Introduction
Older adults more commonly suffer from esophageal
squamous cell carcinoma (ESCC), and as populations age, the average
age of those affected also increases (1). Comorbidities and critical dysfunction
in pulmonary, cardiac, or renal organs, for example, are often
present in older patients (2), who
cannot tolerate treatment intensity as easily as younger patients.
However, as the clinical data clearly show statistically, surgery
alone cannot control advanced ESCC (3). In Japan, esophageal cancer in the
surgically resectable stages is generally treated with neoadjuvant
chemotherapy (NAC) and subsequent surgery (4,5).
Recent results from the JCOG1109 randomized clinical study have
changed the standard treatment for patients with clinical stage II
or III ESCC in Japan. Now, neoadjuvant triplet chemotherapy with
docetaxel, cisplatin, and 5-fluorouracil (DCF) is administered in
place of cisplatin (CDDP) plus 5-fluorouracil (5-FU) (6,7).
However, the JCOG1109 study included only patients aged ≤75 years
with an Eastern Cooperative Oncology Group performance status (PS)
of 0 or 1 (8). Deciding how to
treat older patients based on clinical trial results from younger
patients can be difficult in real-world clinical practice. By
dividing the doses of docetaxel (TXT), CDDP, and 5-FU, new regimens
with high completion rates and therapeutic efficacy are being
developed (9–11). Identification of the increasing
number of older patients with ESCC who are intolerant to
preoperative treatment vs. those who should be treated
preoperatively could speed the development of appropriate
therapeutic strategies.
We therefore conducted a multicenter retrospective
study to determine the indications for divided-dose DCF (biweekly
DCF) in patients aged ≥70 years with ESCC in comparison to upfront
surgery (US).
Patients and methods
Patient eligibility
Data were retrieved from a prospective database of
patients who had undergone esophagectomy at Gifu University
Hospital, Gifu Prefectural General Hospital, and Gifu Municipal
Hospital. Eligibility criteria included subtotal esophagectomy
performed for curative intent between January 2015 and December
2021; primary ESCC confirmed histologically; age ≥70 years; and
clinical stage II/III disease as defined by the International Union
Against Cancer TNM classification system, 8th edition (12), which includes clinical stage IV (no
distant organ metastasis other than supraclavicular lymph node
metastasis). Exclusion criteria were clinical T4 tumor, conversion
to definitive chemoradiotherapy, and salvage surgery. Patients
unable to undergo esophagectomy with no known reason for
discontinuation were excluded. The eligible patients were divided
into the NAC group and the US group for comparison of long-term
outcomes. The Gifu University School of Medicine Ethics Committee
and all participating centers approved the study protocol (ID:
2022-232).
Preoperative neoadjuvant chemotherapy
and surgical treatment
The PS of all patients who underwent NAC (biweekly
DCF) was 0–2. All had adequate bone marrow, liver, renal, and
cardiovascular function. The anticancer drugs were TXT (35
mg/m2), CDDP (40 mg/m2), and 5-FU (400
mg/m2). TXT and CDDP were administered intravenously on
days 1 and 15, and 5-FU was administered on days 1–5 and 15–19,
with all patients scheduled for two cycles. Computed tomography
(CT) or magnetic resonance imaging was used to evaluate all
measurable lesions other than the primary tumor. Lesions were
assessed with Response Evaluation Criteria in Solid Tumors Criteria
version 1.1 (13). Four weeks
following completion of the two chemotherapy cycles, response was
confirmed by esophagogastroduodenoscopy and CT. Adverse events were
defined according to the National Cancer Institute's Common
Terminology Criteria for Adverse Events version 5.0.
In all patients, subtotal esophagectomy with
mediastinal lymphadenectomy was performed via right thoracoscopy or
thoracotomy. Follow-up included esophagogastroduodenoscopy and CT
performed every 4–6 months each year postoperatively.
Endpoints
The primary endpoints were 3-year overall survival
(OS) and recurrence-free survival (RFS). OS was calculated from the
first examination day to the day of death or last follow-up day.
