Introduction
Epstein-Barr virus-positive (EBV+)
extranodal T and NK cell lymphoma (ENKTL) is a common peripheral T
cell lymphoma in East Asia and South America (1). CD20 is typically considered to be a
specific marker for B cells, playing a crucial role in B lymphocyte
differentiation, signal transduction and cell cycle regulation. It
also targets monoclonal antibody therapies against B cell lymphoma
(2). Therefore, CD20 is an
essential marker for the diagnosis and treatment of B cell
lymphoma. However, CD20 expression in T cell lymphomas is rare and
can pose diagnostic challenges. CD20 expression in T cell lymphomas
is primarily observed in peripheral T cell lymphoma and is rarely
seen in ENKTL (3,4).
CD30, a tumor necrosis factor receptor superfamily
member, was first discovered in Hodgkin's lymphoma (HL), but it is
also expressed in normally activated B and T cells. CD30 is also
expressed in hematological diseases such as anaplastic large-cell
lymphoma, mediastinal large B cell lymphoma, mycosis fungoides,
infectious mononucleosis and embryonal carcinoma (5–7). CD30
is expressed in 20–50% of ENKTL cases and, in some cases, up to 70%
(8,9). Brentuximab vedotin targets
CD30-positive cells in patients with lymphoma (9). Furthermore, CD15 is typically
expressed in HL or chronic myeloid leukemia and, in rare cases, in
peripheral T cell lymphoma not otherwise specified (PTCL-NOS).
However, to the best of our knowledge, there have been no relevant
reports in ENKTL.
The present case describes a rare case of ENKTL with
high CD20 and CD30 expression and partial CD15 expression in tumor
cells, which posed a significant diagnostic challenge. The patient
died of multiple organ failure shortly after diagnosis. To the best
of our knowledge, there have been no similar cases reported before.
This study discusses the clinical and pathological features of the
tumor, as well as the possible molecular mechanisms and therapeutic
targets.
Case report
A patient with lymphadenopathy, a sore throat and
malaise for 1 month was admitted to Lanzhou University Second
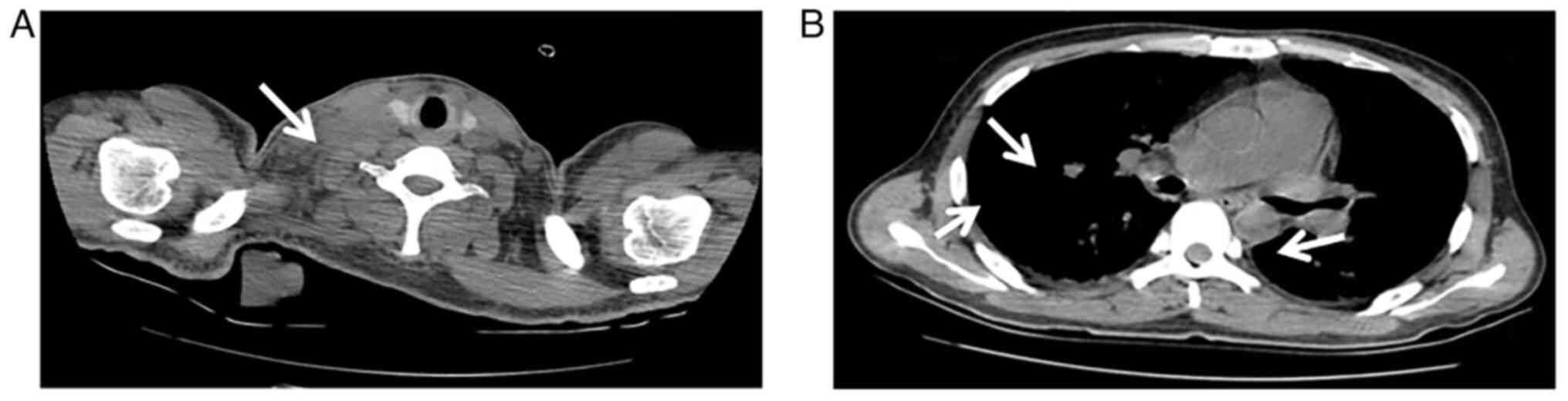
Hospital (Lanzhou, China) in October 2020. A chest computed
tomography (CT) scan revealed multiple enlarged lymph nodes in the
mediastinum, hilum, armpit and neck, with no abnormalities in the
nasopharynx (Fig. 1). Blood tests
showed severe anemia with the following parameters: WBC count,
1.7×109/l (normal range, 3.50–9.50×109/l);
RBC count, 2.94×1012/l (normal range,
4.30–5.80×1012/l); and HGB, 77 g/l (normal range,
