Introduction
Colorectal cancer (CRC) was the third most commonly
occurring cancer and the second leading cause of cancer-associated
deaths worldwide in 2022 (1). For
patients with locoregional CRC, surgical resection is the primary
treatment with curative potential (2). However, most patients undergoing the
resection of CRC experience postoperative pain and require
postoperative analgesic management (3,4).
Opioids, such as morphine and sufentanil, are the main agents used
for postoperative analgesia in patients with CRC undergoing tumor
resection (5–8). However, the use of opioids potentially
induces adverse events, including hyperalgesia and physical
dependence, which can lead to opioid use disorder and reduced
patient satisfaction (9–11).
Dezocine is an amino-tetrahydronaphthalene
derivative widely used in China as an analgesic, which produces a
pain-relieving effect through the partial activation of µ and κ
receptors as well as the inhibition of norepinephrine reuptake
(12,13). The specific pharmacological
mechanisms of dezocine provide a similar analgesic effect to those
provided by typical opioids, including morphine and sufentanil, but
with fewer adverse effects, such as respiratory depression,
immunosuppression, and the disruption of intestinal motility
(14–17). In addition to being used as a
monotherapy, dezocine is commonly combined with nonsteroidal
anti-inflammatory drugs in clinical settings, and studies suggest
that such combinations allow a reduced dosage of dezocine to
achieve enhanced analgesic effects (18–20).
Therefore, the combination of dezocine and nonsteroidal
anti-inflammatory drugs appears to be a promising analgesic regimen
for patients undergoing surgery.
Flurbiprofen axetil is a prodrug of flurbiprofen
with a high affinity for inflammatory tissues and is one of the
most commonly used nonsteroidal anti-inflammatory drugs for
analgesia in China (12,21,22).
It relieves pain by inhibiting the production of prostaglandin; it
also reduces pro-inflammatory cytokines, such as tumor necrosis
factor-α (TNF-α) and interleukin 6 (IL-6), which further alleviates
inflammatory pain (23,24). Given the promising analgesic effects
and distinct analgesic mechanisms of flurbiprofen axetil and
dezocine, their combination may be a promising regimen for
postoperative pain management (25,26).
Recently, several studies have suggested that the postoperative
analgesic effect of dezocine plus flurbiprofen axetil (DFA) is
greater than that of opioids for patients undergoing surgery
(25–27). For instance, one study found that
postoperative pain was reduced in patients who had undergone
abdominal surgery when treated with DFA compared with opioids
(26). In another study,
postoperative pain and the level of TNF-α in patients with
resectable non-small cell lung cancer were reduced by a greater
extent by DFA than by dezocine (25). Therefore, it is speculated that DFA
may provide analgesia superior to that of opioids in patients with
CRC undergoing tumor resection. However, this hypothesis remains
unverified.
Therefore, the current prospective, observational
study aimed to compare the postoperative analgesic effect, levels
of pro-inflammatory cytokines, patient satisfaction and safety
profiles between DFA and sufentanil in patients with CRC following
tumor resection.
Materials and methods
Patients
Between February 2020 and September 2023, the
current prospective observational study included 107 patients with
CRC who received postoperative patient-controlled anesthesia (PCA)
with DFA or sufentanil. The patients were enrolled at the Fifth
Affiliated Hospital of Wenzhou Medical University (also known as
Lishui Hospital of Zhejiang University; Lishui, China). The
inclusion criteria comprised the following: i) Confirmed as having
CRC; ii) >18 years old; iii) American Society of
Anesthesiologists (ASA) physical status I or II; iv) underwent
tumor resection surgery; v) received postoperative analgesia with a
DFA or sufentanil PCA pump; vi) had normal cognitive function and
were able to complete all required assessment scales; and vii) were
willing to provide blood samples for the detection of
pro-inflammatory cytokines. The exclusion criteria comprised: i)
Known allergies to the study drugs; ii) distant metastases; iii)
severe hepatic, renal or cardiorespiratory abnormalities; iv)
history of abdominal surgery or other malignant diseases; v)
history of psychoactive drug dependence; and vi) women who were
pregnant or lactating. The study was approved by the Ethics
Committee of Lishui Hospital of Zhejiang University (approval no.,
2017056). All patients provided written informed consent.
Postoperative analgesia
This was a prospective, observational and
non-interventional study, so the patients' treatment was not
intervened with. The anesthetists decided the medication based on
their experience, patient's comorbidities, patient's age and
patient's weight. According to the medications received, patients
who received DFA via PCA pump were considered as the DFA group,
while patients who received a sufentanil via a PCA pump were
considered as the control group. The patients in the DFA group were
given an intravenous infusion of 5 mg dezocine and 50 mg
flurbiprofen axetil as loading dose, followed by the continuous
administration via PCA pump of a composition containing 30 mg
dezocine, 200 mg flurbiprofen axetil and 8 mg ondansetron. All
medications were diluted to 100 ml with saline. The PCA pump was
set up to provide automatic infusion at a background dose of 2
ml/h, and patients were able to self-administer additional single
1-ml doses as required, with a 15-min lock time after each
additional dose to prevent overdose. Patients in the control group
were given 5–10 µg sufentanil as an intravenous infusion loading
dose, followed by a PCA pump containing 100 µg sufentanil and 8 mg
ondansetron, also diluted in saline to 100 ml. The PCA pump
settings in the control group were the same as those in the DFA
group. For some patients, the dosage was adjusted according to
their body weight to meet the demands of personalized
treatment.
