Introduction
Ewing sarcoma is a round cell sarcoma commonly found
in younger patients (children and young adults), and standard
therapy includes vincristine, doxorubicin, and cyclophosphamide
alternating with ifosfamide and etoposide (VDC/IE) chemotherapy in
combination with surgical resection and/or radiation therapy
(1). Meanwhile, round cell sarcomas
which resemble Ewing sarcoma are referred to as ‘Ewing-like
sarcomas’. According to the latest edition of the World Health
Organization classification, in undifferentiated round cell
sarcomas of the bones and soft tissues, Ewing-like sarcoma has been
classified into three categories: round cell sarcoma with
EWSR1::non-ETS fusions, capicua transcriptional repressor
(CIC)-rearranged sarcoma, and sarcoma with B cell lymphoma 6
corepressor (BCOR) genetic alterations (2). CIC-rearranged sarcomas and sarcomas
with BCOR genetic alterations are well-established
clinicopathologically, whereas round cell sarcomas with
EWSR1::non-ETS fusions are a heterogeneous and premature
group.
Since the discovery of EWSR1::NFATC2 sarcoma
as an EWSR1::non-ETS sarcoma by Szuhai et al
(3), new clinical and pathological
information on this sarcoma has accumulated. Yoshida et al
(4) reported that, in addition to
the co-expression of CD99, NKX2-2, and PAX7, NKX3-1 is a useful
immunohistochemical marker of EWSR1::NFATC2 sarcomas,
similar to Ewing sarcoma (5–7). In
addition, Perret et al (8)
demonstrated the utility of aggrecan immunohistochemistry for the
identification of NFATC2-rearranged sarcomas, including
EWSR1::NFATC2 and FUS::NFATC2 fusions. Furthermore,
unlike Ewing sarcoma, these sarcomas often form focal nests, cords,
or trabeculae within a fibrotic, hyalinized, or myxoid stromal
background, mimicking myoepithelial tumors (4,9).
EWSR1::NFATC2 sarcoma is very rare but is
considered to be the most common subtype of ESWR1::non-ETS
sarcoma. Regarding pathological diagnosis, the histological
features of EWSR1::NFATC2 sarcoma are similar to those of
other sarcomas such as Ewing-like adamantinoma, which was first
reported by Lipper et al (10) as a variant of adamantinoma of the
long bones. Notably, Makise et al (11) reported the case of a patient
previously diagnosed with Ewing-like adamantinoma who was finally
diagnosed with EWSR1::NFATC2 sarcoma using fluorescence
in situ hybridization (FISH)-amplified fusion signals of
EWSR1 and NFATC2.
EWSR1::NFATC2 sarcoma is thought to have a
more indolent clinical course than Ewing sarcoma, despite poor
responses to Ewing sarcoma chemotherapy regimens (7); therefore, metastatic
EWSR1::NFATC2 sarcoma is very rare. Thus, there is
insufficient information regarding chemotherapeutic treatments for
metastatic EWSR1::NFATC2 sarcoma. Herein, we report a case
of a man with EWSR1::NFATC2 sarcoma, initially diagnosed as
Ewing-like adamantinoma, who received a series of chemotherapy
treatments of for Ewing sarcoma, including eribulin, trabectedin,
and pazopanib.
