Introduction
Primary squamous cell carcinoma of the thyroid
(PSCCT) is a progressive and highly invasive tumor, accounting for
~0.1% of all primary thyroid malignancies (1). The origin of PSCCT is unclear, as the
squamous epithelium is usually absent from the thyroid gland under
normal physiological conditions. Currently, three theories have
been proposed to explain the origin of PSCCT. First, PSCCT is
hypothesized to originate from a residual branchial arch or
thyroglossal ducts of embryonic origin (2). Second, underlying diseases such as
Hashimoto's thyroiditis and inflammatory reactions trigger squamous
metaplasia (3). Third,
dedifferentiation of pre-existing primary thyroid cancers, such as
medullary, papillary or anaplastic carcinomas, may cause PSCCT
(4). The World Health Organization
(WHO) reclassified PSCCT as a subtype of anaplastic carcinoma
rather than a separate entity in 2022 (5).
Patients with PSCCT are frequently diagnosed at an
advanced stage. An early stage diagnosis is challenging due to the
rare occurrence of this malignancy and the absence of typical
imaging manifestations. The current study presents a case of PSCCT
and describes the process of its diagnosis based on findings from
ultrasound (US) combined with contrast-enhanced US (CEUS). To the
best of our knowledge, the present case study is the most
thoroughly documented profile among the six instances of PSCCT
recorded in the history of the Affiliated Hospital of Guangdong
Medical University (Zhanjiang, China). In addition, the available
literature on PSCCT diagnosis and treatment is summarized.
Case report
Patient
A 69-year-old woman presented at the Affiliated
Hospital of Guangdong Medical University in October 2022 with a
painless right-sided neck mass that had been rapidly enlarging for
the last 2 months (Fig. 1). In
addition, the patient had lost 13 kg of weight within this short
duration. As the disease progressed, local ulceration of the skin
was observed on the right side of the neck, with yellowish ooze and
hyperpigmentation. At 5 months prior to this presentation, the
patient had been diagnosed with poorly differentiated
adenocarcinoma of the stomach and underwent a laparoscopic distal
gastrectomy for gastric cancer (Billroth II procedure). The patient
did not have any family history of thyroid cancer or any other type
of cancer, nor had neck radiation ever been administered.
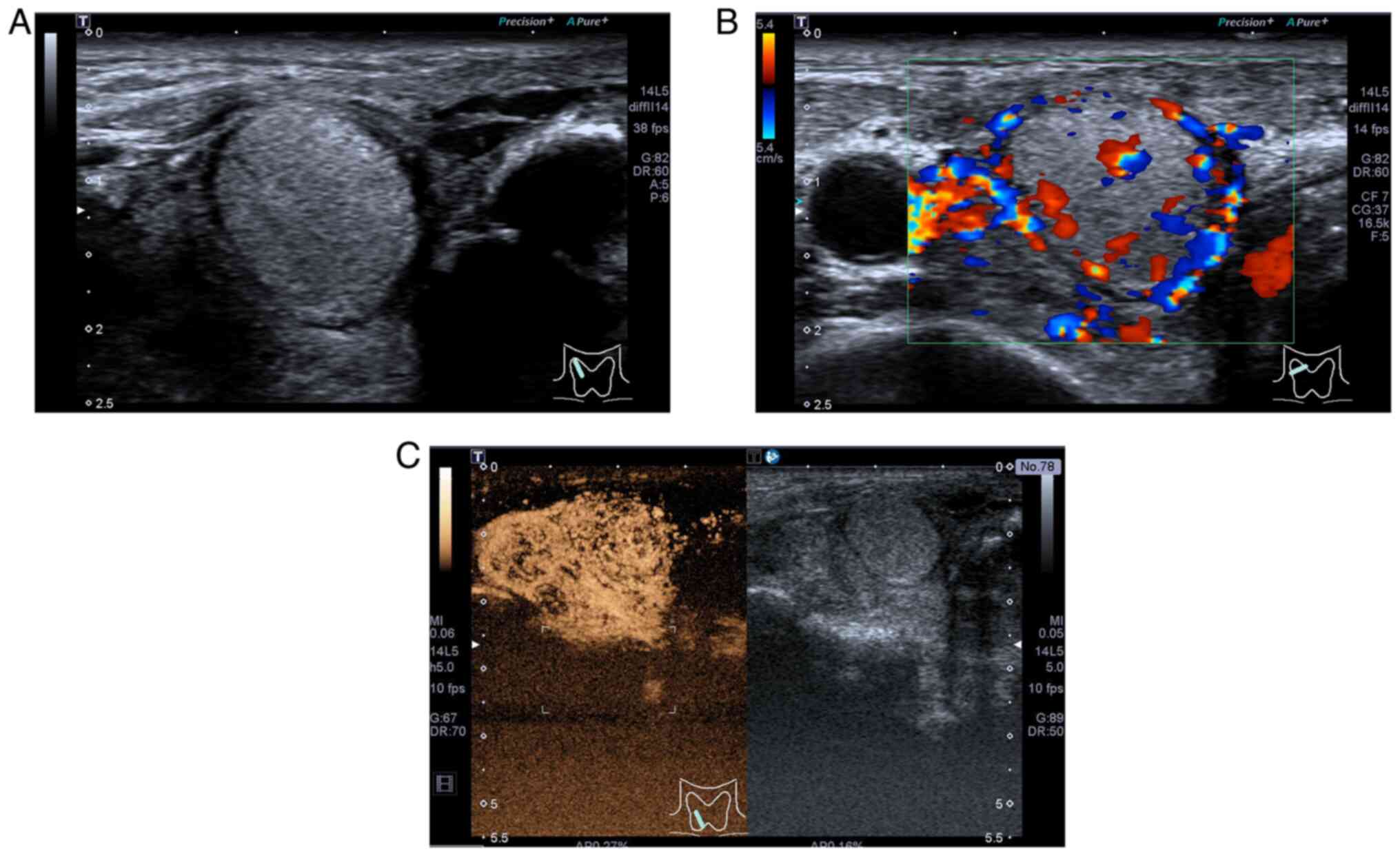
Two heterogeneous echogenic masses in the right lobe
of the thyroid gland were visible on thyroid US in October 2022.
