Introduction
Prostate cancer incidence is considerably high in
Western and developed Asian countries. Although the 5-year survival
rate of prostate cancer is ~97%, which is one of the highest among
cancers, mortality due to prostate cancer is substantilly high,
ranking it second among all cancer-related deaths (1). Chemotherapeutic agents such as
docetaxel and cabazitaxel and androgen signaling inhibitors such as
abiraterone and enzalutamide have successfully been used to treat
prostate cancer. However, the recurrence of prostate cancer
resistant to these therapies, such as castration-resistant prostate
cancer (CRPC), and the side effects of these therapies, which
includes impotence, continue to affect the quality of life of
patients (2,3).
Although safe and effective methods for treating
prostate cancer are being investigated, effective
androgen-independent therapies for advanced prostate cancer remain
unavailable. Similar to other tumor types, a very small proportion
(<1%) of cancer stem cells (CSCs) are present in prostate cancer
(4,5). Prostate CSCs differentiate into
androgen-dependent and androgen-independent carcinomas.
Androgen-independent carcinomas are more resistant to anticancer
therapy. Thus, in addition to conventional chemotherapy, novel
therapies targeting CSCs are required to prevent CSCs from
continuously supplying androgen-independent carcinomas.
Accordingly, it is necessary to elucidate the characteristics of
prostate CSCs and identify methods to mitigate their properties to
effectively treat patients with CRPC (6,7).
Because the elimination of CSCs in various tumor
types using conventional anticancer agents has been ineffective,
the differentiation of CSCs into nonmalignant cells has been
attempted for different cancer types (8–10).
Previous studies on other cancer types have reported the use of
differentiation therapy to convert prostate CSCs into more
differentiated cells (11).
However, these studies were unsuccessful. Further understanding of
signal transduction involved in the differentiation of prostate
CSCs can help develop anticancer therapies, including
differentiation therapies. ZEP1, YAP1, and TMPRSS4, which are
involved in maintaining the properties of prostate CSCs, are
potential targets for the differentiation therapy of prostate
cancer (12–14).
Previous studies have suggested the use of dopamine
receptor 2 (DRD2) antagonists as putative anticancer agents
(15,16). Neoplastic human pluripotent stem
cells (hPSCs) possessing CSC-like properties were differentiated
into cells that lost their pluripotency due to treatment with
dopamine receptor (DR) antagonists, such as thioridazine (10). Our previous study using CSC-like
cells derived from PC-3 cell lines (human prostate cancer cells)
and thiordazine yielded results very similar to those obtained
using the hPSCs described above (17). In addition, our previous study
suggested that thioridazine induces the differentiation of
PC-3-derived CSC-like cells via AMPK inhibition. However,
thioridazine inhibits other types of receptors, including
histamine, muscarine, and serotonin receptors, in addition to DRD2
(18–20). Accordingly, it must be clarified
that the differentiation of PC-3-derived CSC-like cells using
thioridazine was induced by DRD2 inhibition and not by the
nonspecific inhibition of different receptors. Herein, PC-3-derived
CSC-like cells were transfected with siRNA or treated with highly
specific antagonists against five different DR subtypes (DRD1-DRD5)
and the effects of the inhibition of each receptor subtype were
compared. In addition, the involvement of DRD2 in the formation of
PC-3-derived CSC-like cells was confirmed using the heterozygous
knockout of the DRD2 gene in PC-3 cells via the CRISPR/Cas9 method
and by investigating the effects of DRD2 knockdown in the
cells.
Materials and methods
Reagents
LE300, L-741,626, PG 01037, PD 168568, and SCH 39166
were purchased from Tocris. Antibodies against Akt, phosphor-Akt,
AMPKa, phospho-AMPKa, mTOR, phospho-mTOR, SAPK/JNK,
phospho-SAPK/JNK, Oct4, Klf4, c-Myc, and b-actin were purchased
from Cell Signaling Technology. Unless otherwise stated, all other
reagents were purchased from Merck KGaA.
Cell lines
The human prostate cancer cell line PC-3 was
obtained from the American Type Culture Collection and cultured in
RPMI-1640 medium supplemented with 10% (v/v) heat-inactivated fetal
bovine serum (FBS). Cell cultures enriched for CSC-like cells
derived from PC-3 cells were prepared according to a previously
described protocol (17). Briefly,
PC-3 cells were trypsinized, harvested after washing with
phosphate-buffered saline (PBS), and then suspended in serum-free
DMEM/F12 medium (R&D Systems) supplemented with 100 IU/ml
penicillin, 100 µg/ml streptomycin, 10 ng/ml human recombinant
epidermal growth factor (hrEGF; R&D Systems), 10 ng/ml human
recombinant basic fibroblast growth factor (hrbFGF; R&D
Systems), and 2% B27 supplement (Thermo Fisher Scientific, Inc.).
These suspended cells were cultured in ultralow attachment culture
dishes at a density of 1×106 cells/dish and then allowed
to form tumorspheres for 7 days. Single cells obtained via the
trypsinization of the tumorspheres were collected and allowed to
form an adherent monolayer culture in a regular animal cell culture
ware in the abovementioned serum-free medium and maintained in
serum-free DMEM/F12 medium supplemented with 100 IU/ml penicillin,
100 µg/ml streptomycin, 10 ng/ml hrEGF, 10 ng/ml hrbFGF, and 2% B27
supplement.
Immunocytochemistry
The cells were seeded into a four-chamber plate
(Falcon) at a density of 1×104 cells/well, incubated for
72 h, and fixed with 3.7% formaldehyde at room temperature for 10
min. Fixed cells were permeabilized with 0.1% Tween-20 in PBS (pH
7.4) for 5 min, blocked with PBS containing 1% bovine serum albumin
(BSA) for 30 min, and incubated with a rabbit monoclonal antibody
against DRD1, DRD2, DRD3, DRD4, and DRD5 at 4°C overnight. The
cells were washed with PBS and incubated in the presence of goat
antirabbit IgG conjugated with Alexa Fluor™ 594 (Thermo
Fisher Scientific, Inc.) for 1 h in the dark. Alexa
Fluor™ 488 Phalloidin (Thermo Fisher Scientific, Inc.)
diluted to a ratio of 1:40 using PBS was added to the wells and
incubated for another 20 min. The cells were washed with PBS,
stained with DAPI for 5 min, and observed under an LSM 700 confocal
microscope (Zeiss).
Human tumor xenografts in nude
mice
The Institutional Animal Care & Use Committee
(IACUC) of Korea University (Seoul, Korea), reviewed and approved
the animal study protocol (protocol number: KUIACUC-2021-0028).
Six- to seven-week-old female Balb/c nude mice (Charles River
Laboratories) were maintained as previously described (21). Cancer cells (5×106
cells/200 µl/mouse) suspended in PBS (pH 7.4) were injected
subcutaneously into the right flank of a mice. Tumor volumes were
measured 2–3 times weekly using a Vernier caliper and were
calculated using the following equation: Tumor
volume=Dmin2 × Dmax × 0.5 (Dmin: short axis, Dmax: long
axis of mass). The experiments were stopped before the tumor volume
reached 1,000 mm3 and the mice were euthanized by
introducing CO2 into the euthanasia chamber at a rate
sufficient to fill 50% of the chamber's volume per minute. The mice
were continuously monitored for visible signs of death, such as
cessation of breathing and lack of movement. Even after observing
the visible signs of death, CO2 exposure were continued
for an additional 1 min. After then, the tumors were collected from
the mice.
siRNA transfection
PC-3-derived CSC-like cells were seeded into tissue
culture plates at a density of 3×104 to 1×105
cells/ml depending on the type of experiment using antibiotic-free
DMEM/F12 medium with supplements and growth factors. To silence the
expression of the DRD subtype genes, the cells were transfected for
6 h with 50–100 pmol/ml of control siRNA and DRD1, DRD2, DRD3,
DRD4, and DRD5 siRNA separately using the siRNA Reagent System
(Santa Cruz Biotechnology, Inc.) following the manufacturer's
instructions (see Table SI for
more information about siRNA).
Semiquantitative reverse transcription
(RT)-polymerase chain reaction (PCR)
Total RNA was extracted from cells using a GeneAll
kit (GeneAll Biotechnology), and cDNA was prepared and amplified
using a One Step RT-PCR kit (SolGent). Table SII shows the primer sequences for
the dopamine receptor subtypes (DRD1, DRD2, DRD3, DRD4, and DRD5)
and GAPDH. The PCR products were subjected to electrophoresis using
1.5–1.8% agarose gel and visualized after staining with ethidium
bromide.
cAMP assay
PC-3-derived CSC-like cells were transfected with
siRNA against DRD1-DRD5 and seeded in 96-well plates at a density
of 5×104 cells/well. After 24 h of incubation, the
intracellular cAMP levels were assayed using cAMP-Glo™ assay
(Promega) following the manufacturer's instructions. Luminescence
was measured using a Centro LB 960 microplate luminometer (Berthold
Technologies).
Cell growth assay
To measure the growth of PC-3-derived CSC-like cells
transfected with DRD siRNAs, the WST-8 assay (Biomax) was used.
