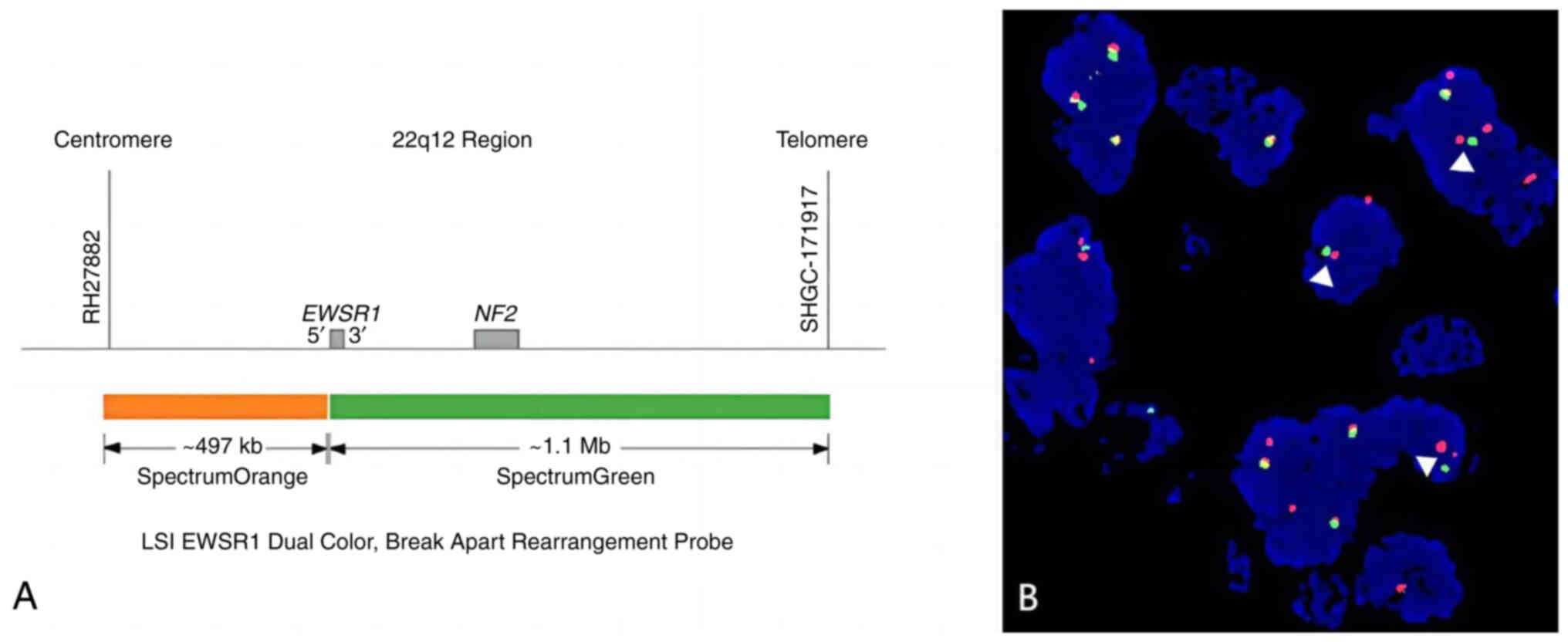
Fluorescence in situ hybridization (FISH) was
performed on formalin-fixed, paraffin-embedded tissue sections
(4-µm thickness). Sections were deparaffinized in xylene,
rehydrated in graded ethanol and subjected to proteolytic digestion
using pepsin solution (cat. no. P7000; MilliporeSigma) at 37°C for
15 min. Hybridization was conducted overnight at 37°C with a
dual-color break-apart probe targeting the Ewing sarcoma RNA
binding protein 1 (EWSR1; ZytoLight® 22q12.2;
ZytoVision) using the ZytoLight FISH-Tissue Implementation Kit
(ZytoVision) according to the manufacturer's protocol.
Post-hybridization washing was carried out in 0.4X SSC at 72°C for
2 min, followed by 2X SSC at room temperature for 2 min. FISH
signals were visualized using a Leica DM6 B fluorescence microscope
(Leica Microsystems) equipped with a DAPI filter at ×1,000
magnification. Images were captured and analyzed using CytoVision
software (version 7.6; Leica Microsystems). The dual-color
break-apart probe consists of fluorescently labeled regions
flanking the EWSR1 breakpoint, allowing rearrangements to be
identified by the separation of red and green signals. EWSR1
rearrangement was identified in the analyzed sample through FISH
(Fig. 5). The final pathological
diagnosis was HCCC of the trachea, with full-thickness tracheal
wall invasion. The surgical margins were negative for tumor
invasion.