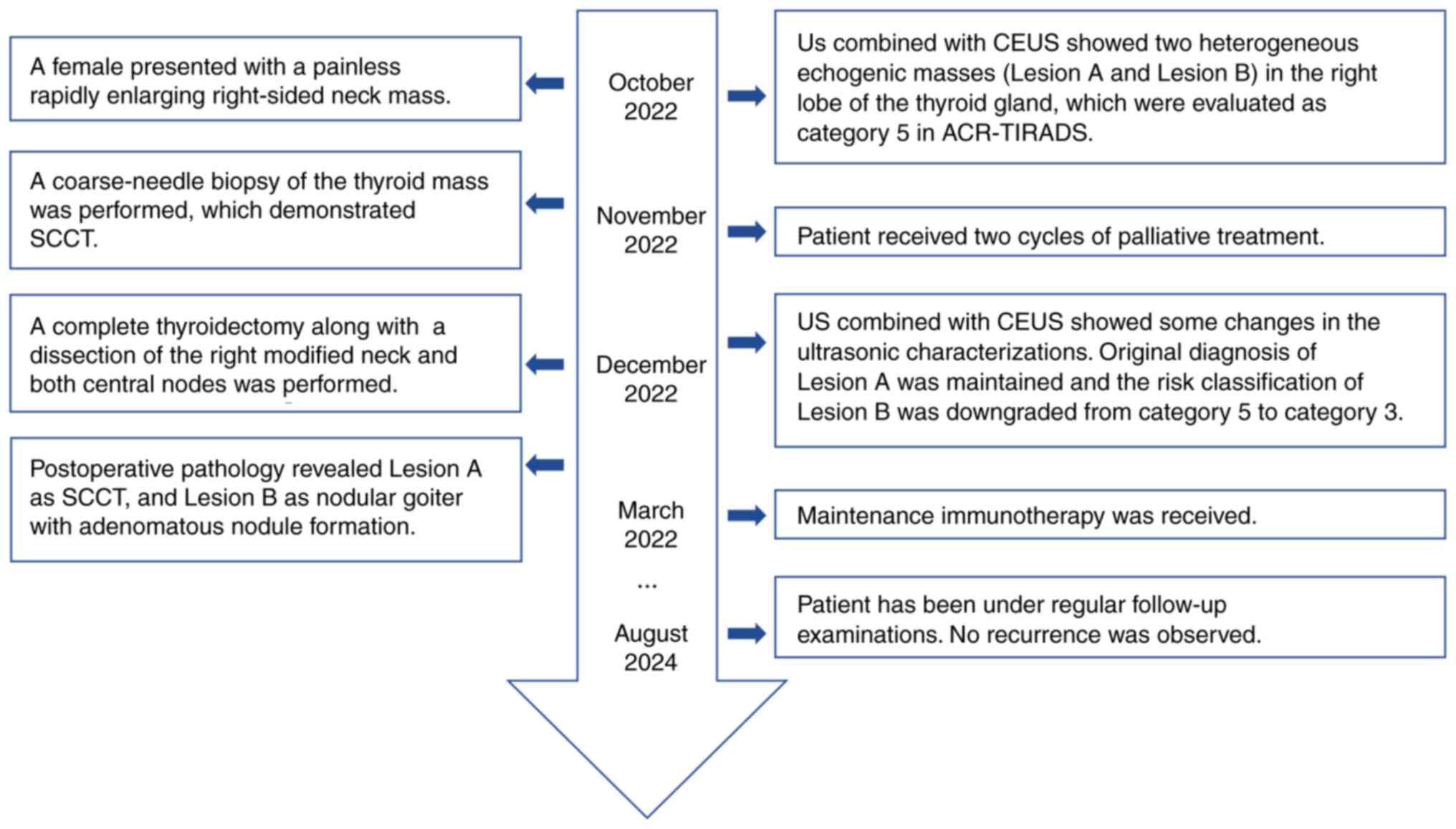
Lesion A, a mixed cystic-solid mass measuring 5.7×4.3×5.6 cm, was
situated in the lower middle region of the right lobe of the
thyroid gland (Fig. 2A). The lower
edge extended beyond the superior sternal fossa. Lesion B was a
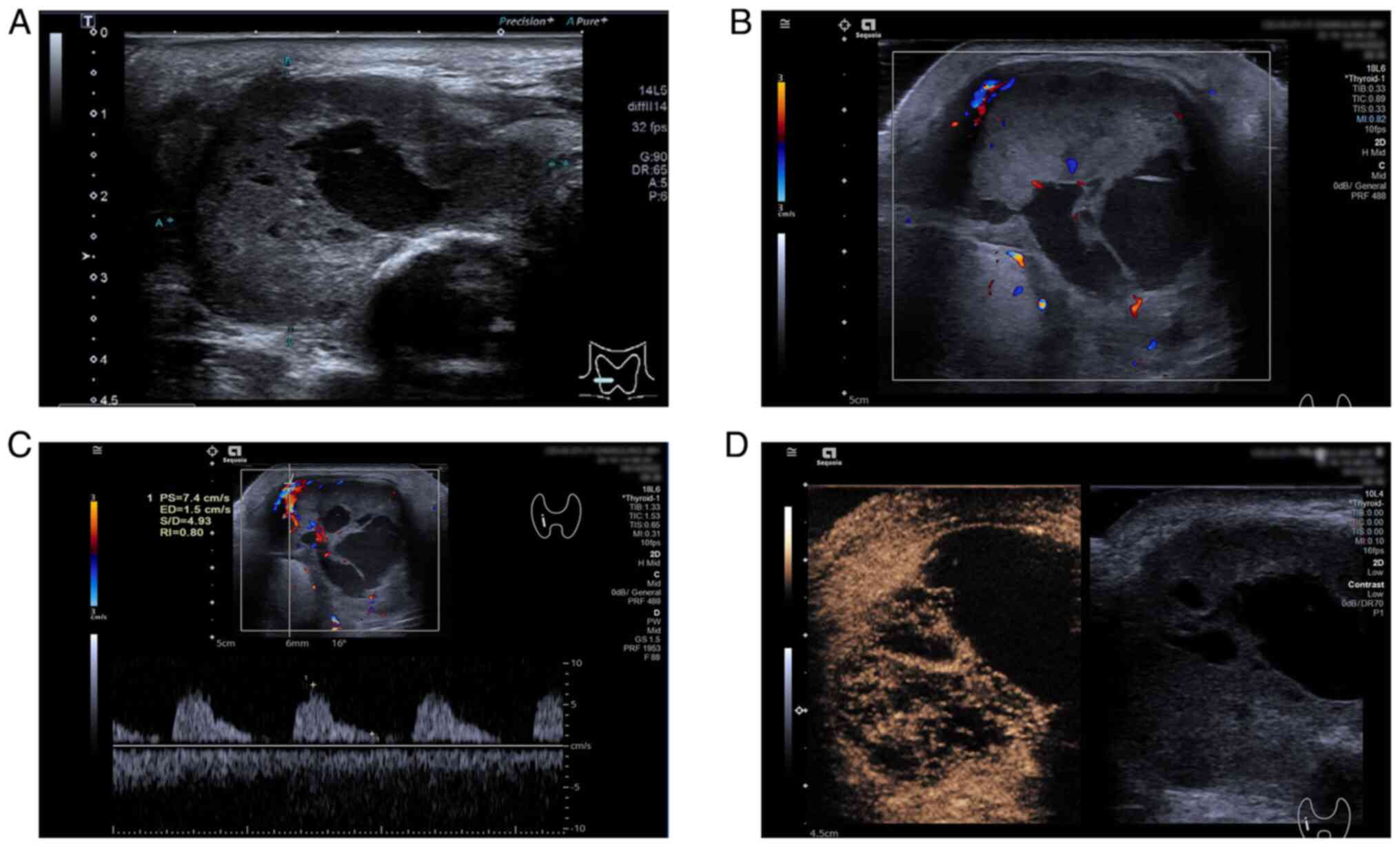
solid, slightly hyperechoic nodule measuring 2.6×1.7×2.3 cm,
occupying the upper part of the ipsilateral lobe (Fig. 3A). These lesions showed some of the
malignant ultrasonic characterizations, such as irregular margins
and unclear boundaries with adjacent soft-tissue layers. In
addition, two irregular macrocalcifications and microcalcifications
were observed in Lesion A. Color Doppler flow imaging (CDFI)
displayed abundant blood flow signals inside the lesion (Fig. 2B), with a resistive index (RI) of
0.80 in one of the arteries (Fig.
2C). Lesion B showed an abundant blood flow signal in and
around the nodule on CDFI (Fig.
3B). CEUS was performed after injecting 2.0 ml
SonoVue® (Bracco).
Lesion A presented as an inhomogeneous hypo-enhanced
thyroid nodule with a broad central area of non-enhancement and
blurred borders (Fig. 2D). Lesion
B, observed in reperfusion mode, had unclear boundaries with
