Introduction
Esophageal cancer is the eighth most common cancer
and sixth leading cause of mortality worldwide (1). Unresectable advanced or recurrent
esophageal cancer remains an intractable malignant disease, and
>10,000 people die annually from esophageal cancer in Japan
(2). In Japan, fluoropyrimidines
plus platinum-based chemotherapy was used as the first-line
therapy, followed by taxanes as the second-line therapy for
advanced esophageal cancer until the launch of immune checkpoint
inhibitors (ICIs). Nivolumab, an anti-programmed cell death protein
1 (PD-1) antibody that reverts the ability of T cells to recognize
and kill tumor cells, showed a survival benefit over taxane for
esophageal squamous cell cancer (ESCC) patients who were refractory
to fluoropyrimidines- and platinum-based chemotherapy in the phase
III ATTRACTION-3 trial (3). Based
on these results, nivolumab has been approved in Japan since
February 2020 for ESCC patients who have progressed after
chemotherapy.
The ATTRACTION-3 trial showed a promising efficacy
with acceptable toxicity profiles for nivolumab; however, this
study excluded patients with a poor Eastern Cooperative Oncology
Group performance status (ECOG-PS≥2) and consequently did not
include patients >70 years of age in the nivolumab group. A
small number of real-world data on nivolumab as second- or
further-line therapy has been reported (4); however, data on patients with
conditions that were excluded from the phase III ATTRACTION-3 trial
are still required. Thus, its efficacy and safety in these patients
are still unknown and worthy of further exploration in real-world
settings. Although ICIs, including nivolumab, demonstrate marked
and durable responses in some patients, they can occasionally cause
immune-related adverse events (irAEs), including fatalities.
Therefore, it is imperative to predict the efficacy of ICI therapy
before treatment initiation. Several reports suggest the usefulness
of biomarkers, such as PD-L1 expression with CD8+
tumor-infiltrating lymphocytes (5)
and inflammatory markers (CRP/albumin ratios (CAR)) (6), in predicting nivolumab efficacy in
ESCC; nonetheless, this information remains limited and lacking,
with no established biomarkers as of yet. Accumulating evidence has
shown that liver metastasis reduces the efficacy of ICI (7–11).
However, the potential value of liver metastasis as a predictor of
ICI efficacy for ESCC remains unclear. The identification of a
convenient and reliable biomarker for the ICI treatment of ESCC
remains a challenge.
Herein, we present the efficacy and safety of
nivolumab monotherapy as second or further-line therapy for ESCC
patients from a real-world data, with an analysis of the predictive
factors for treatment efficacy.
Patients and methods
Study design and patients
We conducted a retrospective analysis of consecutive
patients with unresectable advanced or recurrent esophageal cancer
treated with nivolumab as a second- or later-line treatment at the
Kyoto Prefectural University of Medicine between February 2020 and
December 2021. The data used in this study were extracted in
January 2022. All patients were pathologically confirmed to have
esophageal squamous or adenosquamous cell carcinoma and were
refractory or intolerant to at least one line of fluoropyrimidine-,
platinum-, or taxane-based chemotherapy. This study was approved by
the Medical Ethics Review Committee of Kyoto Prefectural University
of Medicine (approval no. ERB-E-42). All procedures were performed
in accordance with the ethical standards of the Medical Ethics
Review Committee of the Kyoto Prefectural University of Medicine
and the Declaration of Helsinki. An opt-out approach was employed
to obtain informed consent from patients, and personal information
was protected during data collection.
Treatment and evaluation
Intravenous nivolumab was administered at a dose of
240 mg every 2 weeks or 480 mg every 4 weeks. Treatment was
continued until disease progression, unacceptable toxicity, or
patient refusal. Information was collected from the electronic
medical records of the hospital. The data collected for this study
included sex, age, height, weight, ECOG PS, number and duration of
nivolumab administration, smoking and alcohol consumption,
histopathology, TNM at diagnosis, metastatic organ at diagnosis,
best response to primary therapy, overall survival (OS),
progression-free survival (PFS), blood test data (albumin, CRP,
neutrophils, lymphocytes), presence of surgery, presence of
radiation therapy, presence of treatment-related adverse events,
severity of treatment-related adverse events, and time to onset of
treatment-related adverse events. The overall response was
evaluated based on the Response Evaluation Criteria in Solid Tumors
version 1.1. The severity of adverse events was graded according to
CTCAE 4.0 criteria.
