Introduction
Primary lymphoma of the central nervous system (CNS)
is a rare and aggressive extranodal type of non-Hodgkin lymphoma
that can originate in the brain, eyes, meninges or spinal cord. The
diagnosis of primary CNS lymphoma requires the exclusion of
systemic lymphoma. Primary CNS lymphomas account for 3% of all CNS
tumors, 4% of all primary brain tumors, 5% of all extranodal
lymphomas and <1% of all non-Hodgkin's lymphomas. The tumors can
occur in both immunocompromised and immunocompetent hosts, but have
a low incidence in the general immunocompetent population, with an
incidence rate of 0.4 per 100,000 people per year. The 5-year
survival rate of patients with these tumors remains low, with a
recent estimate of ~22%. The median survival time is estimated to
be 26 months. High-dose methotrexate is the primary treatment
(1). The main pathological subtype
of these tumors is diffuse large B-cell lymphoma (DLBCL) (2,3).
Primary CNS lymphoma originating in the meninges is a rare type of
meningioma that is not associated with parenchymal or systemic
spread. Natural killer (NK)/T-cell lymphoma of the CNS accounts for
only 2% of all extranodal lymphomas, and primary NK/T-cell lymphoma
of the meninges is even rarer, with no accurate incidence data
available so far (4). The
radiological presentation of this rare tumor can be misleading,
often mimicking other brain tumors, infections or inflammatory
diseases. Hence, the diagnosis of primary NK/T-cell lymphoma of the
meninges is challenging and typically requires the identification
of lymphoma cells in a brain tissue specimen obtained using
stereotactic biopsy. However, lymphoma cells can also be detected
in the cerebrospinal fluid (CSF), which can be collected via a
lumbar puncture (5). The present
study reports the case of a patient who was diagnosed with primary
NK/T-cell meningeal lymphoma using CSF cytological examination and
flow cytometric analysis. The present study aimed to explore the
basis and significance of CSF examination as a type of ‘fluid
biopsy’ for the diagnosis of primary CNS lymphoma of the meninges
(5).
Case report
Clinical history
A 55-year-old male patient presented with
intermittent dizziness and periodic headaches for the past month
and gait instability for 9 days prior to presentation, and was
admitted to Shaanxi Provincial People's Hospital (Xi'an, China) in
October 2023. The patient reported that the episodes of dizziness
and headaches began after catching a cold, and that no fever,
vomiting or seizures were experienced during this time. A
neurological examination demonstrated bilateral hearing loss and
left-sided tinnitus. The patient's medical history was unremarkable
and there was no evidence of hereditary diseases. A comprehensive
medical history, epipharyngoscope examination, magnetic resonance
imaging (MRI) and positron-emission tomography/computed tomography
(PET/CT) indicated no sinonasal or nasopharyngeal primary lesions.
The patient's white blood cell count (7.54×109/liter),
platelet count (217×109/liter) and hemoglobin levels
(144 g/l) were within the reference ranges (6,7). A
quantitative-PCR assay for circulating Epstein-Barr virus DNA was
positive (data not shown).
CSF cytology
Cytological examination of the CSF with
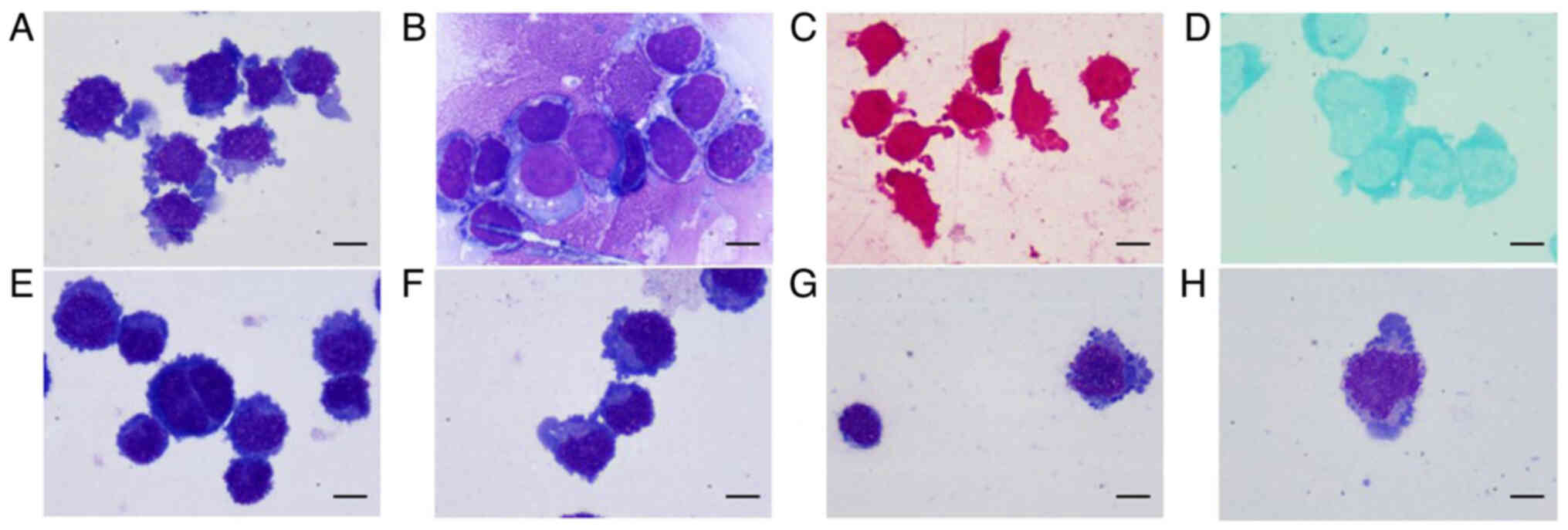
Wright-Giemsa staining (Appendix
S1) showed that cells with abnormal morphology accounted for
~99% of all cells in the patient's CSF sample. The abnormal cells
consisted of a mixture of two main cell types. The first cell type
was characterized by an irregular appearance with a slightly
serrated border, with long pseudopods, a puffy and large or
petal-like nucleus, scant basophilic cytoplasm and a number of
cytoplasmic azurophilic granules. The second type of cells appeared
round or oval, with flat and coarse granular chromatin, one or two
hazy nucleoli in a number of cells, scant dark blue cytoplasm and
vacuolar degeneration (Fig. 1A and
B). The cells were negatively stained with leukocyte
peroxidase, while a few cells were positively stained with periodic
acid-Schiff stain (Fig. 1C and D).
