Introduction
Acute myeloid leukemia (AML) is a diverse group of
hematologic malignancies that are characterized by the abnormal
growth and differentiation of precursor cells, and to clinical
manifestations such as infection, anemia and bleeding (1). The clonal expansion results in the
accumulation of immature myeloid precursors in the bone marrow,
peripheral blood and/or other organs and tissues (2,3). AML
usually occurs in older individuals and incidence increases with
age, with the median age at diagnosis being 68 years (4). The development of genome sequencing
technology has made it possible to deeply sequence AML samples to
describe the mutation spectrum and understand the biological
heterogeneity (5). Although
increased understanding of the pathophysiology, next-generation
sequencing and the recent approval of numerous treatment options,
including BCL2, FMS-like tyrosine kinase 3 (FLT3) inhibitor,
isocitrate Dehydrogenase 1/2 (IDH1/2) inhibitors and allogeneic
hematopoietic stem cell transplantation have transformed the way
AML is approached, the estimated 5-year survival rate is 62% for
patients under the age of 50 years, 37% for patients aged 50–64
years old and only 9.4% for patients ≥65 years at diagnosis,
leaving much room for improvement (6,7). The
biological and clinical heterogeneity of the disease makes AML
difficult to treat, and selective targeted inhibitors combined with
chemotherapy may contribute towards improving efficacy.
Signal transducer and activator of transcription 3
(STAT3), a member of the STAT family, has an important function in
controlling the proliferation of healthy and cancerous cells
(8–10). STAT3 has four recognized subtypes,
including STAT3α (92 kDa), STAT3β (83 kDa) and STAT3γ (72 kDa) and
STAT3δ (64 kDa), of which STAT3α and STAT3β are produced by
alternative splicing (11). The
constitutive activation of STAT3α plays a key role in the
activation of carcinogenic pathways and involves in the regulation
of apoptosis, proliferation, differentiation and evolution of
numerous neoplasms, while STAT3β is generally considered to be a
dominant negative regulator of cancer (12). STAT3γ and STAT3δ are derived from
proteolytic processing and have no transcriptional effect (13).
In hematopoietic cells, STAT3 facilitates aberrant
signal transduction by enlisting receptor complexes that have
undergone tyrosine phosphorylation. Upon being phosphorylated, the
STAT3 protein forms dimers and migrates to the nucleus, where it
initiates transcription proteins, ultimately resulting in
tumorigenesis (14). Since STAT3 is
an important transcription factor in pathogenesis and chemotherapy
resistance, a number of studies have been conducted targeting
STAT3. Constitutive STAT3 activation is observed in ~ 50% of newly
diagnosed AML. In addition, patients with leukemia cells
demonstrating constitutive STAT3 activation have a shorter
disease-free survival rate (15,16).
The antitumor effects of blocking STAT3 activity have been
demonstrated in both solid tumors (17–20)
and AML (21,22). Therefore, STAT3 has been recognized
as a promising protein target for the development of broad-spectrum
therapeutic drugs (23).
Structure and function of STAT3
STAT3 is a signaling molecule that relays
information from cell surface receptors to the nucleus. It is
activated by a variety of soluble mediators, such as interleukins
(IL-2, IL-3, IL-5, IL-6, IL-7, IL-9, IL-11), cytokines [granulocyte
colony-stimulating factor (G-CSF), epidermal growth factor,
platelet-derived growth factor] and hormones (growth hormone,
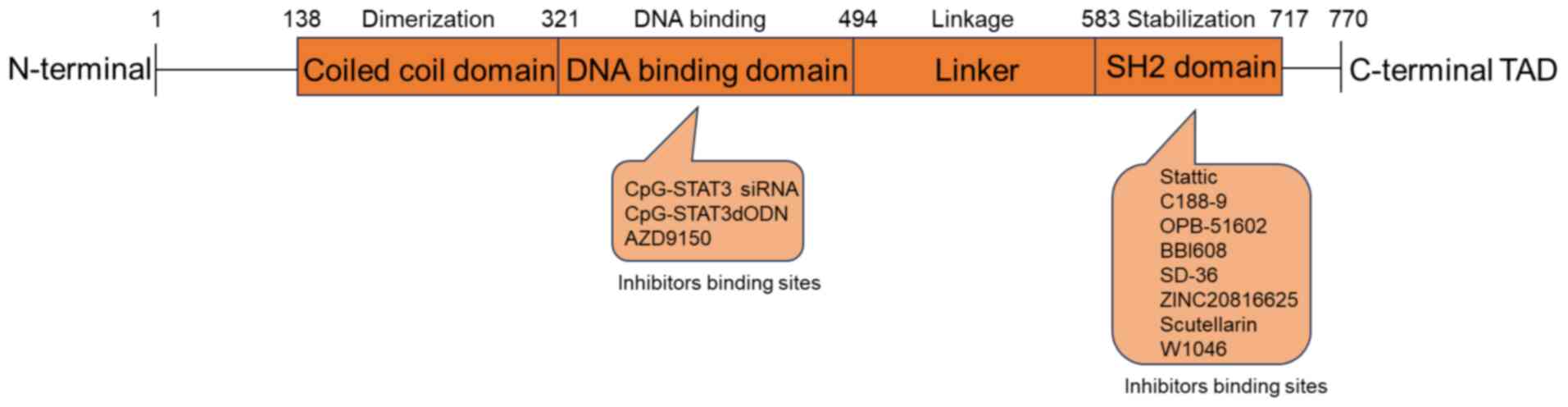
prolactin, leptin) (24,25). Fig.
1 illustrates the various functional domains of the STAT3,
including an N-terminal domain, a DNA-binding domain, a linker
domain, an Src homology 2 (SH2) domain and a C-terminal
transactivation domain (26).