130–175 g/l). An elevated ferritin count of >200,000 ng/ml
(normal range, 30.00–400.00 ng/ml) and an elevated vitamin B12
count, of 1,960.00 pg/ml (normal range, 197.00–771.00 pg/ml) were
also observed. A cervical lymph node biopsy was performed after
admission. Tissues were fixed with 4% neutral formalin (12 h at
25°C) and embedded in paraffin. Consecutive tissue sections (4-µm
thick) were prepared and stained with hematoxylin and eosin (8 h at
25°C). The biopsy revealed destruction of the lymph node structure
by tumor cells, which had diffused into the surrounding fibrous and
adipose tissues. Furthermore, nerve invasion and vascular damage
were observed. The tumor cells were medium- to large-sized
heterotypic lymphocytes with moderate cytoplasm, irregular nuclei,
small nucleoli and obvious coagulative necrosis.
Immunohistochemical staining was performed on the Ventana automated
staining system, BenchMark ULTRA (Roche Tissue Diagnostics).
Antigens were detected using the assay kit ultraView Universal DAB
Detection Kit (cat no. 760-500). After deparaffinisation on a
BenchMark ULTRA, antigen repair was performed with repair solution
at 99°C. The primary antibody was added dropwise and incubated at
37°C for 32 min, the horseradish peroxidase (HRP)-labeled secondary
antibody was incubated for 8 min, and then DAB was used to develop
the colours. Hematoxylin was used to return the blue lining. In all
the processes, the buffer reaction buffer was used for rinsing, and
finally the film was blocked by gradient alcohol. The cells
strongly expressed CD20 (ready-to-use; clone L26; cat. no.
kit-0001), CD3 (ready-to-use; clone MX036; cat. no. MAB-0740), CD43
(ready-to-use; clone MX099; cat. no. MAB-0892), CD30 (ready-to-use;
clone MX080; cat. no. MAB-0868), TIA-1 (ready-to-use; clone
2G9A10F5; cat. no. MAB-0798) and Granzyme (ready-to-use; clone
GZB01; cat. no. MAB-0352), and partially expressed CD15
(ready-to-use; clone MMA; cat. no. MAB-0779). The CD30-positive
rate in large tumor cells was ~80%. In addition, >90% of cells
were positive for Ki-67 (ready-to-use; clone SP6; cat. no.
RMA-0542). The tumor cells were negative for CD79a (ready-to-use;
clone MX076; cat. no. MAB-0864), PAX5 (ready-to-use; clone MX017;
cat. no. MAB-0706), CD4 (ready-to-use; clone SP35; cat. no.
RMA-0620), CD56 (ready-to-use; cloneMX039; cat. no. MAB-0743), CD8
(ready-to-use; clone MX117; cat. no. MAB-1031) and ALK
(ready-to-use; clone MX064; cat. no. MAB-0848) (all Fuzhou Maixin
Biotech Co., Ltd.). In situ hybridization revealed EBV in
the tumor cells (Fig. 2).