Briefly, 24 h after seeding the cells in a six-well culture plate
at a density of 1×104 cells/ml, WST-8 assay was
conducted at designated times and absorbance was measured at 450
nm. To determine the growth of DRD2 heterozygous knockout PC-3
cells, the cells were seeded into a six-well culture plate at a
density of 1×104 cells/ml and grown in RPMI-1640 medium
supplemented with 10% heat-inactivated FBS at 37°C. The cells were
trypsinized and harvested at designated times and counted using an
automated cell counter (Countess™ II #AMQX1000).
In vitro cell invasion assay
The insert polycarbonate membranes (8 mm in pore
size) of the upper compartments of the 24-well Transwell Boyden
chamber were coated with Matrigel® (BD Biosciences)
diluted using a serum-free medium. Depending on the cell type, 300
µl of cells (1.5×105 cells/ml) suspended in RPMI-1640 or
DMEM/F12 medium were placed in the upper compartment. The lower
compartment was then filled with 500 µl of RPMI-1640 medium
supplemented with 10% FBS or serum-free DMEM/F12 medium
supplemented with 2% B-27 supplements and growth factors. The cells
on the lower side of the insert membrane were fixed with 10%
trichloroacetic acid (TCA) and stained with 0.5% crystal violet in
2% ethanol for 2 h. The insert was washed with PBS and air dried.
The upper side of the insert was wiped using a cotton swab, and
images of the cells that had migrated to the underside of the
insert membrane were taken. The dye that stained the cells was
extracted with 0.2 ml of 30% acetic acid, and the absorbance was
measured at 590 nm.
Western blotting analysis
Protein extracts (20 µg) of cell lysates were
resolved on 8–10% SDS-polyacrylamide gels and transferred to
Immobilon-P transfer membranes as described previously (22). The membranes were blocked with
Tris-buffered saline containing 0.1% Tween-20 (TBST) supplemented
with 0.5–2% BSA and probed with primary antibodies. After washing
with TBST, the membranes were probed with species-specific
horseradish peroxidase (HRP)-conjugated secondary antibodies and
developed using an Immobilon western chemiluminescent HRP
substrate.
Staining of cells to observe
morphological changes
The cells incubated for 6 days in the presence and
absence of compounds were fixed with 50% TCA and stained with 0.4%
sulforhodamine B (SRB) in 0.1% acetic acid. Excess staining was
removed by washing the cells with 1% acetic acid. The morphology of
the stained cells was observed using an inverted microscope and
photographed (Nikon).
Preparation of DRD2 heterozygous
knockout (DRD2+/−) PC-3 cells
PC-3 cells were seeded into 60-mm dishes at a
density of 1.5×105 cells/ml and cultured in an
antibiotic-free medium. When the cells were at ~80% confluency,
they were transfected with an all-in-one vector (Macrogen, Seoul,
Korea) containing the DRD2 sgRNA sequence
(5′-GGTATGATGATGATCTGGAGAGG-3′), puromycin resistance gene, and
CAS9 expression gene using the TransIT-LT1 transfection
reagent (Mirus Bio) following the manufacturer's instructions (see
Fig. S1 for the all-in-one vector
map). After 48 h, the cells were cultured on RPMI medium
supplemented with 100 IU/ml penicillin, 100 µg/ml streptomycin, and
1 µg/ml puromycin for 6 days. During this period, the medium was
changed daily. The surviving cells were amplified, and genomic DNA
was isolated using Exgene™ Cell SV mini kit (GeneAll
Biotechnology, Seoul, Korea). DNA fragments were then amplified by
PCR with the DRD2 primers used in the T7 endonuclease assay and
sequenced using the Sanger sequencing method (Cosmogenetech) (See
the DNA sequences in Fig. S2).
T7 endonuclease assay
To validate DRD2 heterozygous knockout in PC-3
cells, genomic DNA isolated from wild-type and all-in-one
vector-transfected PC-3 cells were amplified using PCR with the
DRD2 primers (forward: 5′-TGTGTTTGCTCATTTGTCCTACC-3′, reverse:
5′-AGGAAACAATCTACCCATTTCGT-3′). The amplified DNA products were
incubated at 37°C for 20 min in the presence of T7 endonuclease
(Goldbio). The reaction products were then subjected to
electrophoresis using 1.2% agarose gel and visualized after
staining with ethidium bromide.
Sphere formation assay
Wild-type, mock-transfected, and DRD2+/−
PC-3 cells grown in RPMI medium containing 10% heat-inactivated FBS
were trypsinized and cultured at a density of 1×104
cells/well in an ultralow attachment six-well plate containing
DMEM/F12 medium supplemented with 2% B27, 10 ng/ml hrEGF, and 10
ng/ml hrbFGF. Round cell clusters of >40 µm were classified as
spheres.
Statistical analysis
GraphPad Prism 5.03 (GraphPad Software, Boston,
Massachusetts, USA) was used for all statistical analyses.
Statistical differences among experimental groups were analyzed
using a unpaired Student's t-test or one-way/two-way ANOVA with
Dunnett's post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
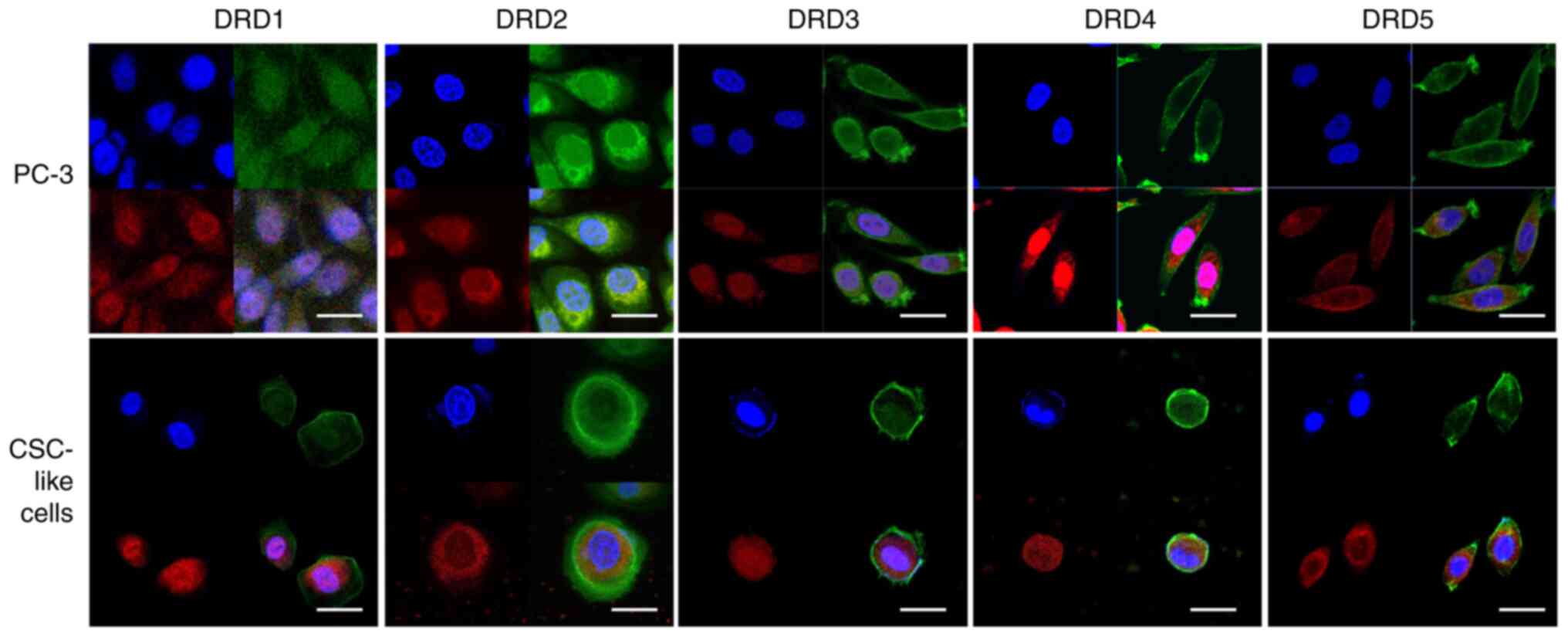
Detection of DR subtype receptors in
PC-3 and PC-3-derived CSC-like cells via immunocytochemistry
Our previous study showed that cell cultures
prepared for enriching CSC-like cells from PC-3 cells possessed
several properties characteristic of CSC-like cells, such as the
expression of Oct4, Klf4, and Sox2. These PC-3-derived CSC-like
cells were more resistant to some agents with anticancer activity
such as dasatinib and saracatinib, tyrosine kinase inhibitors, and
tirbanibulin, a tubulin polymerization inhibitor, than PC-3 cells
(Fig. S3). In addition, the mRNA
of all DR subtypes, which were of primary interest in our study,
were expressed in PC-3 cells and PC-3-derived CSC-like cells
(17). Immunocytochemical analysis
revealed that the proteins of the DR subtypes were also expressed
in these cells (Fig. 1). Capturing
stained images of DRD3 was challenging, as expected from the
extremely low expression of its mRNA; however, the presence of all
five subtypes was detected in both the cell types.