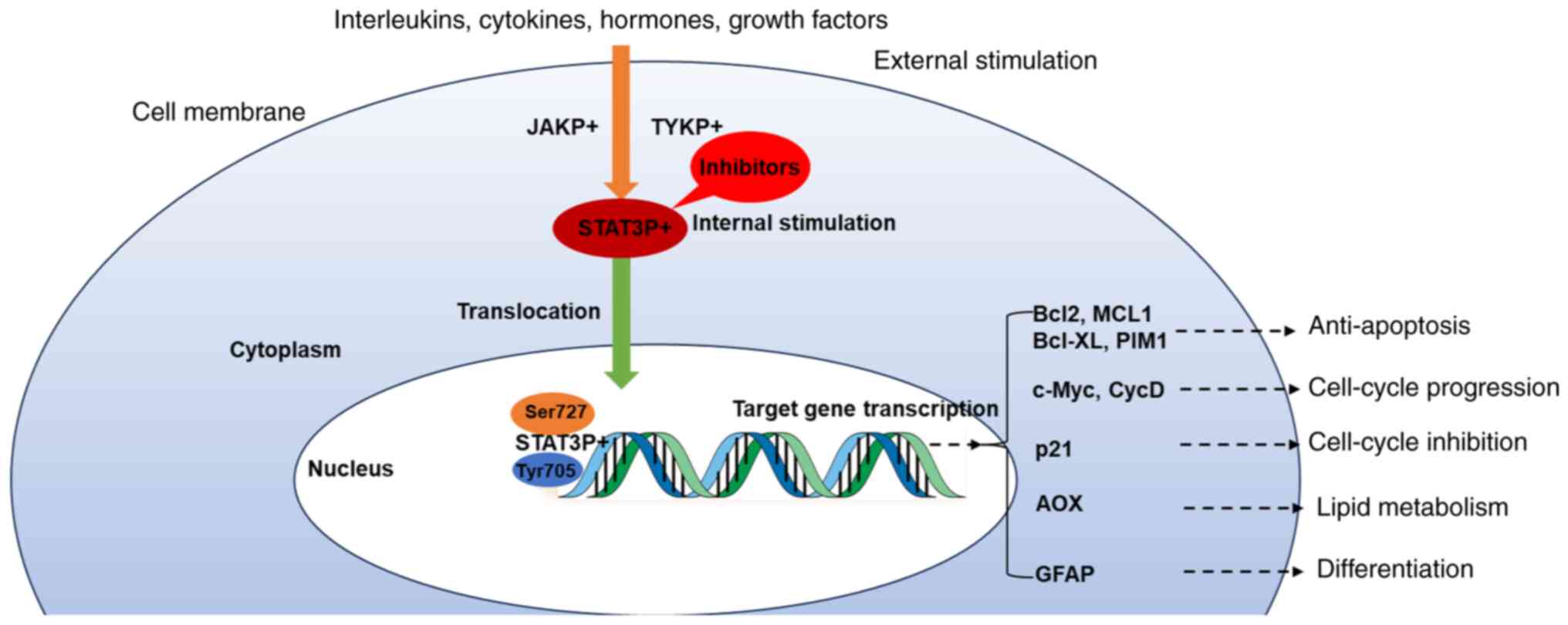
The activation of STAT3 involves the interaction
between specific cytokines and its receptors on the cell membrane,
which triggers the activation of tyrosine kinase. The
receptor-kinase complex undergoes phosphorylation and subsequently
serves as a docking site for STAT3. The recruited STAT molecules
are phosphorylated at Tyr705 and Ser727 of mitochondrial STAT3.
Following activation, STAT3 is transferred to the nucleus where it
acts as a transcription activator, stimulating the transcription of
genes responsible for regulating cell proliferation,
differentiation and apoptosis (Fig.
2) (27–31).
The constitutive STAT3 activity is often associated
with adverse outcomes in human cancer, as it promotes tumor cell
proliferation, survival and metastasis, while impairing antitumor
immune responses (32,33). The phosphorylation of STAT3 can be
activated in various cancerous cells, including multiple AML cell
lines and primary cells obtained from AML patients (14,16).
STAT3 in AML
STAT3 is crucial for maintaining the balance of
myeloid cells, as it can block myeloid differentiation and plays an
important role in leukemogenesis (21,26,34).
STAT3 regulates cell survival and proliferation through its target
genes (MYC, cyclin D1, baculoviral IAP repeat-containing 5 and
BCL2). The inhibition of STAT3 can induce cell apoptosis, and
constitutive STAT3 activity is associated with adverse prognosis in
patients with AML (16,35).
Genetic mutations or external factors can frequently
disrupt the normal STAT3 signaling pathway (36–38).
In 28–44% of patients with AML, constitutive STAT3 activation has
been observed at the initial stage of diagnosis, whereas when
patients experience a recurrence, constitutive activity is absent
or decreased (15,16,38).
The factors contributing to the increased activation of
constitutive STAT3 in AML cells seem to differ among individuals. A
potential factor is the continual activation of the IL-6 signaling
pathway, and another factor contributing to this is the occurrence
of activating mutations in the SH2 domain of STAT3, specifically
between residues 585 and 688 (15,37).
Based on these findings, STAT3 has become a
desirable focus for AML treatment. However, Lee et al
(39) revealed that feedback
activation of STAT3 in PC-9 NSCLC cells promotes drug resistance
through the activation of multiple kinases, including EGFR, MET and
KRAS. Therefore, the combination of STAT3 inhibitors with other
chemotherapies (cytarabine + mitoxantrone/idarubicin/etoposide) may
be effective against refractory or relapse AML.