Furthermore, the T cell receptor gene and immunoglobulin heavy
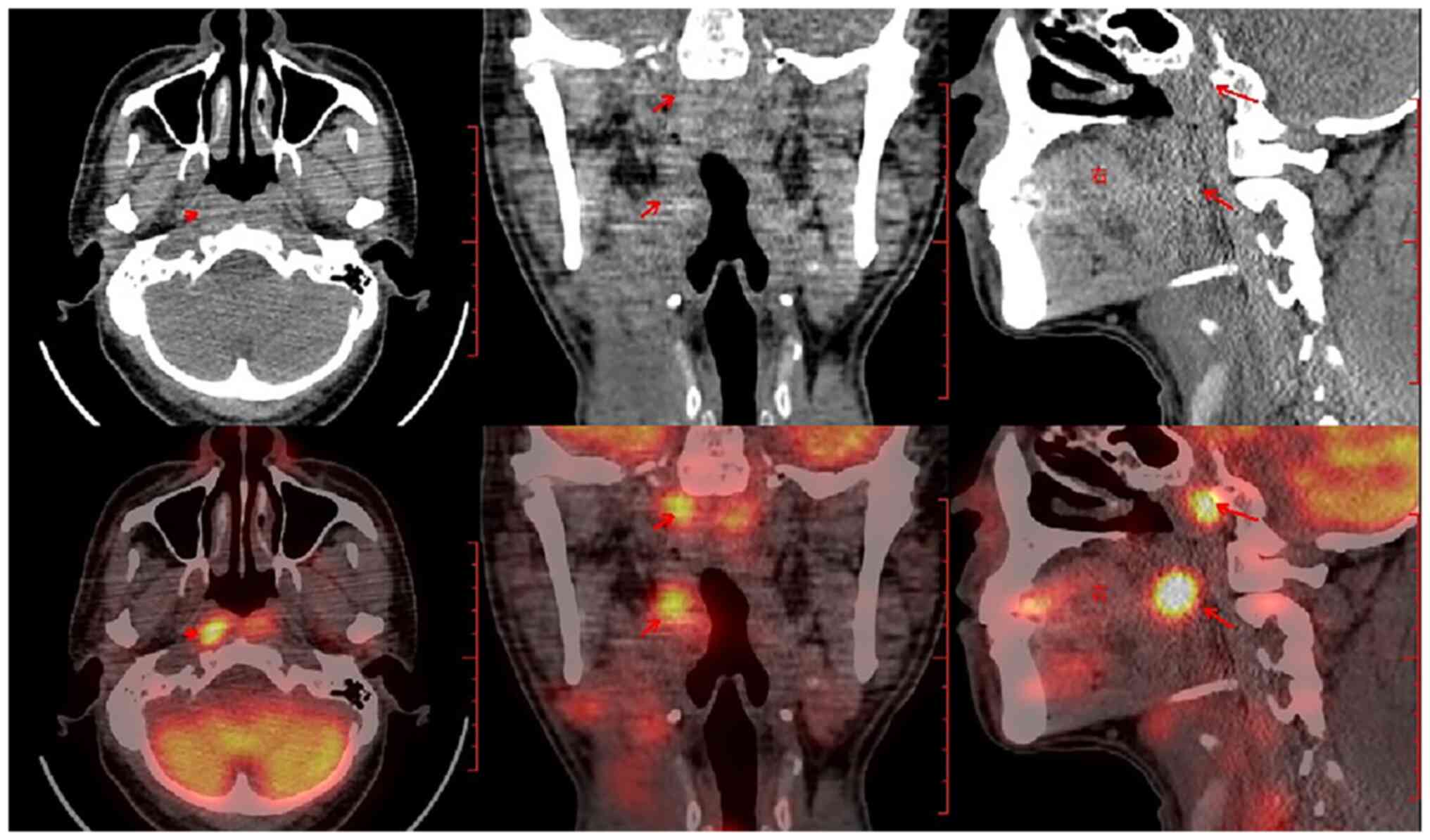
chain exhibited a polyclonal pattern. A whole-body positron
emission tomography-CT scan subsequently revealed a high metabolic
area in the nasopharynx (Fig. 3),
and the patient was diagnosed with ENKTL with an abnormal
expression of CD30, CD15 and CD20 (Ann Arbor Stage IV) (10). Following the diagnosis, the patient
underwent treatment with a CHOP chemotherapy regimen consisting of
1,200 mg cyclophosphamide, 40 mg doxorubicin and 2 mg vincristine
on day 1, and 100 mg prednisone on days 1–5. However, after one
cycle of chemotherapy, the patient died of multiple organ
failure.