Morphological changes and loss of
pluripotency markers in PC-3-derived CSC-like cells caused by a
specific DRD2 antagonist
Thioridazine used in our previous study (17) is a DRD2 antagonist but it also binds
and inhibits various other receptors, including serotonin and
cholinergic receptors. Thus, more specific antagonists against each
DR subtype, such as LE300, L-741,626, PG 01037, PD 168568, and
SCH39166, were applied to PC-3-derived CSC-like cells at a
concentration of 3 µM. Subsequently, the morphological changes of
the cells and the expression of pluripotency markers such as Oct4,
Klf4, and c-Myc were analyzed (Fig.
2). L-742,626, a DRD2 antagonist, strongly induced
morphological changes in the cells, a strong decrease in Oct4 and
Klf4 protein expression, and a strong increase in c-Myc protein
expression, indicating the loss of CSC properties. These results
are consistent with those of our previous study (17). Meanwhile, PD 168568, a DRD4
antagonist, also induced changes in the morphology and Oct4, Klf4,
and c-Myc protein expression in the cells, although the effects
were weaker than those induced by L0741,626. PG-01037 induced very
weak morphological changes in the cells and a slight decrease in
Oct4 protein expression; however, further studies are needed to
determine whether DRD2 is involved in maintaining the properties of
PC-3-derived CSC-like cells.
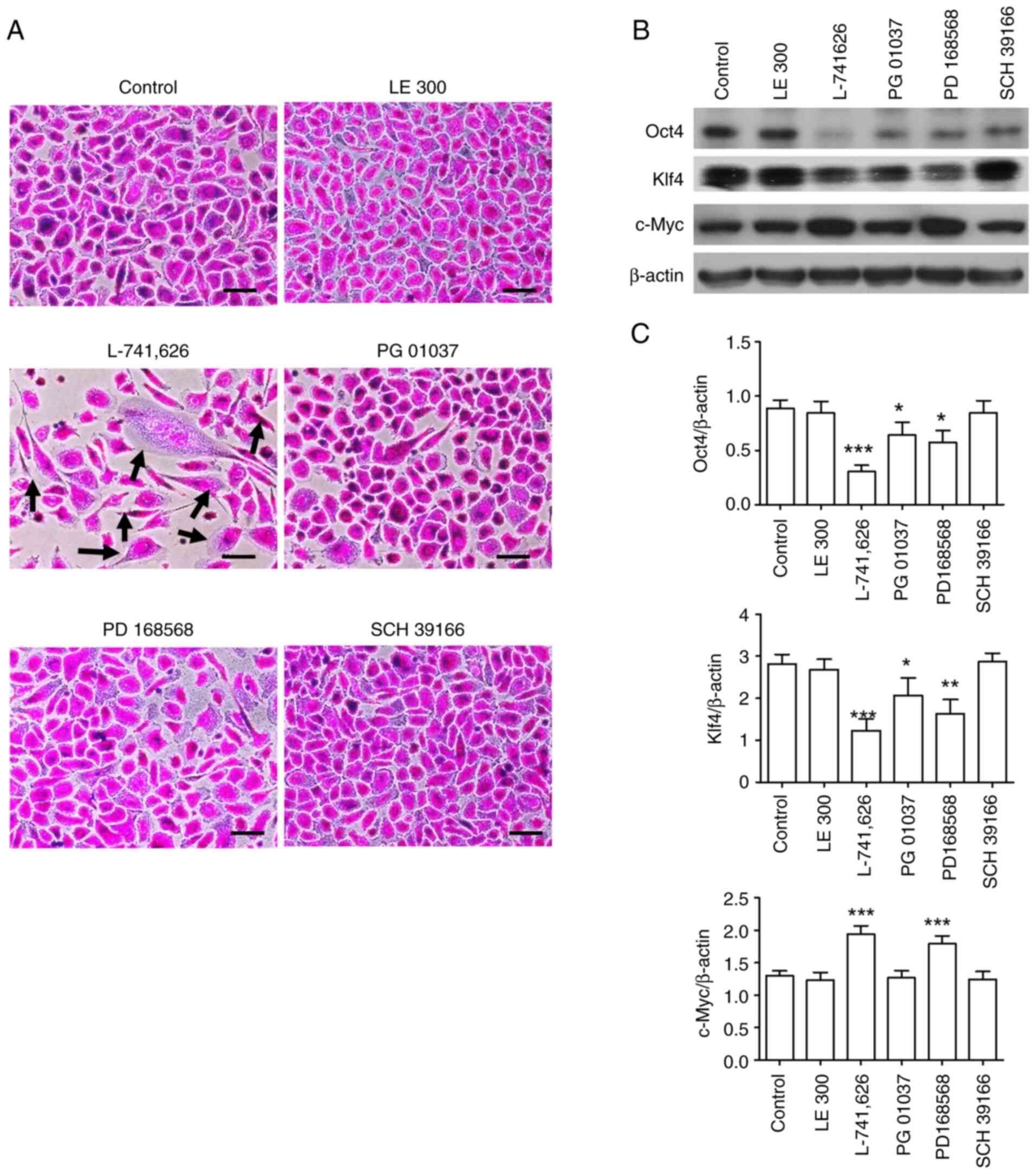 | Figure 2.Effects of DR subtype antagonists on
the morphology and expression of pluripotency markers in
PC-3-derived CSC-like cells. (A) PC-3-derived CSC-like cells were
incubated in the presence and absence of 3 µM of antagonists
against DR subtypes (LE 300, L-741,626, PG 01037, PD 168568, and
SCH 39166 for DRD1, DRD2, DRD3, DRD4, and DRD5, respectively) for 6
days and stained with 0.4% SRB. Black arrows indicate cells with
altered morphology. Scale bar: 100 µm. (B) Lysates of cells
incubated as described in (A) were subjected to western blotting.
(C) Band densities of western blot images (n=3) were measured using
ImageJ software. Data are presented as mean ± SD. *P<0.05,
**P<0.01, ***P<0.001 vs. control. DRD, dopamine receptor;
CSC, cancer stem cell. |
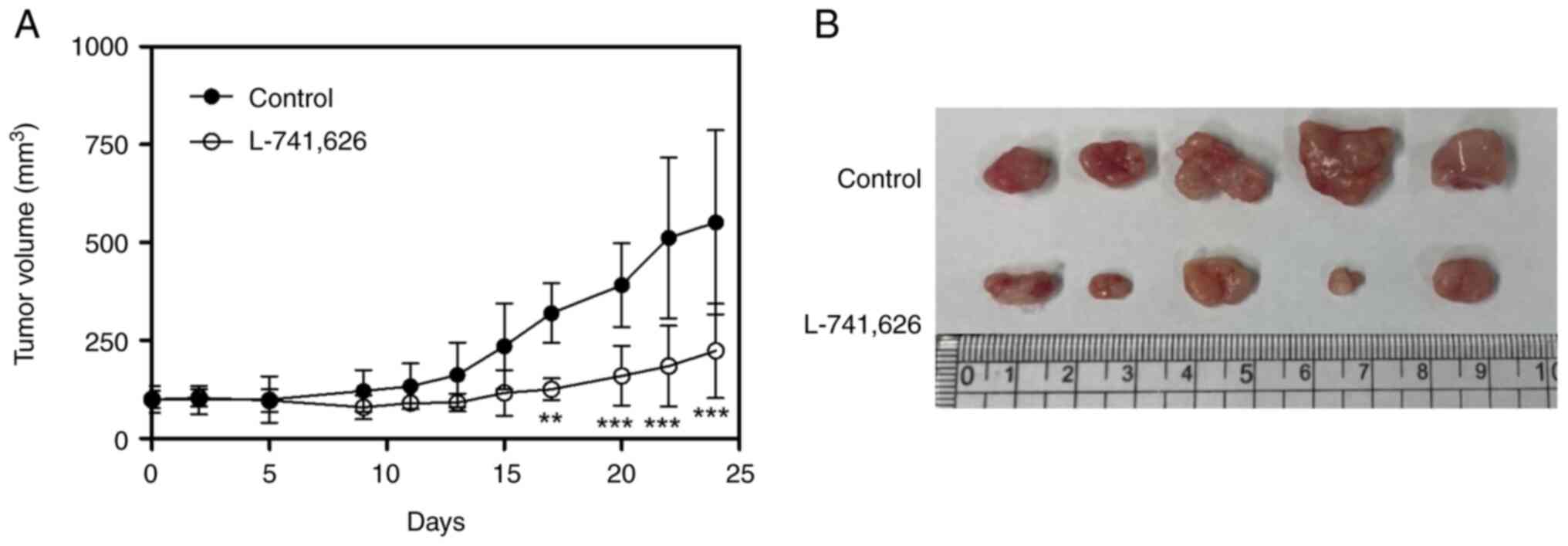
Inhibition of the growth of tumors
induced by PC-3-derived CSC-like cells by a specific DRD2
antagonist
To determine whether a specific DRD2 antagonist
inhibits tumor growth in vivo, L-741,626 (20 mg/kg) was
administered intraperitoneally to nude mice with tumors formed from
PC-3-derived CSC-like cells daily. L-741,626 induced 59.5% tumor
growth inhibition compared with the control group without
significant changes in body weight (Fig. 3).
