Introduction
Glioblastoma multiforme (GBM), the most common primary brain tumor in adults, accounted for approximately one-half of all central nervous system (CNS) cancers according to data from the Central Brain Tumor Registry of the United States for the period between 2012 and 2016 (1). Despite aggressive initial treatment strategies, such as complete surgical removal and chemoradiotherapy, adult GBM often recurs shortly after initial treatment, indicating its high propensity for relapse (2). GBM has been identified as the most common and malignant type of glial tumor [World Health Organization (WHO) grade IV astrocytoma] (3) in the United States, with an annual incidence rate of ~3 per 100,000 individuals (4,5). Several studies have demonstrated that GBM is more likely to be located in the frontal lobe and insula, and patients with GBM have a worse prognosis in terms of progression-free survival (PFS) when the tumor is located on the left side of the brain (6–8). Additionally, predicting the location of the tumor and its likelihood to spread can provide preliminary information about the prognosis of the disease.
Known for its aggressive behavior, GBM is characterized by rapid proliferation, extensive invasion of adjacent brain tissue, molecular variability, resistance to standard treatments and difficulty in delivering effective chemotherapy to the CNS (9). Despite the use of aggressive therapeutic approaches such as surgical resection followed by chemotherapy and radiotherapy, the prognosis of GBM remains poor, with a median survival of ~15 months after diagnosis and a 5-year survival rate of <10% (10,11).
Cognitive decline is a well-documented consequence of GBM (12,13). While the physical effects of tumor expansion, such as mechanical dysfunction and brain compression, are recognized contributors, the specific mechanisms driving this cognitive impairment, other than the effects of brain radiotherapy, chemotherapy and neuroinflammation, remain largely unidentified. GBM involves complex cellular and molecular interactions that contribute to brain dysfunction, since glioblastoma cells are capable of secreting neurotoxic and immunomodulatory factors that affect nearby neural tissues (14). These factors may exert their effects directly through paracrine signaling or indirectly through exosomes released by the cancer cells, and are likely to be a major factor in the neurotoxic processes leading to cognitive decline in affected individuals (14).
Previous research suggests a marked interplay between immunotherapy and anti-angiogenic treatments across various cancer types, including GBM (15). Understanding this interaction could lead to the development of novel and more effective therapy combinations. The tumor microenvironment (TME) has been identified as an obstacle that diminishes the efficacy of immune checkpoint inhibitors (ICIs) (16–18). Within the TME, the interaction between cancer-promoting immune cells and tumor blood vessels creates a harmful cycle that hampers antitumor immunity and promotes cancer growth (19–21).
During tumor-induced angiogenesis, the newly formed vasculature has an abnormal structure that lacks the specific barrier function of normal blood vessels of the blood-brain barrier (BBB) (22). This effect is stronger in high-grade gliomas, which almost completely lack the BBB barrier function, and weaker in diffuse and low-grade gliomas (23). All gliomas, including glioblastomas, have areas of intact BBB, especially in the tumor periphery, which is one of the main obstacles to their response to drug therapies (22). The formation of abnormal tumor vessels enables tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) to evade detection by adopting an immune-suppressive phenotype within the TME. TAMs release factors like VEGF, IL-10, and TGF-β to suppress antitumor immune responses and promote angiogenesis, while MDSCs inhibit T-cell activation and recruit additional suppressive immune cells (22,24).
This interaction between the immune system and vasculature not only establishes a barrier preventing T cell infiltration into the tumor but also impairs T cell function and may lead to T cell death within the TME (25–27). Therefore, targeting the tumor vasculature is emerging as a promising strategy to enhance antitumor immunity and overcome ICI resistance. The present review examines the interaction between immune and anti-angiogenic therapies in the treatment of GBM. It demonstrates how the sequential use of immunotherapy and bevacizumab therapy could induce marked changes in the tumor vasculature, highlighting the reciprocal regulation of blood vessels and immune cells within the TME and supporting a combined immunotherapy approach that simultaneously targets the tumor vasculature and immune defenses.
Case report
Case 1
A 45-year-old female patient with a hypervascular mass lesion (Fig. 1A), located close to the right parietal vertex, underwent surgery at The Department of Neurosurgery, Hacettepe University Faculty of Medicine (Ankara, Turkey), in September 2018 (Fig. 1A and B), and the pathology indicated glioblastoma, isocitrate dehydrogenase (IDH) wild-type (GBM). Immunohistochemical analysis was performed on paraffin-embedded tissue samples using primary antibodies against ATRX, IDH1, glial fibrillary acidic protein (GFAP), Ki67 and p53. The Ki-67 proliferation index was 30–35%, ATRX expression was preserved, GFAP staining was positive and p53 was overexpressed in 80% of the tumor cells. The patient received 60 Gy intensity-modulated radiotherapy simultaneously with temozolomide, and then adjuvant temozolomide treatment was initiated. The patient completed the first course of temozolomide treatment in December 2018. Progression was detected on the magnetic resonance imaging (MRI) captured afterwards (Fig. 1C), and the patient underwent surgery again in January 2019 (Fig. 1D). The pathology was compatible with GBM, and postoperative changes and a residual mass were revealed on the postoperative MRI. The first course of treatment with nivolumab (200 mg every 2 weeks) was started in January 2019. After the second course of nivolumab, spinal MRI was performed because the patients' lower back pain was severe. After a collapse fracture was detected in multiple-level vertebrae, in February 2019, L2-L4 vertebroplasty surgery was performed. The third cycle of nivolumab began in April 2019. The treatment of the patient was discontinued after the third cycle due to severe pneumonitis. In June 2019, a cranial MRI (Fig. 1E) showed an increase in the size of the right parietal lesion, perilesional edema with heterogeneous contrast enhancement and a lack of increased perfusion on the cerebral blood volume map, indicative of pseudoprogression. These findings extended to the right parietal periventricular area and involved the corpus callosum splenium.