Case report
A 55-year-old male patient with Ewing-like
adamantinoma, who had a durable response with trabectedin
monotherapy as third-line therapy, was recently definitively
diagnosed as having EWSR1::NFATC2 sarcoma using direct
sequencing. The patient was admitted to Department of Medical
Oncology, Cancer Institute Hospital of Japanese Foundation for
Cancer Research (Tokyo, Japan) in July 2022. He initially
experienced pain in his left lower leg and consulted a local clinic
at 38 years of age. The patient also exhibited disc space narrowing
and osteolytic changes in the proximal tibia. The patient had no
significant medical history. One month later, he underwent biopsy
of the tumor in the proximal part of the left tibia at our
hospital. The pathological diagnosis was ‘epithelioid malignant
tumor of the left tibia suggesting Ewing-like adamantinoma’. A
month thereafter, he underwent wide excision of the tumor in his
left tibia with left total knee arthroplasty and a medial
gastrocnemius muscle flap. The patient survived with no evidence of
recurrent or metastatic disease. However, five years after surgery,
the patient underwent right thyroid lobectomy and lymph node
dissection for papillary thyroid cancer. In addition, 14 years
after surgery, multiple new lesions were detected radiographically
in the left lung, left pleural dissemination, and mediastinal, left
hilar, and juxtaesophageal lymph nodes. The lesion, which was
biopsied by transbronchial lung biopsy (TBLB), was diagnosed as
‘Ewing-like adamantinoma’. At 52 years of age, he started systemic
chemotherapy with five cycles of vincristine, doxorubicin, and
cyclophosphamide (VDC) combination chemotherapy, with the maximum
doxorubicin dosage, resulting in stable disease (SD). Next, he
received eight cycles of vincristine, actinomycin D, and
cyclophosphamide (VAC) combination chemotherapy. In addition, he
received radiation therapy (60 Gy/30 fractions) for the tumor in
the left lower lobe. However, he developed radiation pneumonitis
and steroid therapy was initiated at a dose of 40 mg prednisolone
per day. The prednisolone dosage was gradually reduced, and once a
dosage of 5 mg/day was reached, the patient started receiving
eribulin monotherapy as second-line therapy. Prednisolone was
administered until the completion of 16 cycles of eribulin.
Microsatellite instability (MSI) tests using the TBLB specimens
yielded negative results. In addition, a BCL2L1
amplification, previously reported in EWSR1::NFATC2 sarcoma,
was identified using FoundationOne® CDx (12). Although EWSR1 rearrangement
was not detected using FoundationOne® CDx, FISH analysis
using an EWSR1 break apart probe revealed amplification of
the 5′-end of EWSR1, indicating the rearrangement of the
EWSR1 gene, compatible with EWSR1::NFATC2 sarcoma.
Reverse transcription-polymerase chain reaction (RT-PCR) using a
pair of primers (forward: 5′-GAGAGAACCGGAGCATGAGTG-3′ and reverse:
5′-CTTGGGCTGCACCTCGATCCGC-3′) followed by direct sequencing
revealed an in-frame EWSR1::NFATC2 fusion where exon 8 of
EWSR1 (ENST00000397938.7) was fused to exon 3 of
NFATC2 (ENST00000371564.8), as previously shown by Sadri
et al (9). We also used a
pair of primers for glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) (forward: 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse:
5′-GAAGATGGTGATGGGATTTC-3′) as internal control for RT-PCR. In
addition, the tumor mutational burden (TMB) was found to be 1
Mut/Mb. Three months later, he had progressive disease (PD): left
pleural dissemination progressed, left pleural effusion increased,
and peritoneal dissemination in the left paracolic gutter was
suspected. The next treatment was trabectedin monotherapy. Although
he also had a bone infection in the postoperative region of the
left tibia, he continued the trabectedin monotherapy with
concurrent oral antibiotics. The patient remained stable for 16
months, however, after 18 cycles of trabectedin monotherapy, the
patient developed PD. He was subsequently administered pazopanib
(800 mg/day). After 2 months, he once again had PD due to the
progression of the pleural effusion.
Discussion
In recent years, EWSR1::NFATC2 sarcoma has
become recognizable using various pathological and genetic tests.
Our patient was diagnosed by additional immunohistochemical
staining for NKX3-1, FISH, and RT-PCR followed by direct
sequencing. EWSR1::NFATC2 sarcoma is classified as
ESWR1::non-ETS sarcoma, a wastebasket diagnosis; however, it
comprises the majority of this class of Ewing-like sarcomas, and
the number of case reports and studies have been accumulating.