The results of flow cytometric analysis showed that a large
proportion of the cells were of the NK/T-cell lineage. Considering
the aforementioned results combined with those of the flow
cytometric analysis, a lymphoma was initially suspected. However,
to eliminate infection-induced morphological changes in
lymphocytes, anti-viral (0.8 g acyclovir orally, daily) and
anti-tuberculosis (0.6 g rifampicin, 500 mg pyrazinamide and 0.25 g
ethambutol hydrochloride orally, daily) therapies were administered
to the patient. To observe the therapeutic effects, a second CSF
cytological analysis was conducted after 1 week of anti-infection
therapy, but the results were unchanged (Fig. 1E). Furthermore, ink stain and
acid-fast stain tests of CSF were negative (data not shown). Hence,
chemotherapy was administered, after which, the total number of
nucleated cells in the CSF decreased notably, with only 17%
lymphoma cells (Fig. 1F-H). The
cell morphology also changed; the cells were elliptical, with
coarse clumpy nuclear chromatin, vaguely visible nucleoli, light
blue cytoplasm, purple-red cytoplasmic granules in a number of
cells and almost no pseudopodia.
From our previous clinical experience, it can be
suggested that secondary CNS lymphoma, including DLBCL and nasal
NK/T-cell lymphoma, is more common than primary CNS lymphoma. The
present case is the first time a primary CNS NK/T-cell lymphoma of
the meninges was encountered at Shaanxi Provincial People's
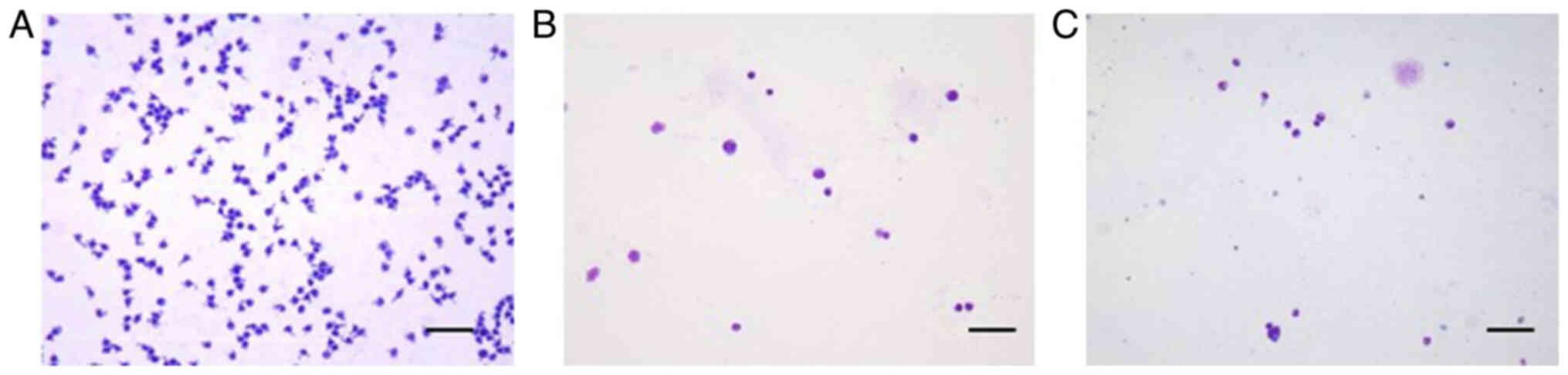
Hospital. The total number of nucleated cells in the CSF was higher
in the present patient than in CSF samples obtained from patients
with DLBCL and nasal NK/T-cell lymphoma (Fig. 2A-C). Moreover, the CSF was almost
entirely composed of tumor cells, whereas in the case of DLBCL and
nasal NK/T-cell lymphoma, the CSF included a number of normal
lymphocytes, monocytes and neutrophils, as well as tumor cells.