Statistical analysis
OS was defined as the time from the initiation of
nivolumab therapy until death by any cause or final follow-up. PFS
was defined as the time from the administration of the first dose
of nivolumab to disease progression or death. OS and PFS were
estimated using the Kaplan-Meier method. The log-rank test was used
to compare groups, and Cox regression models were used to calculate
hazard ratios (HR) and 95% confidence interval (95% CI). The
effects of liver metastases and possible factors, such as PS,
smoking status, and inflammatory markers (CAR,
neutrophil/lymphocyte ratio (NLR), and prognostic nutritional index
(PNI)), on PFS and OS were analyzed using COX regression models.
The cutoff values for NLR and PNI were determined based on previous
reports (12,13), and median value was employed for the
others.
PFS and OS were estimated with a 95% confidence
interval (CI) using the Kaplan–Meier method and compared using the
log-rank test. Cox regression models were used to calculate the
hazard ratio (HR) and 95% CI. Significant variables in the
univariate analysis, P<0.05 for PFS and P≤0.01 for OS, were
included in the multivariate analysis. All statistical tests were
two-sided, and P<0.05 was set as the level of significance.
Statistical analyses were performed using the JMP Pro 17 software
(SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The characteristics of the 42 enrolled patients are
summarized in Table I. The median
age was 70 years old, with 22 patients (52%) aged 70 years or
older, a demographic not represented in the ATTRACTION-3 trial. The
proportion of males was 85%. The patients had ECOG-PS scores of 0
(n=21), 1 (n=18), or 2 (n=3). Histopathological findings included
squamous cell carcinoma in 41 patients (97%) and adenosquamous cell
carcinoma in one patient (3%). Nivolumab was used in 16 (38%)
second-line cases and 26 (61%) third-line or later cases. All the
patients received at least one regimen of fluoropyrimidine-,
platinum-, or taxane-based chemotherapy. Twenty-eight (66%) and 19
(45%) patients underwent surgery and radiation therapy,
