Introduction
Malignant lymphomas constitute a biologically
heterogeneous group of diseases resulting from the clonal expansion
of lymphocytes (1) and they are
categorized based on their presumed cell of origin (either B-cell
or T-cell), their morphology (Hodgkin or non-Hodgkin), and their
level of cell differentiation (2).
Approximately one fourth of all lymphomas originate from extranodal
tissues, with the gastrointestinal tract and skin being the most
common sites (3). Lymphomas that
arise from the gynecological tract are less common, representing
around 1% of extranodal lymphomas. Of these, less than 1% emerge
from the uterine cervix (4).
The symptoms of primary lymphoma of the uterine
cervix (PLUC) often mimic those of cervical epithelial tumors. A
notable number of patients present with atypical symptoms such as
bleeding and pain, and pelvic exam usually reveals cervical oedema.
Since PLUC tend to develop in the cervical stroma rather than the
mucosa, it has been suggested that cytology cannot serve as a
reliable diagnostic tool, which creates a diagnostic challenge for
clinicians (5). This characteristic
limits the utility of traditional cytological screening methods,
such as the Pap smear, which are designed to detect epithelial
abnormalities. As a result, cytology often produces false negatives
in PLUC cases and therefore has limited efficacy as an initial
diagnostic tool. Given this, reliance on histopathological
examination through biopsy and the use of imaging modalities, such
as MRI and CT scans, is crucial for accurate diagnosis (6). PLUC bear histopathological resemblance
to lymphomas that occur in other anatomical sites. However, the
characterization of the malignancy as primary can be difficult
because the disease often involves contiguous sites including the
uterus and the vagina (6).
The optimal therapeutic management of patients with
female genital tract lymphomas and PLUC comprise another matter of
debate due to the rarity of the disease, the presence of different
histopathological subtypes that respond differently to treatment
and the lack of prospectively designed studies on the disease.
According to a body of evidence which is mainly based on case
reports and series, treatment options may encompass surgery,
chemotherapy, radiotherapy, or various combinations of the above
(7). Multidisciplinary co-operation
between gynecologists, pathologists, hematologists and medical
oncologists has been strongly recommended for the management of
patients diagnosed with the disease.
Given the limited clinical data on PLUC, we sought
to thoroughly review the existing literature and summarize the
reported cases. Our focus was on the clinicopathological features,
diagnosis, and treatment approaches associated with this
condition.
Materials and methods
Registration
This review was reported based on the ‘Preferred
Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA)
guidelines (8). The protocol was
registered in PROSPERO (CRD42023424206).
Literature search
Title screening was managed using Covidence. Two
reviewers (SB, AS) independently searched PubMed, Web of Science
and Scopus library databases from inception until August 2023. The
search included the following terms: ‘Lymphoma’ AND (‘cerv*’ OR
‘vagina*’ OR ‘uter* OR ‘gynecological tract’). No restrictions
regarding study design, geographic region or language were applied.
To identify any overlooked studies, we also conducted a manual
search of the references cited in the selected articles and
relevant published reviews. Additionally, Google Scholar, tailored
Google searches, and consultations with experts were employed to
locate further articles from grey literature sources. Any
discrepancies during the search process were addressed by involving
a third investigator (KSK).
Eligibility criteria
All study designs were considered eligible for
inclusion only if they provided data for cases diagnosed with PLUC.
The definition of PLUC was based on the definitions provided by the
authors of the included studies (lymphoma presenting only in the
uterine cervix with no visible lymphadenopathy on imaging or larger
involvement of the uterine cervix in case of lymphoma presence on
contiguous sites). Review articles, conference abstracts, and
non-peer reviewed sources were not eligible for inclusion. We also
excluded published dissertations, studies in vitro and
animal models.
Data extraction and handling
Non-English articles were manually translated when
possible (9–15). In all studies, patient data was
retrieved and handled by two authors (SB, AS) who conducted the
data extraction independently. We collected the following
information when available: Sex, age, menopausal status,
comorbidities, family history of cancer, presenting symptoms and
duration of symptoms, laboratory tests, primary diagnosis,
histopathological markers, imaging findings, staging, therapeutic
management, follow up time and clinical outcome (overall survival
and disease-free survival). Any disagreements were discussed and
resolved by a third investigator (KSK).
Quality assessment
The risk of bias (RoB) was evaluated independently
by two authors (SB, AS). To assess the overall quality of the case
reports and case series, we utilized the critical appraisal
checklist from the Joanna Briggs Institute (JBI) (16), which has been reliably employed in
similar studies. The evaluation focused on eight key elements:
patient demographics, medical history, current health status,
physical examination and diagnosis, concurrent therapies,
post-intervention health outcomes, and the interaction between drug
administration and patient response. Each study was rated as ‘Yes’,
‘No’, or ‘Unclear/Not Applicable’ based on the availability and
clarity of the information for each element.
Data synthesis
This review employs descriptive statistics to
present the demographics and clinical features, including symptoms,
imaging methods, treatment approaches, staging, follow-up duration,
and outcomes. Continuous variables are summarized using means,
while binary variables are presented as rates or percentages. The
duration of symptoms is reported as a range to account for the
inconsistent and approximate reporting of this data across
different studies.
Kaplan-Meier curves were generated to estimate
overall survival irrespective of treatment used using GraphPad
PRISM 9.0. Data was pooled when duration of follow up (in months)
and outcome (alive or deceased) were provided. Duration of follow
up was converted to months and the definition for overall survival
was based on the definitions provided by the authors of the
included studies. The patients were divided into different
subgroups based on menopausal status, therapeutic management,
hysterectomy type, use of adjuvant treatments and staging at
diagnosis (supplementary material). Further sensitivity analyses
were performed, to ensure homogeneity of data, excluding studies
with high risk of bias, excluding pre-menopausal patients and
excluding patients with high stage disease based on Ann Arbor
staging system. Data with a P<0.05 was considered statistically
significant. Missing and unidentifiable data were excluded from the
statistical analysis. Both the log-rank test and the
Gehan-Breslow-Wilcoxon method were used to compare the survival
trend between the different subgroups.