Assessment
Clinical characteristics were collected for study
analysis, including age, sex, weight, ASA grade, tumor location,
and T (tumor size and extent), N (nearby lymph node involvement)
and tumor-node-metastasis (TNM) stages (28,29).
The operation time and intraoperative blood loss were also
documented. The pain was evaluated on a numerical rating scale
(NRS) at 2, 6, 12, 24 and 48 h after surgery. The pain score on the
NRS scale ranged from 0–10 as follows: 0, no pain; 1–3, mild pain;
4–6 moderate pain; and 7–10, severe pain. Moderate-to-severe pain
was defined as a pain NRS score of 4–10. The number of PCA boluses
was also recorded. A patient satisfaction score was also collected,
ranging from 1–5, with a higher rating indicating greater
satisfaction as follows: 1, very dissatisfied; 2, dissatisfied; 3,
neutral; 4, satisfied; and 5, very satisfied. Total satisfaction
was defined as satisfied or very satisfied. In addition, patient's
blood was collected 24 and 48 h after surgery and the serum TNF-α
and IL-6 levels were detected using enzyme-linked immunosorbent
assay kits (TNF-α, cat. no. PT518; IL-6, cat. no. PI330) from
Beyotime Institute of Biotechnology. In addition, any adverse
events were recorded for safety assessment.
Statistical analysis
Statistics were conducted using SPSS v.26.0 (IBM
Corp.). Data are presented as the mean ± standard deviation, median
(interquartile range) or n (%) as appropriate. Comparisons between
the DFA and control groups were analyzed using unpaired Student's
t-test, Wilcoxon's rank-sum test, χ2 test or
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant result.
Results
Comparison of baseline characteristics
between the DFA and control groups
The DFA group included 45 patients, with a mean age
of 55.1±11.6 years. There were 11 (24.4%) female and 34 (75.6%)
male patients. Regarding the TNM stage, 6 (13.3%) patients were at
stage I, 16 (35.6%) patients were at stage II and 23 (51.1%)
patients were at stage III. The control group comprised 62 patients
with a mean age of 57.9±10.7 years, among whom 21 (33.9%) patients
were female and 41 (66.1%) were male. Regarding the TNM stage, 10