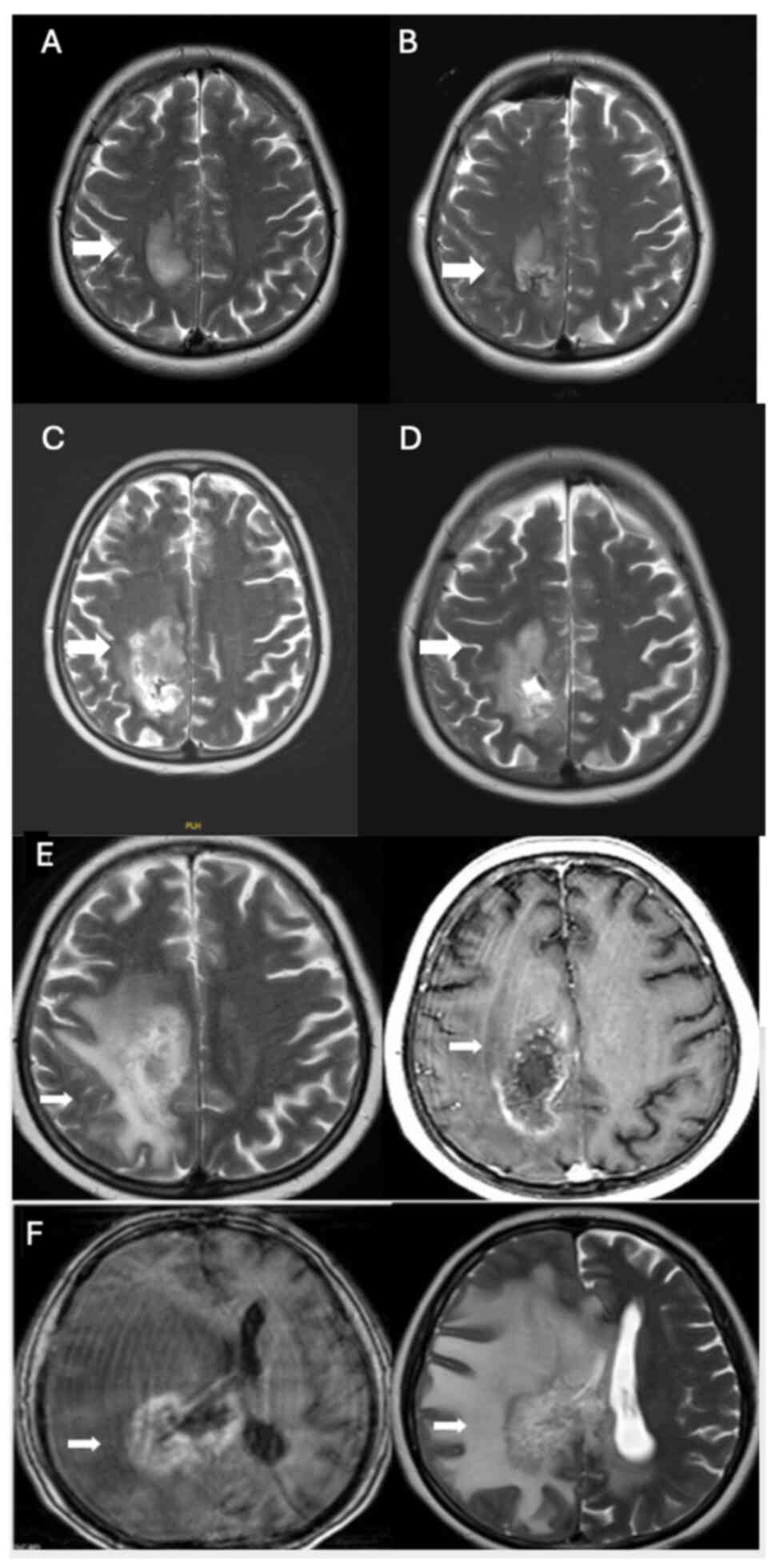 |
Figure 1.