Results
Study characteristics
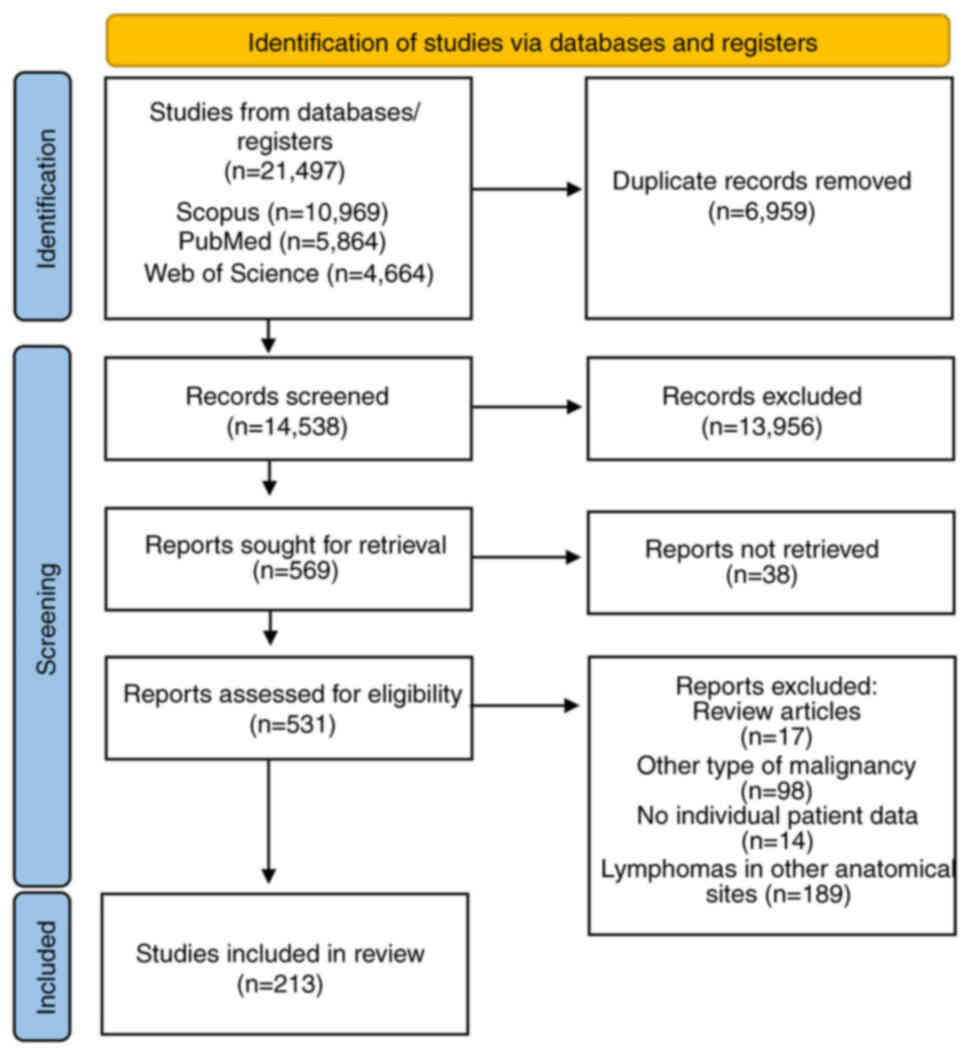
We identified 21,497 articles through the literature
search; 6,959 bibliographic references were removed as duplicates
and 13956 articles were excluded through title and abstract
screening as irrelevant. Two authors reviewed 569 full text
articles for eligibility. Finally, 213 studies, published between
1964 and 2023, were found eligible for the systematic review
(Figs. 1 and S1). Regarding continent, 84 of the
studies were conducted in Asia, 74 in Europe, 49 in Americas, 5 in
Africa and 1 in Oceania. In terms of design, 162 studies were case
reports and 51 were case series (Table
SI).
Patient characteristics
We identified a total of 339 cases of PLUC (Table SI). The mean age of the patients
was 48.5 years (median: 46, age range: 15–88). Data regarding
menopausal status was provided for 146 patients. For the remaining
cases menopausal status was based on the age. In total, 129
patients were classified as premenopausal (129/339, 38.1%) and 129
as postmenopausal (129/339, 38.1%) (Table SI).
Data regarding symptomatology was available for 318
cases. The most common presenting symptom was vaginal bleeding
(189/318, 59.4%) and its duration ranged from 4.5 days to 24
months. Vaginal bleeding was characterized in many cases as
post-coital (33/3018, 10.4%) or inter-menstrual (12/318, 3.8%). The
second most common symptom was vaginal discharge (44/318, 13.8%)
followed by abdominal pain (34/318, 107) and pelvic pain (12/318,
3.8%). A few patients remained asymptomatic (42/318, 13.2%) and
were diagnosed incidentally. Only a small fraction of patients
developed ‘B’ symptoms (28/318, 8.8%) including night sweats
(5/318, 1.5%), fever (9/318, 2.7%) and weight loss (14/318, 4.1%)
(Tables SI and SII).
Although all cases were confirmed by
histopathological examination, data regarding diagnostics was
provided for 278 patients. Biopsy (either excisional or punch
biopsy) was the most commonly used initial diagnostic modality
(78/278, 28.1%) followed by ultrasound (59/278, 21.2%), cervical
cytology (54/267, 20.2%) and computerized tomography (24/278,
8.6%). Data regarding histopathological types was available for all
cases. Among the 339 cases analyzed, the most common
histopathological subtype of PLUC was diffuse large B-cell lymphoma
(DLBCL) (39/339). Other predominant subtypes included non-Hodgkin's
B-cell lymphoma (13/339), large lymphoma (6/339), and intermediate
lymphoma (6/339). Less common but notable types observed were
lymphoma-like lesions (5/339) and several other rare subtypes,
reflecting the histopathological diversity within PLUC. Data for
histopathological markers was provided for 136 cases. The most
commonly encountered markers included CD3 (61/136, 44.9%), CD10
(48/136, 35.3%), CD20 (15/136, 11%) cyclin D1 (34/136, 25%), CD5
(29/136, 21.3%) and BCL-2 (16/136, 11.8%) (Table SI).
For staging the Ann Arbor system was used in 179
patients. The most common stages included stage I (93/179, 52%) and
stage II (47/179, 26.3%) followed by stage IV (29/179, 16.2%) and
stage III (8/179, 4.5%). FIGO staging for cervical cancer was also
utilized in 21 cases. The most common stages included FIGO stage I
(12/21, 57.1%), FIGO stage II (4/21, 19%), FIGO stage III (1/21,
4.8%) and FIGO stage IV (4/21, 19%). Data regarding the presence of
distant metastases at the time of diagnosis was provided for 245
patients with metastases being present in only a small fraction of
patients (26/245, 10.6%) mainly affecting retroperitoneal lymph
nodes and the kidney.