STAT3-targeted inhibitors for AML
In previous years, multiple inhibitors targeting
STAT3 for patients with AML have been studied (Table I). The present review will briefly
review some of the most impressive developments regarding these
inhibitors in the following paragraphs.
 | Table I.Inhibitors of STAT3 for AML. |
Table I.
Inhibitors of STAT3 for AML.
| Inhibitors | Target sites | Types | Results | Clinical trial (ID
no.) | Years | (Refs.) |
|---|
| Stattic | SH2 | Small molecule | Inhibits cell
proliferation and promotes apoptosis | No | 2006, 2021 | (40,41) |
| C188-9 | SH2 | Small molecule | Induces
apoptosis | No | 2011 | (21) |
| OPB-51602 | SH2 | Small molecule | Suppresses cell
proliferation | Stage I
(NCT01344876) | 2015 | (43,44) |
| BBI608 | SH2 | Small molecule | Suppresses cancer
stemness | Stage Ib/II and
stage II (NCT02352558) | 2019 | (45,46) |
| SD-36 | SH2 | Small molecule | Promotes growth
inhibition and induces apoptosis | No | 2019 | (47–50) |
| W1046 | SH2 | Small molecule | Inhibits cell
proliferation and promotes cell apoptosis | No | 2023 | (53) |
| CpG-STAT3
siRNA | DBD | Nucleotide
based | Increases
immunogenicity of primary AML cells | No | 2014 | (55) |
| CpG-STAT3dODN | DBD | Nucleotide
based | Eliminates leukemia
stem/progenitor cells | No | 2016 | (56) |
| AZD9150 | DBD | Nucleotide
based | Promotes
hematopoietic differentiation | Stage I trials
(NCT05986240) | 2018, 2024 | (57,58) |
| ZINC20816625 | SH2 | Natural
compounds | Artificial
intelligence screening, not yet validated | No | 2020 | (59) |
| Scutellarin | SH2 | Natural
compounds | Hinders the growth
of AML cells, triggering cell cycle arrest and apoptosis | No | 2020 | (62–64) |
Small-molecule inhibitors
Stattic
Stattic is a small molecule compound screened by
Schust et al (40) from
Maybridge (https://maybridgechem.lookchem.com/). It can
effectively inhibit STAT3 activation and dimerization by
selectively acting on the STAT3 SH2 domain and induces the
apoptosis of STAT3-dependent cancer cells. Luo et al
(41) found that stattic can
inhibit the proliferation of AML cell lines, promote apoptosis and
arrest the cell cycle at G0/G1. The possible
mechanism is that stattic can inhibit the function of DNA damage
repair and block the repair of DNA double-strand breaks (DSBs),
thus enhancing the sensitivity to chemotherapy drugs. Because most
traditional chemotherapeutic drugs kill cancer cells by inducing
DSBs (42). In addition, a study
found that stattic can inhibit the homologous recombination
pathway, which may also be the mechanism of static-induced
apoptosis (41). Although stattic
has shown good antitumor effects in vitro experiments, its
specific mechanism and clinical trials still need to be further
evaluated for future clinical applications.
C188-9
The small molecule inhibitor known as C188-9 has
demonstrated its ability to effectively induce apoptosis in AML
cell lines and primary cells derived from patients with AML by
inhibiting the STAT3 activation caused by G-CSF. The compound
inhibits the binding sites between the phosphotyrosine peptide and
the SH2 domain, consequently impeding the interaction and
dimerization with tyrosine kinase. C188-9 exerts antileukemia
effects by inhibiting ligand-induced STAT3 phosphorylation
(21). However, whether C188-9 can
improve the efficacy of chemotherapy for patients with AML still
needs further confirmation through experiments in vivo and
clinical trials.
OPB-51602
OPB-51602 is a novel small molecule compound that
binds to the SH2 domain of STAT3 with high affinity and can
effectively inhibit the proliferation of various cancer cells in
vitro and in vivo by targeting the Tyr705 and Ser727
phosphorylation sites of mitochondrial STAT3 (43). The results of phase I trials
demonstrated that 20 patients with recurrent or resistant
hematological malignancies received OPB-51602 treatment on the
basis of chemotherapy. Common side effects including nausea,
peripheral sensory neuropathy and diarrhea were reported, and they
were well tolerated and did not pose any safety concerns.
Nevertheless, the patient cohort showed limited success in
responding to the treatment, except for two individuals diagnosed
with AML and one individual with multiple myeloma, both of whom
exhibited persistent stable disease (44). In this diverse group of patients,
the authors stated that determining the most effective dosage and
frequency for long-term treatment is challenging.
BBI608
BBI608 (Napabucasin) is a compound that hinders the
process of gene transcription mediated by the STAT3 protein. The
characteristics of this compound have been verified when used alone
and combined with paclitaxel during stage Ib/II and stage II trials
in solid tumors (45). A previous
study found that BBI608 demonstrates antileukemia properties in
both the MOLM-13 cell line and primary cells obtained from patients
with AML, and also had potent effects in immunodeficient mice
xenograft models of AML in vivo (46). Meanwhile, the coadministration of
BBI608 and venetoclax resulted in an increased cell death efficacy
in Kasumi-1 cells that developed resistance to BBI608 (46).
Based on the available information, BBI608 shows
promise as a potential treatment option for AML but needs to be
further validated for its effectiveness in AML primary cells and
clinical trials.