respectively, prior to treatment. Twenty-five patients (40%) had
metastases in more than two organs, with the most prevalent
metastatic organs being the lymph nodes (88%), lungs (35%), and
liver (28%), followed by the bone (11%). Except for two patients
(5%) who were non-smokers, 40 patients (95%) had a history of
smoking (at least once).
 | Table I.Characteristics of patients
(n=42). |
Table I.
Characteristics of patients
(n=42).
| Characteristics | n (%) |
|---|
| Agea, years |
|
|
<65 | 12 (28) |
| ≥65 | 30 (71) |
| of which
≥70 | 22 (52) |
| Sex |
|
| Male | 36 (85) |
|
Female | 6 (14) |
| ECOG Performance
status |
|
| 0 | 21 (50) |
| 1 | 18 (42) |
| 2 | 3 (7) |
| Pathological
tissue |
|
| Squamous
cell carcinoma | 41 (97) |
|
Adenosquamous cell
carcinoma | 1 (3) |
| Treatment lines |
|
| 2nd | 16 (38) |
| 3rd or
lator | 26 (61) |
| Previous
therapies |
|
|
Surgery | 28 (66) |
|
Radiotherapy | 19 (45) |
| Systemic
anticancer therapy | 42 (100) |
| Number of organs with
metastases |
|
|
<2 | 17 (59) |
| ≥2 | 25 (40) |
| Site of
metastases |
|
| Lymph
node | 37 (88) |
|
Liver | 12 (28) |
| Lung | 15 (35) |
| Bone | 5 (11) |
| History of
smoking |
|
|
Never | 2 (5) |
| Not
never | 40 (95) |
Efficacy of nivolumab
The median observation period after nivolumab
therapy was 7.9 months (0.6–23.8), and the median number of doses
was nine (1–53). As shown in
Table II, two patients (4%) had a
complete response; nine (21%), a partial response; 22 (52%), stable
disease; and nine (21%), progressive disease. Response and disease
control rates were 26 and 78%, respectively. Four patients (9%)
responded to nivolumab and continued treatment. The median PFS and
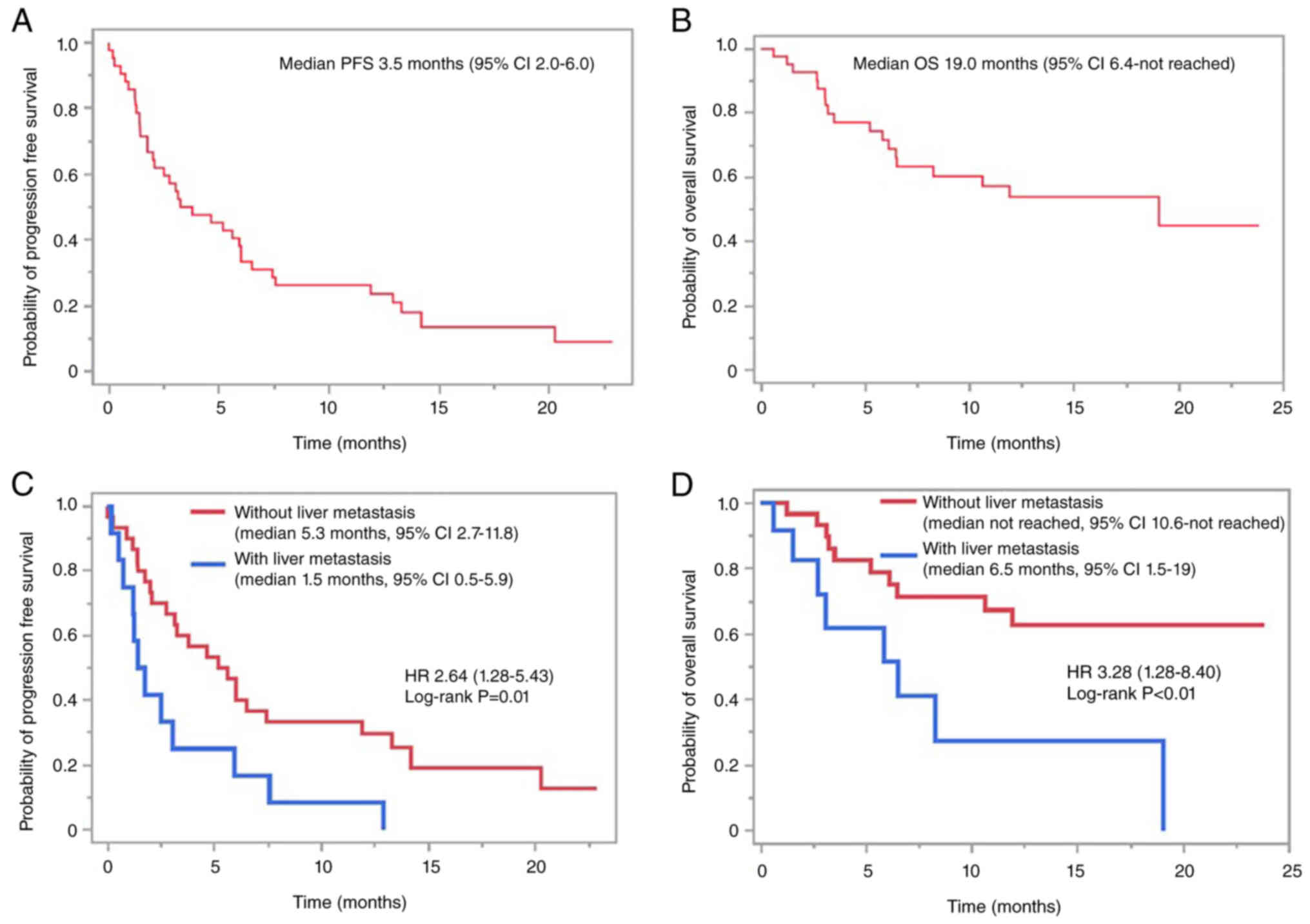
OS were 3.5 (95% CI 2.0–6.0, Fig.
1A) and 19 (95% CI 6.4-not reached, Fig. 1B) months, respectively. In a
comparison of PFS and OS based on liver metastatic status, PFS and
OS in patients without liver metastases were significantly better
than those with liver metastases (median PFS 5.3 vs. 1.5 months,
P=0.01, Fig. 1C; median OS not
reached vs. 6.5 months, P<0.01, Fig.