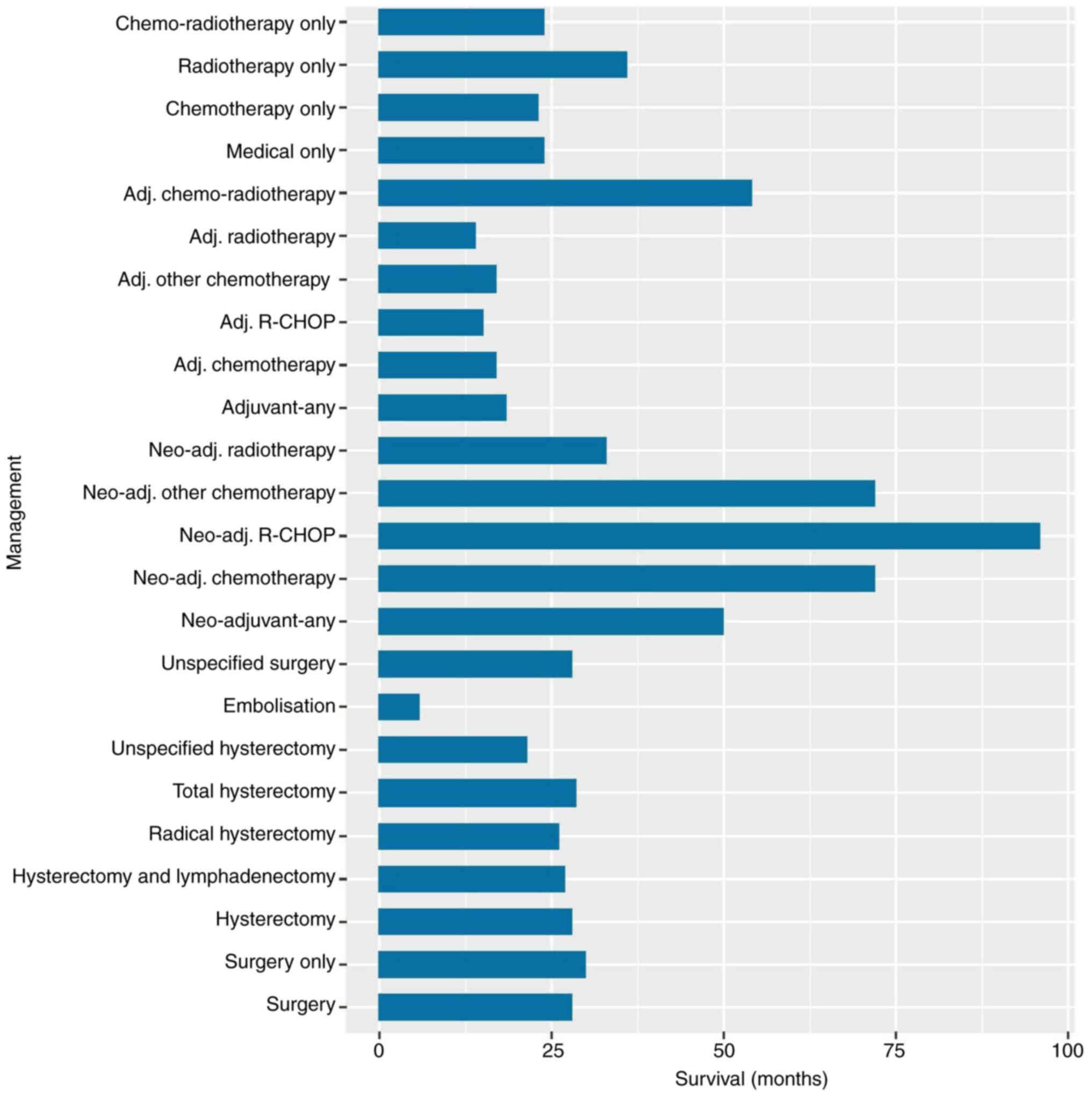
Information regarding management was provided for
309 patients (Table I). Almost one
third of the patients were managed surgically (115/309, 35.5%),
with most receiving adjuvant treatment (62/115), some receiving
surgery alone (34/115), and a few receiving neo-adjuvant treatment
(19/115). Of those that received surgery, the majority underwent
hysterectomy (85/115); which included radical hysterectomy (9/115),
total hysterectomy (58/85) and unspecified hysterectomy (18/85). A
small number of patients underwent hysterectomy and lymphadenectomy
(23/115). Of those that received neo-adjuvant treatment (19/115),
the majority received neo-adjuvant chemotherapy (15/115), with a
very small fraction of those receiving ‘R-CHOP’ chemotherapy
(2/15), and the remaining receiving neo-adjuvant radiotherapy
(4/19). More than half of those that underwent surgery received
adjuvant treatment (62/115); the majority of this was chemotherapy
(35/62), with a small amount receiving ‘R-CHOP’ chemotherapy
(9/35), and the remainder receiving radiotherapy (18/62) or
chemo-radiotherapy (9/62) (Table
SIII).
 | Table I.Median survival according to
therapeutic management type. |
Table I.
Median survival according to
therapeutic management type.
| A, Surgery |
|---|
|
|---|
| Management | n/N (%) | Median survival,
months (range) |
|---|
| Surgery | 115/309 (37.2) | 28 (1.5–228) |
| Surgery only | 34/309 (11.0) | 30 (6–204) |
| Hysterectomy | 85/115 (73.9) | 28 (2–228) |
| Hysterectomy and
lymphadenectomy | 23/115 (20.0) | 27 (4–108) |
| Radical
hysterectomy | 9/85 (10.6) | 26 (10–60) |
| Total
hysterectomy | 58/85 (68.2) | 28.5 (2–228) |
| Hysterectomy
(unspecified) | 18/85 (21.2) | 21.5 (3–204) |
| Embolisation | 3/115 (2.6) | 5.75 (1.5–10) |
| Surgery
(unspecified) | 14/115 (12.2) | 28 (6–156) |
|
| B, Neo-adjuvant
treatment |
|
|
Management | n/N (%) | Median survival,
months (range) |
|
| Neo-adjuvant | 19/115 (16.5) | 50 (8–156) |
| Chemotherapy | 15/19 (78.9) | 72 (36–156) |
| R-CHOP | 2/15 (13.3) | 96 (36–156) |
| Other
chemotherapy | 14/15 (93.3) | 72 (36–132) |
| Radiotherapy | 4/19 (21.1) | 33 (8–40) |
|
| C, Adjuvant
treatment |
|
|
Management | n/N (%) | Median survival,
months (range) |
|
| Adjuvant | 62/115 (53.9) | 18.5 (1.5–228) |
| Chemotherapy | 35/62 (56.5) | 17 (1.5–228) |
| R-CHOP | 9/35 (25.7) | 15 (4–48) |
| Other
chemotherapy | 26/35 (74.3) | 17 (1.5–228) |
| Radiotherapy | 18/62 (29.0) | 14 (2–60) |
|
Chemo-radiotherapy | 9/62 (14.5) | 54 (10–89) |
|
| D, Medical
treatment only |
|
|
Management | n/N (%) | Median survival,
months (range) |
|
| Medical only | 194/309 (62.8) | 24 (1–246) |
| Chemotherapy
only | 109/194 (56.2) | 23 (3–168) |
| Radiotherapy
only | 23/194 (11.9) | 36 (3–240) |
| Combination
therapy: Chemo-radiotherapy only | 62/194 (32.0) | 24 (1–246) |
The largest group of patients were managed without
surgery and received only medical therapies (194/309). This
comprised chemotherapy alone (109/194) with the commonest regime
being ‘R-CHOP’, followed by chemo-radiotherapy (62/194), and
radiotherapy (23/194).