Statistically significant differences in the protein and glucose,
and no significant difference in the CSF concentration of chloride
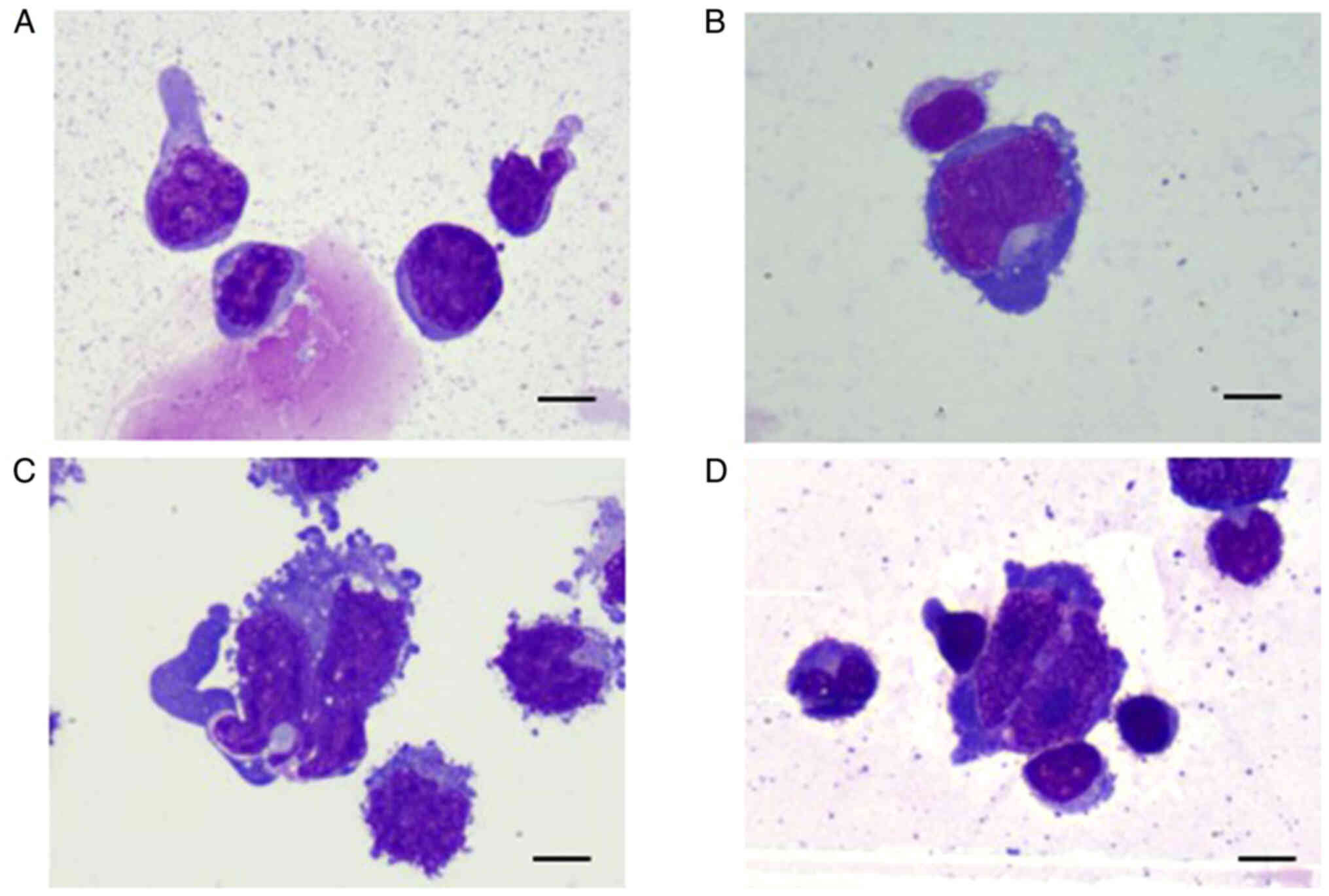
were observed among the three types of lymphomas (Table SI). However, the cell morphology
was slightly different among the three tumor types (Fig. 3A-D). In contrast to primary CNS
NK/T-cell lymphoma, nasal NK/T-cell lymphoma exhibits regular,
round or oval cells, with coarse granular nuclear chromatin and
visible nucleoli. The cytoplasmic volume is moderate with a few
pseudopod-like protrusions on one side of the cytoplasmic border.
DLBCL exhibits cells with notably large cell bodies, irregular hazy
nuclei and chromatin condensation. The cytoplasm appears dark blue
on Wright-Giemsa staining with medium volume. Most of the
cytoplasmic edges are irregular with pseudopods and vacuolar
degeneration.