abundant contrast agent inside the lesion. Based upon the
conventional malignant findings in US and CEUS patterns, these two
nodules merited 10 points (mixed cystic-solid, 1 point; hypoechoic,
2 points; irregular margins and unclear boundaries with adjacent
soft-tissue layers, 3 points; macrocalcifications, 1 point; and
microcalcifications, 3 points) and were evaluated as category 5 in
the 2017 American College of Radiology-Thyroid Imaging and
Reporting Data System (ACR-TIRADS) (6). Therefore, these nodules were
preliminarily diagnosed as malignant tumors.
Following these findings, the clinician performed a
coarse-needle biopsy of the thyroid mass, which showed an SCCT.
Immunohistochemical findings were positive for cytokeratin (CK)19,
CK5/6, p63, p53, Ki67 and paired box protein Pax-8 (PAX-8), and
negative for thyroid peroxidase and thyroid transcription factor 1
(TTF-1). A diagnosis of PSCCT was eventually established only after
excluding all other possible primary tumor sites. Considering the
large size of the tumor and the fact that the patient was in the
postoperative period of gastric cancer, the patient was started on
palliative treatment with oral anlotinib (12 mg daily on days
1–14), intravenous paclitaxel (200 mg on day 1 every 3 weeks) and
intravenous tislelizumab (300 mg on day 1 every 3 weeks) (for two
cycles lasting 21 days).
The patient underwent another US and CEUS
examination after the second cycle of palliative treatment in
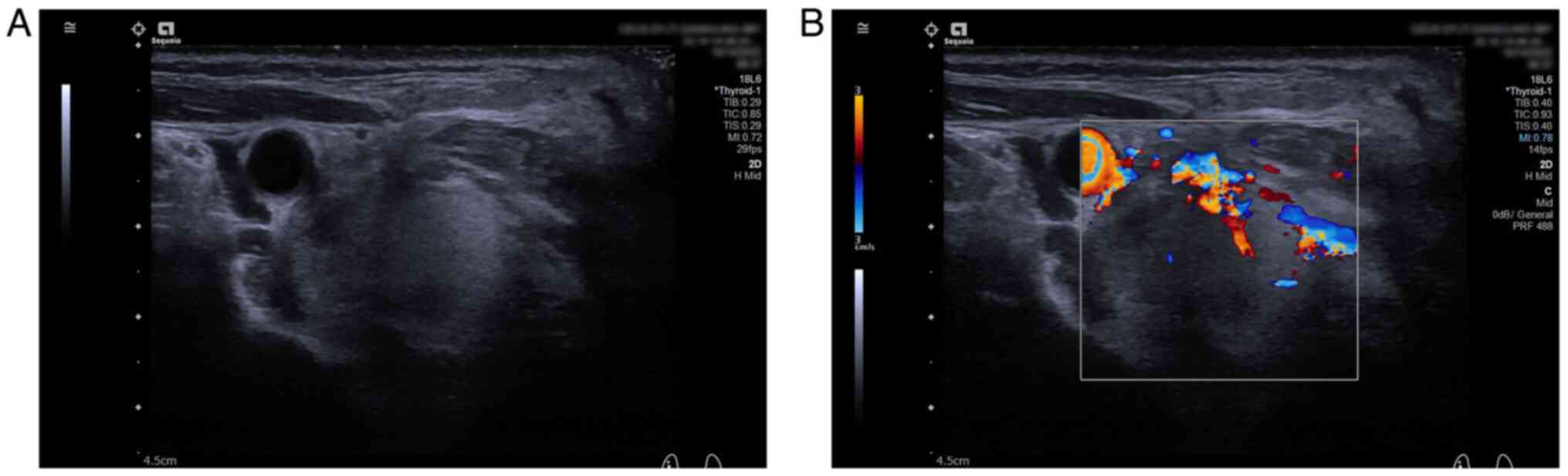
December 2022. Lesion A was smaller (3.5×2.6×3.3 cm) than its
initial size, and the RI (0.65) was decreased compared with its
initial value (Fig. 4A-C). CEUS
still showed heterogeneous hypo-enhancement of the nodule with
blurred boundaries, but the percentage of internal non-enhancing
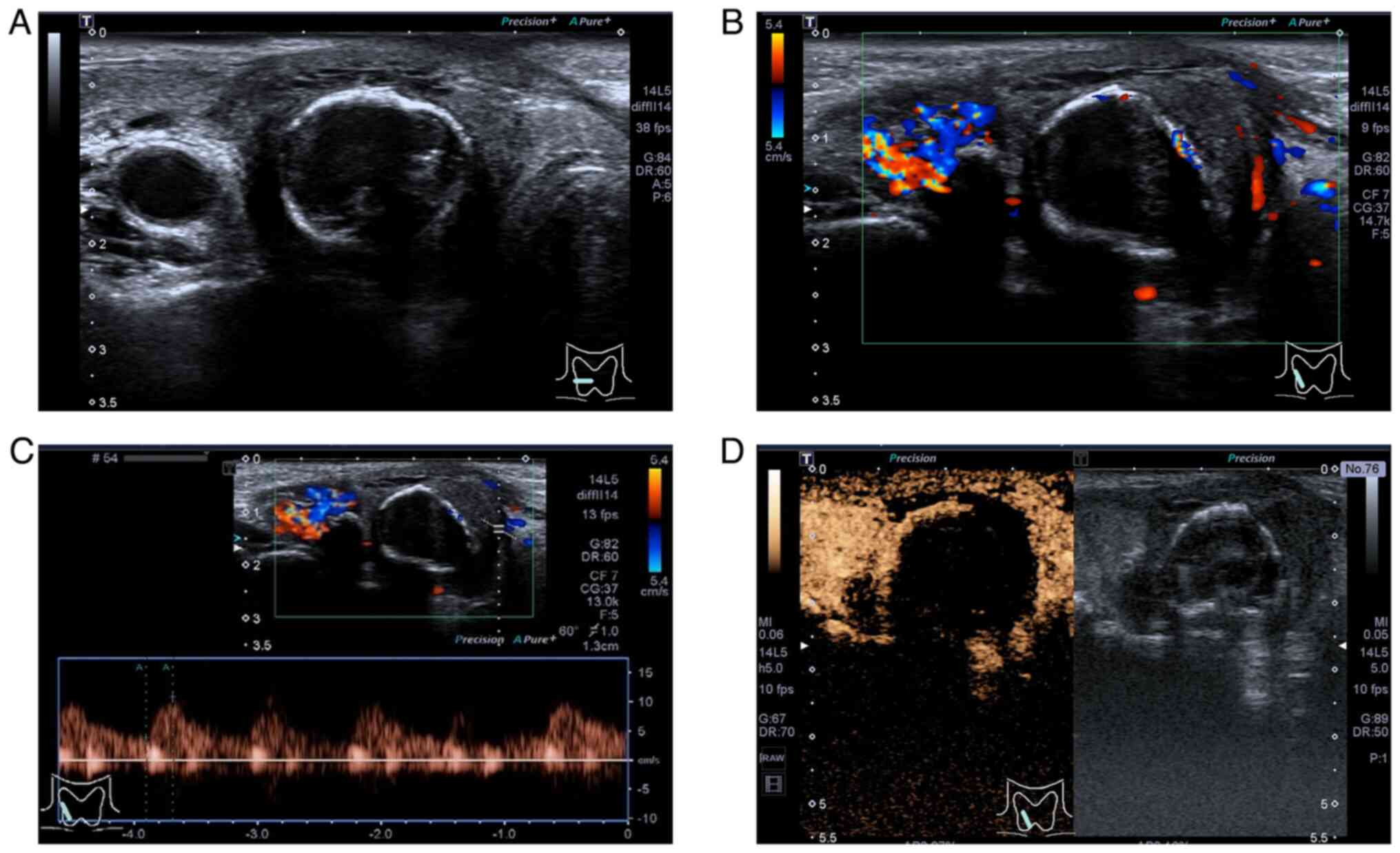
areas was higher than the initial percentage (Fig. 4D). The boundary of Lesion B became
clearer with a uniform and continuous hyperechoic thin halo visible
around it, and there was no marked change in its size (Fig. 5A). CDFI showed an increase in the
internal blood flow signal of the nodule, and the periphery
appeared to have a circumferential blood flow signal (Fig. 5B). Correspondingly, CEUS showed a
uniformly high enhancement of the nodule with a clearer boundary
(Fig. 5C). Finally, the original
diagnosis of Lesion A was maintained and the risk classification of
Lesion B was downgraded from category 5 to category 3 in
ACR-TIRADS.