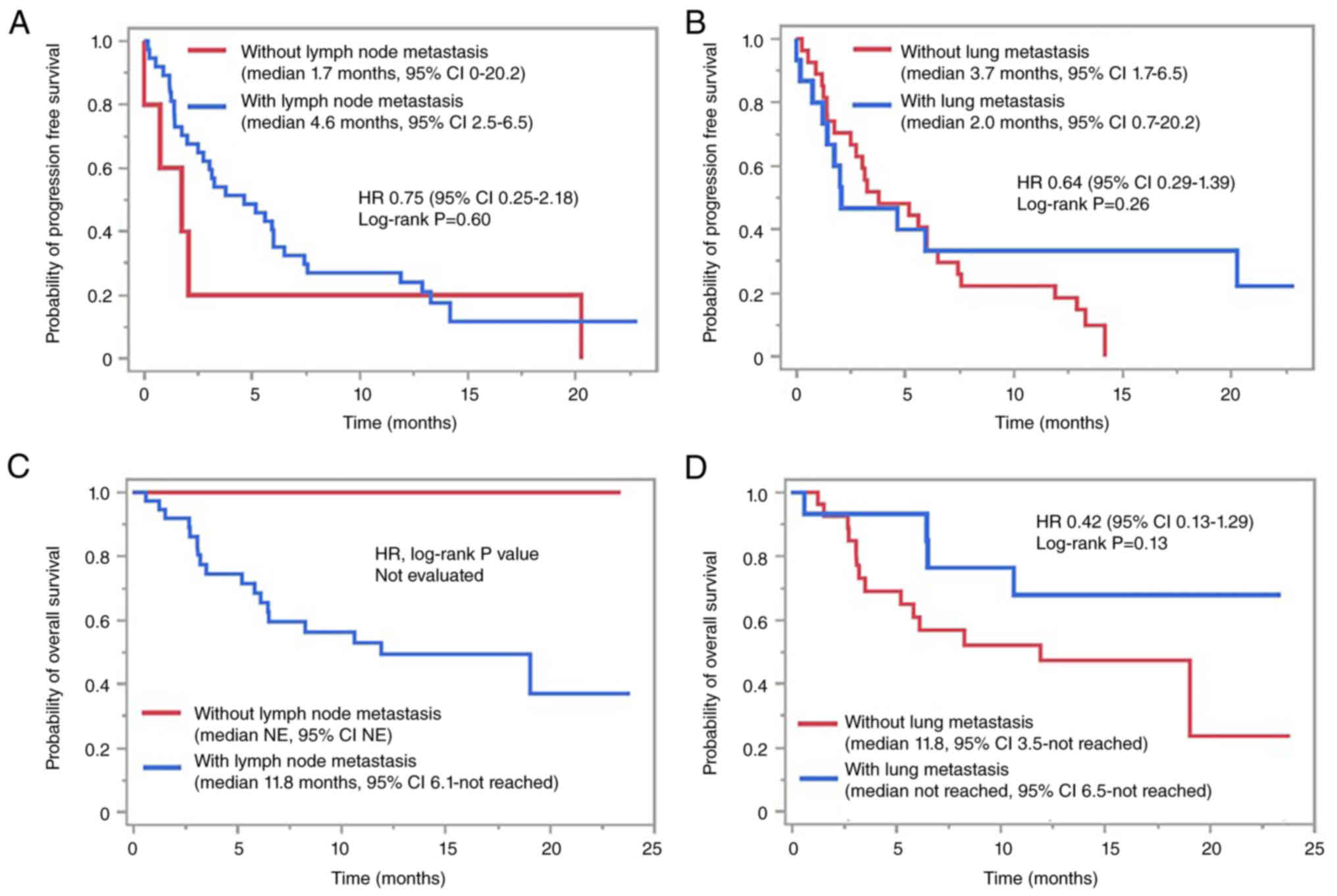
1D). In contrast, a comparison based on lymph node and lung
metastasis status showed no significant differences in PFS and OS
depending on the metastatic status, although there was a trend
toward longer OS in patients without lymph node metastasis
(Fig. 2).
 | Table II.Treatment outcome (n=42). |
Table II.