EWSR1::NFATC2 sarcoma often occurs in the
diaphyseal medulla of long bones, similar to Ewing sarcoma. In the
present case, the tumor was located at the proximal site of the
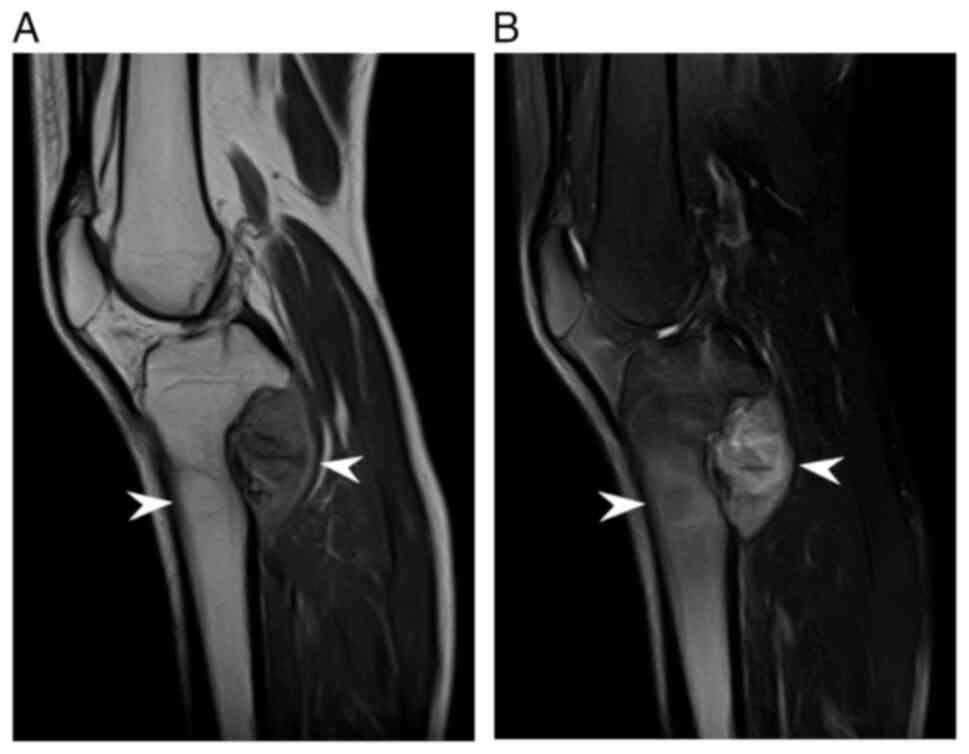
metaphysis of the left tibia (Fig. 1A
and B). Notably, no tibial tumors, including those in our case,
occurred on the anterior side of the cortex, which is the most
common site of classic adamantinoma of the long bones, as shown in
Table SI (4,8,13–17).
EWSR1::NFATC2 sarcomas tend to grow slowly and are
relatively indolent in nature. The metastatic tumors in our case
were also characteristic because of their slow growth.
Ewing-like adamantinoma is composed of relatively
uniform, small round or epithelioid cells arranged in nests, cords,
or trabeculae with fibrous or myxoid stroma. Our patient had an
epithelioid malignant tumor of the left tibia, suggesting a
Ewing-like adamantinoma 19 years previously. This case was similar
to the EWSR1::NFATC2 sarcoma case reported by Makise et
al (11).
When we reanalyzed the tissue samples, hematoxylin
and eosin staining revealed relatively uniform round cells in a
trabecular arrangement within a fibrous background (Fig. 2A) and epithelioid tumor cells with
abundant clear cytoplasm, forming nests or trabeculae (Fig. 2B). Immunohistochemical staining
revealed that the tumor cells were weakly and focally positive for
AE1/AE3 (Fig. 2C), weakly positive
for CD99 (Fig. 2D), and diffusely
positive for NKX3.1 (Fig. 2E).