(A) In September 2018, before surgery, a hyperintense expansile lesion with prominent enhancement was observed in the right paracentral lobule on axial T2-weighted imaging and post-contrast axial T1-weighted imaging (white arrows). (B) Postoperative MRI after the first surgical resection in September 2018, confirming GBM pathology (white arrow). (C) MRI after the first course of temozolomide in December 2018, showing progression (white arrow). (D) Postoperative MRI after the second surgery in January 2019, revealing residual GBM and postoperative changes (white arrow). (E and F) The right parietal lesion exhibited an increase in size, perilesional edema and heterogeneous contrast enhancement (white arrows). There was no evidence of increased perfusion on the cerebral blood volume map (not shown), indicating pseudoprogression. MRI, magnetic resonance imaging; GBM, glioblastoma multiforme.
|
The treatment of the patient was switched to bevacizumab-irinotecan (10 mg/kg bevacizumab, 125 mg/m2 irinotecan, every 2 weeks) in July 2019; however, after the second dose of bevacizumab, the patient was admitted with gastrointestinal bleeding, and based on colonoscopy, colitis was detected. In December 2019, an MRI (Fig. 1F) showed no evidence of tumor progression, but rather pseudoprogression due to radiation necrosis. The patient died in October 2020.
Case 2
In October 2019 (Fig. 2A), a 42-year-old male patient underwent surgery for an intracranial mass at The Department of Neurosurgery, Hacettepe University Faculty of Medicine, and was diagnosed with glioblastoma, IDH wild-type, WHO grade IV. Immunohistochemical analysis was performed on paraffin-embedded tissue samples using primary antibodies against GFAP, Ki67, IDH1, p53 and ATRX. GFAP staining was positive, ATRX expression was preserved, IDH1 (R132H) and IDH2 were negative, confirming IDH wild-type status. The Ki-67 proliferation index was ~30%, p53 was overexpressed in 10–15% of tumor cells. Total resection was achieved during the operation, as confirmed by pre- and post-operative MRI (Fig. 2A and B). Following surgery, the patient received chemoradiotherapy in the post-operative period. Subsequently, adjuvant temozolomide treatment was initiated in November 2019. An increase in headache complaints was noted after the fifth cycle of temozolomide treatment. The MRI findings were indicative of progression (data not shown).
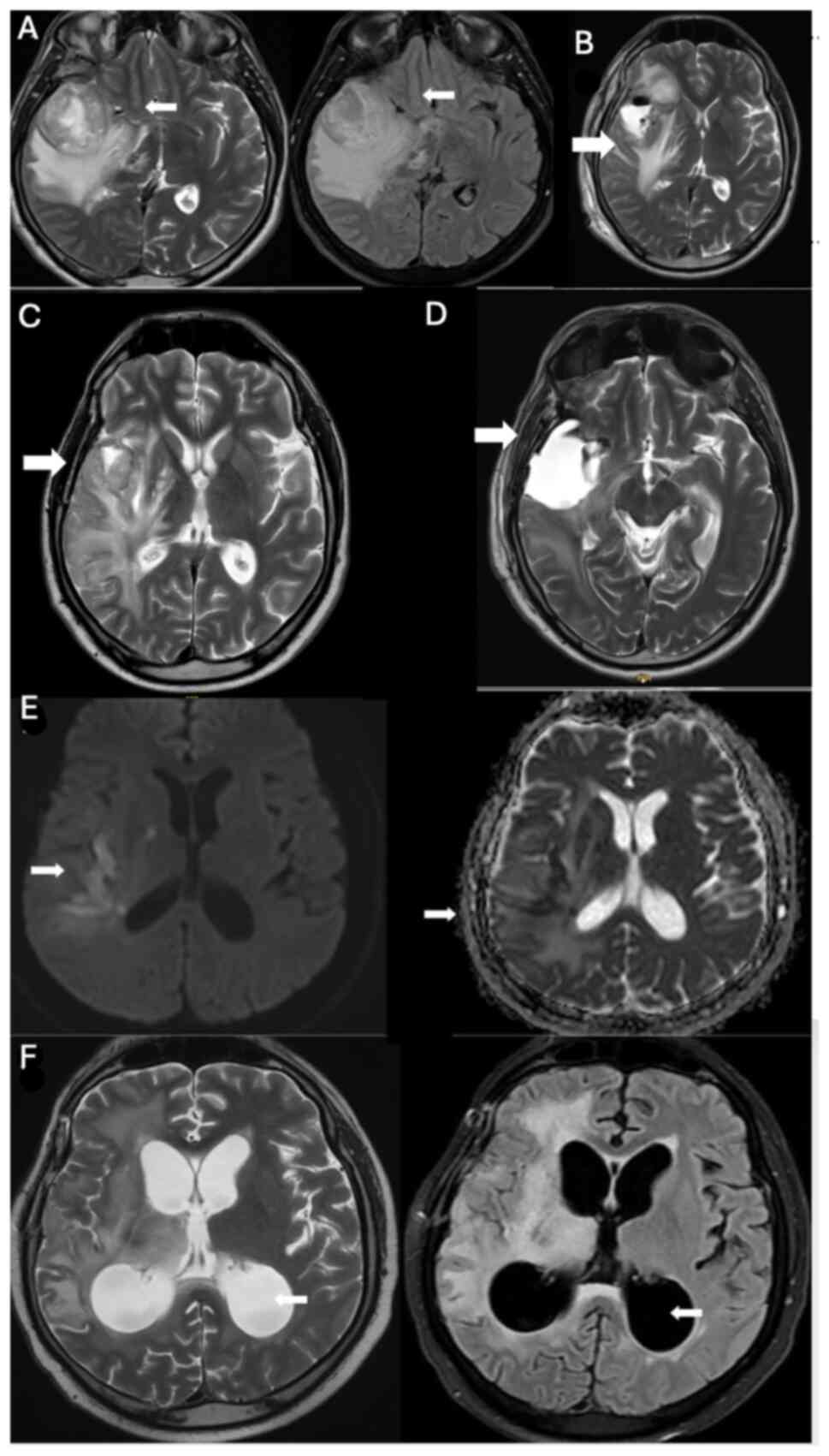 |
Figure 2.