MRI and PET/CT diagnosis
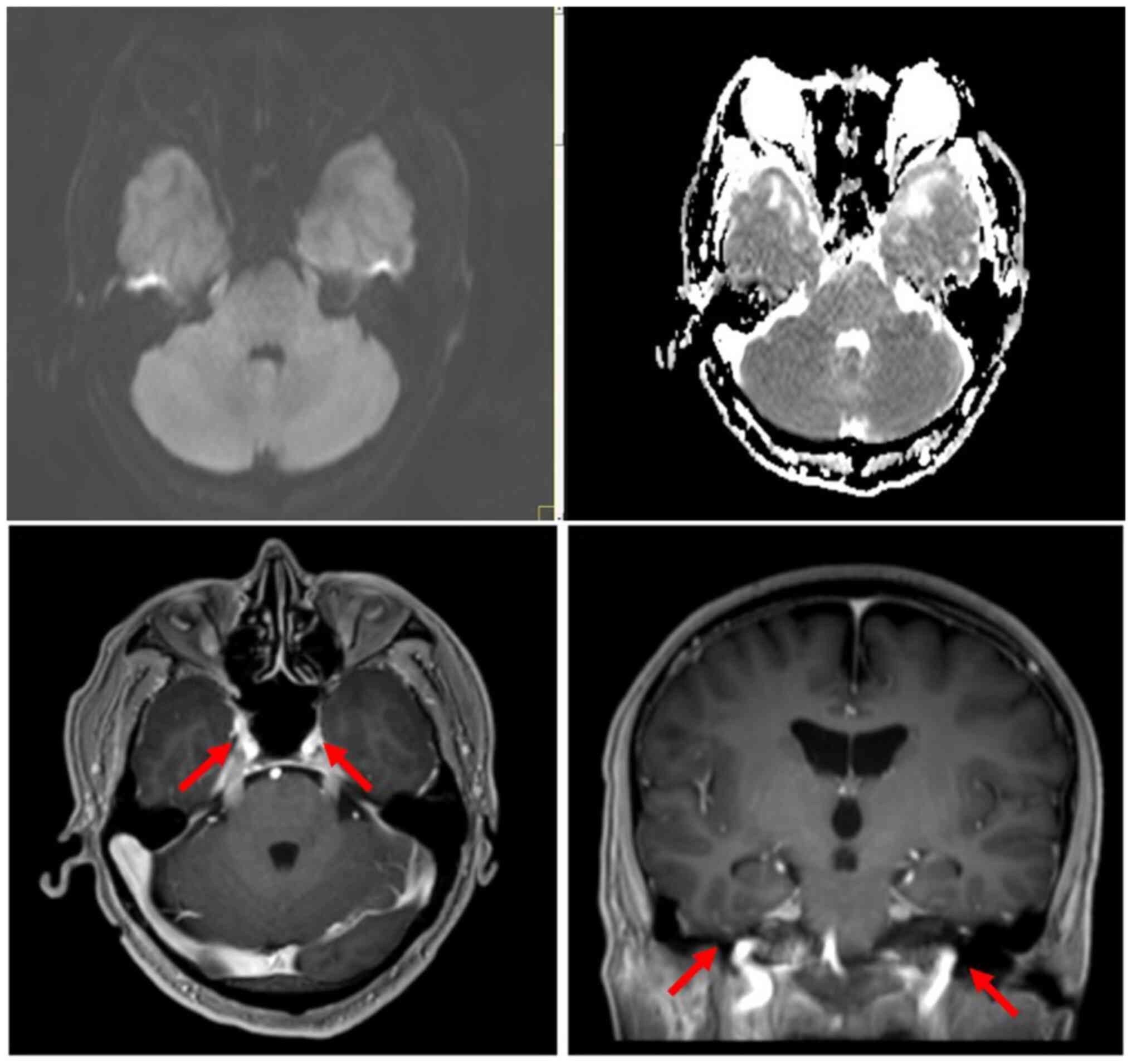
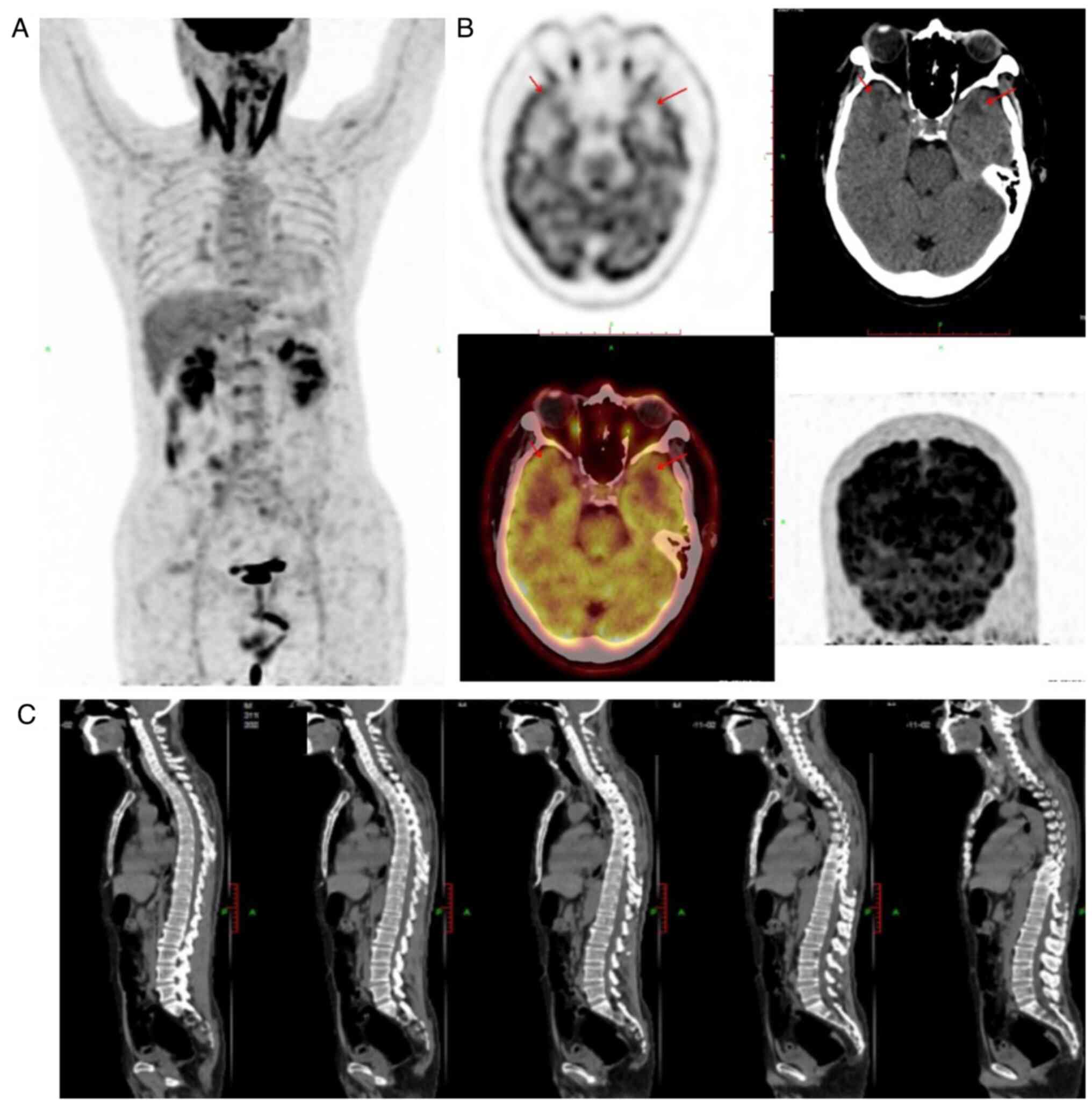
MRI of the brain showed slight bilateral thickening
of the trigeminal nerves, and bilateral deep and lobar distribution
of lesions in the cerebral white matter, with slightly low-density
or equal-density shadows on plain CT and slightly longer signals on
T1- and T2-weighted MRI. Multiple patches were visible in the
subcortical white matter in the frontal and parietal lobes, with
slightly longer signals on T1- and T2-weighted MRI, and
hyperintensity on fluid-attenuated inversion/recovery scans
(Fig. 4). No sinonasal or
nasopharyngeal primary lesions were found by means of medical
history, epipharyngoscope examination and PET/CT. Additionally, no
significant abnormalities such as a tumor mass were found on
whole-body, brain, nasopharynx and spine PET/CT (Fig. 5). Due to the presence of lymphoma
cells in the CSF and the absence of extracranial primary lesions,
particularly in the nose, sinuses and pharynx, a primary lymphoma
of the leptomeninges was suspected; however, no change was detected
in the leptomeninges early in the course of the disease.