Subsequently, the patient underwent a total
thyroidectomy with right modified radical neck dissection and
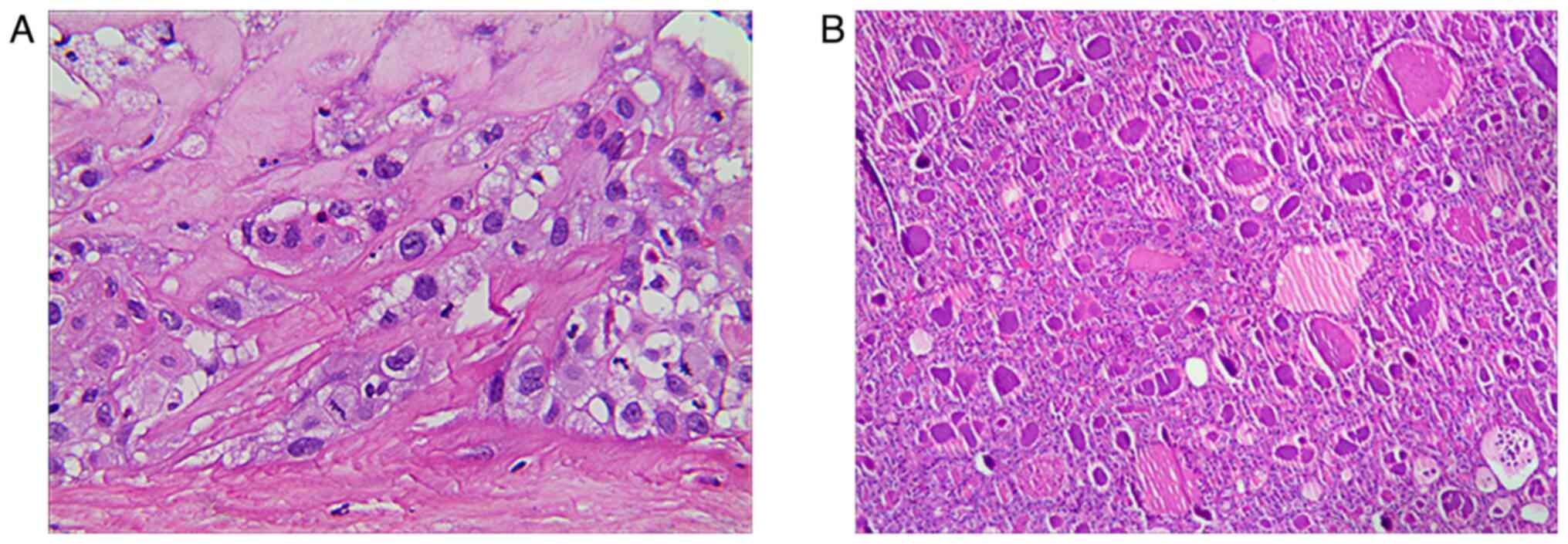
bilateral central node dissection. Postoperative pathology revealed
Lesion A as an SCCT, with mostly coagulative necrosis, and Lesion B
as nodular goiter, with adenomatous nodule formation (Fig. 6). In addition, none of the lymph
nodes were metastatic, and cancerous invasion of the left thyroid
gland had not occurred. Adjuvant radiotherapy was suggested to the
patient and their family, but was refused. Finally, tislelizumab
(200 mg in day 1 of each cycle, every 3 weeks) was administered as
maintenance immunotherapy. Follow-up was performed every 3 months,
and the patient is currently alive with no tumor recurrence after
19 months of surgery.
Immunohistochemistry
Tissues were fixed in 10% neutral formalin fixative
immediately after sampling. For fixation, 10% neutral formalin
(neutral buffered formalin) was used at room temperature, for 24 h.
Conventional paraffin was used for embedding. The section thickness
was 3–5 µm, using standard paraffin sectioning methods. In order to
fully expose the antigen, thermal repair with 0.01 M citrate buffer
(pH 6.0) was performed, heating the sections at 95–100°C for 10–20
min in a microwave or water bath. Primary antibodies (dilution,
1:100 to 1:500; all Abcam) were incubated with the sections
overnight at 4°C. An HRP-conjugated secondary antibody (dilution,
1:200 to 1:1,000; Dako; Agilent Technologies, Inc.) was added and
incubated at room temperature for 30–60 min. 3,3′-diaminobenzidine
was applied for 3–10 min as the chromogenic substrate. Contrast
staining was performed using hematoxylin for 1–3 min, followed by
dehydration and sealing. Observation was carried out using a light
microscope.
Pathology
Post-operative pathology tissues were fixed using
10% neutral formalin at room temperature for 24 h, followed by
paraffin embedding. Tissues were sections at 3–5 µm using a
standard microtome. Hematoxylin and eosin staining was performed to
observe histological features. Hematoxylin was applied at room
temperature for 5 min, and eosin staining was performed at room
temperature for 2 min. This was followed by dehydration,
visualization and sealing. A light microscope was used to observe
the sections.
Discussion
PSCCT is a rare and highly aggressive thyroid
malignancy with a short median survival time that accounts for
~0.1% of all primary thyroid cancer cases (1). Notably, older individuals aged 50 to
60 years are typically affected, and the ratio of women to men is
2.5:1.7 (7). PSCCT manifests as a
fast growing tumor in the anterior neck. Dysphagia, vocal
abnormalities and dyspnea are additional typical symptoms (8). An early diagnosis increases the
probability of timely treatment, prolongs the duration of patient
survival and improves the quality of life for patients. Therefore,
clinical studies should focus on finding improved methods to
determine an early diagnosis.
The preferred imaging technique for thyroid nodule
diagnosis is US. Several ultrasonic characteristics, including
solid components, microcalcifications, hypoechogenicity,
taller-than-wide dimensions and irregular margins, indicate thyroid
malignancy. Nevertheless, the ultrasonic features of PSCCT are not
yet fully understood due to the limited number of reported cases.
Yan et al (7) reported that
~85.7% (6/7) of PSCCTs on US were hypoechoic or mixed echoes with
heterogeneous echogenicity, that 57.1% (4/7) revealed calcification
and that all nodules had blood flow signals within them. Zhang
et al (9) observed the
ultrasonic features of nine PSCCT tumors and concluded that PSCCTs
tended to appear as solid nodules that were relatively large,
hypoechoic or very hypoechoic, with intranodular vascularity and
extrathyroidal extension. Ou et al (10) reported that ultrasonography
characteristics of PSCCT were predominantly the presence of
hypoechoic, hard, solid nodules with rough boundaries and a grade
1–2 blood flow signal, occasionally accompanied by necrosis and
calcification. In the present case, PSCCT, i.e., Lesion A,
manifested as a mixed cystic and solid thyroid nodule with
irregular margins and indistinct borders with adjacent soft-tissue
layers. In addition, macrocalcifications and microcalcifications
were observed inside the tumor, and CDFI showed abundant vascular
signals with a high RI. These malignant US findings were consistent
with those reported in other national and international studies
(7–10).