The main radiological characteristics of
EWSR1::NFATC2 sarcoma are as follows: tendency to arise at
the diaphysis of long bones, cortical expansion with
buttressing-type thickening, and frequent bone surface involvement
with saucer-like erosion without cortical destruction. Adamantinoma
is a primary low-grade malignant bone tumor of epithelial origin
(18). Although predominantly
localized in the mid-tibial diaphysis, cases of synchronous or
isolated lesions in the fibula have been reported. It is a rare
neoplasm, comprising only 0.1–0.5% of all primary bone tumors
(19). Our tibial
EWSR1::NFATC2 sarcoma case is very rare and appears to be
different from classic adamantinomas in terms of localization.
EWSR1::NFATC2 sarcoma carries a
t(20;22)(q13;q12) chromosomal translocation (3). This type of sarcoma is translocation
sarcoma (TRS). In 2015, trabectedin was approved in Japan for the
treatment of patients with soft tissue sarcoma (STS) after a
clinical trial targeting TRS (20).
We previously demonstrated that the median progression-free
survival was been 7.3 months in our single-institution cohort
(21). Kobayashi et al
(22) reported an overall median
progression-free survival (PFS) of 3.7 months in a cohort of 140
patients who underwent trabectedin treatment at 29 Japanese
Musculoskeletal Oncology Group institutions. With respect to the
histological type in their study, the median PFS was 17.4 months
for myxoid liposarcoma, 4.9 months for leiomyosarcoma, 5.6 months
for synovial sarcoma, and 3.7 months for dedifferentiated
liposarcoma, respectively. As the PFS was 16.4 months in our
patient during trabectedin administration, he showed a noteworthy
response to trabectedin without any severe adverse events. However,
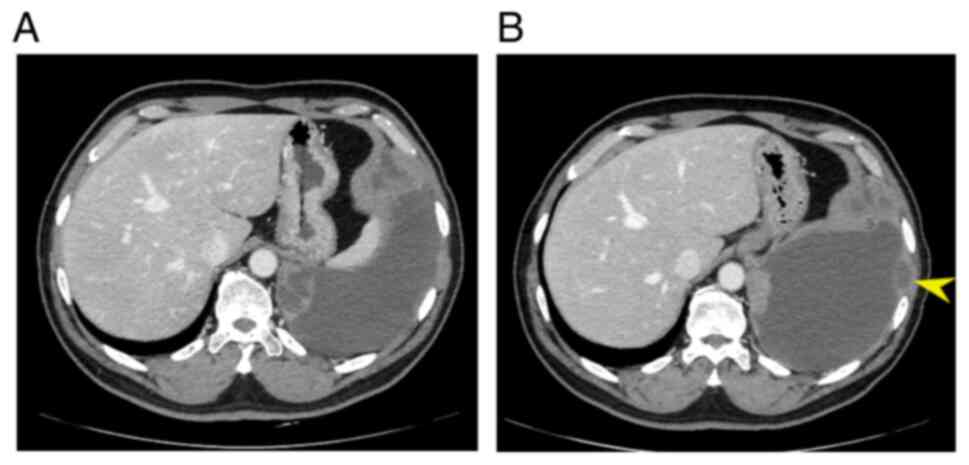
he developed PD after 18 cycles of trabectedin as the left pleural
lesion grew bigger and the pleural effusion increased (Fig. 3A and B), and received best
supportive care. Notably, the patient also had stable disease for
more than a year with eribulin treatment. A future trial with
eribulin in these patients may therefore be worthwhile.