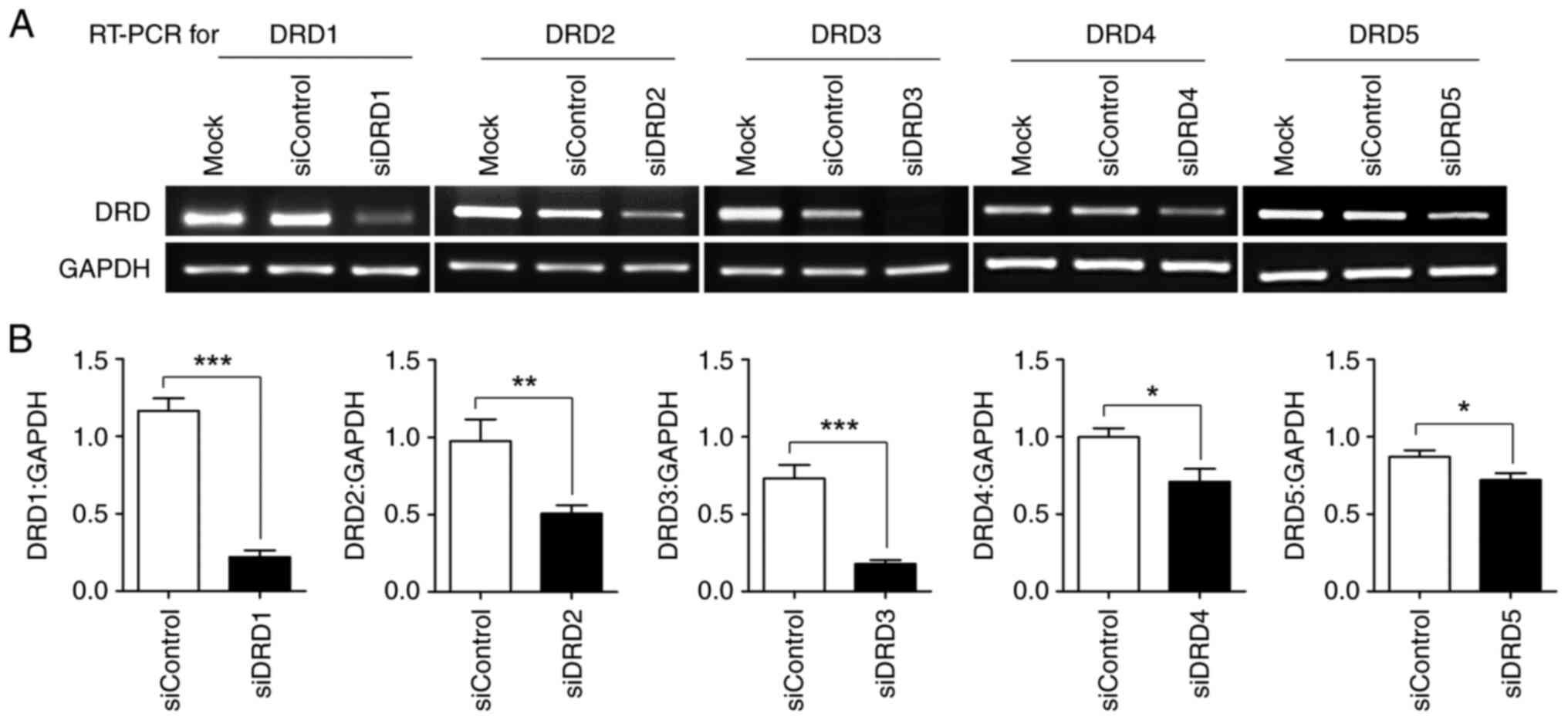
Downregulation of the mRNA expression
of DR subtypes through transfection with siRNA against each
subtype
Because the inhibition of DRD2 in PC-3-derived
CSC-like cells by thioridazine, a DRD2 antagonist, appeared to
induce a loss of CSC characteristics in our previous study
(17), it was necessary to
determine whether this effect could be mimicked by the knockdown of
any DR subtype. siRNA against all five subtypes was transfected
into PC-3-derived CSC-like cells. Semiquantitative RT-PCR revealed
that the mRNA of each subtype was effectively downregulated by its
corresponding siRNA (Fig. 4).
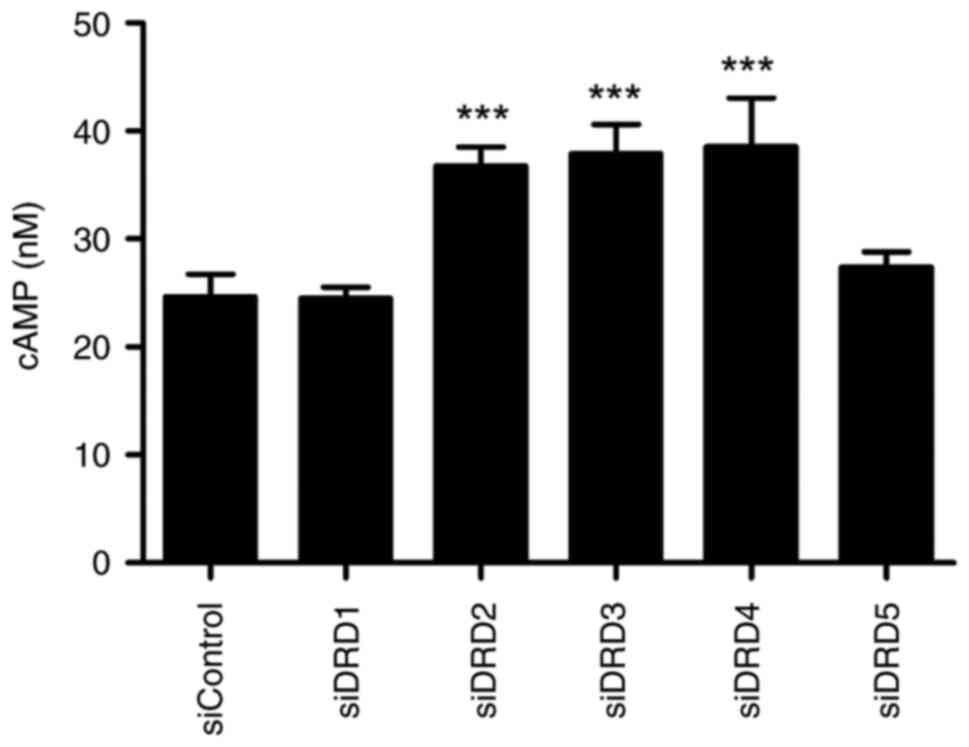
Effects of DR siRNA transfection on
the intracellular cAMP concentration
While DRD2, DRD3, and DRD4 are type II dopamine
receptors coupled to the Gs protein, DRD1 and DRD5 are type I
dopamine receptors coupled to the Gi protein (23). To determine whether the
siRNA-mediated knockdown of DR subtypes is functionally effective,
intracellular cAMP concentrations were measured (Fig. 5). The knockdown of type I dopamine
receptors suppressed intracellular cAMP concentrations to the
control levels, whereas the knockdown of type II dopamine receptors
increased intracellular cAMP concentrations.