(16.1%) patients were at TNM stage I, 24 (38.7%) patients were at
stage II, and 28 (45.2%) patients were at stage III. The age, sex,
weight, ASA grade, tumor location, and T, N and TNM stages were not
significantly different between the DFA and control groups (all
P>0.05). The detailed baseline characteristics of the patients
are listed in Table I.
 | Table I.Clinical characteristics. |
Table I.
Clinical characteristics.
| Items | Control group
(n=62) | DFA group
(n=45) | P-value |
|---|
| Age (years) | 57.9±10.7 | 55.1±11.6 | 0.206 |
| Sex |
|
| 0.293 |
|
Female | 21 (33.9) | 11 (24.4) |
|
|
Male | 41 (66.1) | 34 (75.6) |
|
| Weight, kg | 61.7±8.9 | 63.1±9.4 | 0.443 |
| ASA grade |
|
| 0.510 |
| I | 17 (27.4) | 15 (33.3) |
|
| II | 45 (72.6) | 30 (66.7) |
|
| Tumor location |
|
| 0.315 |
|
Rectum | 19 (30.6) | 18 (40.0) |
|
|
Colon | 43 (69.4) | 27 (60.0) |
|
| T stage |
|
| 0.592 |
| T1 | 4 (6.5) | 0 (0.0) |
|
| T2 | 12 (19.4) | 10 (22.2) |
|
| T3 | 42 (67.7) | 32 (71.1) |
|
| T4 | 4 (6.5) | 3 (6.7) |
|
| N stage |
|
| 0.248 |
| N0 | 34 (54.8) | 22 (48.9) |
|
| N1 | 23 (37.1) | 13 (28.9) |
|
| N2 | 5 (8.1) | 10 (22.2) |
|
| TNM stage |
|
| 0.530 |
| I | 10 (16.1) | 6 (13.3) |
|
| II | 24 (38.7) | 16 (35.6) |
|
|
III | 28 (45.2) | 23 (51.1) |
|
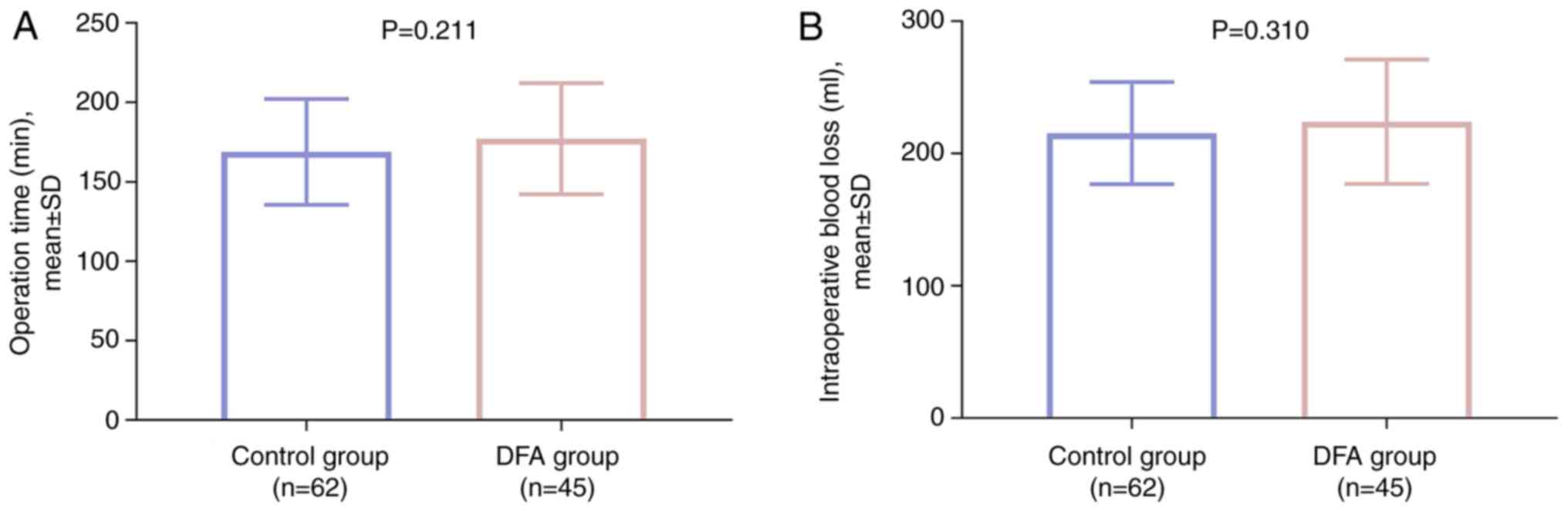
In terms of surgical information, no difference was
observed in operation time (P=0.211; Fig. 1A) or intraoperative blood loss
(P=0.310; Fig. 1B) between the DFA
and control groups.
Comparison of pain and PCA boluses
between the DFA and control groups
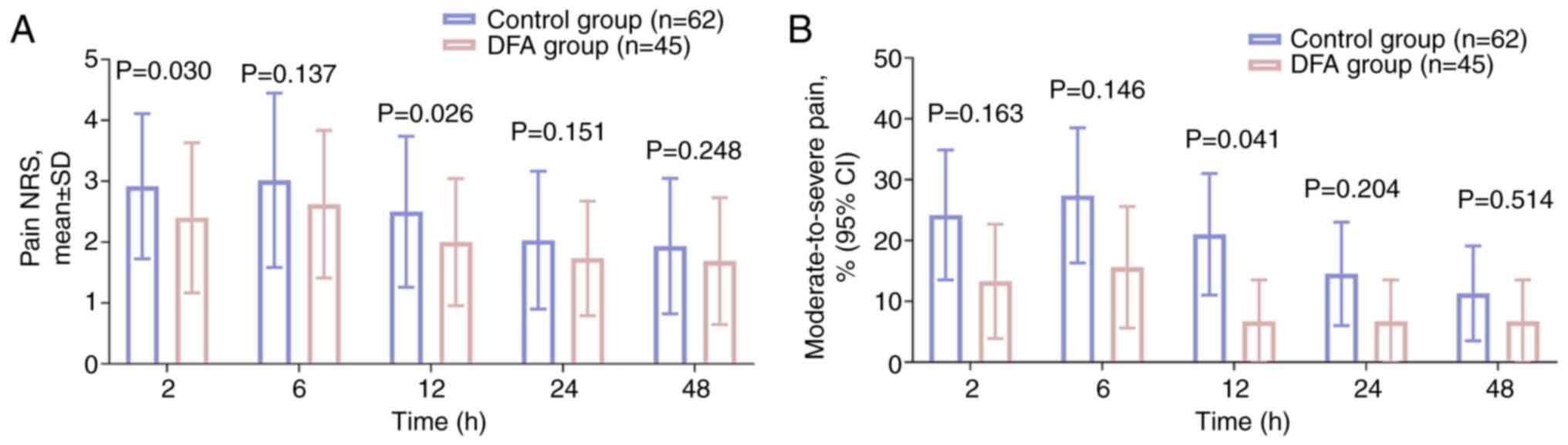
Pain NRS scores at 2 h (P=0.030) and 12 h (P=0.026)
were significantly decreased in the DFA group compared with those
in the control group. However, the scores at 6, 24 and 48 h did not
differ between the two groups (all P>0.05; Fig. 2A). The proportion of patients with
moderate-to-severe pain at 12 h was significantly reduced in the
DFA group compared with that in the control group (6.7 vs. 21.0%,
respectively; P=0.041); whereas the rates at 2, 6, 24 and 4 8 h did
not differ between the two groups (all P>0.05; Fig. 2B).