RFS was calculated from the first examination day to the day of
death, day of disease recurrence, or last follow-up day. At the
last follow-up, patients were contacted to determine if they were
still alive. The secondary endpoints were the between-group
differences in perioperative complications, prognosis by
pathological stage, and the difference in prognosis between
patients with high and low psoas muscle index (PMI), a background
factor assessed in older patients with ESCC who may derive greater
benefit from this NAC regimen. As PMI may influence treatment
effect (14,15), we classified patients into the PMI
high group and PMI low group based on cut-off values of 6.36
cm2/m2 for males and 3.92
cm2/m2 for females (16), which indicate low skeletal muscle
mass in Japan.
Statistical analysis
Patients' characteristics between the NAC and US
groups are summarized by frequencies and percentages for
categorical variables and by interquartile ranges for continuous
variables. Between-group differences were compared with the
chi-square test, Wilcoxon rank-sum test or Fisher's exact test. A
logistic regression model estimated a propensity score representing
the possibility of receiving NAC based on the patients' data at
first examination. This model included the variables listed in
Table I. Stabilized inverse
probability weights were generated using the previously obtained
propensity score. Kaplan-Meier curves adjusted by inverse
probability weighting (IPW) were calculated to graphically compare
OS and RFS between the NAC and US groups. The reported
p-value was estimated using a Cox proportional hazards
model. The hazard ratio (HR) was estimated by Cox IPW regression.
Robust variance was used to avoid underestimating the variance of
the regression coefficients. Subgroup analysis based on
pathological stage and PMI was performed in the unweighted
population.
 | Table I.Patient clinical and background
characteristics. |
Table I.
Patient clinical and background
characteristics.
| Characteristics | NAC group (n=47) | US group (n=39) | P-value |
|---|
| Median age, years
(IQR) | 75.0 (71.5,
78.0) | 76.0 (72.0,
79.0) | 0.310 |
| Sex, n (%) |
|
| 0.863 |
| Male | 38 (80.9) | 33 (84.6) |
|
|
Female | 9 (19.1) | 6 (15.4) |
|
| PS, n (%) |
|
| <0.001 |
| 0 | 14 (29.8) | 1 (2.6) |
|
| 1 | 29 (61.7) | 15 (38.5) |
|
| 2 | 4 (8.5) | 23 (59.0) |
|
| cStage (UICC8th), n
(%) |
|
|
<0.001a |
| II | 8 (17.0) | 22 (56.4) |
|
|
III | 28 (59.6) | 15 (38.5) |
|
|
IVA | 10 (21.3) | 2 (5.1) |
|
|
IVB | 1 (2.1) | 0 (0.0) |
|
| Median Cre, mg/dl
(IQR) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.979 |
| Median WBC, /µl
(IQR) | 6720.0 (5310.0,
9420.0) | 6750.0 (5230.0,
8250.0) | 0.329 |
| Median Hb, g/dl
(IQR) | 13.4 (12.1,
14.1) | 13.5 (12.2,
14.6) | 0.376 |
| Median BMI,
kg/m2 (IQR) | 20.5 (18.6,
23.6) | 21.5 (19.8,
23.6) | 0.450 |
| Median serum Alb,
g/dl (IQR) | 4.1 (3.9, 4.3) | 4.0 (3.7, 4.2) | 0.090 |
| Median T-Cho, mg/dl
(IQR) | 174.0 (148.0,
215.0) | 191.0 (177.5,
206.0) | 0.202 |
| Median CRP, mg/dl
(IQR) | 0.1 (0.1, 0.9) | 0.2 (0.1, 0.4) | 0.900 |
| Median Neut, /µl
(IQR) | 4670.0 (3515.0,
6695.0) | 4323.0 (2917.5,
5536.0) | 0.254 |
| Median Lymph, /µl
(IQR) | 1562.0 (1189.5,
1884.5) | 1584.0 (1275.0,
2077.0) | 0.240 |
| Median Plt,
103/µl (IQR) | 273.0 (219.5,
328.5) | 223.0 (199.0,
255.0) | 0.005 |
Adverse events in the NAC group are summarized by
frequencies and percentages. Surgical results are summarized by
frequencies and percentages for the categorical variables and
medians with interquartile ranges for the continuous variables.