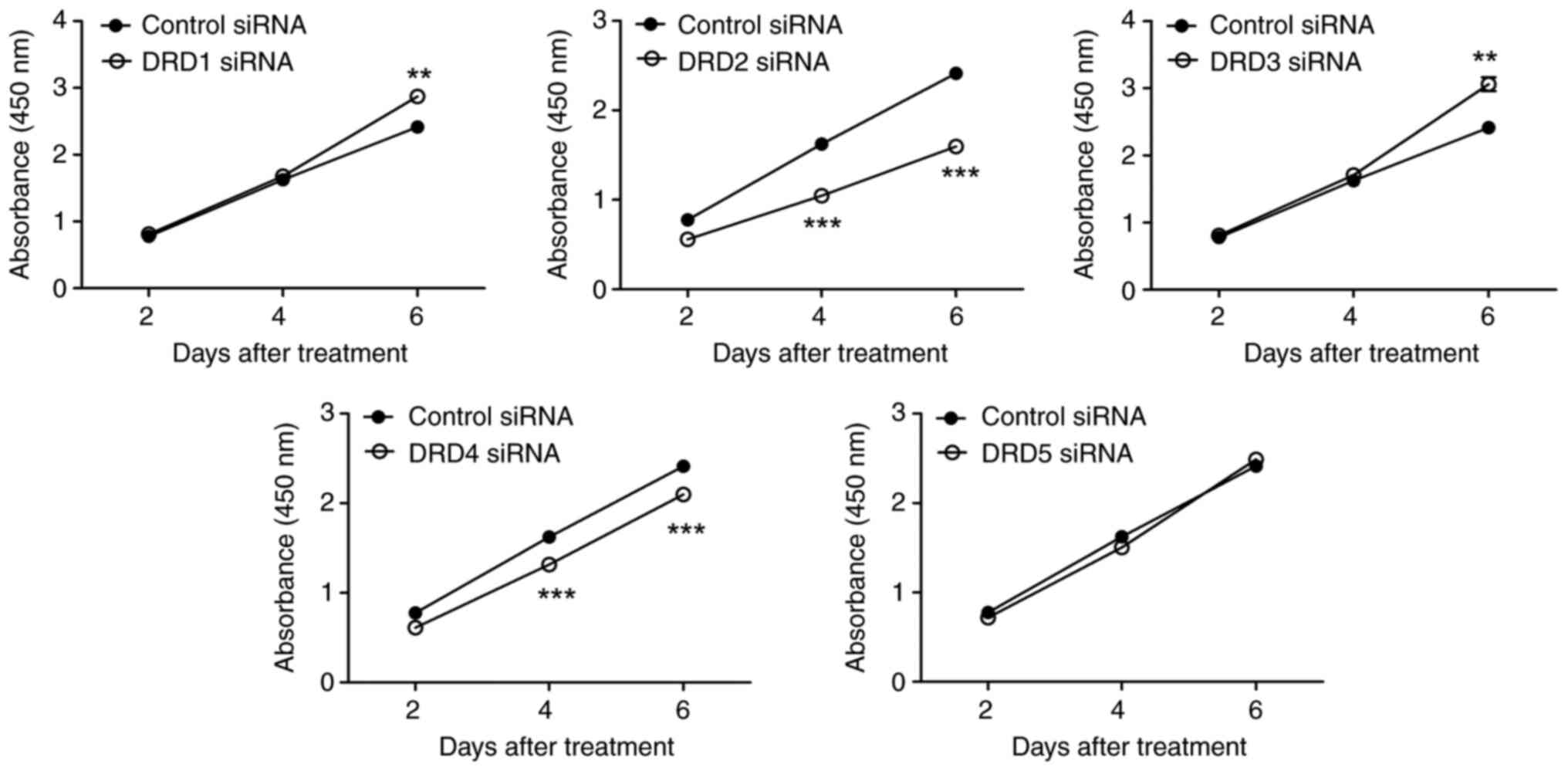
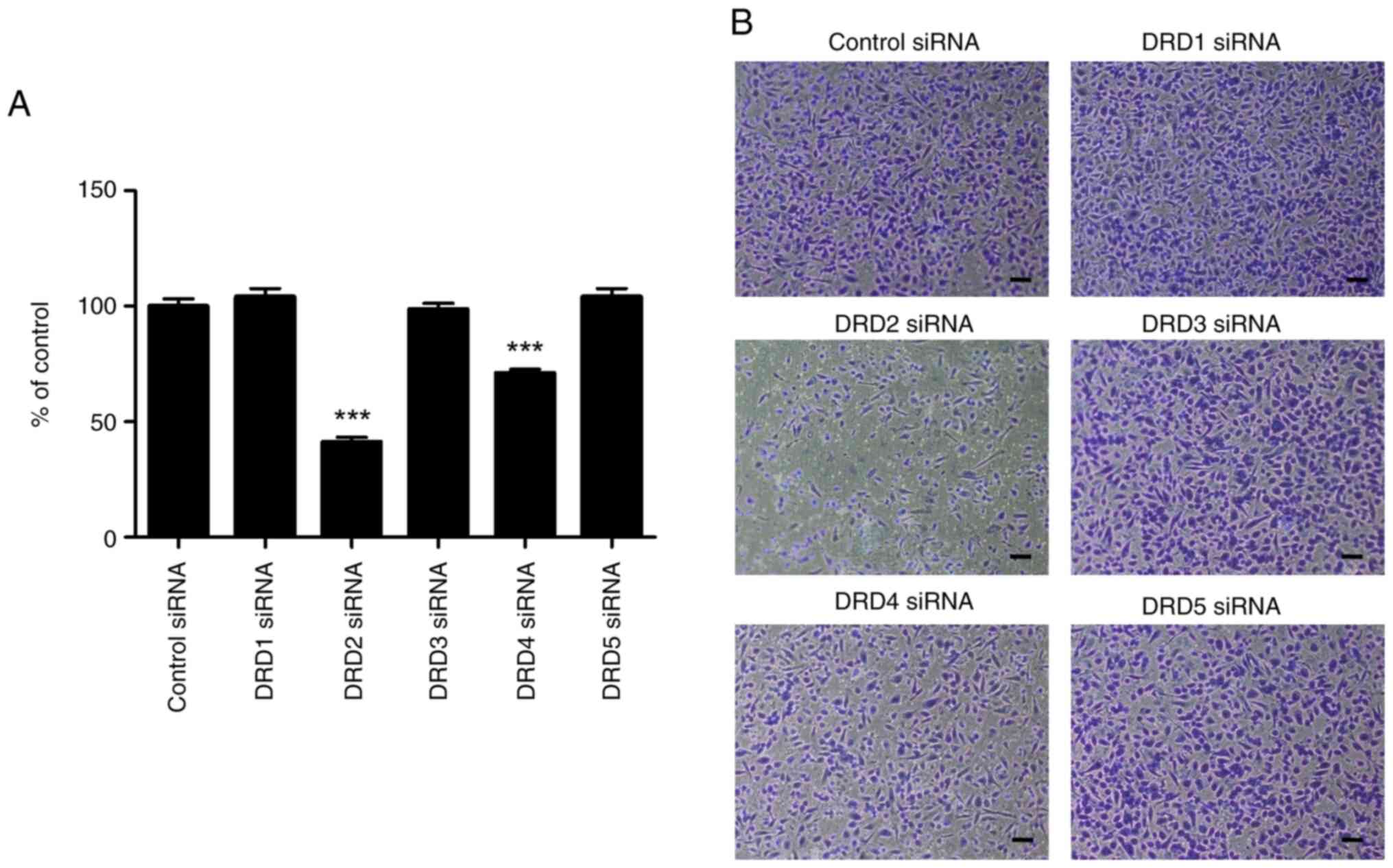
Alteration of growth and in vitro
invasion of PC-3-derived CSC-like cells by DR siRNAs
The growth of PC-3-derived CSC-like cells
transfected with DRD2 siRNA decreased substantially with time
(Fig. 6). Transfection with DRD4
siRNA also inhibited cell growth, but the effect was less
pronounced than that caused by DRD2 siRNA. A slight but
statistically significant increase in the growth of cells
transfected with DRD1 or DRD3 on day 6 was observed. In contrast to
the effects on cell growth, in vitro cell invasion remained
unaffected by DRD1 and DRD3 siRNA (Fig.
7). However, it was substantially inhibited by DRD2 siRNA and
slightly by DRD4 siRNA.
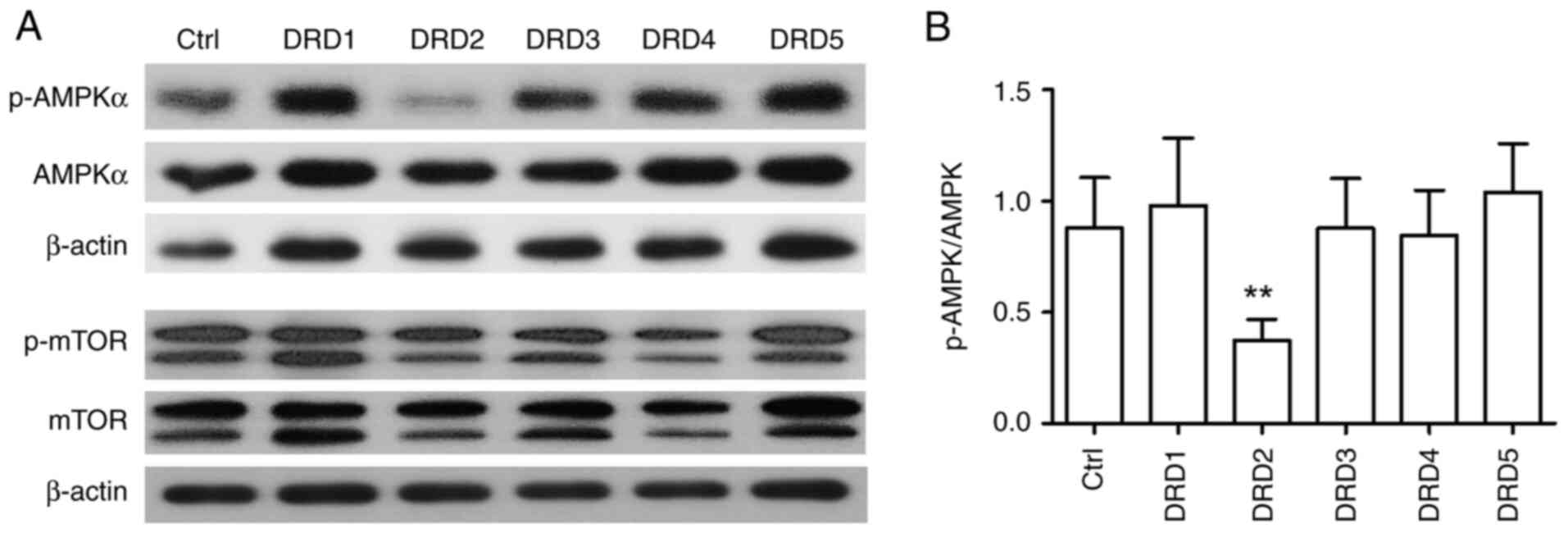
Inhibition of AMPK phosphorylation by
DRD2 siRNA
Our previous study showed that AMPK phosphorylation
was inhibited by thioridazine, a DRD2 antagonist (17). To examine whether AMPK
phosphorylation is inhibited by DR knockdown, PC-3-derived CSC-like
cells were transfected with DR siRNAs. Fig. 8 shows that only DRD2 siRNA
significantly inhibited AMPK phosphorylation. mTOR phosphorylation,
which was unaffected by thioridazine, was also unaffected by
transfection with siRNA against DR subtypes.