Flow cytometry and genetic
testing
Flow cytometric immunophenotypic analysis of the CSF
(Appendix S1) demonstrated that a
large proportion of cells was positive for CD2, CD56, CD38, CD30
and CD45, and negative for surface CD3, CD4, CD5, CD8, CD19, CD20,
CD16, CD7, CD30, κ and λ light chains, and HLA-DR, which is
consistent with the NK/T-cell lineage (Fig. S1). Lymphocytes constituted ~95.33%
of nucleated cells, with CD3−CD56+ NK cells
accounting for ~98.96% of the lymphocyte population.
Furthermore, 14 genes with mutations were detected
upon screening of the CSF for blood system diseases using next
generation sequencing (Appendix
S1), including in Janus kinase 3 (JAK3), PTPN11, BCOR, NOTCH2,
HLA-A, TRRAP, POT1, SETD1B, CNOT3, BCL11A, IKZF3, TYK2, GNAI2 and
FAT1. Among these gene mutations, the JAK3 mutation was closely
related to NK/T-cell lymphoma, with a 30% mutation rate. The
mutation sites in the JAK3 gene included p.Met511, p.Arg657Gln and
p.Ala572Val. All sequencing data are available through the NCBI
Sequence Read Archive under the accession no. PRJNA1171089, and the
raw reads under accession no. SRR30938159.