(A) A heterogeneous T2 hyperintense tumor with prominent perilesional edema was located in the right fronto-temporo-insular region (white arrows). Additionally, signs of uncal herniation and indentation of the right crus cerebri were evident. (October 2019, before surgery). (B) Postoperative MRI confirming total resection after surgery in October 2019 (white arrow). (C) MRI after two cycles of pembrolizumab, showing progression in June 2020 prior to re-surgery (white arrow). (D) Postoperative MRI in July 2020 after re-surgery, following pembrolizumab treatment (white arrow). (E) Increased signal intensity on diffusion-weighted imaging with corresponding hypointensity on the apparent diffusion coefficient map confirmed the presence of acute ischemia in these areas (white arrows). (F) Triventricular hydrocephalus was visible on T2-weighted imaging and fluid-attenuated inversion recovery imaging in January 2021 (white arrows). A wide T2 hyperintensity in the right fronto-temporo-insular region was also observed. MRI, magnetic resonance imaging.
|
Before re-surgery, the patient was started on pembrolizumab at a dosage of 200 mg every 3 weeks, and after two cycles of treatment (Fig. 2C), surgical intervention was performed in July 2020 (Fig. 2D). During screening, acute ischemic areas were observed in the right middle cerebral artery territory, with extension into the insular and temporal regions, located at the junction of the right lateral ventricular trunk and the posterior corona radiata (Fig. 2E). Following the surgery, the patient underwent four cycles of pembrolizumab treatment, and cyberknife treatment was administered in September 2020.
As progression was detected, bevacizumab-irinotecan treatment (10 mg/kg bevacizumab, 125 mg/m2 irinotecan, every 2 weeks) was started in September 2020. In January 2021, a cranial MRI (Fig. 2F) performed after four cycles of treatment showed progressive changes. The patient, who had been admitted to the emergency department since that time complaining of continuous seizures, was last observed in the neurosurgical intensive care unit in April 2021 and died in June 2021.
Case 3
In January 2021, an intracranial mass was detected (Fig. 3A) and operated in a 45-year-old male patient (Fig. 3B) at The Department of Neurosurgery, Hacettepe University Faculty of Medicine, resulting in the diagnosis of glioblastoma, IDH wild-type, WHO grade IV. Immunohistochemical analysis was performed on paraffin-embedded tissue samples using primary antibodies against GFAP, Ki67, IDH1, p53 and ATRX. GFAP staining was positive and ATRX expression was maintained. IDH1 (R132H) staining was negative. The Ki-67 proliferation index was ~30% and p53 was overexpressed in 30% of the tumor cells. Concurrent chemoradiotherapy with temozolomide treatment was initiated for the patient; however, temozolomide treatment had to be discontinued due to the side effects of prolonged thrombocytopenia and neutropenia. Following radiotherapy, adjuvant bevacizumab-irinotecan treatment was started in April 2021. After six cycles of treatment, there was a minimal reduction in tumor size, accompanied by a decrease in contrast enhancement (Fig. 3C).