Changes in the properties of
PC-3-derived CSC-like cells after DRD2 knockdown
Because the suppression of DRD2 activity or
expression with a DRD2 antagonist or siRNA changed the
characteristics of PC-3-derived CSC-like cells, it was necessary to
investigate whether the suppression of DRD2 expression in parental
PC-3 cells affected their intrinsic characteristics, such as sphere
formation ability. DRD2 heterozygous knockout (DRD2+/−)
PC-3 cells were prepared, and the heterozygotic genetic mutation
was validated using the T7 endonuclease digestion method (Fig. 9A). The downregulation of DRD2
expression was observed (Fig. 9B and
C). The growth of DRD2+/− PC-3 cells was
substantially slower than that of mock-transfected PC-3 cells.
Addition of 30 µM of A769662, an AMPK activator, to
DRD2+/− PC-3 cells partially restored the cell growth
(Fig. 9D). The concentration of 30
mM was chosen from the previous study as it showed the highest
effects in restoring cell growth (17). The morphology of the
DRD2+/− PC-3 cells differed substantially from that of
the mock-transfected PC-3 cells (Fig.
9E). The sphere formation assay, an in vitro method for
amplifying and isolating CSCs, revealed that the sphere-forming
ability of the DRD2+/− PC-3 cells was substantially
lower than that of the parental and mock-transfected PC-3 cells
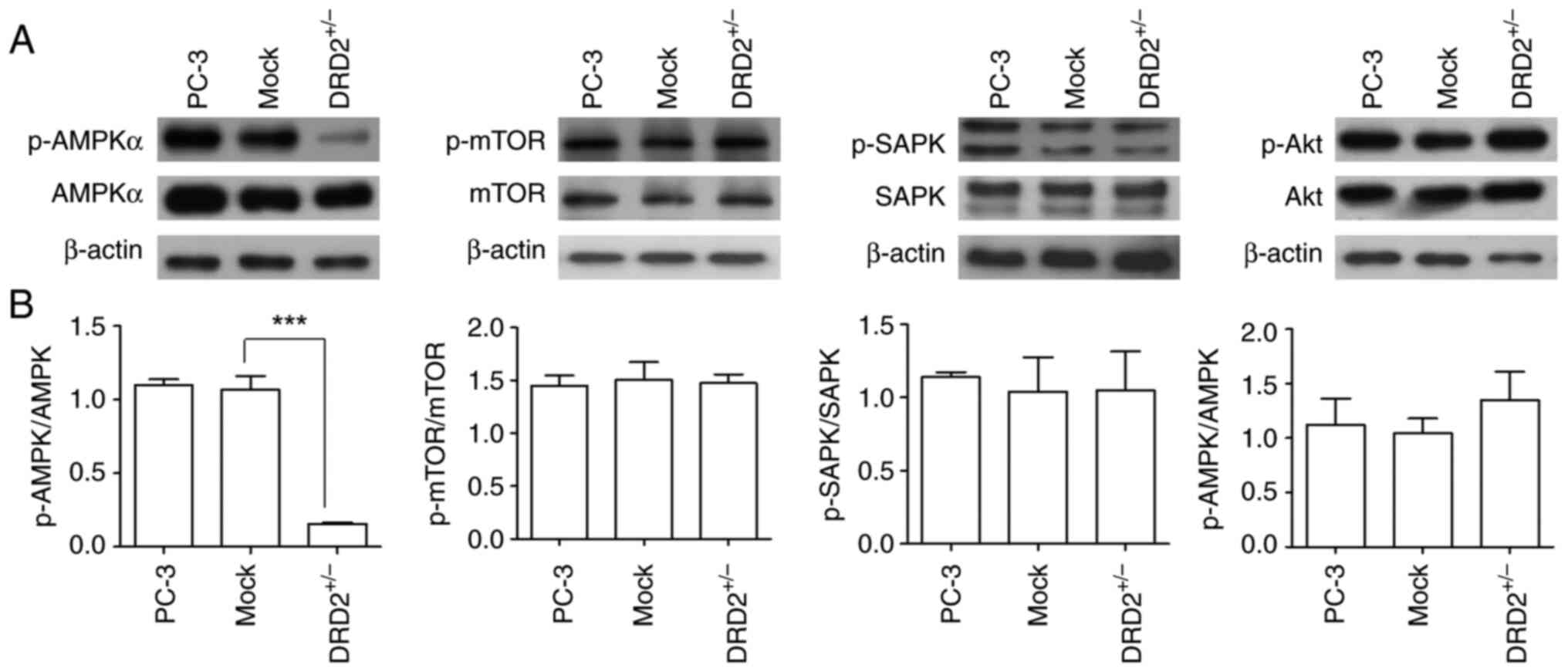
(Fig. 9F and G). Because AMPK
phosphorylation in PC-3-derived CSC-like cells is inhibited by DRD2
siRNA, the effect of heterozygous DRD2 knockout on the
phosphorylation of several signal transduction pathways, including
AMPK, was examined. As shown in Fig.
10, AMPK phosphorylation in DRD2+/− PC-3 cells was
substantially decreased compared with that in the parental and
mock-transfected PC-3 cells, whereas there were no significant
differences in mTOR, SAPK/JNK, and Akt phosphorylation in the
parental, mock-transfected, and DRD2+/− PC-3 cells,
indicating that AMPK activity or phosphorylation is crucial for
maintaining or inducing CSC properties.
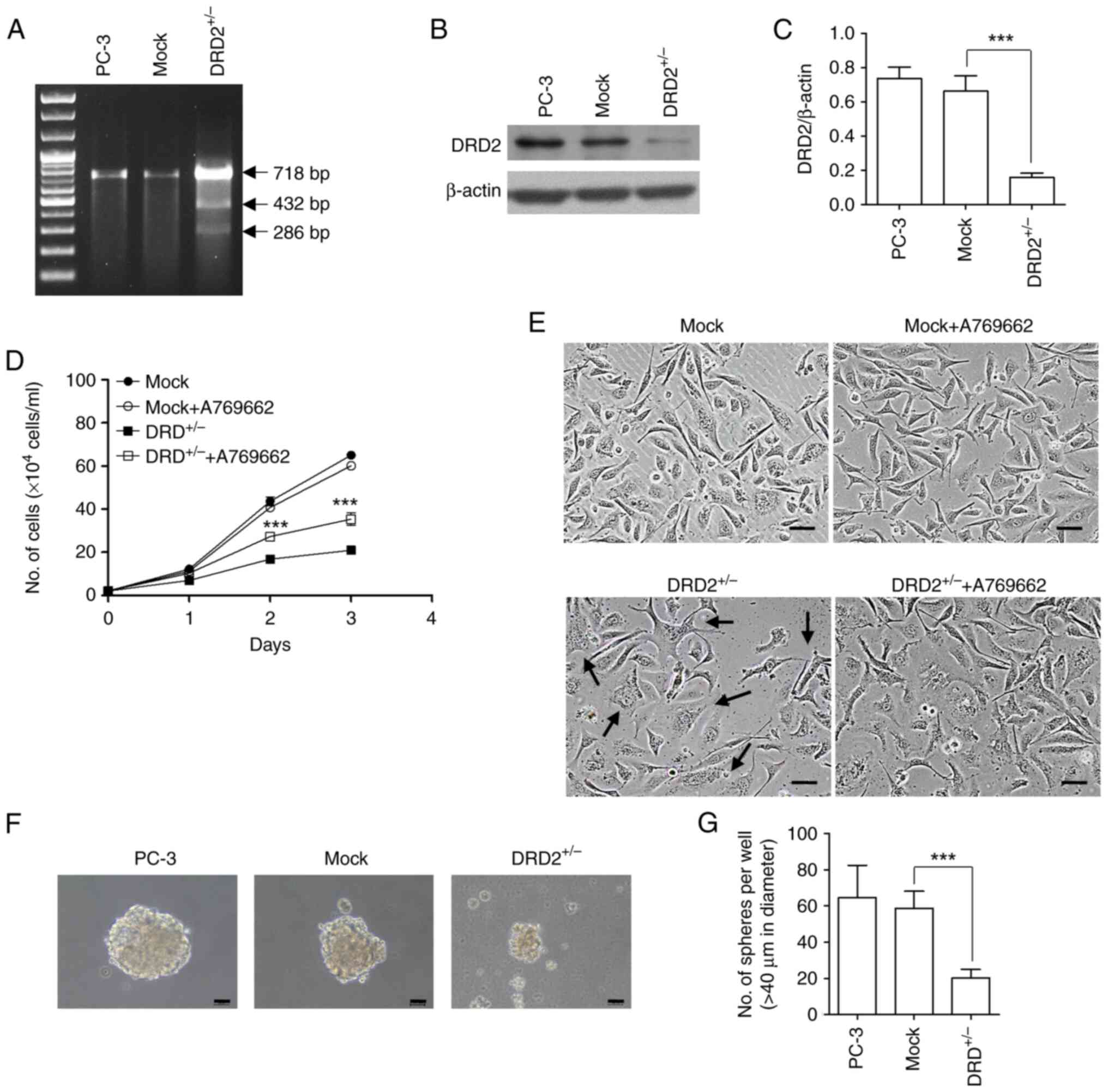 | Figure 9.Changes in the characteristics of
PC-3 cells following the heterozygous knockout of DRD2. (A) Agarose
gel electrophoresis of PCR products amplified using genomic DNA
isolated from wild-type and all-in-one vector-transfected PC-3
cells. (B) Lysates of wild-type, mock-transfected, and
DRD2+/− PC-3 cells were subjected to western blotting to
measure DRD2 protein expression. (C) Band densities of western blot
images (n=3) were measured using ImageJ software. (D and E)
Mock-transfected PC-3 cells and DRD2+/− PC-3 cells
seeded at a density of 0.8×104 cells/ml in a 6-well
plate were incubated for 3 days in the presence and absence of 30
µM A769662 and the number of cells were counted at the indicated
times. Images of the cells were taken at the end of the
experiments. Black arrows indicate cells whose morphologies are
changed. Scale bar: 100 µm. Data are presented as mean ± SD (n=4).