Between-group differences were estimated by Fisher's exact test or
Wilcoxon rank-sum test. All P-values were two-sided, with the level
of significance set at P<0.05. All analyses were performed with
R 4.2.2 (The R Project for Statistical Computing).
Results
Patients and inverse probability
weighting analysis
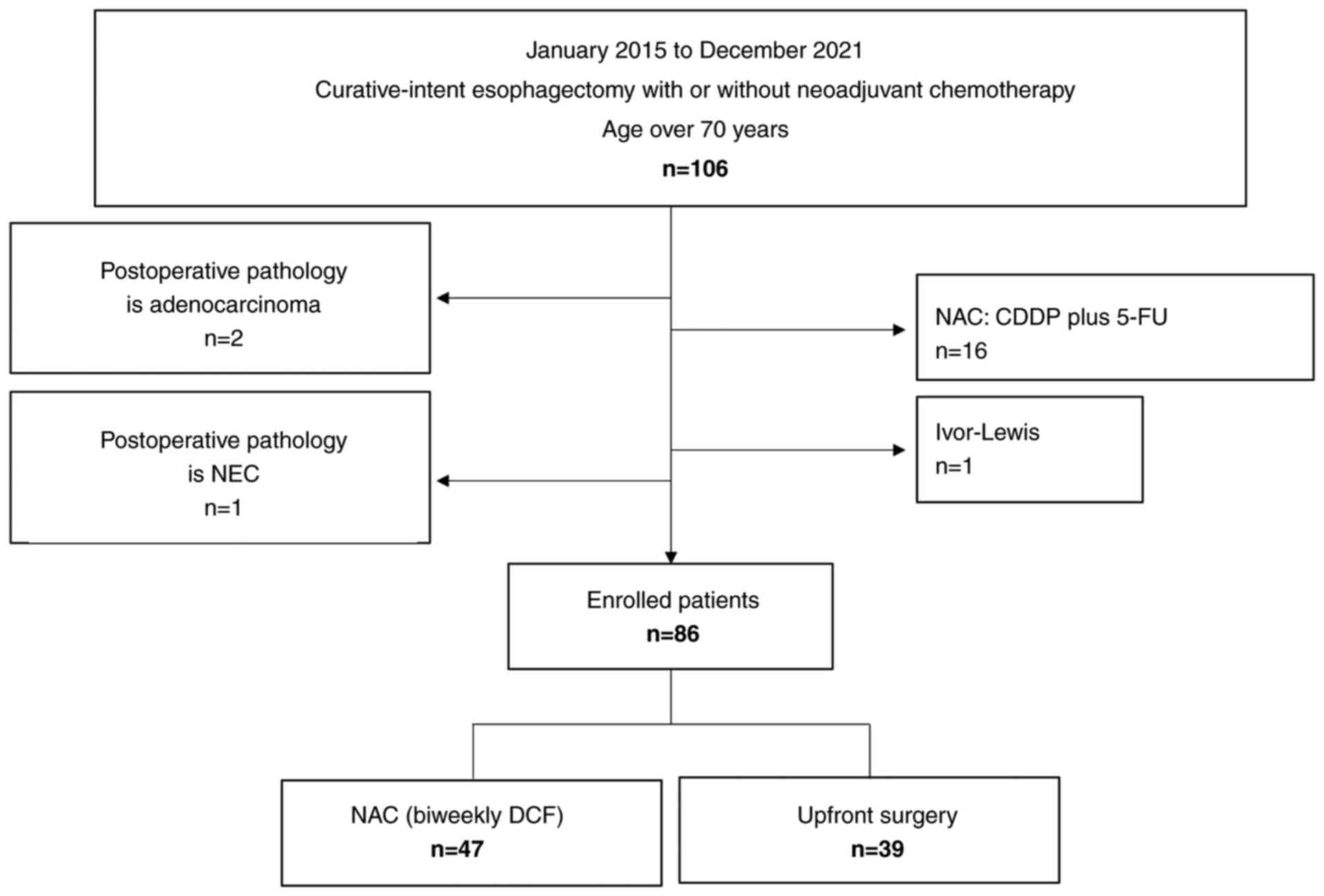
This study included 86 eligible patients (Fig. 1). Table
I summarizes the patient background characteristics of the NAC
group (n=47 patients, 54.7%) and US group (n=39 patients, 44.2%).