With regard to drug therapy, several reports have
suggested pazopanib as an effective treatment (18). A summary of metastatic cases treated
with chemotherapy is presented in Table
I. Seligson et al (12)
demonstrated the mammalian target of rapamycin (mTOR) pathway as a
potential therapeutic target in EWSR1::NFATC2 sarcomas using
multiscale-omic assessment. They also presented the case of a
58-year-old male patient with metastatic EWSR1::NFATC2
sarcomas who achieved 47 months of disease stabilization when
treated with a combination of the mTOR inhibitor, everolimus, and
the vascular endothelial growth factor receptor-tyrosine kinase
inhibitor, pazopanib. EWSR1::NFATC2 sarcomas are molecularly
distinct entities with an overactive mTOR signaling pathway that
may be therapeutically targetable. Gouda et al (18) reported a case of a patient with
EWSR1::NFATC2 sarcoma in which exceptional tumor control was
achieved using pazopanib and surgery, for an overall duration
exceeding 5 years. In addition, Machado et al (23) suggested the possibility of exploring
an immunotherapy approach because the transcriptomes of
EWSR1::NFATC2 sarcomas should be enriched in genes
associated with inflammatory and immune responses (13).
 | Table I.Clinicopathological features of
reported cases of metastatic EWSR1::NFATC2 sarcoma. |
Table I.
Clinicopathological features of
reported cases of metastatic EWSR1::NFATC2 sarcoma.
| First author,
year | Age, years/Sex | Primary site | Surgery | Metastatic
site | Gene
alteration | Chemotherapy | Outcome/duration or
follow-up | (Refs.) |
|---|
| Seligson ND,
2021 | 58/M |
Intraperitoneal | Surgical
excision |
Retroperitoneum, | SPV in
FANCE | ACT (VDC followed
by IE) | Little benefit | (12) |
|
|
| mass | of the mass | lung |
| Pazo alone | Little CB |
|
|
|
|
|
|
|
| Pazo + CPT-11 | Little CB |
|
|
|
|
|
|
|
| Pazo + Eve | SD/26 mo |
|
|
|
|
|
|
|
| Pazo + Pembro | SD/10 mo |
|
|
|
|
|
|
|
| Nivo + Ipi | SD/4 mo |
|
|
|
|
|
|
|
| Pazo + Eve
(re-challenge) | SD/21 mo |
|
| Gouda MA, 2023 | 30sa/M | Left leg | Wedge
resection; | Lung, heart | mTOR
E1799K | PCT (VDC/IE) | NA | (18) |
|
|
|
| surgical
resection |
| mut, TOP1
amp | ACT (high-dose IFM
alternating | NED/24 mo |
|
|
|
|
| of the cardiac |
|
| with ADR and
CDDP) |
|
|
|
|
|
| metastasis |
|
| TMZ + CPT-11 | MR |
|
| Present study | 38a/M | Metaphyseal | Wide resection | Lung, LNs,
pleura | BCL2L1
amp | VDC/VAC | SD/9 mo | - |
|
|
| cortex of
tibia |
| (RT 60 Gy/30
fr |
| Eribulin | SD/15 mo |
|
|
|
| (left) |
| after VDC/VAC) |
| Trabectedin | SD/16 mo |
|
|
|
|
|
|
|
| Pazopanib | PD/2 mo |
|
In conclusion, we described a case of metastatic
EWSR1::NFATC2 sarcoma that was initially diagnosed as a
Ewing-like adamantinoma arising from the left tibia.