The PSCCT lesion measured ~5.7×4.3×5.6 cm before
treatment; this size was comparatively greater than the mean size
of thyroid tumors (2.2±1.9 cm) (11). This may be attributed to the quick
proliferation of tumor cells and the aggressive nature of this
malignancy. The irregular margins and unclear boundaries indicated
possible invasion of adjacent tissue by the nodule. Irregular
macrocalcifications and microcalcifications are also highly
suspicious ultrasonic indicators for thyroid malignancy. Zhang
et al (9) reported that the
mean RI of PSCCTs was 0.84; this value was greater than the RIs of
benign thyroid nodules (0.59) and papillary thyroid carcinomas
(0.70). Approximately 87.5% of the available RIs were >0.70. The
RI values reflect the blood flow resistance of arteries. The newly
generated vascular networks may be prevented from maturing and
pruning, as pro-angiogenic signaling is present continuously within
the tumors. This results in poor vascular organization and
malformations. Furthermore, elevated interstitial fluid pressure is
a result of the high permeability of the tumor vasculature
(12). Vascular structural
malformations and tumor vascular compression may increase arterial
resistance, which may be reflected in the RI value. In the present
patient, intranodal vessels were observed on pre-treatment US with
an RI of 0.80. This feature may reflect the aberrant
neovascularization in the PSCCT.
The human thyroid gland has a rich blood supply;
therefore, CEUS can be potentially useful in identifying and
diagnosing benign and malignant thyroid nodules. However, limited
reports are available on the CEUS imaging of PSCCT. Zhan and Ding
(13) reported on the CEUS
presentation of thyroid nodules. It was found that the majority of
malignant nodules showed slow inhomogeneous hypo-enhancement,
whereas benign nodules showed rapid homogeneous overall
hyper-enhancement, iso-enhancement or peripheral circumferential
enhancement. Chen et al (14) indicated that CEUS displayed
sustained low-peak enhancement of the PSCCT nodule, extending from
its periphery to its center. In the current study, PSCCT presented
as an inhomogeneous hypo-enhanced thyroid nodule with blurred
borders and a broad central area of non-enhancement. This
observation is similar to that reported by Chen et al
(14) and consistent with the
typical CEUS features of most malignant thyroid nodules. PSCCT may
show uneven low enhancement due to several reasons, including the
messy and irregular neovascularization of the malignant nodule and
the uneven distribution of blood vessels. In addition, the
infiltrative growth of the malignant nodule destroys the normal
thyroid tissue and neovascularization in the surrounding area,
which causes changes in the perfusion of the nodule, and makes it
difficult for the contrast agent to enter into the inner part of
the nodule. Rapid growth of tumor tissue can lead to an
insufficient blood supply, resulting in necrosis and defects at the
lesion site (15). In the present
study, CEUS showed a large central area of nodules without
enhancement, which was also consistent with the pathological
changes of SCC and necrotic lesions. Notably, pathological necrosis
is not always visible as anechoic/cystic areas on two-dimensional
US sonograms but may also appear as solid inhomogeneous hypoechoic
areas (16). In the present study,
it was observed that ~97% of Lesion A showed coagulative necrosis
with focal calcification and only a small amount of localized
residual tumor tissue, whereas the percentage of anechoic/cystic
areas and calcifications was <97% on US.
A solid nodule with decreased echogenicity is
considered suspicious for malignancy (17). Unexpectedly, in the present study,
Lesion B presented as a hypoechoic nodule but showed some of the
malignant ultrasonic features, such as solid components, irregular
margins and poor demarcation from adjacent soft-tissue layers, on
the pre-treatment US. In a retrospective study, Liu et al
(18) mentioned that some PSCCTs
could appear as scattered hyperechoic nodules with a blood flow
signal. Therefore, Lesion B was considered to be a malignant
nodule. However, the size of Lesion B did not change markedly after
two courses of treatment, indicating that it was not sensitive to
antitumor drugs. By contrast, the boundaries of Lesion B became
clearer than before, and a uniform and continuous hyperechoic thin
halo appeared in the periphery. A homogeneous continuous
hyperechoic thin halo of solid nodules is an important feature of
benign nodules (19).
Correspondingly, Lesion B presented as a homogeneous
hyper-enhancement with well-defined borders on CEUS, indicating a
benign lesion. The US and CEUS findings before and after antitumor
therapy were compared, and the risk classification of Lesion B was
finally downgraded from category 5 to category 3 based on the
observations. Postoperative pathology showed that Lesion B was a
nodular goiter with adenomatous nodule formation, validating the
decision to adjust the grading.
Lesion B was misdiagnosed as a malignant nodule on
initial US due to several reasons. First, Lesion A was considerably
larger than Lesion B, and both lesions were adjacent to each other.
Therefore, Lesion B could not be clearly distinguished from Lesion
A, and they were tentatively considered of the same type.
Additionally, a small percentage of malignant thyroid nodules are
aggressive with rapid growth, and the internal blood supply may be
hyper-enhanced on CEUS. Currently, typical CEUS imaging findings
are not available due to the rarity of PSCCT, and a hyper-enhanced
contrast pattern may be one of its imaging manifestations. It was
impossible to exclude the possibility of a malignant lesion based
on a hyper-enhanced contrast pattern.
Although imaging examinations have certain
diagnostic value for PSCCT, the gold standard for its diagnosis is
still a postoperative pathological tissue biopsy. Furthermore,
immunohistochemistry is an effective technique for accurately
diagnosing PSCCT and differentiating it from other metastatic SCCTs
at different primary locations. Several PSCCT investigations
revealed that TTF-1 is frequently positive in thyroid-originating
cancers, such as papillary and follicular thyroid carcinomas, but
rarely positive in PSCCT (20–22).
CK7 and CK19 are diffusely expressed in PSCCT tissues, whereas CK20
is not expressed (23). PAX-8 is a
significant biomarker of PSCCT. PAX-8 positivity usually indicates
a PSCCT, whereas a negative result in a thyroid tumor generally
indicates a metastatic SCC from another site (24). In the present case, the
postoperative pathology revealed typical squamous cell morphology
without any indication of additional thyroid cancer cells. In
addition, the expression of the aforementioned immunohistochemical
indicators supported the diagnosis of PSCCT. Notably, the patient
had a history of gastric cancer, and the possibility of metastasis
needed to be excluded for the accurate diagnosis of this thyroid
mass.