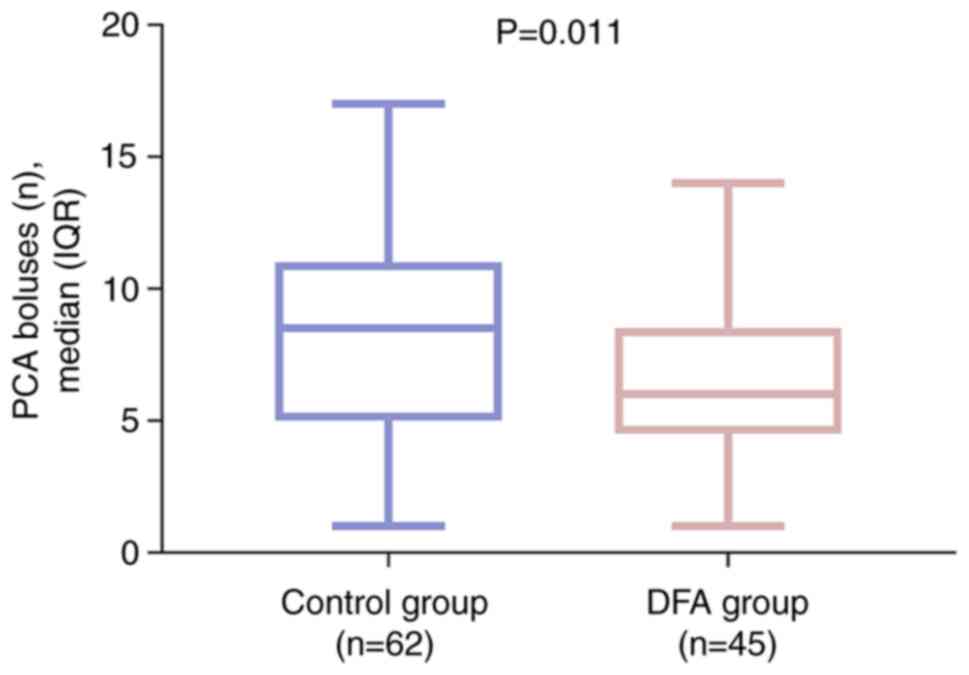
The number of PCA boluses administered was
significantly lower in the DFA group compared with that in the
control group (P=0.011; Fig. 3).
These findings suggest that pain was relieved more effectively in
the DFA group than in the control group.
Comparison of patient satisfaction
between the DFA and control groups
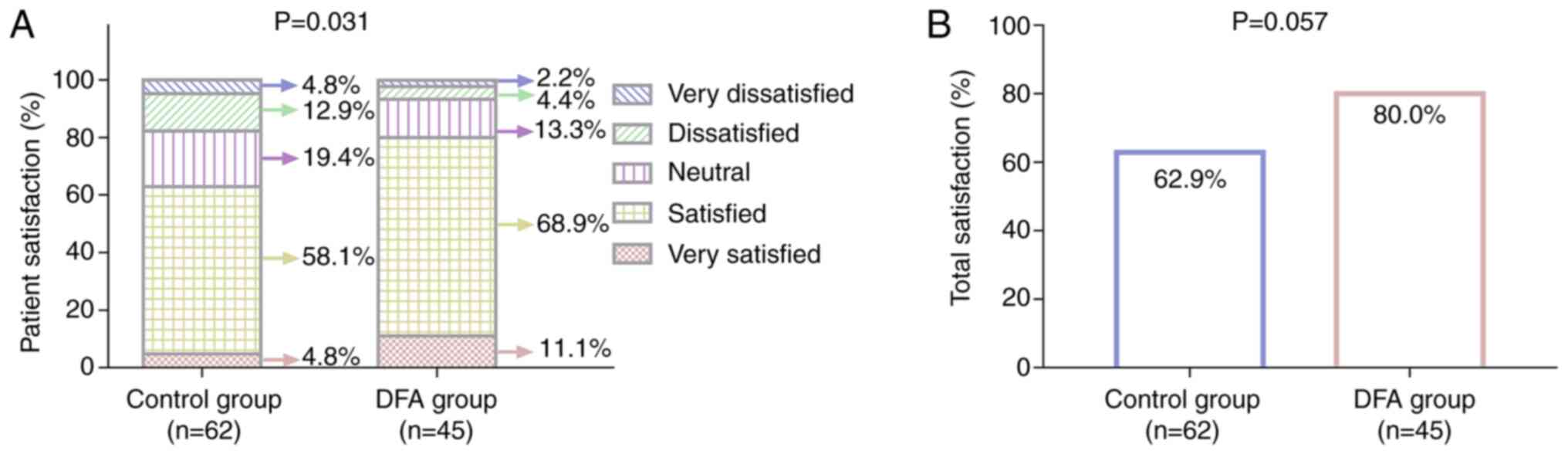
Patient satisfaction varied between the DFA and
control groups (P=0.031; Fig. 4A).