Treatment and follow-up
After the diagnosis of a primary CSF NK/T-cell
lymphoma was confirmed, a total of 7 rounds of chemotherapy were
performed, and the patient was treated with intravenous injections
(iv) of high-dose methotrexate (5,000 mg), tislelizumab (200 mg)
and cyclophosphamide (2.46 g), combined with an intrathecal
injection of methotrexate (15 mg), dexamethasone (10 mg) and
cytarabine (25 mg), along with chidamide (30 mg, orally) and
supportive treatment, including 5% glucose injection,
anti-infective therapy (0.4 g teicoplanin by iv and 0.5 g
levofloxacin orally). Subsequently, the patient also underwent
autologous stem cell transplantation. After the treatment, the
patient showed notable improvement, with symptoms and CSF findings
almost normal at first; however, the patient still died of severe
pneumonia, cardiac insufficiency, and hypohepatia in October
2024.
Discussion
NK/T-cell lymphoma is a rare type of non-Hodgkin
lymphoma originating from activated NK cells or cytotoxic T
lymphocytes. In 2008, NK/T-cell lymphomas occurring in the nasal
cavity and extranasal region were named extranodal NK/T-cell
lymphoma, nasal type, according to the World Health Organization
Classification of Tumors of Hematopoietic and Lymphoid Tissues
(8). Extranodal NK/T-cell lymphoma
of the nasal type predominantly occurs in middle-aged men, and is
associated with Epstein-Barr virus infection and certain
ethnicities, environments and genetic variants, for example, most
patients show monoclonal rearrangements of the TCR genes (9). NK/T-cell lymphoma tends to invade the
midline facial structures such as the nasal cavity and paranasal
sinuses, and extranasal sites such as the skin, soft tissues,
testes and gastrointestinal tract (10). Previous cases of NK/T-cell lymphoma
invading the cauda equina and brain parenchyma have been reported
(10,11); however, primary leptomeningeal
NK/T-cell lymphoma of the CNS is rare.
In the present case, a 55-year-old male patient was
admitted with dizziness and headaches. Routine blood tests yielded
normal results, and no lesions in the brain parenchyma or enlarged
lymph nodes were detected on PET/CT. Hence, diseases of the
hematological system and intracranial mass lesions were
preliminarily excluded. Moreover, the absence of extracranial
disease confirmed the primary nature of the CNS disease. A CSF
examination showed a notable increase in the number of nucleated
cells, but negative results for Mycobacterium tuberculosis
and Cryptococcus neoformans, which excluded these specific
infections. Numerous abnormal lymphocytes were found on CSF
cytomorphological examination, and flow cytometry showed that
98.96% of cells (accounting for all nucleated cells) expressed CD2,
CD56, CD38, CD30 and CD45 on their cell membranes. Since CD2 and
CD56 are NK cell-specific antigens, malignant tumors of NK cell
origin could be identified by immunophenotyping (4). Moreover, treatment using a combination
of chemotherapy with programmed cell death 1 inhibitor
(tislelizumab) and chidamide was effective, as indicated by the
lack of lymphoma cells in the CSF and the improvement in the
patient's CNS symptoms. Considering the aforementioned findings,
the patient was diagnosed with primary CNS NK cell meningeal
lymphoma.
Contrast-enhanced MRI of the head should be
recommended for patients suspected to have meningeal lymphoma. If
the lymphoma has invaded the meninges, contrast-enhanced MRI may
identify overt meningeal thickening and enhancement at the lesion
site (12). However, in the present
study, MRI only showed multiple, patchy and slightly long T1 and T2
signals, and hyperintensity on fluid-attenuated inversion recovery
images in the subcortical white matter of the frontoparietal lobes.
No changes were detected in the meninges. Although CNS lymphomas
can have a characteristic CT or MRI appearance, no imaging
characteristic unequivocally differentiates CNS lymphomas from
other types of tumors. In a number of cases, NK/T-cell lymphoma has
been found to invade the leptomeninges, both temporal lobes, the
cerebellar folia, and the vermis, and even form a mass in the brain
parenchyma (10,13,14).
The most common sites of invasion in the case of primary CNS DLBCL
are the cerebral hemispheres, basal ganglia and corpus callosum
(11). The present patient had
trigeminal nerve thickening, and previous studies suggest that
trigeminal nerve thickening can be seen in chronic inflammatory
demyelinating polyneuropathy (15–17).
It has been reported that trigeminal nerve thickening is associated
with primary CSF NK/T-cell lymphoma (18). A previous study has shown that the
most frequent locations of primary CNS lymphoma are the cerebral
hemispheres, followed by the corpus callosum and basal ganglia
(11). Whether trigeminal nerve
thickening has significance for the diagnosis of primary CNS
NK/T-cell lymphoma needs to be further explored.
A large proportion of primary CNS hematological
diseases invade the leptomeninges, but thickened leptomeninges are
difficult to detect using CT or MRI in the early stage of the
disease. Currently, the diagnosis of primary CNS lymphoma of the
meninges is challenging. Pathological examination of a tissue
biopsy specimen is considered to be the gold standard for
diagnosis, whenever a biopsy is possible. However, as these lesions
mainly involve the meninges, a tissue biopsy is often difficult to
perform and pathological biopsy specimen of the diseased tissue
could not be obtained from the patient. This is a limitation of the
present study; however, as the disease process has an impact on the
morphology of the cells in the CSF, the accurate identification of
the cell types in the CSF may be used for the clinical diagnosis of
primary CNS lymphoma. In practice, however, CSF cytology has
relatively low sensitivity in the diagnosis of primary CNS lymphoma
and only ~20% of diagnoses are made in this way (5). The low sensitivity of CSF cytology is
attributable to the morphological similarity between lymphoma cells
in the CSF and benign reactive cells. Moreover, morphological
variation is observed among different types of lymphomas. Hence,
distinguishing these cells on cytomorphological examination is
difficult.