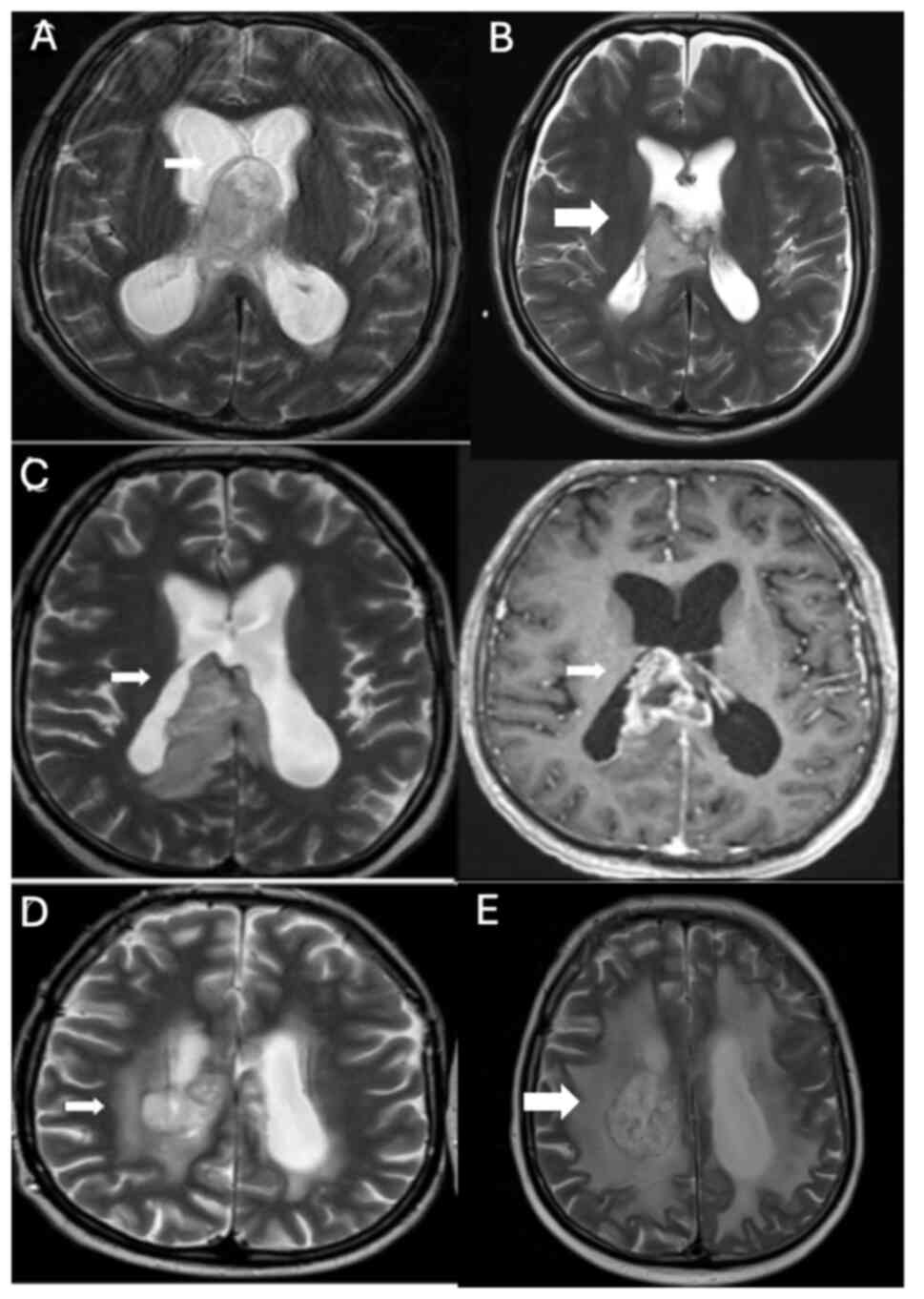 |
Figure 3.
(A) Tumor infiltration into the corpus callosum splenium and protrusion into the intraventricular space, along with involvement of the fornixes, was evident in January 2021 (white arrows). (B) Postoperative MRI confirming GBM diagnosis after surgery (white arrow). (C) GBM showed a slight reduction in size during the interval period (measurements captured at the level of the right part of the corpus callosum splenium and anterior commissure; white arrows). Additionally, there was a decrease in gross contrast enhancement compared with the lesion, which had increased in size and diffusely infiltrated the corpus callosum splenium in March 2021. (D) Increased heterogeneity, minimal shrinkage in the corpus callosum corpus posterior isthmus and splenium part, and prominent necrotic parenchymal changes, particularly on the right, were observed in April 2022 (white arrows). A focal area suggested tumoral infiltration, especially in the presence of bevacizumab, although it did not exhibit increased cerebral blood volume values (not shown). This infiltration extended to the posterior part of the right lateral ventricular corpus and the corona radiata. (E) MRI following three cycles of pembrolizumab and Cyberknife treatment in June 2022, showing stable disease surrounding the posterior part of the right lateral ventricle (white arrow). MRI, magnetic resonance imaging; GBM, glioblastoma multiforme.
|
The patient began to experience numbness in their arms and legs ~10 months into the treatment. In April 2022, a cranial MRI revealed progressive disease (Fig. 3D), prompting the initiation of pembrolizumab treatment at a dosage of 200 mg every 3 weeks and the administration of cyberknife treatment in June 2022. Following three cycles of treatment, the tumor remained stable, surrounding the posterior part of the right lateral ventricle (Fig. 3E).
In October 2022, mild compression fractures on the upper articular surfaces of the L2-S1 vertebral corpuscles were detected, leading to vertebroplasty being performed in the Department of Orthopedics at Hacettepe University Faculty of Medicine. In November 2022, the patient was admitted to the emergency room with loss of consciousness, and after a 2-month stay in the Neurosurgical Intensive Care Unit at Hacettepe University Faculty of Medicine, the patient died in January 2023.
Immunohistochemical methods and parameters
Immunohistochemical analysis was performed on paraffin-embedded tissue samples fixed in 10% neutral buffered formalin at room temperature for 24 h. Sections (4-µm thick) were cut from the paraffin wax-embedded blocks. Antigen retrieval was carried out using citrate buffer (pH 6.0) at 95°C for 20 min, followed by deparaffinization with xylene and rehydration in a descending ethanol series (100, 90 and 70%; and distilled water). Blocking was performed using 5% bovine serum albumin in phosphate-buffered saline at room temperature for 30 min (MilliporeSigma). The following primary antibodies were used: ATRX (1:200; cat. no. HPA001906; MilliporeSigma), IDH1 (1:50; cat. no. DIA-H09; Dianova GmbH), GFAP (1:1,000; cat. no. G9269; MilliporeSigma), Ki67 (1:100; cat. no. ab15580; Abcam) and p53 (1:100; cat. no. 2524S; Cell Signaling Technology, Inc.). Incubation with primary antibodies was performed overnight at 4°C. Sections were counterstained with hematoxylin at room temperature for 2 min. Imaging was performed using a Leica DM2000 light microscope, and ImageJ software (version 1.53t, National Institutes of Health) was used for analysis.