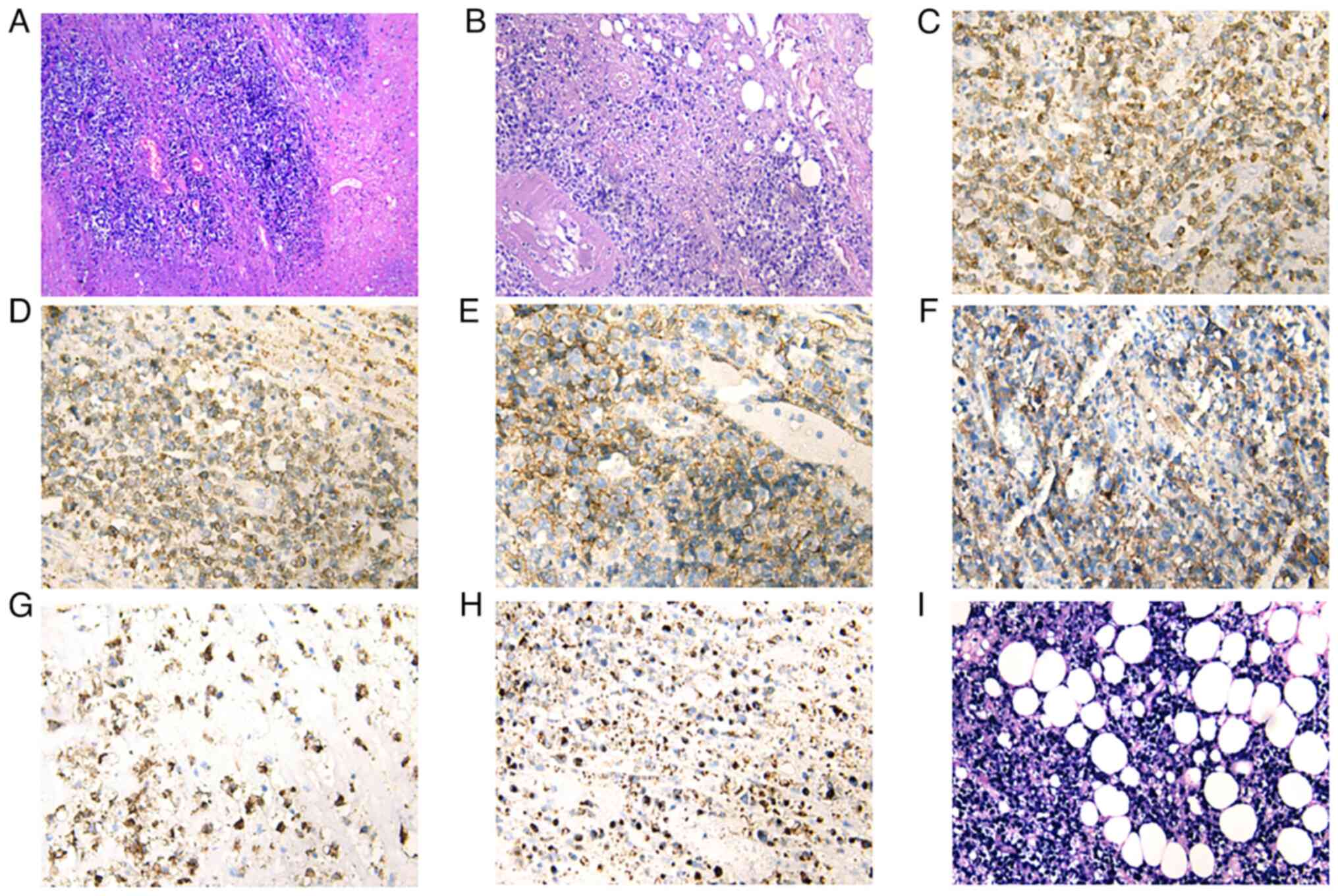 | Figure 2.(A and B) Under the microscope, the
lymph node structure appears to be destroyed by the tumor cells,
which have infiltrated the surrounding fatty and fibrous tissue.
The tumor cells comprise medium to large lymphocytes with irregular
nuclei. Immunohistochemical analysis shows tumor cells positive for
(C) CD3, (D) CD8, (E) CD30, (F) CD20, (G) CD15 and (H) TIA1. (I)
Tumor cells positive for EBER on in situ hybridization. [(A)
HE staining with magnification, ×40; (B) HE staining with
magnification, ×100; (C-H) immunohistochemical staining with
magnification, ×200; (I) In situ hybridization for EBER,
magnification, ×200]. EBER, Epstein-Barr virus small-encoded RNA;
HE, hematoxylin and eosin. |
Discussion
The definition and diagnostic criteria for ENKTL in
the 5th edition of the WHO classification of hematolymphoid
neoplasms remain unchanged (11).