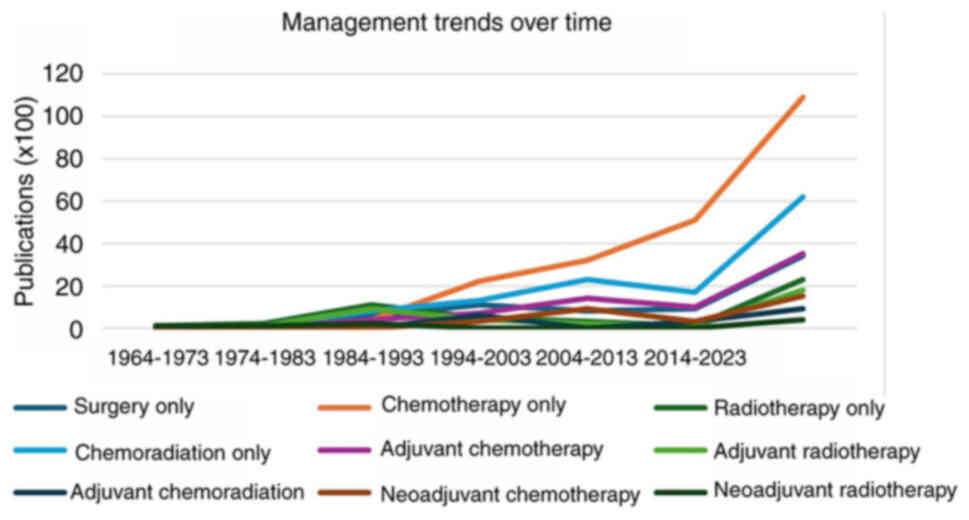
Management trends
The majority of the cases with management data were
published later than 1984 (301/309). By decade, between 1984 and
present day, the proportion of patients treated with radiotherapy
decreased from 25% (11/44) in 1984–1993, to 1% (1/95) in the years
2014–2023. Conversely, the number of patients managed with
chemotherapy alone increased, from 9% (4/44) in the years
1984–1993, to 54% (53/95) in the most recent decade 2014–2023
(Fig. 2; Table SIV).
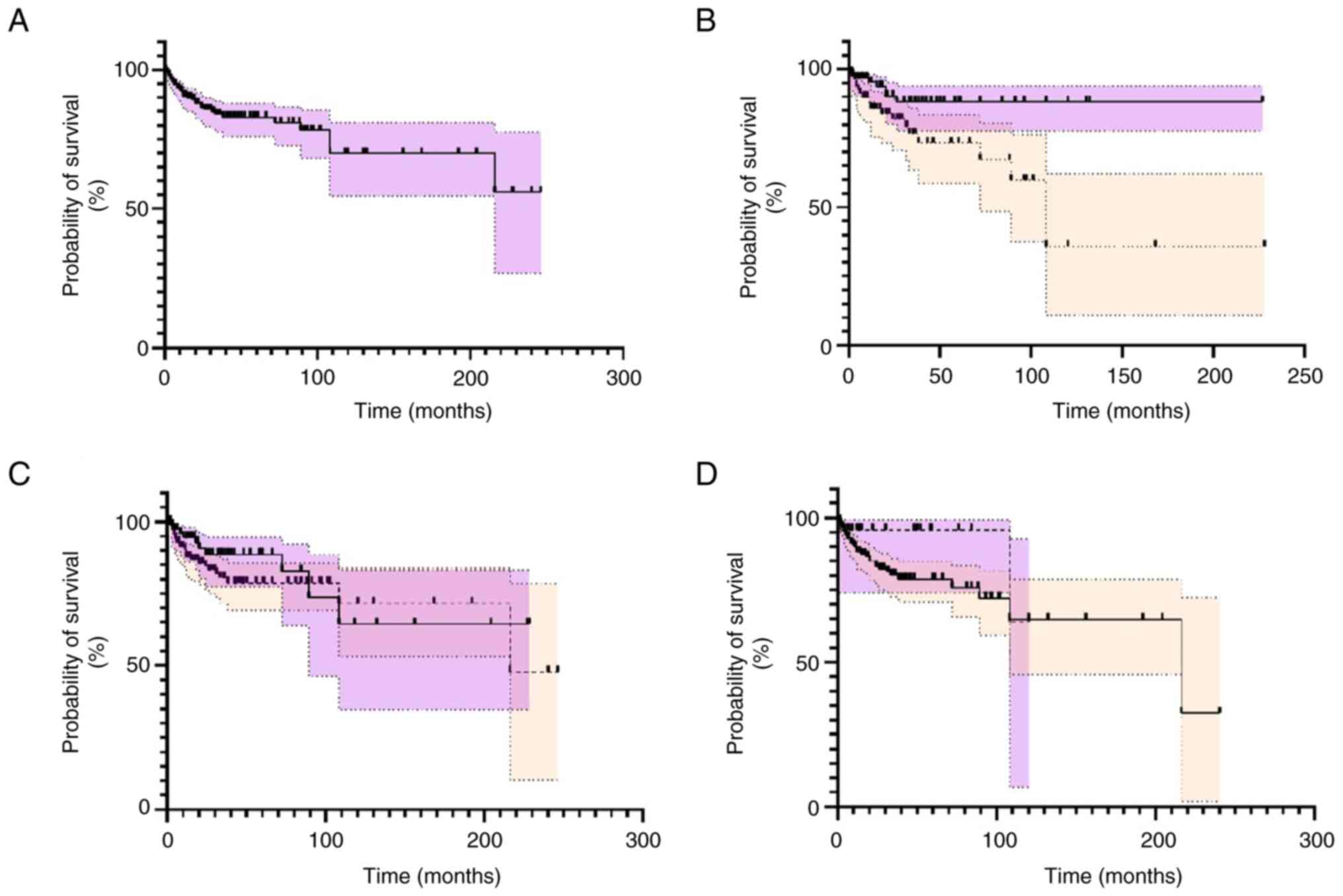
Outcome and survival rates
Data for clinical outcome was provided for 279
patients, of whom 46 died and 233 survived. Almost half of the
deaths occurred within 12 months after the initial diagnosis
(21/45, 46.6%). The follow-up period for survivors ranged from 4
weeks to 246 months and the absolute 5-year and 10-year survival
rates were 86.1 and 85.4%, respectively (Fig. 3A).