Overall median patient age was 75.5 (71–79) years. PS was
significantly better and clinical stage disease was significantly
more advanced in the NAC group vs. US group. Patient
characteristics in both groups were similar following IPW (Table II), and no characteristics were
significantly different. Postoperative adjuvant chemotherapy was
added for 18.4% of the patients in the US group.
 | Table II.Patient clinical and background
characteristics after inverse probability weighting, where the
information of each patient is weighted by their stabilized inverse
probability. |
Table II.
Patient clinical and background
characteristics after inverse probability weighting, where the
information of each patient is weighted by their stabilized inverse
probability.
|
Characteristics | NAC group
(n=30.7) | US group
(n=32.7) | P-value |
|---|
| Median age, years
(IQR) | 75.0 (71.9,
78.0) | 76.4 (72.0,
79.0) | 0.373 |
| Sex, n (%) |
|
| 0.729a |
|
Male | 25.8 (83.8) | 28.8 (87.8) |
|
|
Female | 5.0 (16.2) | 4.0 (12.2) |
|
| PS, n (%) |
|
| 0.032a |
| 0 | 6.7 (22.0) | 1.7 (5.2) |
|
| 1 | 19.4 (63.1) | 16.8 (51.5) |
|
| 2 | 4.6 (15.0) | 14.2 (43.3) |