ENKTL is classified into nasal and non-nasal types depending on the
primary site of the lesion. The nasal type accounts for the
majority (80%) of cases. The remaining 20% of the cases show
lesions in the skin, gastrointestinal tract, testes and other
sites, and these have a worse prognosis than nasal presentations
(12). ENKTL exhibits a diffuse
growth pattern, with growth around blood vessels and their
destruction. Common features of ENKTL include coagulative necrosis
and apoptotic bodies. The cytological spectrum of tumor cells
includes small, medium, large and anaplastic cells. The background
may be accompanied by various inflammatory cells, such as small
lymphocytes, plasma cells, tissue cells and eosinophils, even
resembling inflammation (13,14).
Tumor cells display a T cell or NK cell phenotype, with most cases
expressing CD2, cytoplasmic CD3ε and CD56, along with cytotoxic
molecules such as TIA1, granzyme and perforin. Most tumor cells are
EBV-positive on in situ hybridization (12).
Molecular studies have revealed that ENKTL exhibits
complex molecular mechanisms (15–17).
Recurrent mutations in genes associated with the JAK-STAT pathway
(e.g., STAT3, JAK3, STAT5B), epigenetic regulators (e.g., BCOR,
KMT2D, ARID1A, EP300), tumor suppressor genes (e.g., TP53, MGA) and
RNA helicases (e.g., DDX3X) have been observed in ENKTL.
Furthermore, structural activation of the JAK/STAT pathway through
mutations and phosphorylation plays a crucial role in ENKTL
pathogenesis and represents a potential therapeutic target.
Abnormalities in other signaling pathways, such as NF-κB and PDGFR,
and alterations in genes such as BIRC5, MYC, RUNX3, AURKA and EZH2
are also potential therapeutic targets (15–17).
Immune evasion has been highlighted as a key mechanism for ENKTL
cell survival, which is possibly driven by LMP-1 or STAT3-mediated
upregulation of PD-L1. Immune checkpoint inhibitors targeting the
PD1/PD-L1 axis are promising for ENKTL treatment (15–17).
T cell lymphomas occasionally express B cell
markers, such as CD20 or CD79a, with 5–8% of T cell lymphomas
showing CD20-positivity, with the most common type bringing
PTCL-NOS; some ENKTLs also show aberrant CD20 expression (18,19).
Huang et al (4) reviewed 18
cases of ENKTL with CD20 expression, making it the most
comprehensive case series to date. They noted that abnormal CD20
expression in ENKTL often occurs in elderly patients and is
associated with a highly invasive clinical course and poor
prognosis. Several hypotheses can explain this abnormal CD20
expression. One possible explanation is that during malignant
transformation, T, B and NK cells, which share common progenitor
cells, express markers typical to other cell types, suggesting that
CD20 expression in T cell lymphoma is related to malignant
transformation. Another possibility is that CD20 positivity results
from abnormal antigen expression associated with T cell lymphoma.
In some instances, CD20 expression might be lost upon recurrence,
indicating that it could be a transient phenomenon. In addition,
CD20-positive T cell subsets in the blood may contribute to
abnormal CD20 expression in T cell lymphomas. Normal
CD20+ T cell precursors have been confirmed to exist in
the blood, and possibly account for the abnormal CD20 expression
observed in some T cell lymphomas (20). Overall, the precise mechanism
underlying CD20 expression in T cell lymphoma remains unclear, and
further research is needed to elucidate this phenomenon.
When ENKTL abnormally expresses CD20, CD30 and CD15,
it must be differentiated from HL, anaplastic large-cell lymphoma
and diffuse large B cell lymphoma. Hodgkin's and Reed-Sternberg
cells in HL are characterized by CD30 and CD15 expression, with
heterogeneous CD20 staining. However, T cell atypia, necrosis and
cytotoxic expression typically rule out HL (14). Further, detection of EBV is crucial
for diagnosing both ENKTL and anaplastic large-cell lymphoma. In
diffuse large B cell lymphoma, in addition to CD20, other B cell
markers, such as CD79a and PAX-5, are also expressed.
Studies have shown that CD20 expression may be
associated with a highly aggressive clinical course and a poor
prognosis (3–8 months) (21,22).