SD-36
SD-36 is protein hydrolysis targeted chimeric
protein (PROTAC) that serves as a potent small molecule inhibitor
of the STAT3 SH2 domain and exhibits the ability to selectively
degrade STAT3 in vitro and in vivo (47). In contrast to traditional small
molecule protein inhibitors, PROTAC degraders can effectively
remove a protein target through degradation and block all functions
related to the protein. In addition, a PROTAC degrader can achieve
higher selectivity since the degrader needs to bind and recruit
target proteins and E3 ligases to form a productive ternary complex
for ubiquitination and degradation (48). Studies have provided evidence that
the SD-36 compound demonstrates effective degradation of the STAT3
protein in different types of leukemia and lymphoma cells (49,50).
SD-36 represents a novel PROTAC that specifically targets STAT3,
indicating how advanced technology can enhance the identification
of appropriate inhibitors. Although SD-36 is a selective small
molecule inhibitor, interference with normal STAT3 protein function
may be a challenge. The differential E3 ligase expression levels
between tumor and normal cells should be utilized to develop STAT3
inhibitors with minimal side effects.
W1046
Currently, immune checkpoint blockades (ICBs) have
also shown encouraging responses in a number of types of treatment
for cancer. Mo et al (51)
revealed that STAT3-regulated V-domain immunoglobulin suppressor of
T cell activation (VISTA), a novel immune checkpoint that mediates
immune escape primarily by blocking T cell activation, is highly
expressed in AML (52). Therefore,
a novel inhibitor W1046 was designed with a boronic acid
pharmacophore targeting the STAT3 SH2 domain This inhibitor was
identified as a highly effective inhibitor by replacing the
carboxylic acid in the compound with boric acid, demonstrating
higher binding affinity, better cellular efficacy, more favorable
PK profile and higher in vivo anti-tumor activity (53). W1046 significantly inhibits
proliferation and promotes apoptosis in both AML cells lines and
primary AML with hyperactive STAT3 and has demonstrated anti-AML
efficacy in vivo but lacks sensitivity in cell lines with
low STAT3 activation and STAT3 deletion (51). The development of inhibitors and
monoclonal antibodies that target the STAT3-VISTA axis may provide
a promising therapeutic strategy for immunotherapy of AML.
Nucleotide-based inhibitors
Cytosine-guanine dinucleotide (CpG)-STAT3
Hossain et al (54) investigated the influence of CpG
small interfering RNAs (siRNAs) that specifically targeted
STAT3-silencing in Toll-like receptor 9 (TLR9)-positive
hematopoietic cells. By conjugating the inhibitor with both the
TLR9 ligand and the CpG, efficient targeting of the inhibitor to
TLR9-positive antigen-presenting immune cells was achieved
(54). TLR9 can mediate innate and
adaptive immunity, making it an attractive strategy for enhancing
anticancer therapies (54). The
application of CpG-STAT3 siRNA in a mouse model that mimics human
inv (16) AML leads to regression
of the disease through a mechanism that relies on CD8+ T
cells. STAT3-silencing and TLR9 stimulation results in an increased
immunogenicity of primary AML cells, as observed in a previous
study (55). This finding suggests
the potential to use targeted STAT3 inhibition/TLR9-triggering to
break tumor tolerance and induce immunity against AML and
potentially other TLR9-positive hematological tumors. However,
CpG-STAT3 siRNA has poor serum stability and needs to be optimized
through chemical modification, binding with high molecular weight
polymers or encapsulation to further improve its therapeutic
effect.
CpG-STAT3dODN
The decoy oligodeoxynucleotide (dODN) inhibitor
CpG-STAT3dODN, which acts as a DNA decoy molecule and binds STAT3
within the cytoplasm, effectively blocking STAT3 transcriptional
activity by using this immunostimulatory approach. This study
specifically delivered STAT3dODN to myeloid cells by connecting
STAT3dODN to the TLR9 ligand and CpG. The CpG-STAT3dODN conjugates
are quickly internalized by human and mouse TLR9+ immune
cells and AML primary cells. Following their uptake, CpG-STAT3dODNs
are released from endosomes, which bind and isolate cytoplasmic
STAT3, thereby inhibiting downstream gene expression. In an
experiment using xenografts from patients with AML, CpG-STAT3dODN
achieve immune-mediated AML cell eradication in mice by CD8+/CD4+T
cells. However, the direct cytotoxic effects of STAT3 inhibition
were limited by alternative survival signaling in AML cells, and
the rapid degradation of decoy oligonucleotides also pose a major
therapeutic challenge (56). This
study mainly provides evidence for further development of the
dual-function CpG-STAT3dODN for the treatment of AML.
AZD9150
Due to the swift advancements in next-generation
sequencing technology, an antisense oligonucleotide (ASO)
inhibitor, designed using the genetic sequence of the target gene,
has been arisen. AZD9150 (danvatirsen) is a highly effective
inhibitor of STAT3 ASO that has shown promising results in
preclinical trials involving patients with lymphoma and non-small
cell lung cancer, with adverse events that include transaminase
abnormalities, fatigue, thrombocytopenia, nausea and anemia
(57). A previous study also
verified that AZD9150 promotes hematopoietic differentiation in
myelodysplastic syndrome (MDS) and AML (58). Currently, a Stage I clinical trial
is underway to investigate the safety and efficacy of danvatirsen
as a monotherapy followed by combination with venetoclax in
patients with relapsed/refractory MDS/AML (NCT05986240). Based on
the aforementioned studies, the results of this clinical trial on
relapsed/refractory MDS/AML will be notable.
Natural compounds
ZINC20816625
The swift advancement of artificial intelligence
technology enables its extensive application in various facets of
pharmaceutical research and development. Chen et al
(59) have discovered promising
candidates for STAT3 inhibitors (ZINC20816625) through the
utilization of artificial intelligence models. The nitro group of
ZINC20816625 interacts with the GLU638 and PRO725 of STAT3 through
two hydrogen bonds. Based on in vitro experiments,
researchers have determined that ZINC20816625 exhibits potential as
an efficacious medication for treating AML (59). However, the mechanism and
effectiveness of ZINC20816625 still need to be further verified by
basic experimental methods.