In the present case, a large proportion of CSF cells
observed were of approximately the same size, and exhibited coarse
nuclear chromatin, visible petal-like nuclei, blue cytoplasm,
visible granules and numerous pseudopodia at the cell border. In
viral meningitis, the total cell count and the proportion of
lymphocytes in the CSF are increased, and a number of lymphocytes
exhibit the reactive lymphocyte phenotype, whereby they
morphologically resemble immature lymphocytes (5). In Shaanxi Provincial People's
Hospital, it has been found that although viral infection also
stimulates lymphocytes to produce pseudopodia, the number of
lymphocytes with pseudopodia and the number of pseudopodia
themselves are few. In contrast to a patient with viral meningitis,
the present patient had few normal lymphocytes in the CSF and a
markedly increased number of pseudopodia, which is morphologically
consistent with malignant lymphoma cells. In our experience, in
most primary CNS B-cell lymphomas, the tumor cells in the CSF are
large with rough nuclear chromatin, irregular nuclei, basophilic
cytoplasm and a dark blue appearance. By contrast, in the present
study, the cells were relatively small with petal-like nuclei and
overt cytoplasmic granules. Therefore, the cell type was
preliminarily determined using cellular morphology, which served an
important role in the diagnosis.
Liquid biopsy is a technique that can be used to
examine cell-free DNA extracted from body fluids, such as
peripheral blood plasma. This technique is currently used to
identify genetic mutations in tumor cells in translational research
and detect minimal residual disease in various types of tumors
(19). In the present study,
genetic testing showed three point mutations in the JAK3 gene,
which were closely related to NK/T-cell lymphomas. Studies have
shown that the activation of JAK3 mutations plays a significant
role in the pathogenesis of NK/T-cell lymphoma (20,21).
However, ~90% of primary CNS lymphomas are DLBCL, and recent
studies have shown an accumulation of mutations in genes such as
MYD88 in DLBCL tumors (22,23). The present study also found that
chemotherapy was notably associated with alterations in the number
and morphology of the lymphoma cells in the patient's CSF.
Specifically, the number of lymphoma cells decreased after
chemotherapy and the number of pseudopods around the cytoplasm of
the lymphoma cells was also reduced.
In summary, the present case of a patient with
primary CNS NK/T-cell lymphoma serves to add to the small number of
cases reported in the literature thus far. Multimodal analysis of
the CSF plays an important role in the diagnosis of primary CNS
NK/T-cell lymphoma, and certain imaging methods, such as MRI, can
increase the diagnostic accuracy. Furthermore, future work to
identify diagnostic biomarkers is necessary to aid in the
diagnosis. The morphology of CSF cells plays an important role in
the diagnosis of primary CNS NK/T-cell lymphoma in the absence of
pathological tissue or imaging diagnosis.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Exploration and
Innovation Projects of Xi'an Jiaotong University (grant no.
xzy012022133), the Science and Technology Talent Support Program of
Shaanxi Provincial People's Hospital (grant no. 022JY-56), and the
Science and Technology Development Incubation Funding of Shaanxi
Provincial People's Hospital (grant no. 2022YJY-24).
Availability of data and materials
The data generated in the present study is shown in
the National Center for Biotechnology Information Sequence Read
Archive when the manuscript published under the accession no.
PRJNA1171089, and the raw reads data under the accession no.
SRR30938159 (https://www.ncbi.nlm.nih.gov/sra/?term=SRR30938159).
The remaining data generated in the present study may be requested
from the corresponding author.
Authors' contributions
Flow cytometry was performed by WZ. MRI and PET/CT
diagnosis were performed by ZM. Collection of information, the
analysis of patient data and writing of the original draft was
conducted by XC and JF. CSF cytology, review and editing of the
manuscript was undertaken by SZ. All authors read and approved the
final manuscript. SZ and XC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The report was approved by the Medical Ethics
Committee of Shaanxi Provincial People's Hospital (Xi'an, China;
approval no. R017). All patients provided consent for the use of
CSF samples.