Reconsideration during the durable response to trabectedin revealed
a definitive diagnosis of a translocation-related sarcoma,
EWSR1::NFATC2 sarcoma. Accordingly, physicians should
consider EWSR1::NFATC2 sarcoma in patients who have been
previously diagnosed with Ewing-like adamantinoma with a slow
clinical course. It is important to diagnose it via FISH
analysis using an EWSR1 break-apart probe. Amplification of
the 5′-end of EWSR1 indicates the rearrangement of the EWSR1
gene, which is consistent with EWSR1::NFATC2 sarcoma. In
addition, this sarcoma may have a prolonged response to eribulin
and trabectedin in preventing or delaying the progression of
additional metastasis after adriamycin-based chemotherapy. Further
research is required to identify additional active agents or
sequences for chemotherapy for this type of sarcoma.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Satoko Baba
(Division of Pathology, Cancer Institute of Japanese Foundation for
Cancer Research) for their technical assistance with fluorescence
in situ hybridization.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TU drafted the manuscript. TU, MO, TT and AO
contributed to the management of the clinical case. TU, MO, KY, TT,
KT, SM and KA contributed to conception and design, and analysis
and interpretation of clinical data. KY performed histological
assessment and immunohistochemistry. KY and YT performed RT-PCR and
direct sequencing. HS, RO, XW, NF, YS, KN and JT contributed to the
patient's care and acquisition of data. TN, MS and KH contributed
to the acquisition of data. MO, KY, TT and ST contributed to
interpretation of clinical data and reviewed the manuscript. TU and
MO confirm the authenticity of all the raw data. KT, SM, KA and ST
supervised this study. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of any potentially identifiable images
or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BCOR
|
B cell lymphoma 6 corepressor
|
|
CIC
|
capicua transcriptional repressor
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
IE
|
ifosfamide and etoposide
|
|
MSI
|
microsatellite instability
|
|
mTOR
|
mammalian target of rapamycin
|
|
PD
|
progressive disease
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
SD
|
stable disease
|
|
TBLB
|
transbronchial lung biopsy
|
|
VAC
|
vincristine, actinomycin D, and
cyclophosphamide
|
|
VDC
|
vincristine, cyclophosphamide, and
doxorubicin
|
References
|
1
|
Gaspar N, Hawkins DS, Dirksen U, Lewis IJ,
Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, et
al: Ewing sarcoma: Current management and future approaches through
collaboration. J Clin Oncol. 33:3036–3046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The WHO Classification of Tumours
Editorial Board, . WHO Classification of Tumours: Soft Tissue and
Bone Tumours. 3.5th edition. International Agency for Research on
Cancer; Lyon: 2020
|
|
3
|
Szuhai K, Ijszenga M, de Jong D,
Karseladze A, Tanke HJ and Hogendoorn PC: The NFATc2 gene is
involved in a novel cloned translocation in a Ewing sarcoma variant
that couples its function in immunology to oncology. Clin Cancer
Res. 15:2259–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida KI, Machado I, Motoi T,
Parafioriti A, Lacambra M, Ichikawa H, Kawai A, Antonescu CR and
Yoshida A: NKX3-1 is a useful immunohistochemical marker of
EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma. Am J Surg
Pathol. 44:719–728. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charville GW, Wang WL, Ingram DR, Roy A,
Thomas D, Patel RM, Hornick JL, van de Rijn M and Lazar AJ: EWSR1
fusion proteins mediate PAX7 expression in ewing sarcoma. Mod
Pathol. 30:1312–1320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toki S, Wakai S, Sekimizu M, Mori T,
Ichikawa H, Kawai A and Yoshida A: PAX7 Immunohistochemical
evaluation of ewing sarcoma and other small round cell tumours.
Histopathology. 73:645–652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang GY, Thomas DG, Davis JL, Ng T, Patel
RM, Harms PW, Betz BL, Schuetze SM, McHugh JB, Horvai AE, et al:
EWSR1-NFATC2 translocation-associated sarcoma clinicopathologic
findings in a rare aggressive primary bone or soft tissue tumor. Am
J Surg Pathol. 43:1112–1122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perret R, Escuriol J, Velasco V, Mayeur L,
Soubeyran I, Delfour C, Aubert S, Polivka M, Karanian M, Meurgey A,
et al: NFATc2-rearranged sarcomas: Clinicopathologic, molecular,
and cytogenetic study of 7 cases with evidence of AGGRECAN as a
novel diagnostic marker. Mod Pathol. 33:1930–1944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sadri N, Barroeta J, Pack SD, Abdullaev Z,
Chatterjee B, Puthiyaveettil R, Brooks JS, Barr FG and Zhang PJ:
Malignant round cell tumor of bone with EWSR1-NFATC2 gene fusion.
Virchows Arch. 465:233–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipper S and Kahn LB: Case report 235.