Scutellarin
Traditional Chinese medicine (TCM) has been employed
as a significant therapeutic approach to treat AML for over two
millennia in China (60). TCMs are
known for their intricate system, which consists of several
components, diverse targets and multiple pathways of action
(61). Scutellarin, an ingredient
in TCM, was discovered to exhibit cytotoxic effects on diverse
types of tumor cells. This compound exhibits anticancer properties
by activating various pathways such as inducing apoptosis,
inhibiting cell proliferation and blocking cell invasion,
demonstrating beneficial therapeutic outcomes and minimal toxic
side effects (62–64).
In a recent study, it was found that scutellarin has
potential in hindering the proliferation of AML cells, triggering
cell cycle arrest and apoptosis in a concentration- and
time-dependent manner. This effect is thought to be associated with
the regulation of the JAK2/STAT3 signaling pathway, because the
activation of this pathway contributes to the formation of tumor
inflammatory microenvironment and is closely related to
tumorigenesis and progression (65). Based on the aforementioned studies,
it is suggested that scutellarin may be a promising candidate for
new natural inhibitor and deserves special attention in further
studies.
Conclusions and perspectives
AML is a challenging type of hematological neoplasm
characterized by a diverse range of genetic and cytogenetic
markers, as highlighted in the most recent classification systems
issued by the World Health Organization and International Consensus
Classification (66,67). The use of personalized treatments
based on the specific molecular data of patients is markedly
increasing in the field of oncology, allowing for targeted
therapies aligned with the underlying pathobiology of the disease
(68).
STAT3 is an important regulator in normal
hematopoiesis, and constitutive activation of STAT3 is associated
with the occurrence and prognosis of AML. Currently, targeted STAT3
inhibitors are being tried in the treatment of several cancers,
including AML. Due to STAT3 and its associated upstream JAKs that
play a crucial role in AML, inhibitors targeting this pathway are
an important direction for improving AML efficacy. Selective
targeting of the JAK/STAT pathway has shown promising results both
in vitro and in vivo models (26). Targeted IL-6/JAK/STAT3 inhibitors
have also been revealed to be beneficial for treating ovarian,
prostate and myeloproliferative neoplasms, with potential to
inhibit tumor growth (14).
Furthermore, the proliferation of cancer cells can be driven by a
diverse range of activated kinases, including EGFR, HER2, ALK and
MET. Specifically, inhibition of enzymes can lead to autocrine
activation of STAT3, which suppresses tumor cells through the
FGF/JAK/STAT3 feedback loop (39).
Therefore, these comprehensive summaries may provide new strategies
and insights for targeted STAT3 therapy in AML. Some notable
STAT3-targeting inhibitors in hematological malignancies, such as
BBI608 (trial no. NCT02352558) and AZD9150 (NCT05986240), have been
identified and have undergone clinical trials and achieved good
therapeutic effects. However, despite extensive research and
ongoing clinical trials, the efficacy of STAT3 inhibitors in
clinical studies remains inconclusive (Fig. 3). The present review mainly focused
on various inhibitors targeting STAT3 and systematically elucidated
the mechanism of various STAT3 inhibitors in AML, current research
status and existing challenges, which is expected to contribute to
future research.
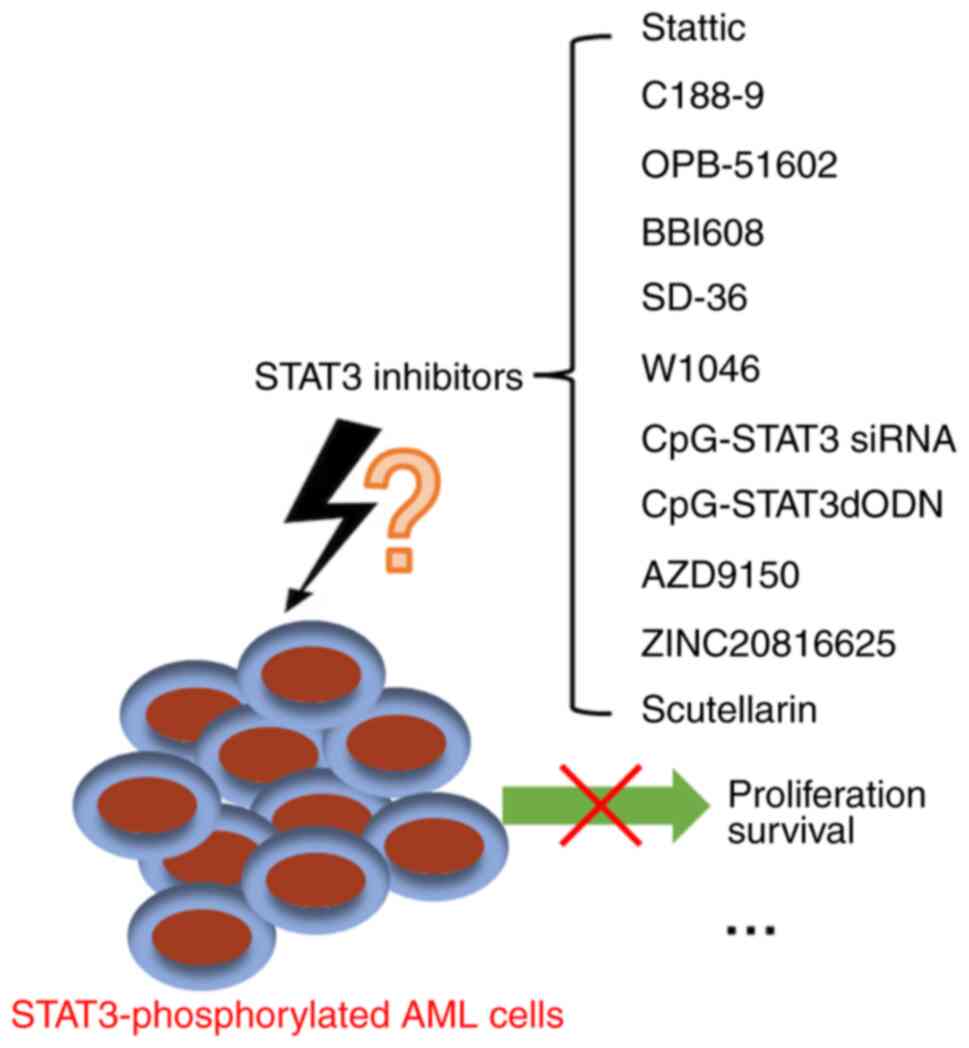 | Figure 3.Targeted STAT3 inhibitors for AML.