Treatment options for ENKTL include CHOP or regimens such as
steroid, methotrexate, ifosfamide, L-asparaginase and etoposide
(SMILE) (23). The efficacy of
targeting CD20 in T cell lymphoma remains uncertain. Although
rituximab, a CD20-targeted therapy, is widely used for
CD20-positive B cell lymphomas, literature on its use for
CD20-positive T cell lymphoma is limited. Some studies suggest that
CD30 is a prognostic factor for overall survival or
progression-free survival in T cell lymphoma (24). A recent study reported that ENKTL
patients with high expression (>40%) exhibit improved overall
survival compared with those with low or negative (0%) CD30
expression levels (24); therefore,
the present study used 40% as a meaningful cut-off value for CD30
positive expression. Furthermore, CD30 expression has been reported
to affect the survival of clinical subgroups of patient (24). However, some studies hypothesize
that CD30 is unrelated to prognosis (8,9,25).
Brentuximab vedotin, an antibody-drug conjugate targeting CD30,
represents a significant advance in lymphoma treatment. It is
approved for relapsed HL and ALCL and has shown effectiveness in
other CD30-expressing lymphomas, such as PTCL-NOS (6).
To the best of our knowledge, this is the first
report in the literature of ENKTL expressing CD20, CD30 and CD15,
which is associated with aggressive clinical behavior. The present
case revealed that differential diagnosis should be cautiously
approached in ENKTL because the aforementioned markers are
typically expressed in B cell lymphoma or HL. In addition to
rigorous histological and comprehensive immunohistochemical
staining, whole-body imaging and molecular testing can assist with
diagnosis. Understanding the molecular mechanisms of CD30 and CD20
expression in ENKTL can also bring novel ideas for treatment.
Acknowledgements
Not applicable.
Funding
This research was supported by the Gansu Provincial Natural
Science Foundation of China (grant no. 21JR7RA427), and the Cuiying
Inovation Technology Foundation of Lanzhou University Second
Hospital (grant no. CY2021-QN-B08).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributors
PZ and JY conceived the study idea and drafted the
manuscript. PZ and JY carried out data collection. QZ, CX and BZ
performed the CT/PET scans. PZ and SY performed the biopsy. PZ and
YL interpreted the data and revised the manuscript. PZ and JY
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective study was not applicable for
ethics approval and written informed consent to participate was
provided.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hue SS, Oon ML, Wang S, Tan SY and Ng SB:
Epstein-Barr virus-associated T- and NK-cell lymphoproliferative
diseases: An update and diagnostic approach. Pathology. 52:111–127.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavlasova G and Mraz M: The regulation and
function of CD20: An ‘enigma’ of B-cell biology and targeted
therapy. Haematologica. 105:1494–1506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Wang K, Tan Q, Yang K, Wu D, Xu
Y, Zhao X and Jiang Z: Primary breast CD20-positive extranodal NK/T
cell lymphoma with stomach involvement: A case report and
literature review. Diagn Pathol. 16:1032021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Chen S, Wei R, Guo X, Yang X, Cao
Q, Yang Y and Yun J: CD20-positive extranodal NK/T cell lymphoma:
Clinicopathologic and prognostic features. Virchows Arch.
477:873–883. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng J, Zhu H and Choi JK: CD30
expression in pediatric neoplasms, study of 585 cases. Pediatr Dev
Pathol. 20:191–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pierce JM and Mehta A: Diagnostic,
prognostic and therapeutic role of CD30 in lymphoma. Expert Rev
Hematol. 10:29–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu ML, Gabali A, Hsi ED, Fedoriw Y, Vij K,
Salama ME, Ramchandren R, O'Malley D, Wick MR, Battistella M and
Gru AA: Practical approaches on CD30 detection and reporting in
lymphoma diagnosis. Am J Surg Pathol. 44:e1–e14. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawamoto K, Miyoshi H, Suzuki T, Sasaki Y,
Yamada K, Yanagida E, Muto R, Kiryu M, Sone H, Seto M, et al:
Frequent expression of CD30 in extranodal NK/T-cell lymphoma:
Potential therapeutic target for anti-CD30 antibody-based therapy.