The percentage of patients reporting total satisfaction in the DFA
group was increased compared with that in the control group, but
the difference between the groups did not achieve statistical
significance (80.0 vs. 62.9%, respectively; P=0.057; Fig. 4B). These findings indicate that
patient satisfaction was improved to some extent in the DFA group
compared with that in the control group.
Comparison of pro-inflammatory
cytokines between the DFA and control groups
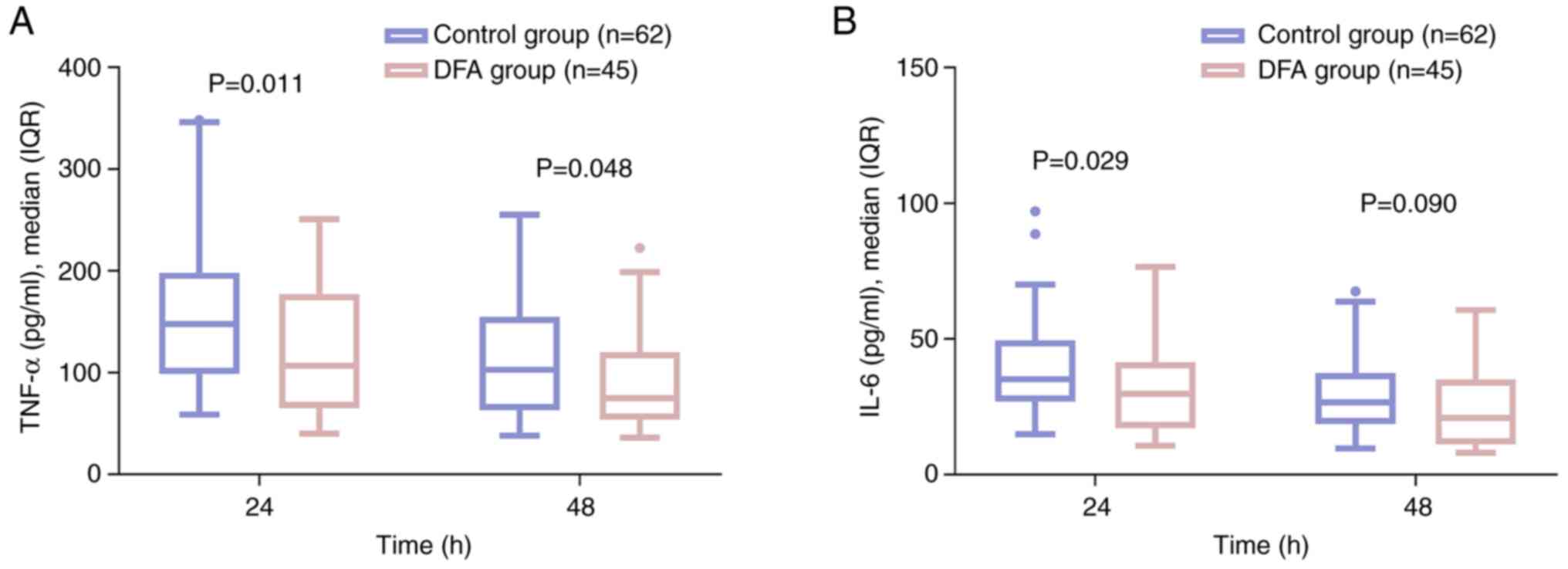
The DFA group had decreased levels of TNF-α at 24 h
(P=0.011) and 48 h (P=0.048) compared with those in the control
group (Fig. 5A). In addition, the
level of IL-6 at 24 h in the DFA group was lower than that in the
control group (P=0.029), but its level at 48 h did not vary between
the two groups (P=0.090) (Fig. 5B).
These results indicate that pro-inflammatory cytokines were
generally lower in the DFA group than in the control group.
Comparison of adverse events between
the DFA and control groups
Adverse events in the DFA group included nausea
(8.9%), pruritus (6.7%), vomiting (4.4%), dizziness (4.4%) and
drowsiness (2.2%). In the control group, adverse events included
nausea (14.5%), pruritus (8.1%), drowsiness (8.1%), vomiting
(6.5%), dizziness (4.8%), constipation (3.2%), respiratory
depression (1.6%) and hypotension (1.6%). The incidences of adverse
events did not differ between the two groups (all P>0.05;
Table II). These findings indicate
that the safety of DFA was acceptable.
 | Table II.Adverse events. |
Table II.
Adverse events.
|
| Incidence, n
(%) |
|
|---|
|
|
|
|
|---|
| Items | Control group
(n=62) | DFA group
(n=45) | P-value |
|---|
| Nausea | 9 (14.5) | 4 (8.9) | 0.379 |
| Pruritus | 5 (8.1) | 3 (6.7) | 1.000 |
| Vomiting | 4 (6.5) | 2 (4.4) | 1.000 |
| Dizziness | 3 (4.8) | 2 (4.4) | 1.000 |
| Drowsiness | 5 (8.1) | 1 (2.2) | 0.397 |
| Constipation | 2 (3.2) | 0 (0.0) | 0.508 |
| Respiratory
depression | 1 (1.6) | 0 (0.0) | 1.000 |
| Hypotension | 1 (1.6) | 0 (0.0) | 1.000 |
Discussion
Given the negative effects of postoperative pain on
the physical function, recovery from surgery, quality of life and
psychological status of patients, effective analgesic management is
crucial for improving patient outcomes (30). In previous studies, flurbiprofen
axetil combined with opioids was indicated to be more effective
than opioid alone for the alleviation of postoperative pain in
patients undergoing tumor resection (25,31).