Discussion
GBM is the most common and aggressive form of glial tumor in adults (1). GBM is characterized by rapid growth and resistance to standard treatments, and the prognosis of GBM remains poor (10,11).
Cognitive decline in GBM is well defined, but the factors underlying this pathology, independent of brain radiotherapy and chemotherapy and separate from neuroinflammation, are not well understood (14). It is likely that GBM cells secrete factors that can exert neurotoxic effects that cause cognitive impairment. Among these factors, glutamate released by primary brain tumors has been associated with epileptic seizures (28). Wei et al (29) showed that human glioma stem cells secrete microvesicles and exosomes containing coding and non-coding RNA molecules that interact with the surrounding neuronal structures. In another study, GBM-derived exosomes were shown to increase oxidative stress in cerebellar neurons by decreasing cellular antioxidant defenses and increasing oxidative damage. In brain tissues, these vesicles may create a proinflammatory microenvironment by promoting the differentiation of neural stem cells into astrocytes or cause cognitive decline through multiple mechanisms (14).
The natural environment of the brain tends to suppress immune responses (30), a characteristic that the resistance of GBM to immunotherapy exploits through various mechanisms to enhance immunosuppression (31–35). GBM cells frequently express checkpoint proteins such as programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte associated protein 4, with higher levels being associated with poorer patient outcomes (36). Unlike other cancer types where a high tumor mutational burden (TMB) indicates an immunosuppressive environment conducive to an effective CD8+ T-cell response, GBM typically presents with a lower TMB (37). This feature contributes to its poor prognosis, which results from a lack of immunogenic neoantigens to trigger immune responses. Notably, in GBM, a higher TMB does not translate into improved survival or response to immunotherapy, a departure from the pattern observed in other malignancies (38–40). GBM cells secrete a number of cytokines, such as IL-1, IL-6 and IL-10, which suppress immune cell activity (41–43), and chemokines, such as C-C motif chemokine 22, which attract regulatory T cells (44,45), further enhancing the immunosuppressive environment of the tumor. Transforming growth factor-β serves a role in promoting tumor evasion of immune surveillance by enabling tumor cell transformation and reducing T-cell migration to the tumor by decreasing intercellular adhesion molecule expression on endothelial cells (46–48). This immunosuppression is not confined to the tumor site, but extends systemically, as evidenced by altered blood T-cell levels (decreased levels of circulating T cells and an increased proportion of regulatory T cells measured in peripheral blood) (49) and a high neutrophil-to-lymphocyte ratio, which adversely affects patient survival (50).
Preclinical investigations present a rationale for merging anti-angiogenesis therapy with ICIs. The interplay between tumor immunity and angiogenesis suggests that remodeling tumor vasculature might augment the effectiveness of cancer immunotherapy. The combination not only increases tumor sensitivity to anti-PD-L1 therapy, but also facilitates the infiltration of activated T cells into tumors by normalizing tumor vasculature and increasing pericyte coverage (51). In addition, the formation of high endothelial venules within tumors further promotes immune cell infiltration (52). Combination treatment with anti-VEGF and ICI reverses T-cell depletion, enhances therapeutic efficacy and increases vascular perfusion, potentially improving therapeutic efficacy (53,54).
Despite efforts to develop novel treatments targeting angiogenesis and the immune system, no anti-VEGF and ICI combination has extended survival in patients with GBM, either newly diagnosed or recurrent (15,55,56). Pembrolizumab in combination with bevacizumab was ineffective in prolonging overall survival (OS) or PFS in recurrent GBM (55). In a phase II study by Nayak et al (55), 80 patients with recurrent glioblastoma who had not previously received bevacizumab were randomized into two groups: One receiving a combination of pembrolizumab and bevacizumab (cohort A; n=50) and the other receiving pembrolizumab alone (cohort B; n=30). In cohort A, the 6-month PFS rate was 26.0%, with a median OS of 8.8 months. In cohort B, the 6-month PFS rate was 6.7% and the median OS was 10.3 months. Poor survival was associated with increased baseline dexamethasone use and increased post-therapy plasma VEGF levels, which should be evaluated as potential markers in future combination trials. A total of 369 patients were enrolled in an open-label, randomized phase 3 clinical trial, with 184 patients assigned to receive nivolumab and 185 patients assigned to receive bevacizumab. OS was generally comparable across patient subgroups (15). The addition of durvalumab to bevacizumab also did not improve the outcome of patients with recurrent GBM compared with the use of durvalumab alone (56).