Treatment outcome (n=42).
| Outcome | N |
|---|
| Doses of nivolumab
administered, n (range) | 9 (1–53) |
| Best overall
response, n (%) |
|
| Complete
response | 2 (4) |
| Partial
response | 9 (21) |
| Stable
disease | 22 (52) |
|
Progressive disease | 9 (21) |
| Not
evaluated | 0 |
| Disease control, n
(%) | 33 (78) |
| Response, n (%) | 11 (26) |
Predictive role of liver metastasis
and the other possible predictive factors
Factors, including liver metastatic status and
inflammatory markers, that may contribute to the PFS and OS of
nivolumab were analyzed. The following factors were included in the
analysis: age, ECOG-PS, smoking history, alcohol consumption, NLR,
CAR, hemoglobin level, PNI, and metastatic status. As shown in
Table III, the PFS was
significantly worse in patients with PS≥2 (HR 7.23, 95%CI
1.87–27.95, P=0.004), history of no smoking (HR 7.20, 95%CI
1.54–33.48, P=0.01), NLR≥4 (HR 3.33, 95%CI 1.65–6.73, P=0.0008),
bone metastasis (HR 3.63, 95%CI 1.29–10.2, P=0.01), and liver
metastasis (HR 2.64, 95%CI 1.28–5.43, P=0.008) in the univariate
analyses. In the multivariate analyses, liver metastasis (HR 2.37,
95%CI 1.07–5.24, P=0.03), history of no smoking (HR 5.81, 95%CI
1.07–31.35, P=0.04), and NLR≥4 (HR 2.53, 95%CI 1.21–5.76, P=0.02)
were shown to be possible worse predictive factors for PFS.
Table IV shows the univariate and
multivariate analyses of OS. In the univariate analyses, PS, NLR,
PNI, and liver metastasis were predictive factors. Because of the
small number of events occurring in the analysis of OS, fewer than
two explanatory variables in the multivariate analysis was
considered desirable; however, given the importance of liver
metastasis in previous reports (7–11), a
multivariate analysis was performed using three explanatory
variables including liver metastasis. Multivariate analysis
revealed that liver metastasis (HR 2.75, 95%CI 1.00–7.53, P=0.04)
and NLR≥4 (HR 3.15, 95%CI 1.17–8.49, P=0.02) were statistically
associated with worse OS.
 | Table III.Univariate and multivariate analysis
for PFS. |
Table III.
Univariate and multivariate analysis
for PFS.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Categories | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | ≥65 vs. <65 | 1.22
(0.58–2.58) | 0.59 |
|
|
| ECOG PS | ≥2 vs. <2 | 7.23
(1.87–27.95) | <0.01 | 2.62
(0.42–16.03) | 0.29 |
| Smoking | Never vs. Current
or past | 7.20
(1.54–33.48) | 0.01 | 5.81
(1.07–31.35) | 0.04 |
| Alcohol
drinking | Yes vs. No | 0.55
(0.07–4.15) | 0.56 |
|
|
| NLR | ≥4 vs. <4 | 3.33
(1.65–6.73) | <0.01 | 2.53
(1.21–5.76) | 0.02 |
| PNIa | ≥40 vs. <40 | 0.58
(0.30–1.15) | 0.12 |
|
|
| CAR | ≥0.30 vs.
<0.30 | 1.19
(0.48–2.93) | 0.70 |
|
|
| Lung
metastasis | Yes vs. No | 0.64
(0.29–1.39) | 0.26 |
|
|
| Lymph node
metastasis | Yes vs. No | 0.75
(0.25–2.18) | 0.60 |
|
|
| Bone
metastasis | Yes vs. No | 3.63
(1.29–10.2) | 0.01 | 1.27
(0.28–5.65) | 0.75 |
| Liver
metastasis | Yes vs. No | 2.64
(1.28–5.43) | <0.01 | 2.37
(1.07–5.24) | 0.03 |
 | Table IV.Univariate and multivariate analysis
for OS. |
Table IV.
Univariate and multivariate analysis
for OS.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Categories | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | ≥65 vs. <65 | 1.57
(0.51–4.79) | 0.42 |
|
|
| ECOG PS | ≥2 vs. <2 | 12.68
(2.07–77.42) | <0.01 | 5.39
(0.82–35.34) | 0.07 |
| Smoking | Never vs. Current
or past | 3.88
(0.87–17.15) | 0.07 |
|
|
| Alcohol
drinking | Yes vs. No | 0.13
(0.01–1.17) | 0.06 |
|
|
| NLR | ≥4 vs. <4 | 3.56
(1.38–9.13) | <0.01 | 3.15
(1.17–8.49) | 0.02 |
| PNIa | ≥40 vs. <40 | 0.36
(0.13–0.94) | 0.03 |
|
|
| CAR | ≥0.30 vs.