Similarly, the present study found that DFA resulted in improved
analgesia compared with that provided by sufentanil in patients
following CRC resection. It is hypothesized that the possible
reason for this could be that flurbiprofen axetil inhibits
cyclooxygenase enzymes to reduce prostaglandin levels, which
subsequently alleviates pain and improves the pain threshold
(24), while dezocine activates
µ-opioid receptors to relieve pain, and suppresses noradrenaline
reuptake to restrain pain transmission (13). By contrast, sufentanil only
activates the µ-opioid receptor to provide an analgesic effect
(32). Therefore, DFA exerts both
central and peripheral analgesic effects, whereas sufentanil only
provides central analgesia. This dual action of DFA may provide
superior analgesic outcomes than those of sufentanil in patients
following CRC resection. Additionally, previous studies have
indicated that flurbiprofen axetil plus opioids provide increased
patient satisfaction compared with that provided by opioids in
patients after surgery (25,27).
In the present study, patient satisfaction following DFA treatment
was improved compared with that following sufentanil treatment in
patients after CRC resection, which is consistent with the previous
studies (25,27). This may be attributed to the
alleviation of postoperative pain by DFA being more effective than
that of sufentanil, and pain being negatively associated with
satisfaction in patients after surgery (33,34).
Consequently, postoperative analgesia and patient satisfaction in
the patients treated with DFA were elevated compared with those in
the patients treated with sufentanil following CRC resection.
Previously, studies have reported reductions in
postoperative circulating TNF-α and IL-6 levels in patients
receiving flurbiprofen axetil plus opioids (25,35,36).
Consistent with this, the present study showed that DFA decreased
the levels of TNF-α and IL-6 compared with those in patients
treated with sufentanil following CRC resection, suggesting that
DFA had a superior anti-inflammatory effect in these patients.
However, pro-inflammatory cytokines, including TNF-α and IL-6,
activate nociceptor terminals, triggering inflammatory pain and
sensitizing the nociceptors, which lowers the pain threshold and
contributes to postoperative pain (23,37).
Therefore, the improved analgesic effect of DFA compared with that
of sufentanil in patients after CRC resection might be that the
reduction of pro-inflammatory cytokine levels by DFA reduced
inflammatory pain and attenuated the lowering of the pain threshold
(23).
Both opioids and nonsteroidal anti-inflammatory
drugs exhibit adverse effects on the gastrointestinal tract
(38,39). Previous studies have reported that
the adverse events of flurbiprofen axetil plus opioids include
nausea, pruritus, vomiting, dizziness and drowsiness in patients
undergoing gastrointestinal surgery (40–42).
The present study did not identify any novel or unexpected events
in the patients who received DFA after CRC resection. In addition,
the incidence of adverse events did not differ between the patients
who received postoperative DFA and those who received sufentanil,
consistent with previous studies (25,43).
These findings suggest that the safety profile of DFA is acceptable
in patients after CRC resection.
However, there were some limitations to the current
study. First, the sample size was relatively small, which weakened
its statistical power. Therefore, studies with a larger sample size
are warranted for validation. Second, given that sufentanil is one
of the most frequently used opioids in China (12), patients receiving postoperative
sufentanil in this study served as the control group. However, the
analgesic effect of DFA compared with that of other opioids in
patients after CRC resection remains uncertain and requires further
investigation. Third, PCA was used in the present study and the
results might not be applicable to other administration methods,
such as intravenous injection. Thus, the analgesic effect of DFA
using other administration methods remains to be investigated.
Fourth, randomization was unable to be performed in this study,
which could have introduced selection bias and limited the
generalizability or reliability of these findings. Therefore,
future randomized, controlled studies are required for
validation.
In conclusion, the postoperative administration of
DFA exhibits an improved analgesic effect, improves patient
satisfaction and reduces the levels of pro-inflammatory cytokines
compared with those of sufentanil in patients following CRC
resection, but has comparable adverse effects. These findings
provide evidence for the application of DFA as an analgesic option
in with patients with CRC following resection of the tumor.