The longest median survival was for those that
received chemotherapy alone (72 months, range 36–156), and
particularly for the two patients that received neo-adjuvant
‘R-CHOP’ (96 months, range 36–156). The shortest median survival
was for the three patients that received uterine artery
embolization as treatment (5.75 months, range 1.5–10). Median
survival for those who received any surgery (28 months, range
1.5–228) was not dissimilar to those who received surgery alone (30
months, range 6–24), any medical therapy alone (24 months, range
1–246), including specifically chemotherapy alone (23 months, range
3–168), radiotherapy alone (36 months, range 3–240), or
chemo-radiotherapy (24 months, range 1–246). The type of
hysterectomy did not appear to affect median survival; with median
survival for radical hysterectomy (26 months, range 10–36)
calculated to be similar to those that underwent hysterectomy and
lymphadenectomy (27 months, range 4–108), total hysterectomy (28.5,
range 2–228), or any unspecified hysterectomy (21.5 months, range
3–204) (Table I).
Survival analysis indicated that premenopausal
patients exhibited better prognosis compared to postmenopausal
patients (P<0.0001) (Fig. 3B).
Moreover, surgical management with or without adjuvant/neoadjuvant
treatments was not superior to non-surgical management including
chemotherapy, radiotherapy or chemoradiotherapy (P=0.29) (Fig. 2C). Additionally, we did not find
statistically significant differences in overall survival when we
compared surgery and adjuvant chemotherapy with chemotherapy alone
as well as surgery and adjuvant chemoradiation with chemoradiation
alone (Fig. S2; Table SIV). The presence of symptoms was
not associated with a reduced overall survival rate (P=0.24)
(Fig. 3D). The median survival and
ranges for each management type are presented in Table I and displayed in Fig. 4.
Quality of the studies
The quality assessment of the eligible studies
indicated that the majority of the included studies (138/204) met
most of the recommended criteria, categorizing them as having a low
risk of bias. In contrast, 64 studies were classified with a medium
risk of bias, while 2 studies fell into the high-risk category. A
total of 20 studies achieved a perfect score. The aspect most
frequently reported as ‘Not/Applicable’ among the studies was the
patient's past medical history (Table
SV, Table SVI, Table SVII). Sensitivity analyses were
performed using the log-rank test and the Gehan-Breslow-Wilcoxon
method while excluding potential confounding variables such as high
risk of bias studies, premenopausal women, and patients with stages
III and IV PLUC (Fig. S3, Fig. S4, Fig.
S5). The survival curves produced show late-stage crossover
which limits interpretation but have been included as part of
sensitivity analysis.
Discussion
In this systematic review of the literature, we
identified cases of primary lymphoma of the uterine cervix and
examined their clinicopathological characteristics, diagnosis and
therapeutic management. Our study included 213 reports which
comprised 339 patients diagnosed with primary lymphoma of the
uterine cervix. Our findings revealed that most patients presented
with non-specific symptoms including vaginal bleeding and discharge
with a mean age of 48.5. The menopausal status of the patients
varied and most of them were diagnosed at an early stage, according
to the Ann Arbor staging system. The follow-up period ranged from 4
weeks to 246 months and the absolute 5- and 10-year survival rates
were 86.1 and 85.4%, respectively. Although a higher concentration
of cases was noted in Asia and Europe in terms of geographical
distribution, the small volume of case reports and series analyzed
does not allow the identification of any geographical variations or
patterns. Broader studies on extranodal lymphomas suggest that
factors such as genetic predispositions, environmental influences,
or differences in healthcare systems and diagnostic practices may
contribute to regional distribution patterns (3).
The existence and recognition of primary genital
tract lymphomas was initially widely debated in the literature
(3) until the identification of
normal lymphoid tissue in female reproductive tract (17) and the reporting of patients with
malignant lymphomas located exclusively in gynecological organs
(18). While the exact causes and
mechanisms behind these lymphomas remain elusive, chronic
inflammation is suggested as a potential triggering factor
(19). However, further mechanistic
studies are required to elucidate the aforementioned theory. The
increase in the incidence of extra-nodal lymphomas in different
body sites, during the last decades has been also linked with the
increase in immunosuppressive therapies, environmental toxins as
well as, improved diagnostic techniques (20).
Despite the presence of published cases of primary
lymphomas of the female genital tract in the scientific literature
their definition remains a matter of controversy. A number of
investigators have discussed cases of lymphomas in different parts
of the female reproductive tract without presenting specific
criteria to define primary tumors (5,21–23).
Others have applied either the Fox and More criteria for uterine
lymphoma (24) or the Harris and
Scully modification of the Ann Arbor staging system for ovarian
lymphomas (25). Currently,
different staging systems are used for PLUC, which may contribute
to inconsistencies in diagnosis and treatment planning. Developing
a standardized staging and diagnostic framework specifically for
PLUC would reduce this variability and facilitate more consistent
management of the disease across clinical settings (26,27).
Regarding PLUC, the definition that has been proposed by Kosari
et al is the most comprehensive and includes the following
criteria: i) Lymphomas confined to the cervix at the time of
initial diagnosis, ii) lymphomas that are localized in the cervix
without any evidence of involvement of other body parts on full
investigation, and iii) the absence of abnormal cells indicating
leukemia in the peripheral blood and bone marrow (28).
The presentation of the disease usually involves
vaginal bleeding or discharge as cervical lesions can easily become
ulcerated and/or infected (29), a
phenomenon described in different histopathological types of
cervical malignancies (30). In
line with the results of our study, the presence of ‘B’ symptoms is
considered uncommon and affects only a relatively small number of
patients with PLUC (31), while
cases of asymptomatic patients have been also reported. Despite the
fact that clinical presentation of PLUC is very similar to other
cervical tumors, the disease is considered to be human
papillomavirus (HPV)-independent and distinct compared to cervical
epithelial malignancies (32–36).
While the co-existence of PLUC with other synchronous histological
types of cervical cancer is pathophysiologically feasible, in this
review, we only identified sporadic cases.
As far as diagnosis is concerned, histopathology
plays a pivotal role while cytology cannot be utilized reliably as
PLUC mainly affects the stroma rather than the epithelium of the
uterine cervix. This was also reflected in our results, with only
one fifth of the investigators using cytology as the first modality
for diagnosis. The histopathological diversity within PLUC, with
DLBCL as the most common subtype, mirrors patterns observed in
other extranodal lymphomas (27,37).
The range of subtypes suggests that while broad treatment protocols
may be effective, subtype-specific approaches may be beneficial for
optimizing outcomes. Less common subtypes, such as lymphoma-like
lesions, further underscore the need for careful diagnostic
differentiation, as histopathology plays a critical role in guiding
treatment decisions (38). Future
research should focus on identifying and characterizing genetic
profiles in PLUC, as this could lead to more tailored and effective
treatment options, similar to the advancements seen in other
lymphomas.
Immunohistochemistry is another important diagnostic
tool and lymphoma markers used for other types of lymphocyte
malignancies can be also found consistently in PLUC. These marker
include among others CD20 and Ki-67 for B-cell and CD3 and CD20 for
T-cell lymphomas (37). Although
such markers are widely used in diagnosing PLUC, variability in
their expression across cases can impact diagnostic accuracy. This
variability likely stems from the biological diversity of
lymphomas, with different lymphocyte subtypes displaying distinct
immunophenotypic profiles (26).