Hematol Oncol. 36:166–173. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim WY, Nam SJ, Kim S, Kim TM, Heo DS, Kim
CW and Jeon YK: Prognostic implications of CD30 expression in
extranodal natural killer/T-cell lymphoma according to treatment
modalities. Leuk Lymphoma. 56:1778–1786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morley-Jacob C and Gallop-Evans E: An
update on lymphoma in children and young adults. Paediatr Child
Health. 22:92–97. 2012. View Article : Google Scholar
|
|
11
|
Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D,
Calaminici M, et al: The 5th edition of the World Health
Organization classification of haematolymphoid tumours: Lymphoid
neoplasms. Leukemia. 36:1720–1748. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tse E and Kwong YL: The diagnosis and
management of NK/T-cell lymphomas. J Hematol Oncol. 10:852017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldman AL, Laurent C, Narbaitz M,
Nakamura S, Chan WC, de Leval L and Gaulard P: Classification and
diagnostic evaluation of nodal T- and NK-cell lymphomas. Virchows
Arch. 482:265–279. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montes-Mojarro IA, Kim WY, Fend F and
Quintanilla-Martinez L: Epstein-Barr virus positive T and NK-cell
lymphoproliferations: Morphological features and differential
diagnosis. Semin Diagn Pathol. 37:32–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mundy-Bosse BL, Weigel C, Wu YZ, Abdelbaky
S, Youssef Y, Casas SB, Polley N, Ernst G, Young KA, McConnell KK,
et al: Identification and targeting of the developmental blockade
in extranodal natural killer/T-cell lymphoma. Blood Cancer Discov.
3:154–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim H and Ko YH: The pathologic and
genetic characteristics of extranodal NK/T-cell lymphoma. Life
(Basel). 12:732022.PubMed/NCBI
|
|
17
|
de Mel S, Hue SS, Jeyasekharan AD, Chng WJ
and Ng SB: Molecular pathogenic pathways in extranodal NK/T cell
lymphoma. J Hematol Oncol. 12:332019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang YH, Huang CT, Tan SY and Chuang SS:
Primary gastric extranodal natural killer/T-cell lymphoma, nasal
type, with acquisition of CD20 expression in the subcutaneous
relapse: Report of a case with literature review. J Clin Pathol.
68:943–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao SH, Wang Y, Dai XY, Xiao YJ, Guan JJ,
Lin DL, Wang JG, Li YJ, Xing XM and Zhao P: CD20-positive T cell
lymphoma: Clinicopathological features of five cases. Zhonghua Bing
Li Xue Za Zhi. 49:1021–1026. 2020.(In Chinese). PubMed/NCBI
|
|
20
|
Lee AYS: CD20+ T cells: An
emerging T cell subset in human pathology. Inflamm Res.
71:1181–1189. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon YK, Kim JH, Sung JY, Han JH and Ko
YH; Hematopathology Study Group of the Korean Society of
Pathologists, : Epstein-Barr virus-positive nodal T/NK-cell
lymphoma: an analysis of 15 cases with distinct clinicopathological
features. Hum Pathol. 46:981–990. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato S, Asano N, Miyata-Takata T, Takata
K, Elsayed AA, Satou A, Takahashi E, Kinoshita T and Nakamura S:
T-cell receptor (TCR) phenotype of nodal Epstein-Barr virus
(EBV)-positive cytotoxic T-cell lymphoma (CTL): A clinicopathologic
study of 39 cases. Am J Surg Pathol. 39:462–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SJ, Park S, Kang ES, Choi JY, Lim DH,
Ko YH and Kim WS: Induction treatment with SMILE and consolidation
with autologous stem cell transplantation for newly diagnosed stage
IV extranodal natural killer/T-cell lymphoma patients. Ann Hematol.
94:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen Z, Wang Y, Xie R, Zhang Q, Xing X,
Zhang S, Liu H and Sang W: Clinicopathologic features and survival
outcomes of CD30 expression in extranodal natural killer/T-cell
lymphoma. Am J Clin Pathol. 162:95–102. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li P, Jiang L, Zhang X, Liu J and Wang H:
CD30 expression is a novel prognostic indicator in extranodal
natural killer/T-cell lymphoma, nasal type. BMC Cancer. 14:8902014.
View Article : Google Scholar : PubMed/NCBI
|