However, future studies with a larger sample size and other
administration methods are warranted to validate the analgesic
effect of DFA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JH designed the study. Material preparation, data
collection and analysis were performed by SY and JH. The first
draft of the manuscript was written by SY and both authors
commented on previous versions of the manuscript. SY and JH confirm
the authenticity of all the raw data. Both authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study received approval from the Ethics
Committee of Lishui Hospital of Zhejiang University (Lishui, China;
approval no., 2017056). All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwon JH, Park HJ, Sim WS, Park JH, Jung
KH, Oh MS, Seon HJ and Lee JY: Evaluation of the intraoperative
perfusion index for correlation with acute postoperative pain in
patients undergoing laparoscopic colorectal cancer surgery. J Clin
Med. 8:12992019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang WK, Tai YH, Lin SP, Wu HL, Tsou MY
and Chang KY: An investigation of the relationships between
postoperative pain trajectories and outcomes after surgery for
colorectal cancer. J Chin Med Assoc. 82:865–871. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pirie K, Traer E, Finniss D, Myles PS and
Riedel B: Current approaches to acute postoperative pain management
after major abdominal surgery: A narrative review and future
directions. Br J Anaesth. 129:378–393. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitakaze M, Uemura M, Kobayashi Y, Paku M,
Miyo M, Takahashi Y, Miyake M, Kato T, Ikeda M, Fujino S, et al:
Postoperative pain management after concomitant sacrectomy for
locally recurrent rectal cancer. Surg Today. 52:1599–1606. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang M, Xv X, Ren C, Yao Y and Gao X:
Effect of ultrasound-guided transversus abdominis plane block with
rectus sheath block on patients undergoing laparoscopy-assisted
radical resection of rectal cancer: A randomized, double-blind,
placebo-controlled trial. BMC Anesthesiol. 21:892021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gedda C, Nygren J, Garpenbeck A, Hoffström
L, Thorell A and Soop M: Multimodal analgesia bundle and
postoperative opioid use among patients undergoing colorectal
surgery. JAMA Netw Open. 6:e23324082023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volkow ND and Blanco C: The changing
opioid crisis: Development, challenges and opportunities. Mol
Psychiatry. 26:218–233. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zare N, Sharafeddin F, Montazerolghaem A,
Moradiannezhad N and Araghizadeh M: NLRs and inflammasome signaling
in opioid-induced hyperalgesia and tolerance. Inflammopharmacology.
32:127–148. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christie NC, Vojvodic V, Meda P and
Monterosso JR: Changes in social, romantic, and general life
satisfaction over the course of a substance use disorder. Front
Psychiatry. 12:7343522021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi H, Chen X, Liu X, Zhu H, Yu F, Ung
COL, Chan WS, Hu H and Han S: National drug utilization trend of
analgesics in China: An analysis of procurement data at 793 public
hospitals from 2013 to 2018. J Pharm Policy Pract. 14:452021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye RR, Jiang S, Xu X, Lu Y, Wang YJ and
Liu JG: Dezocine as a potent analgesic: Overview of its
pharmacological characterization. Acta Pharmacol Sin. 43:1646–1657.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu X, Luo B, Qiu L, Chen S, Wu Q, Chen Q,
Liu X, Ling C, Deng S, Yuan M and Hu P: Dezocine has the potential
to regulate the clinical and biological features of tumors. Drug
Des Devel Ther. 16:1121–1129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bian X, Zhou R, Yang Y, Li P, Hang Y, Hu
Y, Yang L and Wen D: Divergent effect of dezocine, morphine and
sufentanil on intestinal motor function in rats. Int J Med Sci.
12:848–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng M, Feng Q, Chen Y, Liu G, Gao Z, Xiao
J and Feng C: Effect of dezocine on the ratio of Th1/Th2 cytokines
in patients receiving postoperative analgesia following
laparoscopic radical gastrectomy: A prospective randomised study.
Drug Des Devel Ther. 15:2289–2297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Zhang X, Wang H and Liu Y: Effects
of dezocine and sufentanyl for postoperative analgesia on activity
of NK, CD4+ and CD8+ cells in patients with
breast cancer. Oncol Lett. 17:3392–3398. 2019.PubMed/NCBI
|
|
18
|
Wu Y, Cai Z, Li Y, Kang Y, Fu B and Wang
J: Effect of ketorolac tromethamine combined with dezocine prior
administration on hemodynamics and postoperative analgesia in
patients undergoing laparoscopic hernia repair. Medicine
(Baltimore). 101:e293202022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao ZZ, Li F, Zhang ZZ, Feng Y and Ma DY:
Effect of parecoxib sodium combined with dezocine on
gastrointestinal function and analgesia in patients after
laparoscopic gastric cancer surgery. J Guizhou Med Univ.
48:239–243. 2023.(In Chinese).
|
|
20
|
Li JZ, Xue LJ, Liu QY, Ren WR and Sun B: A
clinical study of patient-controlled intravenous analgesia with
dezocine combined with lornoxicam in cardia cancer patients after
radical surgery. Chin J Postgrad of Med. 36:73–74. 2013.(In
Chinese).
|
|
21
|
He Y, Qin M, Li M, Zhi D, Tian B and Qin
F: Comparison of in vivo pharmacokinetic behaviors of R- and
S-flurbiprofen after intravenous injection of flurbiprofen axetil.
Chirality. 35:247–255. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Luo J, Zheng L and Luo T:
Preoperative flurbiprofen axetil administration for acute
postoperative pain: A meta-analysis of randomized controlled
trials. J Anesth. 31:852–860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bell A: The neurobiology of acute pain.