|
| cStage (UICC8th), n
(%) |
|
| 0.052a |
| II | 8.5 (27.5) | 17.3 (52.9) |
|
|
III | 15.3 (49.7) | 13.2 (40.2) |
|
|
Iva | 6.6 (21.4) | 2.3 (7.0) |
|
|
IVb | 0.5 (1.5) | 0.0 (0.0) |
|
| Median Cre, mg/dl
(IQR) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.882 |
| Median WBC, /µl
(IQR) | 6407.7 (5071.2,
8629.9) | 6465.5 (4524.7,
8038.3) | 0.474 |
| Median Hb, g/dl
(IQR) | 13.8 (12.3,
14.3) | 13.4 (11.7,
14.6) | 0.920 |
| Median BMI,
kg/m2 (IQR) | 20.8 (19.3,
23.7) | 21.6 (18.8,
23.6) | 0.879 |
| Median serum Alb,
g/dl (IQR) | 4.1 (3.7, 4.3) | 4.0 (3.8, 4.2) | 0.535 |
| Median T-Cho, mg/dl
(IQR) | 174.4 (156.1,
215.0) | 185.6 (177.6,
198.0) | 0.547 |
| Median CRP, mg/dl
(IQR) | 0.1 (0.0, 0.8) | 0.1 (0.1, 0.3) | 0.978 |
| Median Neut, /µl
(IQR) | 4033.1 (3202.4,
5696.8) | 4127.4 (2539.1,
5441.0) | 0.351 |
| Median Lymph, /µl
(IQR) | 1629.5 (1320.3,
1882.7) | 1610.9 (1244.7,
2014.5) | 0.573 |
| Median Plt,
103/µl (IQR) | 239.6 (208.9,
316.3) | 218.2 (203.4,
249.2) | 0.046 |
Patient outcomes and survival
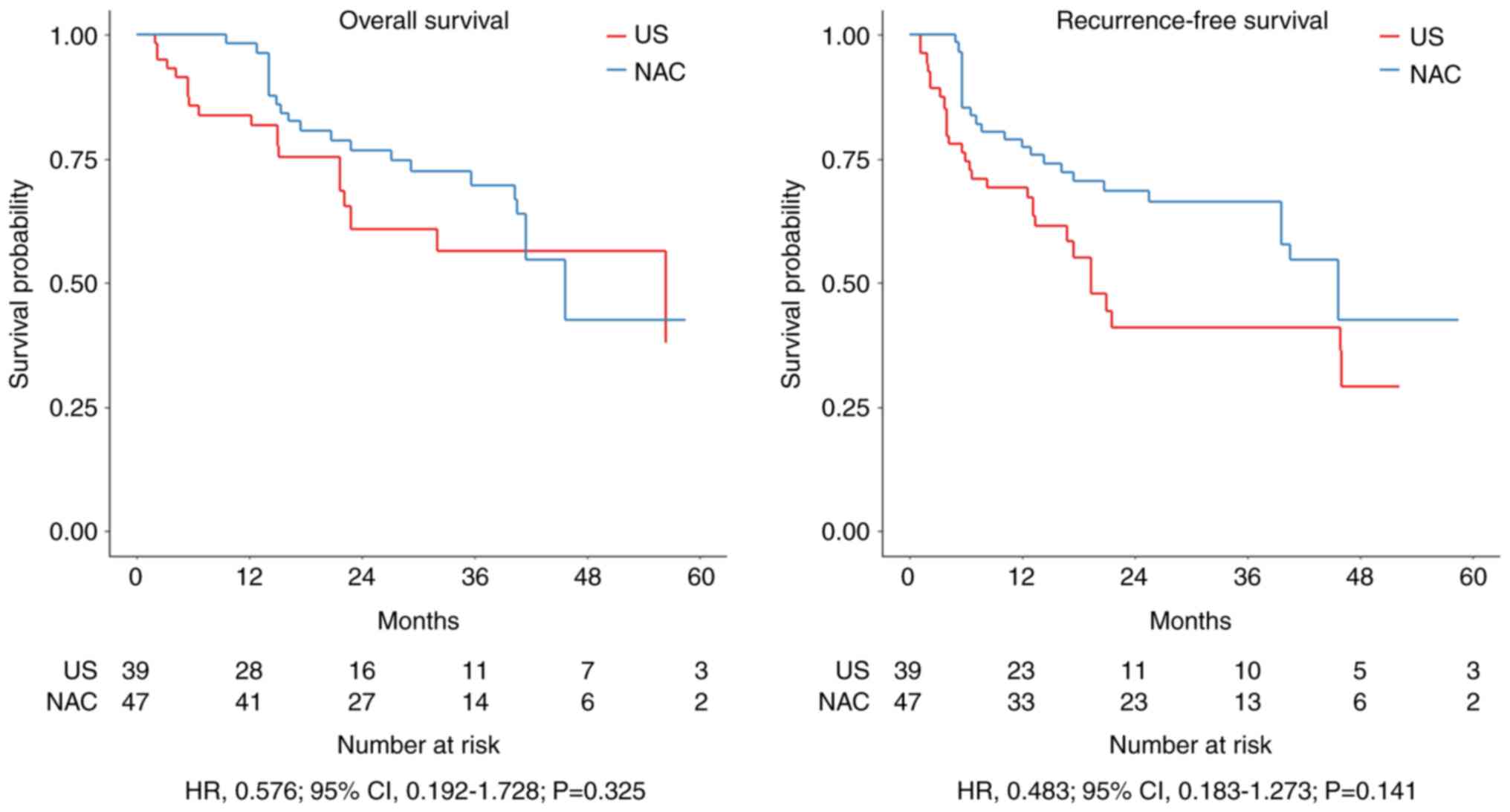
Kaplan-Meier survival curves for OS and RFS in the
IPW cohort are shown in Fig. 2.