Limited information is available on the molecular
genetics of PSCCT. BRAF is a serine/threonine-specific protein
kinase responsible for regulating cell division and survival
(25). In a multi-institutional
study, BRAFV600E mutations were found in 87.5% of PSCCT cases
irrespective of thyroid differentiation status, and the prognosis
of PSCCT was similar to that of anaplastic thyroid carcinoma
(26). This supports the 2022 WHO
classification of PSCCT as a subtype of anaplastic carcinoma
(5). However, Ye et al
(27) performed whole-exome
sequencing of 15 PSCCT tissue samples from 15 different patients
and reported the absence of BRAF mutations in these samples.
Therefore, more research is required to understand the molecular
genetics of PSCCT.
SCCT comprises PSCCT and secondary SCCT (SSCCT;
metastasis or adjacent invasion), and their identification is a
major challenge. PSCCT, the rarer form, typically affects one or
both lobes of the thyroid gland, whereas SSCCT is usually
multifocal (28). In the present
case, PSCCT involved the right lobe of the thyroid gland, and the
left lobe and isthmus were not involved, consistent with the
findings of Ding et al (28). The ultrasonography characteristics
of SCCT include a solid or nearly solid composition, hypoechoic and
very hypoechoic echogenicity, irregular/lobulated margins,
microcalcification and particularly extra-thyroidal invasion
(16). PSCCT and SSCCT have similar
clinicopathological and highly suspicious malignant ultrasonic
features; therefore, it is difficult to differentiate them on
thyroid imaging alone. One of the guidelines recommended for
distinguishing SSCCT from PSCCT is to locate the primary tumor.
Diagnostic tests, such as computed tomography, endoscopy and
immunohistochemistry, can help to exclude SSCCT originating from
the head and neck, chest, upper gastrointestinal tract and pelvis
(28). Overall, the findings of US
or CEUS cannot be used to identify the pathological type of thyroid
cancer. However, the possibility of PSCCT should be considered if
certain ultrasonic features are observed. These features include a
large mass that is solid or has both solid and cystic parts,
appears hypoechoic on the scan, has irregular edges, contains
internal microcalcifications, particularly extends beyond the
thyroid, and shows uneven enhancement with blurred borders on
CEUS.
The treatment of PSCCT is not standardized due to
the lack of sufficient research evidence. Surgery can increase
survival times by lowering tumor load and local invasion, and has
been recognized as the treatment of choice. The median overall
survival time of patients with complete macroscopic resection is
increased by ~7 months compared with that of patients who undergo
incomplete macroscopic resection (29). However, whether adjuvant
chemoradiation benefits patients with PSCCT is still controversial.
A population-based study summarized that extensive surgical
treatments combined with adjuvant radiotherapy showed the best
prognosis compared with surgery alone, radiotherapy alone, and no
surgery and radiotherapy, with a median survival time of 11 months
(1). Ou et al (10) indicated that the addition of
radiotherapy and chemotherapy to surgical treatment may partially
stop the growth of PSCCT. However, Au et al (30) demonstrated that neither adjuvant
radiotherapy nor chemotherapy was associated with the survival
prognosis in patients with PSCCT. In the present case,
re-examination of the US after the second cycle of palliative
treatment showed a significant reduction in the size of Lesion A
and a decrease in the RI. CEUS findings revealed that the
percentage of non-enhancing areas within the nodule after treatment
was higher than the percentage before treatment. These sonographic
changes indicated that the treatment was effective. This may be
related to the benefit of preoperative neoadjuvant chemotherapy
combined with targeted therapy and immunotherapy. At present, the
patient has survived disease-free for 19 months without tumor
recurrence, which is well beyond the median survival time for PSCCT
(6–9 months) (7). This indicates
that the treatment plan of surgery combined with immunotherapy
benefits the patient. Overall, the survival rate of patients with
PSCCT remains low even after aggressive surgical treatment and
adjuvant chemotherapy, and immunotherapy and molecularly targeted
therapy may be considered in the future.
In conclusion, PSCCT is an extremely aggressive
malignant tumor that has a low incidence rate but a poor prognosis.
Therefore, developing effective treatment strategies and raising
survival rates require an early and precise diagnosis. Despite the
limitations of US and CEUS in identifying the pathological type of
thyroid cancer, the possibility of PSCCT should be considered based
on the following ultrasonography characteristics: A large mass, a
solid or mixed cystic-solid mass, presentation of hypoechoic or
very hypoechoic echogenicity, irregular margins,
microcalcifications observed internally, particularly
extra-thyroidal extension, and CUES presenting with inhomogeneous
hypo-enhancement and blurred borders. Additionally, CEUS
demonstrates significant advantages in differentiating between
benign and malignant thyroid nodules. Inhomogeneous
hypo-enhancement is a reliable predictor of malignancy, whereas
homogeneous hyper-enhancement, iso-enhancement or peripheral
circumferential enhancement are more commonly observed in benign
nodules.
US combined with CEUS should be extensively used in
the early diagnosis of thyroid nodules. An early diagnosis will
lead to better therapeutic prospects for the affected patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
CC, QX and LL were responsible for the conception
and design of the manuscript. CC and QX drafted and wrote the
manuscript. YD, JP and XH assisted in acquisition, analysis and
revision of the associated figures. LL revised and proofread the
manuscript. CC, QX, JP and LL confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The research was conducted ethically in conformity
with the World Medical Association Declaration of Helsinki. Ethical
approval for this case report was waived as the patient provided
consent and the report contains nothing that may be considered a
risk to patient privacy and integrity.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case presentation and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PSCCT
|
primary squamous cell carcinoma of the
thyroid
|
|
WHO
|
World Health Organization
|
|
US
|
ultrasound
|
|
CEUS
|
contrast-enhanced ultrasound
|
|
CDFI
|
color Doppler flow imaging
|
|
RI
|
resistive index
|
|
ACR-TIRADS
|
American College of Radiology-Thyroid
Imaging and Reporting Data System
|
|
SSCCT
|
secondary SCCT
|
References
|
1
|
Yang S, Li C, Shi X, Ma B, Xu W, Jiang H,
Liu W, Ji Q and Wang Y: Primary squamous cell carcinoma in the
thyroid gland: A population-based analysis using the SEER database.