Ewing-like adamantinoma of the left radial head and neck. Skeletal
Radiol. 10:61–66. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Makise N, Yoshida K, Iijima T, Yoshida A,
Ushiku T and Ishida T: Skeletal EWSR1-NFATC2 sarcoma previously
diagnosed as ewing-like adamantinoma: A case report and literature
review emphasizing its unique radiological features. Pathol Int.
71:614–620. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seligson ND, Maradiaga RD, Stets CM,
Katzenstein HM, Millis SZ, Rogers A, Hays JL and Chen JL:
Multiscale-omic assessment of EWSR1-NFATc2 fusion positive sarcomas
identifies the mTOR pathway as a potential therapeutic target. NPJ
Prec Oncol. 5:432021. View Article : Google Scholar
|
|
13
|
Watson S, Perrin V, Guillemot D, Reynaud
S, Coindre JM, Karanian M, Guinebretière JM, Freneaux P, Le Loarer
F, Bouvet M, et al: Transcriptomic definition of molecular
subgroups of small round cell sarcomas. J Pathol. 245:29–40. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaheen M, Wurtz LD, Brocken EG and Warmke
LM: EWSR1: NFATC2-rearranged sarcoma in bone-case report and review
of the literature. Hum Pathol. 30:3006802022.
|
|
15
|
Diaz-Perez JA, Nielsen GP, Antonescu C,
Taylor MS, Lozano-Calderon SA and Rosenberg AE: EWSR1/FUS-NFATc2
rearranged round cell sarcoma: Clinicopathological series of 4
cases and literature review. Hum Pathol. 90:45–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bode-Lesniewska B, Fritz C, Exner GU,
Wagner U and Fuchs B: EWSR1-NFATC2 and FUS-NFATC2
gene fusion-associated mesenchymal tumors: Clinicopathologic
correlation and literature review. Sarcoma. 2019:93863902019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuda Y, Zhang L, Meyers P, Tap WD, Healey
JH and Antonescu CR: The clinical heterogeneity of round cell
sarcomas with EWSR1/FUS gene fusions: Impact of gene fusion type on
clinical features and outcome. Genes Chromosomes Cancer.
59:525–534. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gouda MA, Zarzour MA, Vaporciyan AA,
Kairemo K, Chuang HH and Subbiah V: Activity of pazopanib in
EWSR1-NFATC2 translocation associated bone sarcoma.
Oncoscience. 10:44–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kosemehmetoglu K, Rekhi B, Erdem ZB,
Yildiz AE and Comunoglu N: Clinicopathological features of three
rare EWSR1::NFATC2 sarcomas of bone and soft tissues. Int J
Surg Pathol. 32:1275–1285. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawai A, Araki N, Sugiura H, Ueda T,
Yonemoto T, Takahashi M, Morioka H, Hiraga H, Hiruma T, Kunisada T,
et al: Trabectedin monotherapy after standard chemotherapy versus
best supportive care in patients with advanced,
translocation-related sarcoma: A randomized, open-label, phase 2
study. Lancet Oncol. 16:406–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawaguchi K, Nakano K, Urasaki T, Fukuda
N, Taira S, Ono M, Tomomatsu J, Nishizawa M, Ae K, Matsumoto S and
Takahashi S: Retrospective analysis of trabectedin therapy for soft
tissue sarcoma. In Vivo. 33:1609–1614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi H, Iwata S, Wakamatsu T,
Hayakawa K, Yonemoto T, Wasa J, Oka H, Ueda T and Tanaka S:
Efficacy and safety of trabectedin for patients with unresectable
and relapsed soft-tissue sarcoma in Japan: A Japanese
musculoskeletal oncology group study. Cancer. 126:1253–1263. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Machado I, Llombart-Bosch A, Charville GW,
Navarro S, Domínguez Franjo MP, Bridge JA and Linos K: Sarcomas
with EWSR1::Non-ETS fusion (EWSR1::NFATC2 and EWSR1::PATZ1). Surg
Pathol Clin. 17:31–35. 2024. View Article : Google Scholar : PubMed/NCBI
|