Genetic mutations and the tumor microenvironment also contribute to
these differences, highlighting the need for a more comprehensive
marker panel to improve accuracy (38).
In the analysis it was evident that premenopausal
women had better oncological outcomes when compared to the
postmenopausal women. Although such an outcome could have been
expected as premenopausal status could be associated with a
younger, healthier population, the actual reasons for this
disparity remain unclear. Potential factors may include differences
in hormonal status, comorbidities, or variations in treatment
response. Further research is necessary to investigate the
underlying mechanisms and how they may influence survival outcomes
in PLUC (38,39).
Due to the rarity of the disease, a consensus
regarding therapeutic management has not been established. Older
reports utilized radiotherapy as monotherapy. However, more recent
approaches included either surgery (115/309), with or without
neo-adjunctive or adjunctive therapy, chemotherapy alone (109/309),
or a combination of chemotherapy and radiotherapy (62/310). In our
analysis, there was no statistically significant difference in the
overall survival when surgery alone was compared with chemotherapy
alone. However, the longest calculated median survival was for
those who received neo-adjuvant chemotherapy (15 patients, median
survival 72 months, range 36–156). Additionally, surgery followed
by adjunctive chemotherapy resulted in similar overall survival
when compared to surgery alone. The question is whether PLUC should
be managed in accordance with treatment guidelines for lymphoma of
other sites (26), or whether it
should be site-specific; an anatomical site such as the uterine
cervix is feasible to remove surgically with relatively little
morbidity. Our study does not provide sufficient evidence to
confirm the idea that PLUC should be managed non-surgically. Based
on the extremely limited present data, which may also be influenced
by the age of some of the studies and geographical variation, there
is possibly a tentative suggestion that a combination of
neo-adjuvant chemotherapy and surgery may represent a reasonable
treatment option.
Current guidelines for the management of non-Hodgkin
lymphomas typically recommend chemotherapy as the first-line
approach. Specifically, for diffuse B-cell lymphomas, it is
preferable to utilize a doxorubicin-based regimen, such as a course
of six to eight cycles of R-CHOP (rituximab, cyclophosphamide,
vincristine, prednisolone) (40).
In contrast, when dealing with indolent B-cell lymphomas, such as
marginal zone lymphomas, there is no single standardized regimen
that is universally recommended. Treatment options vary, ranging
from rituximab alone to more intricate and complex combinations
like R-hyper C-VAD (cyclophosphamide, vincristine, doxorubicin, and
dexamethasone alternating with methotrexate and cytarabine)
(41). The consideration of
radiotherapy as a treatment option is typically reserved for
instances of very low-stage lymphomas that exhibit localized
characteristics and can be easily targeted. For example, this
approach may be appropriate for early-stage follicular lymphoma, as
indicated in the NICE guidelines (42). Treatment guidelines for squamous
cell carcinoma or adenocarcinoma of the cervix recommend surgery in
cases where the disease is at an early stage (43). In our study the overall survival was
not significantly different in patients treated with chemotherapy
alone. It is possible that chemotherapy alone represents a safe
treatment option for younger premenopausal patients diagnosed at an
early stage who wish to retain their fertility. Del Valle-Rubido
et al published the only case included in this study where
the patient received fertility-sparing management; a 28-year-old
woman received ‘CHOP’ chemotherapy, after transplantation of the
ovaries; this patient went on to survive at least 10 years and was
still alive at the time of writing (9). However, the results of our analysis in
this study, including the lack of difference in survival between
management options, should be interpreted with caution considering
the potential limitations associated with the analysis including
the low number of patients, the heterogeneity between the included
studies and the scarcity of events.
To the best of our knowledge, our study is the most
comprehensive review on the reported cases of PLUC. Our findings
present pooled data from 328 patients and highlight published data
with quality assessment of included studies.
However, several limitations should be acknowledged.
The inclusion of low-quality case reports and case series limits
the validity and generalizability of our conclusions. These studies
inherently carry a significant risk of bias, being particularly
susceptible to overinterpretation and selection bias. Although many
of the studies were rated as low risk of bias according to our
assessment tool, their fundamental design flaws make them prone to
publication bias, especially since negative findings are rarely
reported. Consequently, the data presented, while potentially
insightful, may not accurately reflect real-world outcomes.
Additionally, although this study identifies various subtypes of
PLUC, the limited data available restricts our ability to
thoroughly examine the treatment response and prognosis for each
subtype, as also reflected in the lack of statistical power of the
Kaplan-Meier graphs. Given the rarity of PLUC and its subtypes,
further research is needed to understand these subtype-specific
outcomes, including recurrence rates and disease-free survival,
which may guide more tailored treatment strategies. Quality of life
metrics are also essential for providing insight into the impact of
PLUC treatments in the long-term. Therefore, data from larger,
prospectively designed studies are essential for drawing more
reliable conclusions about optimizing diagnostic and therapeutic
strategies for patients with PLUC.
A further limitation in the classification of PLUC
arises from the concurrent use of the Ann Arbor staging system,
which is traditionally used for lymphomas, and the FIGO staging
system, which is applied to cervical cancers. The use of these two
distinct staging systems can create inconsistencies and challenges
in classification, which may impact treatment decisions and hinder
efforts to further develop comparative studies. Developing a
unified staging system specifically tailored to PLUC could improve
clarity in both clinical practice and research, allowing for a more
standardized approach to diagnosis and treatment planning.
Another notable limitation is our inability to
estimate the effect size of outcomes and quantify uncertainty. It
is noteworthy that a majority of clinical cases lack sufficient
information for concentrating study results into a mean, median, or
proportion with a confidence interval. This underscores the
significance of initiatives aimed at standardizing and enhancing
the quality of information reported in case reports. Overall, a
pooled integrated analysis of case reports and series cannot
replace evidence provided by clinical trials. Therefore, subject
recruitment in rare diseases and personalized medicine represents a
critical task in clinical research (40,41,43,44).
Finally, the conclusions drawn from our integrated
analysis are inevitably constrained by the small number of patients
included, the difficulty to assess homogeneity between studies and
the inherent rarity of the events under investigation. Statistical
significance is typically determined by observing patterns or
outcomes that are unlikely to occur by chance alone (45). However, in the context of rare
events, the occurrence itself is infrequent, making traditional
measures of statistical analysis and significance less applicable.
The late crossover of survival curves observed in some of the
supplementary sensitivity analyses highlights a statistical
manifestation of this key limitation. Overall, the scarcity of
events can lead to challenges in sample size, limiting the
statistical power to detect meaningful differences or associations
(46–47).