<0.30 | 0.82
(0.32–2.11) | 0.68 |
|
|
| Lung
metastasis | Yes vs. No | 0.42
(0.13–1.29) | 0.13 |
|
|
| Lymphnode
metastasis | Yes vs. No | Unreached | - |
|
|
| Bone
metastasis | Yes vs. No | 3.90
(0.77–19.77) | 0.09 |
|
|
| Liver
metastasis | Yes vs. No | 3.28
(1.28–8.40) | 0.01 | 2.75
(1.00–7.53) | 0.04 |
Frequency of immune-related adverse
events
IrAEs occurred in 12 of 42 patients (28%) of all
grades, with three (7%) patients experiencing grade 3 or higher
irAEs. Treatment discontinuation was required in three patients
(7%) who developed grade 3 pneumonitis (n=2) and thyroiditis (n=1).
No adverse events resulted in mortality. The most common irAEs were
thyroiditis and rash in four patients (9%), followed by diarrhea,
pneumonia, adrenal insufficiency, and pituitaryitis (Table V).
 | Table V.Characteristics of immuno-related
adverse events (n=42). |
Table V.
Characteristics of immuno-related
adverse events (n=42).
|
Characteristics | All Grade, n
(%) | Grade 3/4, n
(%) |
|---|
| All events | 12 (28) | 3 (7) |
| Events leading to
discontinuation | 3 (7) | 3 (7) |
| Events leading to
death | 0 | 0 |
| Event details |
|
|
| Rash | 4 (9) | 0 |
| Diarrhea | 2 (4) | 0 |
| Fatigue | 0 | 0 |
| Thyroiditis | 4 (9) | 1 (2) |
| Pituitaryitis | 1 (2) | 0 |
| Interstitial
pneumonia | 2 (4) | 2 (4) |
| Adrenal
insufficiency | 1 (2) | 0 |
Discussion
In the present study, the clinical efficacy and
safety of nivolumab as second- or third-line treatments for ESCC
were confirmed using real-world data. Notably, both treatment
efficacy and safety profiles were comparable to or better than
those of the phase 3 ATTRACTION-3 trial, despite the fact that our
study included a relatively large number of patients with poor PS
and aged >70 years, a demographic not included in the
ATTRACTION-3 trial. Furthermore, analysis of the predictors of PFS
and OS demonstrated that the presence of liver metastases is a
possible predictor of worse PFS and OS. Although there have been
several reports on the possibility that liver metastases may
attenuate the effects of ICIs (7–11),
this is the first report on ESCC to clearly demonstrate this
fact.
This study included patients over 70 years of age
(22 cases, 52%) and patients with PS2 (3 cases, 7%), both of which
were not included in the ATTRACTION-3 trial; however, the treatment
efficacy was rather favorable compared to that of the ATTRACTION-3
trial (overall response rate (ORR), 26% vs. 19%; disease control
rate (DCR) 78% vs. 37%; median PFS 3.5 vs. 1.7 months; median OS
19.0 vs. 10.9 months). Comparing the patient profile to that of the
ATTRACTION-3 trial, this study included fewer non-smokers (5% vs.
14%) and more patients over 65 years of age (71% vs. 47%). There
were no marked differences in the proportion of male patients,
number of metastatic organs, sites of metastasis, or prior
treatment. Smoking history has been reported to affect the efficacy
of ICI in non-small cell lung carcinoma (14–16),
and it is possible that the small number of non-smokers in this
study contributed to this favorable result. Since this was a
retrospective study with a small number of patients, coupled with
unknown PD-L1 status in most cases, discussing the reasons for more
favorable results than in the ATTRACTION-3 trial is constrained by
these limitations. Notably, the findings of this study, drawn from
real-world data that included a larger proportion of patients in
relatively poor condition, were comparable to or better than those
of the ATTRACTION-3 trial. Due to the small number of cases in this
study, we did not explore the difference in efficacy of nivolumab
by the treatment line (2nd vs. 3rd line or later), which should be
explored in future studies with larger sample sizes.
IrAEs were observed in 12 cases (28%), most of which
were mild. Grade 3 or higher irAEs were observed in 7% of patients,
and there were no treatment-related deaths. In patients aged
>70, five cases (22.7%) had irAEs of any grade, indicating no
increase in irAEs compared to younger patients. Only one case had a
grade 3 or higher adverse event (AE) (4%, interstitial pneumonia).
Although the retrospective nature of the study may have resulted in
an underestimation in assessing AEs, especially the mild ones, we
believe that this study provided evidence of the safety of this
treatment in real-world clinical practice.