OPB-51602, AZD9150 and BBI608 have entered phase I clinical trials.
Stattic, C188-9, SD-36, CpG-STAT3, CpG-STAT3dODN, ZINC20816625,
scutellarin and W1046 are in preclinical studies. STAT3, signal
transducer and activator of transcription 3; dODN, decoy
oligodeoxynucleotide; siRNA, small interfering RNA; AML, acute
myeloid leukemia. |
In order to enhance our understanding of STAT3 in
AML, it is crucial to gain deeper insights into the biological
traits and roles of STAT3 within primary AML cells. The
investigation of STAT3 regulatory effects on the proliferation,
apoptosis and cell cycle of AML primary cells, especially in AML
cells with high STAT3 phosphorylation, may be of great use.
Incorporating STAT3 inhibitors into chemotherapy has the potential
to revolutionize clinical practice and improve treatment outcomes
for patients with AML due to numerous ongoing studies in this
field.
Acknowledgements
Not applicable.
Funding
This work was supported by the Clinical Research Innovation Team
Project (grant no. CTCCR-2019B03).
Availability of data and materials
Not applicable.
Authors' contributions
HC and LC drafted the manuscript and created the
figures. CH edited the manuscript. All authors have read and
approved the final manuscript, took responsibility for the content
and approved the publication. Data authentication is not
required.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shimony S, Stahl M and Stone RM: Acute
myeloid leukemia: 2023 Update on diagnosis, risk-stratification,
and management. Am J Hematol. 98:502–526. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colmone A, Amorim M, Pontier AL, Wang S,
Jablonski E and Sipkins DA: Leukemic cells create bone marrow
niches that disrupt the behavior of normal hematopoietic progenitor
cells. Science. 322:1861–1865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Kouchkovsky I and Abdul-Hay M: ‘Acute
myeloid leukemia: A comprehensive review and 2016 update’. Blood
Cancer J. 6:e4412016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Juliusson G, Antunovic P, Derolf A,
Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A and
Höglund M: Age and acute myeloid leukemia: Real world data on
decision to treat and outcomes from the Swedish acute leukemia
registry. Blood. 113:4179–4187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2022 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Institute of Health, .
Surveillance and Epidemiology, End Results (SEER) US county
populations 1969–2020. February;2022.Available from. www.seer.cancer.gov/popdataNovember 9–2022
|
|
7
|
DiNardo CD, Erba HP, Freeman SD and Wei
AH: Acute myeloid leukaemia. Lancet. 401:2073–2086. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ihle JN: The Stat family in cytokine
signaling. Curr Opin Cell Biol. 13:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levy DE and Lee CK: What does Stat3 do? J
Clin Invest. 109:1143–1148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aigner P, Just V and Stoiber D: STAT3
isoforms: Alternative fates in cancer? Cytokine. 118:27–34. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang HX, Yang PL, Li EM and Xu LY:
STAT3beta, a distinct isoform from STAT3. Int J Biochem Cell Biol.
110:130–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hendry L and John S: Regulation of STAT
signaling by proteolytic processing. Eur J Biochem. 71:4613–4620.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia Z, Baer MR, Block AW, Baumann H and
Wetzler M: Expression of signal transducers and activators of
transcription proteins in acute myeloid leukemia blasts. Cancer
Res. 58:3173–3180. 1998.PubMed/NCBI
|
|
16
|
Benekli M, Xia Z, Donohue KA, Ford LA,
Pixley LA, Baer MR, Baumann H and Wetzler M: Constitutive activity
of signal transducer and activator of transcription 3 protein in
acute myeloid leukemia blasts is associated with short disease-free
survival. Blood. 99:252–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Ji M, Zhang S, Xue N, Xu H, Lin S
and Chen X: Bt354 as a new STAT3 signaling pathway inhibitor
against triple negative breast cancer. J Drug Target. 26:920–930.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geiger JL, Grandis JR and Bauman JE: The
STAT3 pathway as a therapeutic target in head and neck cancer:
Barriers and innovations. Oral Oncol. 56:84–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bharadwaj U, Eckols TK, Xu X, Kasembeli
MM, Chen Y, Adachi M, Song Y, Mo Q, Lai SY and Tweardy DJ:
Small-molecule inhibition of STAT3 in radioresistant head and neck
squamous cell carcinoma. Oncotarget. 7:26307–26330. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JH, van Wyk H, McMillan DC, Quinn J,
Clark J, Roxburgh CSD, Horgan PG and Edwards J: Signal transduction
and activator of transcription-3 (STAT3) in patients with
colorectal cancer: Associations with the phenotypic features of the
tumor and host. Clin Cancer Res. 23:1698–1709. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB
and Tweardy DJ: Stat3 signaling in acute myeloid leukemia:
Ligand-dependent and -independent activation and induction of
apoptosis by a novel small-molecule Stat3 inhibitor. Blood.