The patient was monitored with regular follow-up
visits, including chest CT scans, every 3 months. At the most
recent follow-up in June 2024, which was 3 years post-surgery,
there was no evidence of recurrence and the patient remained in
good health.
Discussion
HCCC is a rare, low-grade malignant epithelial tumor
typically originating in the salivary glands, with pulmonary cases
being particularly uncommon. The present study describes the case
of a 34-year-old woman with primary tracheal HCCC, presenting as a
persistent cough and dyspnea, which was successfully treated
through surgical resection. The present report highlights the
diagnostic challenges, and underscores the importance of
comprehensive histopathological and molecular analysis in
identifying this rare type of tumor. Given the rarity and low-grade
malignant nature of HCCC, the necessity for prophylactic lymph node
dissection currently remains uncertain. While the tumor can exhibit
local invasion, its potential for lymphatic spread is not well
documented due to the limited number of cases reported. In the
present case, no regional lymph node metastasis was observed,
supporting a more conservative approach; however, further research
involving larger patient cohorts is needed to establish clear
guidelines on lymph node management in HCCC.
Current knowledge of HCCC is largely derived from
head and neck pathological studies, such as the study in which the
cancer was first characterized by Milchgrub et al in 1994 (7). The initial and subsequent
classifications of HCCC emphasize its unique histological features
and frequent squamous differentiation, identified by markers such
as p63 and 34bE12 (8). In the
present case, diffuse positivity for squamous markers such as p63
and p40, combined with the absence of characteristic clear cell
morphology, led to an initial misdiagnosis of squamous cell
carcinoma, underscoring the risk of diagnostic pitfalls when using
small biopsy samples. Major differential diagnoses included
squamous cell carcinoma with clear cell changes, salivary
gland-type tumors, such as low-grade mucoepidermoid carcinoma, and
metastatic clear cell carcinoma of renal origin. In challenging
patient cases, the EWSR1 gene rearrangement test provides valuable
diagnostic confirmation of the diagnosis. Despite the positivity
for p63, CK5/6 and differential keratins, the bland histological
appearance of the tumor, its low mitotic index and the lack of
keratinization provided evidence against a diagnosis of squamous
cell carcinoma. Differentiating HCCC from low-grade mucoepidermoid
carcinoma can be particularly challenging, as HCCC may exhibit
occasional mucin-positive cells. In complex cases, FISH testing for
mastermind-like transcriptional coactivator 2 and EWSR1
rearrangements can provide critical diagnostic support to
clinicians.
FISH for EWSR1 rearrangement is a valuable
diagnostic tool for HCCC. Between 87 and 91% of HCCC cases in the
head and neck harbor EWSR1 rearrangements, most commonly involving
a fusion with activating transcription factor 1 (ATF1). Reverse
transcription-PCR and sequencing studies have shown that ~93% of
HCCC cases feature an EWSR1-ATF1 fusion (9). In the present case, FISH analysis
using a dual-color break-apart probe for EWSR1 confirmed the
presence of EWSR1 rearrangement. Although EWSR1 rearrangement can
also be observed in other types of tumors, such as Ewing sarcoma,
desmoplastic small round cell tumor, clear cell sarcoma and myxoid
chondrosarcoma, these tumor diagnoses were excluded based on their
distinct clinicopathological characteristics.
The diagnosis and management of HCCC present unique
challenges. Due to its rarity, HCCC is often underrecognized,
leading to potential diagnostic delays. Preoperative biopsies may
not always yield definitive results, necessitating postoperative
pathological confirmation and molecular testing. While there is
currently no standardized treatment protocol due to the rarity of
this tumor, surgical resection with clear margins remains the
mainstay of treatment. Despite its potential for local invasion and
regional metastasis, HCCC generally has a favorable prognosis.
Nevertheless, due to the rarity of this tumor, current knowledge is
predominantly based on case reports, and there is a lack of cohort
studies or long-term follow-up data to provide definitive survival
rates or statistics. With only 22 cases reported worldwide, the
clinical behavior of pulmonary HCCC is not yet completely
understood (10). Tracheal HCCC is
particularly rare, with the present case being only the fourth
currently reported in the literature, to the best of our knowledge.
The three previously reported cases of tracheal HCCC involved
female patients aged 46 to 66 years, with varied smoking histories.
Tumor sizes ranged from 1.3 to 2.5 cm when mentioned, and all were
located in the trachea. Molecular testing in all cases confirmed
EWSR1 gene rearrangement, with one case identifying an EWSR1:ATF1
gene fusion. Treatment strategies included laser therapy and
cryotherapy in one case, while the other two patients underwent
surgical resection, with one receiving additional radiation therapy
and another receiving chemoradiation. Survival outcomes varied: One
patient experienced no recurrence or metastasis at 12 months
post-treatment, while another succumbed 6 years after the initial
diagnosis. Compared with the present case of a 34-year-old female
non-smoker treated with surgical resection, the absence of
recurrence and favorable postoperative recovery align with previous
reports (2–4). The Ki-67 index in the present case was
notably low, consistent with the indolent clinical behavior
observed in the patient, who remained disease-free at the last
follow-up. Low proliferative indices, as indicated by Ki-67, can be
associated with favorable outcomes in rare tumors, including HCCC.