We also examined the predictors of nivolumab
monotherapy. In the present study, no smoking history, liver
metastasis and high NLR (≥4) were independent poor predictive
factors for PFS and NLR and liver metastasis were the same for OS.
The confidence of the results for OS, however, needs to be
evaluated with caution due to the relatively small number of events
in this study. In addition to our results, numerous reports in
non-small cell lung have suggested that smokers treated with ICIs
have a better prognosis because of their stronger immunogenicity
and activated immune microenvironment (14–16).
NLR has the advantage of being easily derived from routine blood
tests and has proven to be useful as an indicator of systemic
immune status (17). Consistent
with our findings, several reports have shown that NLR has the
potential to be a predictive indicator for patients receiving ICIs
for various cancers (18–20). Patients with liver metastases had
significantly worse PFS and OS, whereas lung and lymph node
metastases showed no clear impact on the efficacy of nivolumab.
Furthermore, univariate and multivariate analyses revealed that
liver metastasis was an independent predictor of nivolumab
monotherapy. Consistent with our findings, Yu et al.
reported that liver metastases diminished immunotherapy efficacy in
patients and preclinical models (7). They demonstrated that the
effectiveness of ICIs was attenuated in melanoma and lung cancer
patients with liver metastases, contrasting with molecularly
targeted therapy or cytotoxic chemotherapy. They also demonstrated
that, in a preclinical model, liver metastases induced systemic
tumor-specific CD8+ T cell loss through apoptosis following their
interaction with activated monocyte-derived macrophages, thereby
reducing ICI efficacy. There have been several other reports on the
mechanisms of liver metastasis-induced reduction in the efficacy of
ICI. Liver metastases cause the loss of tumor-specific effector T
cells and loss of function in activated Treg cells and/or liver
macrophages (10,11). Recently, it was reported that in the
1st line treatment for ESCC, liver metastases patients
tended to develop progression disease early after the initiation of
nivolumab plus ipilimumab combination therapy (21). In gastric cancer, hyper progression
after ICI therapy is reported to be significantly more common in
cases with liver metastasis (8).
Therefore, the risk of early disease progression should be also
considered when using nivolumab in esophageal cancer patients with
liver metastases. Taken together, liver metastasis is a potential
biomarker for nivolumab treatment in ESCC. These results should be
confirmed in future prospective studies owing to the inherent
potential biases of our analyses.
This study had several limitations. The main
limitation was that this was a retrospective study with a
relatively small sample size and was conducted at a single center.
Approximately six (14%) patients were currently undergoing
treatment, and 17 (40%) patients were being followed up in the
event of death; therefore, the OS data may be immature.
Furthermore, most cases in this study could not be evaluated for
PD-L1 expression because nivolumab was approved for use regardless
of PD-L1 expression.
In conclusion, this study provided real-world data
on patients with diverse profiles, such as elderly patients and
those with poor PS, unlike the phase 3 ATTRACTION-3 trial, in which
nivolumab provided favorable efficacy with no increase in serious
irAEs. In this study, liver metastases and NLR were identified as
potential predictive biomarkers of nivolumab efficacy in ESCC.
Further studies with larger numbers of patients are warranted to
validate these findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RM and TI confirm the authenticity of all the raw
data. RM, TI, TD, JI, DS, NI, KI, HirK, OD, NY, AS, KU, TT, HF,
HidK and YI contributed to the study conception and design. RM, TI
and TD designed this study. RM contributed to data collection. RM
and TI analyzed the data. TD, JI, DS, NI, KI, HirK, OD, NY, AS, KU,
TT, HF, HidK and YI supervised the study. RM and TI wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Kyoto Prefectural University of Medicine (approval no. ERB-E-42)
and conducted following the declaration of Helsinki principles and
its amendment. All authors read the final manuscript and approved
it for publication. An opt-out approach was employed to obtain
informed consent from patients, and personal information was
protected during data collection.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato K, Cho BC, Takahashi M, Okada M, Lin
CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al:
Nivolumab versus chemotherapy in patients with advanced oesophageal
squamous cell carcinoma refractory or intolerant to previous
chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:1506–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim JH, Ahn B, Hong SM, Jung HY, Kim DH,
Choi KD, Ahn JY, Lee JH, Na HK, Kim JH, et al: Real-world efficacy
data and predictive clinical parameters for treatment outcomes in
advanced esophageal squamous cell carcinoma treated with immune
checkpoint inhibitors. Cancer Res Treat. 54:505–516. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farhood B, Najafi M and Mortezaee K: CD8+
cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell
Physiol. 234:8509–8521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikoma T, Shimokawa M, Matsumoto T, Boku S,
Yasuda T, Shibata N, Kurioka Y, Takatani M, Nobuhisa T, Namikawa T,
et al: Inflammatory prognostic factors in advanced or recurrent
esophageal squamous cell carcinoma treated with nivolumab. Cancer
Immunol Immunother. 72:427–435. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ and Lang X: Liver metastasis
restrains immunotherapy efficacy via macrophage-mediated T cell
elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasaki A, Nakamura Y, Mishima S, Kawazoe
A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, et al:
Predictive factors for hyperprogressive disease during nivolumab as
anti-PD1 treatment in patients with advanced gastric cancer.