117:5701–5709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Minus MB, Liu W, Vohidov F, Kasembeli MM,
Long X, Krueger MJ, Stevens A, Kolosov MI, Tweardy DJ, Sison EAR,
et al: Rhodium(II) proximity-labeling identifies a novel target
site on STAT3 for inhibitors with potent anti-leukemia activity.
Angew Chem Int Ed Engl. 54:13085–13089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takeda K and Akira S: STAT family of
transcription factors in cytokine-mediated biological responses.
Cytokine Growth Factor Rev. 11:199–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rane SG and Reddy EP: JAKs, STATs and Src
kinases in hematopoiesis. Oncogene. 21:3334–3358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bruserud Ø, Nepstad I, Hauge M, Hatfield
KJ and Reikvam H: STAT3 as a possible therapeutic target in human
malignancies: Lessons from acute myeloid leukemia. Expert Rev
Hematol. 8:29–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sellier H, Rébillard A, Guette C, Barré B
and Coqueret O: How should we define STAT3 as an oncogene and as a
potential target for therapy? JAKSTAT. 2:e247162013.PubMed/NCBI
|
|
28
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bar-Natan M, Nelson EA, Xiang M and Frank
DA: STAT signaling in the pathogenesis and treatment of myeloid
malignancies. JAKSTAT. 1:55–64. 2012.PubMed/NCBI
|
|
30
|
Hutchins AP, Diez D and Miranda-Saavedra
D: Genomic and computational approaches to dissect the mechanisms
of STAT3′s universal and cell type-specific functions. JAKSTAT.
2:e250972013.PubMed/NCBI
|
|
31
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spiekermann K, Biethahn S, Wilde S,
Hiddemann W and Alves F: Constitutive activation of STAT
transcription factors in acute myelogenous leukemia. Eur J
Haematol. 67:63–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spiekermann K, Pau M, Schwab R, Schmieja
K, Franzrahe S and Hiddemann W: Constitutive activation of STAT3
and STAT5 is induced by leukemic fusion proteins with protein
tyrosine kinase activity and is sufficient for transformation of
hematopoietic precursor cells. Exp Hematol. 30:262–271. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hankey PA: Regulation of hematopoietic
cell development and function by Stat3. Front Biosci (Landmark Ed).
14:5273–5290. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aoki Y, Feldman GM and Tosato G:
Inhibition of STAT3 signaling induces apoptosis and decreases
survivin expression in primary effusion lymphoma. Blood.
101:1535–1542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koskela HLM, Eldfors S, Ellonen P, van
Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ,
Olson T, Jalkanen SE, et al: Somatic STAT3 mutations in large
granular lymphocytic leukemia. N Engl J Med. 366:1905–1913. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pilati C, Amessou M, Bihl MP, Balabaud C,
Nhieu JT, Paradis V, Nault JC, Izard T, Bioulac-Sage P, Couchy G,
et al: Somatic mutations activating STAT3 in human inflammatory
hepatocellular adenomas. J Exp Med. 208:1359–1366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia Z, Sait SN, Baer MR, Barcos M, Donohue
KA, Lawrence D, Ford LA, Block AM, Baumann H and Wetzler M:
Truncated STAT proteins are prevalent at relapse of acute myeloid
leukemia. Leuk Res. 25:473–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ and
Settleman J: Drug resistance via feedback activation of Stat3 in
oncogene-addicted cancer cells. Cancer Cell. 26:207–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schust J, Sperl B, Hollis A, Mayer TU and
Berg T: Stattic: A small-molecule inhibitor of STAT3 activation and
dimerization. Chem Biol. 13:1235–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo Y, Lu Y, Long B, Lin Y, Yang Y, Xu Y,
Zhang X and Zhang J: Blocking DNA damage repair may be involved in
stattic (STAT3 inhibitor)-induced FLT3-ITD AML cell apoptosis.
Front Cell Dev Biol. 9:6370642021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goldstein M and Kastan MB: The DNA damage
response: Implications for tumor responses to radiation and
chemotherapy. Annu Rev Med. 66:129–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Genini D, Brambilla L, Laurini E, Merulla
J, Civenni G, Pandit S, D'Antuono R, Perez L, Levy DE, Pricl S, et
al: Mitochondrial dysfunction induced by a SH2 domain-targeting
STAT3 inhibitor leads to metabolic synthetic lethality in cancer
cells. Proc Natl Acad Sci USA. 114:E4924–E4933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ogura M, Uchida T, Terui Y, Hayakawa F,
Kobayashi Y, Taniwaki M, Takamatsu Y, Naoe T, Tobinai K, Munakata
W, et al: Phase I study of OPB-51602, an oral inhibitor of signal
transducer and activator of transcription 3, in patients with
relapsed/refractory hematological malignancies. Cancer Sci.
106:896–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hubbard JM and Grothey A: Napabucasin: An
update on the first-in-class cancer stemness inhibitor. Drugs.
77:1091–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bi S, Chen K, Feng L, Fu G, Yang Q, Deng
M, Zhao H, Li Z, Yu L, Fang Z and Xu B: Napabucasin (BBI608)
eliminate AML cells in vitro and in vivo via inhibition of Stat3
pathway and induction of DNA damage. Eur J Pharmacol. 855:252–261.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou H, Bai L, Xu R, Zhao Y, Chen J,
McEachern D, Chinnaswamy K, Wen B, Dai L, Kumar P, et al:
Structure-based discovery of SD-36 as a potent, selective, and
efficacious PROTAC degrader of STAT3 protein. J Med Chem.