Vet J. 237:55–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmadi M, Bekeschus S, Weltmann KD, von
Woedtke T and Wende K: Non-steroidal anti-inflammatory drugs:
Recent advances in the use of synthetic COX-2 inhibitors. RSC Med
Chem. 13:471–496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei X, Wang Z, Chen Y, Wang X, Ma L, Hou J
and Zhao L: Administration of flurbiprofen axetil and dezocine for
the postoperative analgesia in patients with non-small cell lung
cancer: A randomized, controlled study. Oncol Lett. 28:2942024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai R, Cui Y, Guo C, An R, Zheng S and
Shen X: Effect of flurbiprofen axetil on postoperative analgesia
following abdominal surgery: A single-center, prospective
randomized controlled trial. Indian J Surg. 84:124–130. 2022.
View Article : Google Scholar
|
|
27
|
Wang L, Wu LX, Han Z, Ma WH and Geng ZH:
Effect of flurbiprofen axetil combined with ‘Cocktail’ therapy on
opioid dosage in patients after total knee arthroplasty. Pak J Med
Sci. 38:724–729. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Günaydın B: ASA fiziksel durum
siniflandirma sistemi: ASA physical status classification system.
Turk J Anaesthesiol Reanim. 49:192–193. 2021.PubMed/NCBI
|
|
29
|
Mahmoud NN: Colorectal cancer:
Preoperative evaluation and staging. Surg Oncol Clin N Am.
31:127–141. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gan TJ: Poorly controlled postoperative
pain: Prevalence, consequences, and prevention. J Pain Res.
10:2287–2298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Li TT, Yin L, Huang J, Chen YJ,
Xiong LL and Wang TH: Analgesic effects of sufentanil in
combination with flurbiprofen axetil and dexmedetomidine after open
gastrointestinal tumor surgery: A retrospective study. BMC
Anesthesiol. 22:1302022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hughes LM, Irwin MG and Nestor CC:
Alternatives to remifentanil for the analgesic component of total
intravenous anaesthesia: A narrative review. Anaesthesia.
78:620–625. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daifallah A, Salameh H, Suwan B, Rabayaa
M, Khayyat Z, Hasoon M, Nazzal MA, Al-Jabi S and Zyoud SH:
Cancer-related post-treatment pain and its impact on treatment
satisfaction with medication in women with breast cancer: A
cross-sectional study from Palestine. Support Care Cancer.
31:5092023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goshima K, Sawaguchi T, Shigemoto K, Iwai
S, Fujita K, Kataoka T and Taninaka A: Factors associated with
patient satisfaction after opening-wedge high tibial osteotomy.
Orthop J Sports Med. 8:23259671209679642020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen Y, Wang M, Yang J, Wang Y, Sun H, Zhao
J, Liu W, Zhou Z, Deng H, Castillo-Pedraza C, et al: A comparison
of fentanyl and flurbiprofen axetil on serum VEGF-C, TNF-α, and
IL-1ß concentrations in women undergoing surgery for breast cancer.
Pain Pract. 15:530–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geng W, Hong W, Wang J, Dai Q, Mo Y, Shi
K, Sun J, Qin J, Li M and Tang H: Flurbiprofen axetil enhances
analgesic effects of sufentanil and attenuates postoperative
emergence agitation and systemic proinflammation in patients
undergoing tangential excision surgery. Mediators Inflamm.
2015:6010832015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baghaie L, Haxho F, Leroy F, Lewis B,
Wawer A, Minhas S, Harless WW and Szewczuk MR: Contemporaneous
Perioperative inflammatory and angiogenic cytokine profiles of
surgical breast, colorectal, and prostate cancer patients: Clinical
implications. Cells. 12:27672023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang A, Murphy J, Shteynman L, Daksla N,
Gupta A and Bergese S: Novel opioids in the setting of acute
postoperative pain: A narrative review. Pharmaceuticals (Basel).
17:292023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bindu S, Mazumder S and Bandyopadhyay U:
Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A
current perspective. Biochem Pharmacol. 180:1141472020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wan Z, Chu C, Zhou R and Que B: Effects of
oxycodone combined with flurbiprofen axetil on postoperative
analgesia and immune function in patients undergoing radical
resection of colorectal cancer. Clin Pharmacol Drug Dev.
10:251–259. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Wu J, Li H, Ye S, Xu X, Cheng L,
Zhu L, Peng Z and Feng Z: Prospective investigation of intravenous
patient-controlled analgesia with hydromorphone or sufentanil:
Impact on mood, opioid adverse effects, and recovery. BMC
Anesthesiol. 18:372018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang GW, Cheng H, Song XY, Yang YF, Liu H,
Ji FH and Peng K: Effect of oxycodone-based multimodal analgesia on
visceral pain after major laparoscopic gastrointestinal surgery: A
randomised, double-blind, controlled trial. Drug Des Devel Ther.
18:1799–1810. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu TT, Wang ZG, Ou WL, Wang J, Yao GQ,
Yang B, Rao ZG, Gao JF and Zhang BC: Intravenous flurbiprofen
axetil enhances analgesic effect of opioids in patients with
refractory cancer pain by increasing plasma β-endorphin. Asian Pac
J Cancer Prev. 15:10855–10860. 2014. View Article : Google Scholar : PubMed/NCBI
|