***P<0.001 vs. DRD2+/−. (F) Spheres formed from
wild-type, mock-transfected, and DRD2+/− PC-3 cells in
an ultralow attachment six-well plate for 10 days, Scale bar: 100
µm. (G) Round cell clusters larger than 40 µm in diameter were
counted. Data are presented as mean ± SD (n=3). ***P<0.001 vs.
Mock. DRD, dopamine receptor; AMPK, 5′ AMP-activated protein
kinase; mTOR, mammalian target of rapamycin; CSC, cancer stem cell;
p-, phosphorylated. |
Discussion
With the global increase in the incidence of
prostate cancer, the number of patients with intractable prostate
cancer, such as CRPC, is also rising. Various alterations,
including the loss of normal androgen receptor expression in
prostate carcinomas, are reportedly involved in the recurrence of
prostate cancer and the acquisition of metastatic ability by
carcinomas to other tissues, such as bones. CSCs have been reported
to be crucial in these malignant processes (6). Our previous study using PC-3-derived
CSC-like cells semiquantitatively determined the relative mRNA
expression levels of five DR subtypes without providing information
about their protein expression (17). There have been reports regarding the
protein expression of some of the DR subtypes in carcinoma
(10,24,25);
however, to the best of our knowledge, there have been no reports
regarding the protein expression of all five DR subtypes in CSCs or
CSC-like cells. Because the immunocytochemistry methods used in
this study were not quantitative, we could not compare the relative
expression levels of the DR subtypes in the cells. Furthermore, the
stained images of DRD3 were particularly difficult to obtain
compared with those of the other subtypes in both the cell
types.
L-741,626, a DRD2 antagonist, induced significant
changes in the morphology of PC-3-derived CSC-like cells and the
expression of pluripotency markers, including Oct4, Klf-4, and
c-Myc. The DRD4 antagonist PD 168568 produced weak but similar
results to those obtained using L-741,626. These results further
confirm our previous results obtained with siRNA specific for the
DR subtypes (17) and indicate that
DRD2 and DRD4 coupled with Gi protein are involved in
the maintenance of the properties of prostate CSC-like cells. Thus,
in addition to studies on DRD2 and CSCs, further studies on the
relationship between DRD4 and CSCs are required to understand the
effect of dopamine receptors on the properties of CSCs. A previous
study reported that DRD4 inhibition could be an effective tool for
controling the properties of glioblastoma stem cells although it
was not conducted using prostate CSCs (26). Our ongoing study using
DRD4+/− or DRD4−/− is expected to provide a
clearer understanding of the role of DRD4 in PC-3-derived CSC-like
cells. The in vivo study that examined the antitumor effect
of L-741,626, which exhibits stronger in vitro effects than
PD 168568, indicated that a specific DRD2 antagonist could be used
as an antiprostate cancer agent. However, even if a safe and
effective DRD2 antagonist against CPRC is discovered, combination
therapy with conventional therapeutic agents, such as cytotoxic and
androgen-targeted agents, is required because a tumor contains a
heterogeneous population of differentiated carcinomas in addition
to CSCs (11).
Five different types of siRNA specific to each DR
subtype were used to confirm whether the effects of specific
antagonists against the subtypes effectively downregulated the
expression of the mRNA corresponding to each DR subtype. The
downregulation of the mRNA of the DR subtypes produced opposite
effects to those expected from the intrinsic functions of the DR
subtypes on adenylyl cyclase (23).
While the downregulation of DRD2, DRD3, and DRD4 (Gi
protein coupled receptors) appeared to inhibit adenylyl cyclase in
the cells, that of DRD1 and DRD5 (Gs protein coupled
receptors) appeared to cause the cells to lose their ability to
activate adenylyl cyclase. These experiments were designed to
validate whether the downregulation of DR subtypes caused by siRNA
transfected into cells was effective. However, further studies are
warranted to determine whether fluctuations in cAMP concentrations
induced by DRD2 and DRD4 inhibition are related to changes in the
properties of PC-3-derived CSC-like cells. Nevertheless, obtaining
an answer is difficult because DRD3 downregulation did not cause
clear changes in the morphology, growth, and invasion ability of
PC-3-derived CSC-like cells, although it induced an increase in the
cAMP concentration.
As shown in our previous study (17), which revealed the inhibition of AMPK
phosphorylation by thioridazine in PC-3-derived CSC-like cells,
siRNA specific for DRD2 substantially inhibited AMPK
phosphorylation in PC-3-derived CSC-like cells, further confirming
that DRD2 inhibition leads to the loss of PC-3-derived CSC-like
cell properties via AMPK inhibition. To determine whether the lack
of DRD2 itself affects the formation of CSC-like cells and the
regulation of AMPK phosphorylation, we attempted to prepare DRD2
homozygous null (DRD2−/−) PC-3 cells without success.
Complete DRD2 knockout appeared to cause the DRD2−/−
cells to lose their proliferative ability, making the cloning of
DRD2−/− cells impossible (Data not shown here, but our
ongoing study also failed to obtain DRD2−/− DU 145 human
prostate carcinomas). Although we cannot conclusively state that
the presence of DRD2 is essential for the proliferation or survival
of all types of carcinoma cells because we have not yet found other
studies that have prepared and used DRD2−/− carcinoma
cells, DRD2 appears to be important for the proliferation or
survival of certain types of prostate carcinoma cells. Thus,
instead of DRD2−/− cells, heterozygous null
(DRD2+/−) PC-3 cells were prepared and subjected to
further study. The reduced expression of DRD2 protein in
DRD2+/− PC-3 cells correlated well with reduced growth
rates, changes in morphology, and reduced sphere formation
capacity. The fact that A769662, an AMPK activator, partially
restored the growth of DRD2+/− PC-3 cells suggests that
the decreased AMPK activity induced by the heterozygous knockdown
of DRD2+/− PC-3 was partially restored by A769662.
Although the size and number of spheres formed from
DRD2+/− PC-3 cells were smaller and fewer than those
from DRD2+/+ PC-3 cells, respectively, we attempted to
prepare a monolayer culture of CSC-like cells using
DRD2+/− spheres to investigate the effects of reduced
DRD2 expression on signal transduction in PC-3-derived CSC-like
cells. However, this approach was unsuccessful because the growth
rate of single cells prepared from the DRD2+/− spheres
was too low to form a monolayer culture. Thus, we used
DRD2+/− PC-3 cells instead of using DRD2+/−
CSC-like cells to examine changes in signal transduction induced by
reduced DRD2 expression. The formation of spheroid carcinoma
cultures is a well-known method for enriching CSCs in vitro
(27). Accordingly, the fact that
DR+/− PC-3 cells exhibited a reduced ability to form
spheroid cultures and that preparing a monolayer culture of
CSC-like cells from DRD2+/− spheres was almost
impossible indicate that the intact presence of DRD2 is crucial
during the conversion process of parental PC-3 cells to CSC-like
cells.