62:11280–11300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gadd MS, Testa A, Lucas X, Chan KH, Chen
W, Lamont DJ, Zengerle M and Ciulli A: Structural basis of PROTAC
cooperative recognition for selective protein degradation. Nat Chem
Biol. 13:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dale B, Cheng M, Park KS, Kaniskan HÜ,
Xiong Y and Jin J: Advancing targeted protein degradation for
cancer therapy. Nat Rev Cancer. 21:638–654. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bai L, Zhou H, Xu R, Zhao Y, Chinnaswamy
K, McEachern D, Chen J, Yang CY, Liu Z, Wang M, et al: A potent and
selective small-molecule degrader of STAT3 achieves complete tumor
regression in vivo. Cancer Cell. 36:498–511.e17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mo J, Deng L, Peng K, Ouyang S, Ding W,
Lou L, Lin Z, Zhu J, Li J, Zhang Q, et al: Targeting STAT3-VISTA
axis to suppress tumor aggression and burden in acute myeloid
leukemia. J Hematol Oncol. 16:152023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan L, Tatineni J, Mahoney KM and Freeman
GJ: VISTA: A mediator of quiescence and a promising target in
cancer immunotherapy. Trends Immunol. 42:209–227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Deng L, Mo J, Zhang Y, Peng K, Li H,
Ouyang S, Feng Z, Fang W, Wei J, Rong D, et al: Boronic acid: A
novel pharmacophore targeting Src homology 2 (SH2) domain of STAT3.
J Med Chem. 65:13094–13111. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hossain DMH, Dos Santos C, Zhang Q,
Kozlowska A, Liu H, Gao C, Moreira D, Swiderski P, Jozwiak A, Kline
J, et al: Leukemia cell-targeted STAT3 silencing and TLR9
triggering generate systemic antitumor immunity. Blood. 123:15–25.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Krieg AM: Toll-like receptor 9 (TLR9)
agonists in the treatment of cancer. Oncogene. 27:161–167. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang Q, Hossain DMS, Duttagupta P,
Moreira D, Zhao X, Won H, Buettner R, Nechaev S, Majka M, Zhang B,
et al: Serum-resistant CpG-STAT3 decoy for targeting survival and
immune checkpoint signaling in acute myeloid leukemia. Blood.
127:1687–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hong D, Kurzrock R, Kim Y, Woessner R,
Younes A, Nemunaitis J, Fowler N, Zhou T, Schmidt J, Jo M, et al:
AZD9150, a next-generation antisense oligonucleotide inhibitor of
STAT3 with early evidence of clinical activity in lymphoma and lung
cancer. Sci Transl Med. 7:314ra1852015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shastri A, Choudhary G, Teixeira M,
Gordon-Mitchell S, Ramachandra N, Bernard L, Bhattacharyya S, Lopez
R, Pradhan K, Giricz O, et al: Antisense STAT3 inhibitor decreases
viability of myelodysplastic and leukemic stem cells. J Clin
Invest. 128:5479–5488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen X, Chen HY, Chen ZD, Gong JN and Chen
CYC: A novel artificial intelligence protocol for finding potential
inhibitors of acute myeloid leukemia. J Mater Chem B. 8:2063–2081.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen C, Wang L, Li L, Wang A, Huang T, Hu
J, Zhao M, Liu F, Qi S, Hu C, et al: Network-based analysis with
primary cells reveals drug response landscape of acute myeloid
leukemia. Exp Cell Res. 393:1120542020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai T, Zhang L, Dai X, Zhang X, Lu B,
Zheng Y, Shen D, Yan Y, Ji C, Yu J and Sun L: Multimode
participation of traditional Chinese medicine in the treatment of
COVID-19. Integr Med Res. 10 (Suppl 1):S1007812021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chledzik S, Strawa J, Matuszek K and
Nazaruk J: Pharmacological effects of scutellarin, an active
component of genus scutellaria and erigeron: A systematic review.
Am J Chin Med. 46:319–337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
You L, Zhu H, Wang C, Wang F, Li Y, Li Y,
Wang Y and He B: Scutellarin inhibits Hela cell growth and
glycolysis by inhibiting the activity of pyruvate kinase M2. Bioorg
Med Chem Lett. 27:5404–5408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ke Y, Bao T, Wu X, Tang H, Wang Y, Ge J,
Fu B, Meng X, Chen L, Zhang C, et al: Scutellarin suppresses
migration and invasion of human hepatocellular carcinoma by
inhibiting the STAT3/Girdin/Akt activity. Biochem Biophys Res
Commun. 483:509–515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen J, Cai YF, Shao M and Cong H: Effect
of scutellarin on proliferation of acute myeloid leukemia cells and
its related mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
31:358–363. 2023.(In Chinese). PubMed/NCBI
|
|
66
|
Khoury JD, Solary E, Abla O, Akkari Y,
Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, et
al: The 5th of the World Health Organization classifcation of
haematolymphoid tumours: Myeloid and histiocytic/dendritic
neoplasms. Leukemia. 36:1703–1719. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Arber DA, Orazi A, Hasserjian RP, Borowitz
MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S,
et al: International consensus classification of myeloid neoplasms
and acute leukemias: Integrating morphologic, clinical, and genomic
data. Blood. 140:1200–1228. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
DiNardo KW, LeBlanc TW and Chen H: Novel
agents and regimens in acute myeloid leukemia: Latest updates from
2022 ASH annual meeting. J Hematol Oncol. 16:172023. View Article : Google Scholar : PubMed/NCBI
|