In conclusion, malignant lymphomas can arise at
unconventional anatomical sites. In the cervix uteri, the tumor
appearance can vary and thus may be misdiagnosed with a primary
epithelial malignancy. Although primary malignant lymphomas of the
uterine cervix are infrequent, early detection, optimal therapeutic
management, and multidisciplinary involvement may provide benefits
to patients developing the disease. The knowledge of the rare
occurrence of malignant lymphoma in the cervix, its unique
characteristics and the increased awareness of clinicians can
prevent clinical misdiagnoses and ultimately improve the clinical
outcomes of patients developing this uncommon disease. While
evidence on this condition remains limited, leveraging
international registries could significantly strengthen the body of
knowledge surrounding this rare malignancy.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Joana Wagner, Dr
See Hyun Park, Dr Rachel Fitzpatrick, Dr Jorgen Kanters, Dr Irina
Tsibulak, Dr Barbara Chettle, Professor David Chettle, Dr Xingfeng
Li, Ms. Yuki Tokunaga and Mr. Srjdan Saso (Imperial College
Healthcare NHS Trust) for their help with manual translation from
Spanish (9), Polish (10,11)
and Japanese (12–15) into English.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
Conceptualization was performed by KSK. The main
aims were established by KSK, LBE, SJB and AS. Methodology was
performed by KSK, SB, LBE, SJB and AS, and validation by KSK, SB,
LBE, SJB, AS, SMS, IK, DL, FK, AM, AG, MP and MK. All aspects of
the investigation were performed by KSK, SB, LBE, SJB, AS, DL, FK,
AG and MP, specificlly this included protocol writing and
submission (KSK and SB), literature screening (KSK, SB, LBE and
SJB), data extraction (KSK, SB, LBE, SJB, AS, IK, DL, FK, AM, AG
and MP), risk of bias assessement (KSK, SB and SJB), and data
handling (SB, LBE, SJB, IK, DL, AM, AG and MP). Regarding
acquisition of resources, KSK, SB, LBE, SMS, SJB, AS, IK, DL, FK,
AM, AG and MP were responsible. Statistical analysis was performed
by KSK, SB, LBE, SJB and AS, and original draft preparation was
done by KSK, SB, LBE, SMS, SJB, AS, IK, DL, FK, AM, AG and MP.
Review and editing were performed by KSK, SB, LBE, SJB, AS, IK,
SMS, DL, FK, AM, AG, MP and MK, and visualization by KSK, SB, LBE,
SJB, AS, IK, SMS, DL, FK, AM, AG, MP and MK. KSK, LBE and MK
supervised the project, and KSK, LBE and MK were the project
administrators. KSK, SB, LBE, SJB, FK and AS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and patient consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matasar MJ and Zelenetz AD: Overview of
lymphoma diagnosis and management. Radiol Clin North Am.
46:175–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paquin AR, Oyogoa E, McMurry HS, Kartika
T, West M and Shatzel JJ: The diagnosis and management of suspected
lymphoma in general practice. Eur J Haematol. 110:3–13. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freeman C, Berg JW and Cutler SJ:
Occurrence and prognosis of extranodal lymphomas. Cancer.
29:252–260. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan JK, Loizzi V, Magistris A, Hunter MI,
Rutgers J, DiSaia PJ and Berman ML: Clinicopathologic features of
six cases of primary cervical lymphoma. Am J Obstet Gynecol.
193:866–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harris NL and Scully RE: Malignant
lymphoma and granulocytic sarcoma of the uterus and vagina: A
clinicopathologic analysis of 27 cases. Cancer. 53:2530–2545. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen H, Wei Z, Zhou D, Zhang Y, Han X,
Wang W, Zhang L, Yang C and Feng J: Primary extranodal diffuse
large B-cell lymphoma: A prognostic analysis of 141 patients. Oncol
Lett. 16:1602–1614. 2018.PubMed/NCBI
|
|
7
|
Hilal Z, Hartmann F, Dogan A, Cetin C,
Krentel H, Schiermeier S, Schultheis B and Tempfer CB: Lymphoma of
the cervix: Case report and review of the literature. Anticancer
Res. 36:4931–4940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shamseer L, Moher D, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA; PRISMA-P Group,
: Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ.
349:g76472015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
del Valle-Rubido C, Cano-Cuetos A,
Heras-Sedano I, Marcos-González V, Marcos-González M and
Marcos-González A: Linfoma primario de cérvix: Gestación tras
tratamiento conservador Primary lymphoma of the cervix: Pregnancy
after conservative treatment. Progresos de Obstetricia y
Ginecología. 57:225–229. 2014.(In Spanish). View Article : Google Scholar
|
|
10
|
Plewicka M, Szelc S, Smok-Ragankiewicz A,
Wojcieszek A and Szkita D: Non-Hodgkin's lymphoma of the uterine
cervix. Ginekol Pol. 66:246–248. 1995.(In Polish). PubMed/NCBI
|
|
11
|
Makarewicz R, Swirska M and Terlikiewicz
J: Non-hodgkin's malignant lymphoma in the uterine cervix. Wiad
Lek. 46:936–937. 1993.(In Polish). PubMed/NCBI
|
|
12
|
Hashimoto A, Fujimi A, Kanisawa Y, Matsuno
T, Okuda T, Minami S, Doi T, Ishikawa K, Uemura N, Jyomen and
Tomaru U: Primary diffuse large B-cell lymphoma of the uterine
cervix successfully treated with rituximabplus cyclophosphamide,
doxorubicin, vincristine, and prednisone chemotherapy-a case
report. Gan To Kagaku Ryoho. 40:2589–2592. 2013.(In Japanese).
PubMed/NCBI
|
|
13
|
Isosaka M, Hayashi T, Mitsuhashi K, Tanaka
M, Adachi T, Kondo Y, Suzuki T and Shinomura Y: Primary diffuse
large B-cell lymphoma of the uterus complicated with
hydronephrosis. Rinsho Ketsueki. 54:392–396. 2013.(In Japanese).
PubMed/NCBI
|
|
14
|
Murotsuki J, Yazaki S, Hayakawa S and
Yamashita T: A case of malignant lymphoma of the uterine cervix.
Gan No Rinsho. 35:1716–1720. 1989.(In Japanese). PubMed/NCBI
|
|
15
|
Ohkuma Y, Hachisuga T, Fukuda K and
Sugimori H: 23 Examination of lymphoma-like lesion of the uterine
cervix. Journal of the Japanese Society of Obstetrics and
Gynecology. 43:11331991.(In Japanese).
|
|
16
|
Joanna Briggs Institute, . Checklist for
Case Reports Critical Appraisal tools for use in JBI Systematic
Reviews. Joanna Briggs Institute; Adelaide, Australia: 2019,
https://jbi.global/sites/default/files/2021-10/Checklist_for_Case_Reports.docxFebruary
15–2024
|
|
17
|
Woodruff J, Castillo RN and Novak E:
Lymphoma of the ovary. A study of 35 cases from the ovarian tumor
registry of the American gynecological society. Am J Obstet
Gynecol. 85:912–918. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paladugu RR, Bearman RM and Rappaport H:
Malignant lymphoma with primary manifestation in the gonad: A
clinicopathologic study of 38 patients. Cancer. 45:561–571. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dursun P, Gultekin M, Bozdag G, Usubutun
A, Uner A, Celik NY, Yuce K and Ayhan A: Primary cervical lymphoma:
Report of two cases and review of the literature. Gynecol Oncol.