Trials of ICIs (both nivolumab and pembrolizumab) in GBM are active at all stages of glioblastoma treatment. A retrospective case series used single-agent nivolumab to treat progression after bevacizumab in patients with glioblastoma (57). This small retrospective study found a minimal benefit in patients with recurrent GBM after progression on bevacizumab, but the treatment approach at this stage of the disease remains unclear. Due to the lack of clear evidence regarding the best treatment for such patients, post-bevacizumab therapy also represents an unmet need in neuro-oncology for patients with refractory GBM.
The common feature of the present patient group is mainly the sequential use of immunotherapy and bevacizumab treatment rather than combination treatment, and as a result, the patients were observed to have treatment-related changes without tumor progression. The small sample size and retrospective nature of the present case series limit the generalizability of the present findings and warrant further investigation in larger, prospective studies.
The efficacy mechanism of sequential use of drugs is not clear in the literature, and the mechanism of various complications that develop without tumor progression cannot be clearly explained. There are studies showing that vascular structure and inflammatory responses change after treatment. Therefore, more studies are required to determine the mechanism of action of sequential or combined use or whether there is an increase in complications such as severe vascular ischemia.
In conclusion, GBM is characterized by an immunosuppressive microenvironment, with its low TMB contributing to resistance against immunotherapy. The use of immunotherapy in both adjuvant and relapsed settings is being investigated, with studies assessing its combination with bevacizumab. The present review explored whether the sequential use of immunotherapy and bevacizumab can extend PFS. However, treatment-related changes may mimic tumor progression without actual disease progression. Further research is crucial to clarify the underlying mechanisms and potential complications of this sequential therapeutic approach in GBM management.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
NK and GK collected and analyzed the data, performed the investigation and wrote the original draft. IK collected the data. SPS and AII conceptualized and supervised the study. GY conceptualized and supervised the study, and reviewed and edited the original draft. NK and GK confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Written informed consent to participate was obtained from patients or, if they were unable to give consent, from their legal representatives.
Patient consent for publication
Written informed consent for publication was obtained from patients or, if they were unable to give consent, from their legal representatives.
Competing interests
The authors declare that they have no conflicting interests.
Authors' information
Dr Gozde Kavgaci, OCID: 0000-0001-6960-2024.
References
|
1
|
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl 5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, et al: Glioblastoma in adults: A society for neuro-oncology (SNO) and European society of neuro-oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 22:1073–1113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al: The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrom QT, Gittleman H, Stetson L, Virk SM and Barnholtzsloan JS: Epidemiology of gliomas. Cancer Treat Res. 163:1–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, Fuchs HE, Waite KA, Jemal A, Siegel RL and Barnholtz-Sloan JS: Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 71:381–406. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cini NT, Pennisi M, Genc S, Spandidos DA, Falzone L, Mitsias PD, Tsatsakis A and Taghizadehghalehjoughi A: Glioma lateralization: Focus on the anatomical localization and the distribution of molecular alterations (Review). Oncol Rep. 52:1392024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellingson B, Lai A, Harris R, Selfridge J, Yong W, Das K, Pope WB, Nghiemphu PL, Vinters HV, Liau LM, et al: Probabilistic radiographic atlas of glioblastoma phenotypes. AJNR Am J Neuroradiol. 34:533–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kommers I, Bouget D, Pedersen A, Eijgelaar RS, Ardon H, Barkhof F, Bello L, Berger MS, Conti Nibali M, Furtner J, et al: Glioblastoma surgery imaging-reporting and data system: Standardized reporting of tumor volume, location, and resectability based on automated segmentations. Cancers (Basel). 13:28542021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lapointe S, Perry A and Butowski NA: Primary brain tumours in adults. Lancet. 392:432–446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stupp R, Hegi ME, Mason WP, Van Den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-Year analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sinha R, Stephenson JM and Price SJ: A systematic review of cognitive function in patients with glioblastoma undergoing surgery. Neurooncol Pract. 7:131–142. 2020.PubMed/NCBI
|
|
13
|
Kirkman MA, Hunn BH, Thomas MSC and Tolmie AK: Influences on cognitive outcomes in adult patients with gliomas: A systematic review. Front Oncol. 12:9436002022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Genc S, Pennisi M, Yeni Y, Yildirim S, Gattuso G, Altinoz MA, Taghizadehghalehjoughi A, Bolat I, Tsatsakis A, Hacımüftüoğlu A and Falzone L: Potential neurotoxic effects of glioblastoma-derived exosomes in primary cultures of cerebellar neurons via oxidant stress and glutathione depletion. Antioxidants (Basel). 11:12252022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr O, et al: Effect of nivolumab vs. bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 6:1003–1010. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukumura D, Kloepper J, Amoozgar Z, Duda DG and Jain RK: Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol. 15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan KA and Kerbel RS: Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 15:310–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A: Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 18:602019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Kim BY, Chan CK, Hahn SM, Weissman IL and Jiang W: Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 18:195–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahma OE and Hodi FS: The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 25:5449–5457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Palma M, Biziato D and Petrova TV: Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 17:457–474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tate MC and Aghi MK: Biology of angiogenesis and invasion in glioma. Neurotherapeutics. 