To determine whether a signal transduction molecule
was affected by DRD2 suppression, changes in the phosphorylation of
several signal transduction molecules, including AMPK, whose
phosphorylation was decreased by siRNA specific for DRD2 in
PC-3-derived CSC-like cells, were examined in DRD2+/−
PC-3 cells. Consistent with the results obtained using siRNA
specific for DRD2, the reduction in DRD2 expression in
DRD2+/− PC-3 cells resulted in a drastic decrease in
AMPK phosphorylation, further indicating that the inhibition of
AMPK phosphorylation interferes with the maintenance of the intact
properties of PC-3-derived CSC-like cells. The role of AMPK has
been reported in various cancer types. However, it is difficult to
define its role in one sentence because activated AMPK suppresses
or activates cancer or CSCs depending on the cancer type or stage
(28–30). A review on the role of AMPK in
advanced stages of prostate cancer supported the hypothesis that a
complex of activated AMPK and pyruvate kinase 2 (PKM2) participates
in the upregulation of cancer stemness genes by Oct4 (30), supporting the results of this study.
In addition, another of our previous studies showed that AMPK
suppression in PC-3-derived CSC-like cells and various cancer types
using AMPK2a siRNA caused a loss of the properties of these cells
(31).
Overall, DRD2 inhibition with a specific antagonist,
suppression of DRD2 expression by DRD2 siRNA, or the heterozygous
knockout of DRD2 causes PC-3-derived CSC-like cells to lose their
properties and inhibits the formation of PC-3-derived CSC-like
cells, followed by the inhibition of the phosphorylation of AMPK, a
putative downstream signaling molecule of DRD2. Finding ways to
effectively modulate the interrelation between DRD2 and AMPK in
PC-3-derived CSC-like cells will provide an opportunity to identify
new drug targets that can be useful for treating at least some
types of incurable prostate cancer wherein AMPK is constitutively
or highly activated.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by grants [grant nos.
NRF-2019R1F1A1061276 and NRF-2022R1A2C1012921 (to SKP) and grant
no. NRF-2019R1A6A1A03031807 (to YB)] from the National Research
Foundation (NRF) of the Republic of Korea and a Korea University
Grant (to SKP).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JP, HJJ and WKJ contributed to study conception and
performed the experiments of immunocytochemistry, siRNA
transfection, in vitro cell invasion assay, preparation of
DRD2 heterozygous knockout PC-3 cells, cAMP assay, RT-PCR and tumor
xenograft assay. DYK performed western blotting. YLG and HJK
performed cell growth assay and immunocyochemistry experiments. JSK
and JWY contributed to the design of tumor xenograft assays and
cellular signaling studies, and confirmed the authenticity of all
the raw data. YB and SKP designed the study and wrote the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The Korea University IACUC (protocol number:
KUIACUC-2021-0028; Seoul, Republic of Korea) approved the animal
experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Komura K, Sweeney CJ, Inamoto T, Ibuki N,
Azuma H and Kantoff PW: Current treatment strategies for advanced
prostate cancer. Int J Urol. 25:220–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sekhoacha M, Riet K, Motloung P, Gumenku
L, Adegoke A and Mashele S: Prostate cancer review: Genetics,
diagnosis, treatment options, and alternative approaches.
Molecules. 27:57302022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richardson GD, Robson CN, Lang SH, Neal
DE, Maitland NJ and Collins AT: CD133, a novel marker for human
prostatic epithelial stem cells. J Cell Sci. 117:3539–3545. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verma P, Shukla N, Kumari S, Ansari MS,
Gautam NK and Patel GK: Cancer stem cell in prostate cancer
progression, metastasis and therapy resistance. Biochim Biophys
Acta Rev Cancer. 1878:1888872023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gogola S, Rejzer M, Bahmad HF, Alloush F,
Omarzai Y and Poppiti R: Anti-cancer stem-cell-targeted therapies
in prostate cancer. Cancers (Basel). 15:16212023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Thé H: Differentiation therapy
revisited. Nat Rev Cancer. 18:117–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enane FO, Saunthararajah Y and Korc M:
Differentiation therapy and the mechanisms that terminate cancer
cell proliferation without harming normal cells. Cell Death Dis.
9:9122018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sachlos E, Risueño RM, Laronde S,
Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn
A, Graham M, et al: Identification of drugs including a dopamine
receptor antagonist that selectively target cancer stem cells.
Cell. 149:1284–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rane JK, Pellacani D and Maitland NJ:
Advanced prostate cancer-a case for adjuvant differentiation
therapy. Nat Rev Urol. 9:595–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pérez G, López-Moncada F, Indo S, Torres
MJ, Castellón EA and Contreras HR: Knockdown of ZEB1 reverses
cancer stem cell properties in prostate cancer cells. Oncol Rep.
45:582021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee Y, Yoon J, Ko D, Yu M, Lee S and Kim
S: TMPRSS4 promotes cancer stem-like properties in prostate cancer
cells through upregulation of SOX2 by SLUG and TWIST1. J Exp Clin
Cancer Res. 40:3722021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang N, Ke B, Hjort-Jensen K,
Iglesias-Gato D, Wang Z, Chang P, Zhao Y, Niu X, Wu T, Peng B, et
al: YAP1 regulates prostate cancer stem cell-like characteristics
to promote castration resistant growth. Oncotarget.
8:115054–115067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roney MSI and Park SK: Antipsychotic
dopamine receptor antagonists, cancer, and cancer stem cells. Arch
Pharm Res. 41:384–408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosas-Cruz A, Salinas-Jazmín N and
Velázquez MAV: Dopamine receptors in cancer: Are they valid
therapeutic targets? Technol Cancer Res Treat.
20:153303382110279132021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SI, Roney MSI, Park JH, Baek JY, Park
J, Kim SK and Park SK: Dopamine receptor antagonists induce
differentiation of PC-3 human prostate cancer cell-derived cancer
stem cell-like cells. Prostate. 79:720–731. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hill SJ and Young M: Antagonism of central
histamine H1 receptors by antipsychotic drugs. Eur J Pharmacol.
52:397–399. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson DE, Nedza FM, Spracklin DK, Ward
KM, Schmidt AW, Iredale PA, Godek DM and Rollema H: The role of
muscarinic receptor antagonism in antipsychotic-induced hippocampal
acetylcholine release. Eur J Pharmacol. 506:209–219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richtand NM, Welge JA, Logue AD, Keck PE
Jr, Strakowski SM and McNamara RK: Dopamine and serotonin receptor
binding and antipsychotic efficacy. Neuropsychopharmacology.
32:1715–1726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung HS, Lee SI, Kang SH, Wang JS, Yang
EH, Jeon B, Myung J, Baek JY and Park SK: Monoclonal antibodies
against autocrine motility factor suppress gastric cancer. Oncol
Lett. 13:4925–4932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang MR, Park SK, Lee CW, Cho IJ, Jo YN,
Yang JW, Kim JA, Yun J, Lee KH, Kwon HJ, et al: Widdrol induces
apoptosis via activation of AMP-activated protein kinase in colon
cancer cells. Oncol Rep. 27:1407–1412. 2012.PubMed/NCBI
|
|
23
|
Beaulieu JM and Gainetdinov RR: The
physiology, signaling, and pharmacology of dopamine receptors.
Pharmacol Rev. 63:182–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prabhu VV, Madhukar NS, Gilvary C, Kline
CLB, Oster S, El-Deiry WS, Elemento O, Doherty F, VanEngelenburg A,
Durrant J, et al: Dopamine receptor D5 is a modulator of tumor
response to dopamine receptor D2 antagonism. Clin Cancer Res.
25:2305–2313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosas-Cruz A, Salinas-Jazmín N,
Valdés-Rives A and Velasco-Velázquez MA: DRD1 and DRD4 are
differentially expressed in breast tumors and breast cancer stem
cells: Pharmacological implications. Transl Cancer Res.
11:3941–3950. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dolma S, Selvadurai HJ, Lan X, Lee L,
Kushida M, Voisin V, Whetstone H, So M, Aviv T, Park N, et al:
Inhibition of dopamine receptor D4 impedes autophagic flux,
proliferation, and survival of glioblastoma stem cells. Cancer
Cell. 29:859–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bahmad HF, Cheaito K, Chalhoub RM, Hadadeh
O, Monzer A, Ballout F, El-Hajj A, Mukherji D, Liu YN, Daoud G and
Abou-Kheir W: Sphere-formation assay: Three-dimensional in vitro
culturing of prostate cancer stem/progenitor sphere-forming cells.
Front Oncol. 8:3472018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hardie DG: Molecular pathways: Is AMPK a
friend or a foe in cancer? Clin Cancer Res. 21:3836–3840. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonini MG and Gantner BN: The multifaceted
activities of AMPK in tumor progression-why the ‘one size fits all’
definition does not fit at all? IUBMB Life. 65:889–896. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gharibpoor F, Kamali Zonouzi S, Razi S and
Rezaei N: AMPK's double-faced role in advanced stages of prostate
cancer. Clin Transl Oncol. 24:2064–2073. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim TH, Park JH, Park J, Son DM, Baek JY,
Jang HJ, Jung WK, Byun Y, Kim SK and Park SK: Stereospecific
inhibition of AMPK by (R)-crizotinib induced changes to the
morphology and properties of cancer and cancer stem cell-like
cells. Eur J Pharmacol. 911:1745252021. View Article : Google Scholar : PubMed/NCBI
|