Patient consent for publication
All patients provided written informed consent for
the publication of this report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah T and Venur VA: Central nervous
system lymphoma. Semin Neurol. 43:825–832. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernández-Verdin I, Morales-Martínez A,
Hoang-Xuan K and Alentorn A: Primary central nervous system
lymphoma: Advances in its pathogenesis, molecular markers and
targeted therapies. Curr Opin Neurol. 35:779–786. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoang-Xuan K, Deckert M, Ferreri AJM,
Furtner J, Gallego Perez-Larraya J, Henriksson R, Hottinger AF,
Kasenda B, Lefranc F, Lossos A, et al: European association of
neuro-oncology (EANO) guidelines for treatment of primary central
nervous system lymphoma (PCNSL). Neuro Oncol. 25:37–53. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao B, Kamiya-Matsuoka C, Gong Y, Chen M,
Wolf BA and Fowler NH: Primary natural killer/T-cell lymphoma
presenting as leptomeningeal disease. J Neurol Sci. 343:46–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Huang M, Zhang Z, Jing H, Zou Y
and Bu H: Primary meningeal central nervous system lymphoma: A case
report and literature review. Medicine (Baltimore). 101:e325672022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pietras NM, Gupta N, Justiz Vaillant AA
and Pearson-Shaver AL: Immune thrombocytopenia. StatPearls
[Internet] Treasure Island (FL): StatPearls Publishing; 2024
|
|
7
|
Guo Y, Liu X, Zihao Z, Zhang Q, Shi Z and
Zhang N: Blood routine reference value range should be adjusted
according to regional and ethnic characteristics. Front Public
Health. 10:9341012022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: An overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
9
|
Guan H, Huang Y, Wen W, Xu M, Zan Q and
Zhang Z: Primary central nervous system extranodal NK/T-cell
lymphoma, nasal type: Case report and review of the literature. J
Neurooncol. 103:387–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Yu H, Fu X, Zhang L, Li X, Li L,
Wang X, Sun Z, Zhang X, Li Z, et al: Clinical analysis of patients
with primary and secondary extranodal natural killer/T-cell
lymphoma of central nervous system. Hematol Oncol. 41:267–274.
2023. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Küker W, Nägele T, Korfel A, Heckl S,
Thiel E, Bamberg M, Weller M and Herrlinger U: Primary central
nervous system lymphomas (PCNSL): MRI features at presentation in
100 patients. J Neurooncol. 72:169–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capasso R, Negro A, Russo C, Zeccolini F,
Muto G, Caranci F and Prof Pinto A: Conventional and advanced MRI
Techniques in the evaluation of primary CNS lymphoma. Semin
Ultrasound CT MR. 44:126–135. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zing NF, Thais F, Massimo C, Chiattone C
and Ferreri AJM: Diagnosis, prevention and treatment of central
nervous system involvement in peripheral t-cell lymphomas. Crit Rev
Oncol Hematol. 167:1034962021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleinschmidt-DeMasters BK and Gilani A:
Secondary parenchymal CNS involvement by lymphoma including rare
types: Follicular and EBV-positive NK/T cell lymphoma, nasal type.
Ann Diagn Pathol. 53:1517652021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kajtazi NI, Bafaquh M, Ghamdi JA, AlEissa
Z, Shmeikh AA, Alsaeed A, Sulaiman T, Vizcaino MA, Al Hameed M and
Raghunathan A: An unusual case of EBV-negative primary CNS lymphoma
of natural killer/T-cell lineage. Clin Pathol.
14:2632010X2110656922021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ball MK, Morris JM, Wood AJ, Meyer FB,
Kaszuba MC and Raghunathan A: Ventricle-predominant primary CNS
lymphomas: Clinical, radiological and pathological evaluation of
five cases and review of the literature. Brain Tumor Pathol.
37:22–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kan Y, Wang Y, Wang W, Liu J and Yang J:
Unexpected corpus callosum involvement of diffuse large B-cell
lymphoma on FDG PET/CT. Clin Nucl Med. 43:933–935. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guedes BF and Cury RG: Trigeminal nerve
thickening in chronic inflammatory demyelinating polyneuropathy.
Arq Neuropsiquiatr. 73:634–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nikanjam M, Kato S and Kurzrock R: Liquid
biopsy: Current technology and clinical applications. J Hematol
Oncol. 15:1312022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Liang L, Li D, Nong L, Zheng Y,
Huang S, Zhang B and Li T: JAK3/STAT3 oncogenic pathway and PRDM1
expression stratify clinicopathologic features of extranodal
NK/T-cell lymphoma, nasal type. Oncol Rep. 41:3219–3232.
2019.PubMed/NCBI
|
|
21
|
Koo GC, Tan SY, Tang T, Poon SL, Allen GE,
Tan L, Chong SC, Ong WS, Tay K, Tao M, et al: Janus kinase
3-activating mutations identified in natural killer/T-cell
lymphoma. Cancer Discov. 2:591–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi J, Ohka F, Kitano Y, Maeda S,
Motomura K, Aoki K, Takeuchi K, Nagata Y, Hattori H, Tsujiuchi T,
et al: Rapid detection of the MYD88 L265P mutation for pre- and
intra-operative diagnosis of primary central nervous system
lymphoma. Cancer Sci. 114:2544–2551. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iriyama C, Murate K, Iba S, Okamoto A,
Goto N, Yamamoto H, Kato T, Mihara K, Miyama T, Hattori K, et al:
Utility of cerebrospinal fluid liquid biopsy in distinguishing CNS
lymphoma from cerebrospinal infectious/demyelinating diseases.
Cancer Med. 12:16972–16984. 2023. View Article : Google Scholar : PubMed/NCBI
|