6:447–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silantyev AS, Falzone L, Libra M, Gurina OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias PD and Tsatsakis A: Current and future trends on diagnosis and prognosis of glioblastoma: From molecular biology to proteomics. Cells. 8:8632019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hambardzumyan D and Bergers G: Glioblastoma: Defining tumor niches. Trends Cancer. 1:252–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia A, Zhang Y, Xu J, Yin T and Lu XJ: T cell dysfunction in cancer immunity and immunotherapy. Front Immunol. 10:17192019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barsoum IB, Smallwood CA, Siemens DR and Graham CH: A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 74:665–674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chon HJ, Lee WS, Yang H, Kong SJ, Lee NK, Moon ES, Choi J, Han EC, Kim JH, Ahn JB, et al: Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res. 25:1612–1623. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T and Sontheimer H: Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 17:1269–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei Z, Batagov AO, Schinelli S, Wang J, Wang Y, El Fatimy R, Rabinovsky R, Balaj L, Chen CC, Hochberg F, et al: Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 8:11452017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, et al: Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 572:62–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangani D, Weller M and Roth P: The network of immunosuppressive pathways in glioblastoma. Biochem Pharmacol. 130:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LAD, Appin C, Park Y, Scarpace L, Mikkelsen T, Cohen ML, et al: Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 19:4951–4960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, Elsamadicy AA, Cui X, Koyama S, Jackson C, et al: T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 24:4175–4186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA and Fecci PE: T-cell dysfunction in glioblastoma: Applying a new framework. Clin Cancer Res. 24:3792–3802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, Woroniecka K, Elsamadicy AA, Dechant CA, Kemeny HR, et al: Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 24:1459–1468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hao C, Chen G, Zhao H, Li Y, Chen J, Zhang H, Li S, Zhao Y, Chen F, Li W and Jiang WG: PD-L1 expression in glioblastoma, the clinical and prognostic significance: A systematic literature review and meta-analysis. Front Oncol. 10:10152020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khasraw M, Walsh KM, Heimberger AB and Ashley DM: What is the burden of proof for tumor mutational burden in gliomas? Neuro Oncol. 23:17–22. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, Cortes-Ciriano I, Birzu C, Geduldig JE, Pelton K, et al: Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 580:517–523. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Ge J, Lan Y, Shi Y, Luo Y, Tan Y, Liang M, Deng S, Zhang X, Wang W, et al: Tumor mutational burden is associated with poor outcomes in diffuse glioma. BMC Cancer. 20:2132020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riviere P, Goodman AM, Okamura R, Barkauskas DA, Whitchurch TJ, Lee S, Khalid N, Collier R, Mareboina M, Frampton GM, et al: High tumor mutational burden correlates with longer survival in immunotherapy-naïve patients with diverse cancers. Mol Cancer Ther. 19:2139–2145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oft M: IL-10: Master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2:194–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fontana A, Hengartner H, de Tribolet N and Weber E: Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 132:1837–1844. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumari N, Dwarakanath B, Das A and Bhatt AN: Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kang S, Xie J, Ma S, Liao W, Zhang J and Luo R: Targeted knock down of CCL22 and CCL17 by siRNA during DC differentiation and maturation affects the recruitment of T subsets. Immunobiology. 215:153–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Crane CA, Ahn BJ, Han SJ and Parsa AT: Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: Implications for immunotherapy. Neuro Oncol. 14:584–595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wrann M, Bodmer S, de Martin R, Siepl C, Hofer-Warbinek R, Frei K, Hofer E and Fontana A: T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 6:1633–1636. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bryukhovetskiy I and Shevchenko V: Molecular mechanisms of the effect of TGF-β1 on U87 human glioblastoma cells. Oncol Lett. 12:1581–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, et al: Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 17:4296–4308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE II, Bigner DD, Dranoff G and Sampson JH: Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 66:3294–3302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Han S, Liu Y, Li Q, Li Z, Hou H and Wu A: Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 15:6172015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D and De Palma M: Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 9:eaak96702017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP and Bergers G: Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 9:eaak96792017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, et al: Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 71:1247–1261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zheng X, Fang Z, Liu X, Deng S, Zhou P, Wang X, Zhang C, Yin R, Hu H, Chen X, et al: Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest. 128:2104–2115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nayak L, Molinaro AM, Peters K, Clarke JL, Jordan JT, de Groot J, Nghiemphu L, Kaley T, Colman H, McCluskey C, et al: Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin Cancer Res. 27:1048–1057. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reardon D, Kaley T, Dietrich J, Clarke J, Dunn G, Lim M, Cloughesy T, Gan H, Park A, Schwarzenberger P, et al: Atim-38. Phase 2 study to evaluate the clinical efficacy and safety of Medi4736 (Durvalumab, Durva)+ bevacizumab (Bev) in Bev-naïve patients with recurrent glioblastoma (Gbm). Neuro Oncol. 20 (Suppl 6):vi102018. View Article : Google Scholar
|
|
57
|
Chamberlain MC and Kim BT: Nivolumab for patients with recurrent glioblastoma progressing on bevacizumab: A retrospective case series. J Neurooncol. 133:561–569. 2017. View Article : Google Scholar : PubMed/NCBI
|

