This pattern aligns with findings in other uncommon low-grade
malignancies, such as certain types of clear cell tumor, which also
demonstrate low Ki-67 levels and prolonged survival times (11). The low Ki-67 index in HCCC suggests
that, despite its potential for local invasion, the growth rate of
this tumor is generally slow, reinforcing the importance of
complete surgical resection as a viable treatment strategy, with a
favorable prognosis in similar cases.
Unlike the patients in previously reported cases,
the present patient was relatively young, and the tumor presented
with unusual tracheal obstruction, making the present case a rare
example of tracheal involvement in HCCC. The diagnostic challenge
was further compounded by the lack of prominent clear cell
features, which initially suggested squamous cell carcinoma. This
combination of clinical and diagnostic complexities highlighted the
importance of a thorough histopathological and molecular analysis
when identifying such rare cases, and underscored the need for
clinical awareness of the atypical presentations of HCCC.
In conclusion, primary pulmonary HCCC is an
exceptionally rare tumor with distinct pathological features.
Further studies involving larger patient cohorts and extended
follow-up are essential to improve the current understanding of its
clinical behavior and to refine therapeutic strategies.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Tianjin Medical Key
Specialty Construction Project (project no. TJYXZDXK-018A) and the
Tianjin Metrology Science and Technology Project (project no.
2024TJMT001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WZ and ZW were responsible for study conception and
design. XL performed the surgical procedure and contributed to the
revision of the manuscript for important intellectual content. DS
contributed to the formulation of the surgical strategy, provided
insights into patient management, and assisted in the analysis and
interpretation of clinical data. Additionally, DS revised the
manuscript for intellectual content and accuracy. GG assisted with
data acquisition and interpretation. WZ and ZW were responsible for
the provision of study materials or patients, and the collection
and assembly of data. Data analysis and interpretation was
performed by WZ and ZW. All authors helped to write the manuscript.
WZ and ZW confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO Classification of Tumours Editorial
Board, . Thoracic Tumours. WHO Classification of Tumours. 5th
edition. Vol 5. International Agency for Research on Cancer; Lyon:
2021
|
|
2
|
Doxtader EE, Shah AA, Zhang Y, Wang H,
Dyhdalo KS and Farver C: Primary salivary gland-type tumors of the
tracheobronchial tree diagnosed by transbronchial fine needle
aspiration: Clinical and Cytomorphologic features with
histopathologic correlation. Diagn Cytopathol. 47:1168–1176. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gubbiotti MA, Montone K, Zhang P, Livolsi
V and Baloch Z: A contemporary update on hyalinizing clear cell
carcinoma: compilation of all in-house cases at our institution and
a literature review spanning 2015–2020. Hum. Pathol. 111:45–51.
2021.
|
|
4
|
Icard B, Grider DJ, Aziz S and Rubio E:
Primary tracheal hyalinizing clear cell carcinoma. Lung Cancer.
125:100–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Joint Committee on Cancer (AJCC),
. AJCC Cancer Staging Manual. 8th edition. Amin MB, Greene FL, Edge
SB, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton
CC, Hess KR, Sullivan DC, et al: Springer Cham; New York, NY:
2017
|
|
6
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Milchgrub S, Gnepp DR, Vuitch F, Delgado R
and Albores-Saavedra J: Hyalinizing clear cell carcinoma of
salivary gland. Am J Surg Pathol. 18:74–82. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weinreb I: Hyalinizing clear cell
carcinoma of salivary gland: A review and update. Head Neck Pathol.
7 (Suppl 1):S20–S29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thway K and Fisher C: Tumors with
EWSR1-CREB1 and EWSR1-ATF1 Fusions: The current status. Am J Surg
Pathol. 36:e1–e11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YL, Wu F, Cao MF, Lan Y, Du MS, Yu ST,
Wang Y, Yan XC, Bian XW and Duan GJ: Primary pulmonary hyalinizing
clear cell carcinoma with fusions of both EWSR1::CREM and
IRF2::NTRK3: Report of a case with an aggressive behavior. Front
Oncol. 13:11752792023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marković M, Jurišić V, Petrović M, Dagović
A, Stanković V and Mitrović S: Appearance of ductal breast and
colon carcinoma with gastrointestinal stromal tumor (GIST) in a
female patient: An extremely rare case. Rom J Morphol Embryol.
59:613–617. 2018.PubMed/NCBI
|