Gastric Cancer. 22:793–802. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA,
Atkins MB and Leming PD: Five-year survival and correlates among
patients with advanced melanoma, renal cell carcinoma, or non-small
cell lung cancer treated with nivolumab. JAMA Oncol. 5:1411–1420.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JC, Mehdizadeh S, Smith J, Young A,
Mufazalov IA, Mowery CT, Daud A and Bluestone JA: Regulatory T cell
control of systemic immunity and immunotherapy response in liver
metastasis. Sci Immunol. 5:eaba07592020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumagai S, Koyama S, Itahashi K,
Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono
H, et al: Lactic acid promotes PD-1 expression in regulatory T
cells in highly glycolytic tumor microenvironments. Cancer Cell.
40:201–218.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu
B and Wang W: Prognostic nutritional index as a prognostic
biomarker for gastrointestinal cancer patients treated with immune
checkpoint inhibitors. Front Immunol. 14:12199292023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakahama K, Izumi M, Yoshimoto N, Fukui M,
Sugimoto A, Nagamine H, Ogawa K, Sawa K, Tani Y, Kaneda H, et al:
Influence of smoking history on the effectiveness of
immune-checkpoint inhibitor therapy for non-small cell lung cancer:
Analysis of real-world data. Anticancer Res. 43:2185–2197. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Yang Q, Shen J, Wei T, Shen W,
Zhang N, Luo P and Zhang J: The effect of smoking on the immune
microenvironment and immunogenicity and its relationship with the
prognosis of immune checkpoint inhibitors in non-small cell lung
cancer. Front Cell Dev Biol. 9:7458592021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Ricciuti B, Alessi JV, Nguyen T,
Awad MM, Lin X, Johnson BE and Christiani DC: Smoking history as a
potential predictor of immune checkpoint inhibitor efficacy in
metastatic non-small cell lung cancer. J Natl Cancer Inst.
113:1761–1769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giustini N and Bazhenova L: Recognizing
prognostic and predictive biomarkers in the treatment of non-small
cell lung cancer (NSCLC) with immune checkpoint inhibitors (ICIs).
Lung Cancer (Auckl). 12:21–34. 2021.PubMed/NCBI
|
|
18
|
Sacdalan DB, Lucero JA and Sacdalan DL:
Prognostic utility of baseline neutrophil-to-lymphocyte ratio in
patients receiving immune checkpoint inhibitors: A review and
meta-analysis. Onco Targets Ther. 11:955–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie X, Liu J, Yang H, Chen H, Zhou S, Lin
H, Liao Z, Ding Y, Ling L and Wang X: Prognostic value of baseline
neutrophil-to-lymphocyte ratio in outcome of immune checkpoint
inhibitors. Cancer Invest. 37:265–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muhammed A, Fulgenzi CAM, Dharmapuri S,
Pinter M, Balcar L, Scheiner B, Marron TU, Jun T, Saeed A,
Hildebrand H, et al: The systemic inflammatory response identifies
patients with adverse clinical outcome from immunotherapy in
hepatocellular carcinoma. Cancers (Basel). 14:1862021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chau I, Ajani JA, Doki Y, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu C, Adenis A, et al: Nivolumab
(NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) versus chemo
as first-line (1L) treatment for advanced esophageal squamous cell
carcinoma (ESCC): Expanded efficacy and safety analyses from
CheckMate 648. J Clin Oncol. 40 (Suppl 16):S4035. 2022. View Article : Google Scholar
|