98:484–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trenhaile TR and Killackey MA: Primary
pelvic non-Hodgkin's lymphoma. Obstet Gynecol. 97:717–720. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chorlton I, Norris H and King F: Malignant
reticuloendothelial disease involving the ovary as a primary
manifestation. A series of 19 lymphomas and 1 granulocytic sarcoma.
Cancer. 34:397–407. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van de Rijn M, Kamel OW, Chang PP, Lee A,
Warnke RA and Salhany KE: Primary low-grade endometrial B-cell
lymphoma. Am J Surg Pathol. 21:187–194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stroh EL, Besa PC, Cox JD, Fuller LM and
Cabanillas FF: Treatment of patients with lymphomas of the uterus
or cervix with combination chemotherapy and radiation therapy.
Cancer. 75:2392–2399. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fox H and More J: Primary malignant
lymphoma of the uterus. J Clin Pathol. 18:723–728. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monterroso V, Jaffe ES, Merino MJ and
Medeiros LJ: Malignant lymphomas involving the ovary: A
clinicopathologic analysis of 39 cases. Am J Surg Pathol.
17:154–1570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO Classification of Tumours of
Haematopoietic and Lymphoid Tissues. International Agency for
Research on Cancer. 2016.
|
|
27
|
Morton LM, Wang SS, Devesa SS, Hartge P,
Weisenburger DD and Linet MS: Lymphoma incidence patterns by WHO
subtype in the United States, 1992–2001. Blood. 107:265–276. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kosari F, Daneshbod Y, Parwaresch R, Krams
M and Wacker HH: Lymphomas of the female genital tract: A study of
186 cases and review of the literature. Am J Surg Pathol.
29:1512–1520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pretorius R, Semrad N, Watring W and
Fotheringham N: Presentation of cervical cancer. Gynecol Oncol.
42:48–53. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kechagias KS, Zafeiri M, Katsikas
Triantafyllidis K, Kyrtsonis G, Geropoulos G, Lyons D, Burney Ellis
L, Bowden S, Galani A, Paraskevaidi M and Kyrgiou M: Primary
melanoma of the cervix uteri: A systematic review and meta-analysis
of the reported cases. Biology. 12:3982023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stabile G, Ripepi C, Sancin L, Restaino S,
Mangino FP, Nappi L and Ricci G: Management of primary uterine
cervix B-cell lymphoma stage IE and fertility-sparing outcome: A
systematic review of the literature. Cancers (Basel). 15:36792023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Intaraphet S, Farkas DK, Johannesdottir
Schmidt SA, Cronin-Fenton D and Søgaard M: Human papillomavirus
infection and lymphoma incidence using cervical conization as a
surrogate marker: A Danish nationwide cohort study. Hematol Oncol.
35:172–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kyrgiou M, Bowden S, Athanasiou A,
Paraskevaidi M, Kechagias K, Zikopoulos A, Terzidou V,
Martin-Hirsch P, Arbyn M, Bennett P and Paraskevaidis E: Morbidity
after local excision of the transformation zone for cervical
intra-epithelial neoplasia and early cervical cancer. Best Pract
Res Clin Obstet Gynaecol. 75:10–22. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kechagias KS, Kalliala I, Bowden SJ,
Athanasiou A, Paraskevaidi M, Paraskevaidis E, Dillner J, Nieminen
P, Strander B, Sasieni P, et al: Role of human papillomavirus (HPV)
vaccination on HPV infection and recurrence of HPV-related disease
after local surgical treatment: Aystematic review and
meta-analysis. BMJ. 378:e0701352022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kechagias KS, Giannos P, Bowden S,
Tabassum N, Paraskevaidi M and Kyrgiou M: PCNA in cervical
intraepithelial neoplasia and cervical cancer: An interaction
network analysis of differentially expressed genes. Front Oncol.
11:7790422021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bowden SJ, Doulgeraki T, Bouras E,
Markozannes G, Athanasiou A, Grout-Smith H, Kechagias KS, Ellis LB,
Zuber V, Chadeau-Hyam M, et al: Risk factors for human
papillomavirus infection, cervical intraepithelial neoplasia and
cervical cancer: An umbrella review and follow-up Mendelian
randomisation studies. BMC Med. 21:2742023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho J: Basic immunohistochemistry for
lymphoma diagnosis. Blood Res. 57:55–61. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eichenauer DA, Aleman BM, André M,
Federico M, Hutchings M, Illidge T, Engert A and Ladetto M; ESMO
Guidelines Committee, : Hodgkin lymphoma: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29
(Suppl 4):iv19–iv29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chaganti S, Illidge T, Barrington S, Mckay
P, Linton K, Cwynarski K, McMillan A, Davies A, Stern S and Peggs
K; British Committee for Standards in Haematology, : Guidelines for
the management of diffuse large B-cell lymphoma. Br J Haematol.
174:43–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cibula D, Raspollini MR, Planchamp F,
Centeno C, Chargari C, Felix A, Fischerová D, Jahnn-Kuch D, Joly F,
Kohler C, et al: ESGO/ESTRO/ESP guidelines for the management of
patients with cervical cancer-Update 2023. Virchows Arch.
482:935–966. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
National Institute for Health and Care
Excellence, . Non-Hodgkin lymphoma. Updated guidelines.
2023.https://www.nice.org.uk/guidance/ng52/resources/nonhodgkin-lymphoma-pdf-3306777546949March
8–2024
|
|
43
|
Frieden TR: Evidence for health decision
making-beyond randomized, controlled trials. N Engl J Med.
377:465–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Board PATE: Non-Hodgkin Lymphoma Treatment
(PDQ®). PDQ Cancer Information Summaries. National
Cancer Institute; 2023
|
|
45
|
Friede T, Posch M, Zohar S, Alberti C,
Benda N, Comets E, Day S, Dmitrienko A, Graf A, Günhan BK, et al:
Recent advances in methodology for clinical trials in small
populations: The InSPiRe project. Orphanet J Rare Dis. 13:1862018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lane PW: Meta-analysis of incidence of
rare events. Stat Methods Med Res. 22:117–1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cai T, Parast L and Ryan L: Meta-analysis
for rare events. Stat Med. 29:2078–2089. 2010. View Article : Google Scholar : PubMed/NCBI
|