World J Surg. 43:1249–1255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LiVolsi VA and Merino MJ: Squamous cells
in the human thyroid gland. Am J Surg Pathol. 2:133–140. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sahoo M, Bal CS and Bhatnagar D: Primary
squamous-cell carcinoma of the thyroid gland: New evidence in
support of follicular epithelial cell origin. Diagn Cytopathol.
27:227–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kebapci N, Efe B, Kabukcuoglu S, Akalin A
and Kebapci M: Diffuse sclerosing variant of papillary thyroid
carcinoma with primary squamous cell carcinoma. J Endocrinol
Invest. 25:730–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baloch ZW, Asa SL, Barletta JA, Ghossein
RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M,
Tallini G and Mete O: Overview of the 2022 WHO classification of
thyroid neoplasms. Endocr Pathol. 33:27–63. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tessler FN, Middleton WD, Grant EG, Hoang
JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates
MC, et al: ACR thyroid imaging, reporting and data system
(TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll
Radiol. 14:587–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan W, Chen H, Li J, Zhou R and Su J:
Primary squamous cell carcinoma of thyroid gland: 11 case reports
and a population-based study. World J Surg Oncol. 20:3522022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam AK: Squamous cell carcinoma of
thyroid: A unique type of cancer in World Health Organization
Classification. Endocr Relat Cancer. 27:R177–R192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Chen L, Zhang H, Nong L and Wang
F: Ultrasonic characterization of primary squamous cell carcinoma
of the thyroid. J Ultrasound Med. 41:2317–2322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou D, Ni C, Yao J, Lai M, Chen C, Zhang Y,
Jiang T, Qian T, Wang L and Xu D: Clinical analysis of 13 cases of
primary squamous-cell thyroid carcinoma. Front Oncol.
12:9562892022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ben Thayer M, Khanchel F, Helal I, Chiboub
D, Raoueh H, Ben Brahim E, Jouini R and Chadli-Debbiche A:
Epidemiological and histopathological characteristics of thyroid
carcinoma in a Tunisian health care center. World J
Otorhinolaryngol Head Neck Surg. 10:37–42. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhan J and Ding H: Application of
contrast-enhanced ultrasound for evaluation of thyroid nodules.
Ultrasonography. 37:288–297. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Peng Q, Zhang Q and Niu C:
Contrast-enhanced ultrasound of primary squamous cell carcinoma of
the thyroid: A case report. Front Endocrinol. 11:5122020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang
BJ and Wang WP: Diagnostic performances of various gray-scale,
color Doppler, and contrast-enhanced ultrasonography findings in
predicting malignant thyroid nodules. Thyroid. 24:355–363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Wei B, Nong L, Zhang H, Zhang J
and Ye J: To diagnose primary and secondary squamous cell carcinoma
of the thyroid with ultrasound malignancy risk stratification.
Front Endocrinol. 14:12387752023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu G, Xu X, Chen G and Liu Z: Analysis of
primary and secondary squamous cell carcinoma of the thyroid gland:
A retrospective study. Gland Surg. 10:559–566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Unadkat B, Phatak SV, Pavanan A and Patwa
PA: Peripheral halo in a thyroid nodule-a sign of benignity. J Evol
Med Dent Sci. 10:852–854. 2021. View Article : Google Scholar
|
|
20
|
Struller F, Senne M, Falch C, Kirschniak
A, Konigsrainer A and Muller S: Primary squamous cell carcinoma of
the thyroid: Case report and systematic review of the literature.
Int J Surg Case Rep. 37:36–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ordóñez NG: Value of thyroid transcription
factor-1 immunostaining in tumor diagnosis: A review and update.
Appl Immunohistochem Mol Morphol. 20:429–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fassan M, Pennelli G, Pelizzo MR and Rugge
M: Primary squamous cell carcinoma of the thyroid:
Immunohistochemical profile and literature review. Tumori.
93:518–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lam KY, Lo CY and Liu MC: Primary squamous
cell carcinoma of the thyroid gland: An entity with aggressive
clinical behaviour and distinctive cytokeratin expression profiles.
Histopathology. 39:279–286. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki A, Hirokawa M, Takada N, Higuchi M,
Yamao N, Kuma S, Daa T and Miyauchi A: Diagnostic significance of
PAX8 in thyroid squamous cell carcinoma. Endocr J. 62:991–995.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torrez M, Braunberger RC, Yilmaz E and
Agarwal S: Primary squamous cell carcinoma of thyroid with a novel
BRAF mutation and High PDL-1 expression: A case report with
treatment implications and review of literature. Pathol Res Pract.
216:1531462020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu B, Fuchs T, Dogan S, Landa I, Katabi N,
Fagin JA, Tuttle RM, Sherman E, Gill AJ and Ghossein R: Dissecting
anaplastic thyroid carcinoma: A comprehensive clinical, histologic,
immunophenotypic, and molecular study of 360 cases. Thyroid.
30:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye M, Guo Z, Xu J, Jin Y, He X and Ge M:
Primary squamous cell carcinoma of the thyroid has a molecular
genetic profile distinct from that of anaplastic thyroid carcinoma:
A whole exome sequencing and gene expression profiling study. Am J
Surg Pathol. 48:1024–1031. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding W, Gao X and Ran X: Progress in
diagnosing and treating thyroid squamous cell carcinoma under the
5th edition of WHO classification. Front Endocrinol.
14:12734722023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Limberg J, Ullmann TM, Stefanova D,
Finnerty BM, Beninato T, Fahey TJ and Zarnegar R: Prognostic
characteristics of primary squamous cell carcinoma of the thyroid:
A national cancer database analysis. World J Surg. 44:348–355.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Au JK, Alonso J, Kuan EC, Arshi A and St
John MA: Primary squamous cell carcinoma of the thyroid: A
population-based analysis. Otolaryngol Head Neck Surg. 157:25–29.
2017. View Article : Google Scholar : PubMed/NCBI
|