Prognosis was not significantly different between the NAC group and
US group (3-year OS: HR=0.576; P=0.325 and 3-year RFS: HR=0.483;
P=0.141). The incidence of adverse events of Grade 3 or higher in
the NAC group was 20 (42.6%) for hematologic toxicity and 9 (19.1%)
for non-hematologic toxicity (Table
III). In the NAC group, 32 patients (68.1%) underwent
thoracoscopic surgery, and 15 patients (31.9%) underwent open
thoracotomy, whereas in the US group, the numbers were 29 patients
(74.4%) and 10 patients (25.6%), respectively. Operative time,
amount of blood loss, and postoperative complications can be
compared between the two groups in Table IV. In both groups, pneumonia
occurred in about 20% and recurrent nerve palsy in about 10% of the
patients, but the differences were non-significant. However,
anastomotic leakage was significantly more common in the US group.
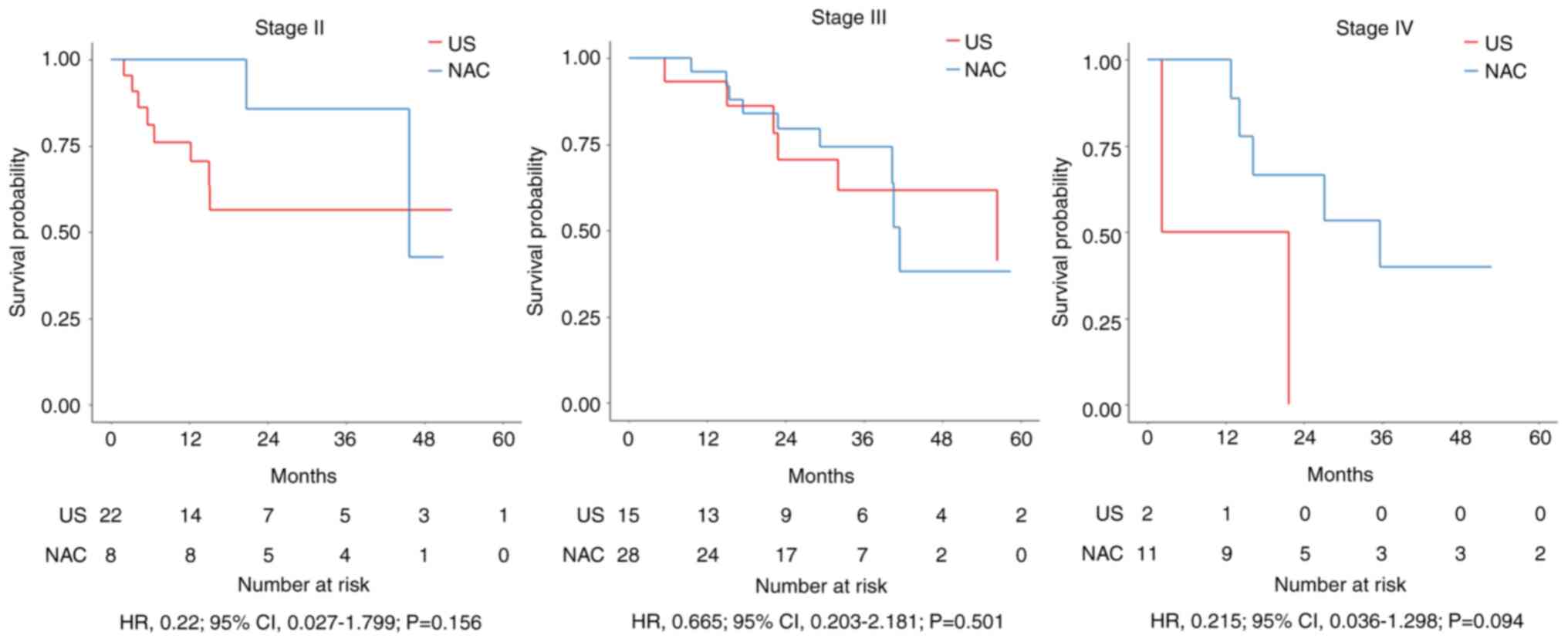
We compared OS by pathological stage between the NAC and US groups
but observed no significant difference for any stage (3-year OS for
stages II, III, and IV: P=0.156, P=0.501, and P=0.094,
respectively) (Fig. 3). There were
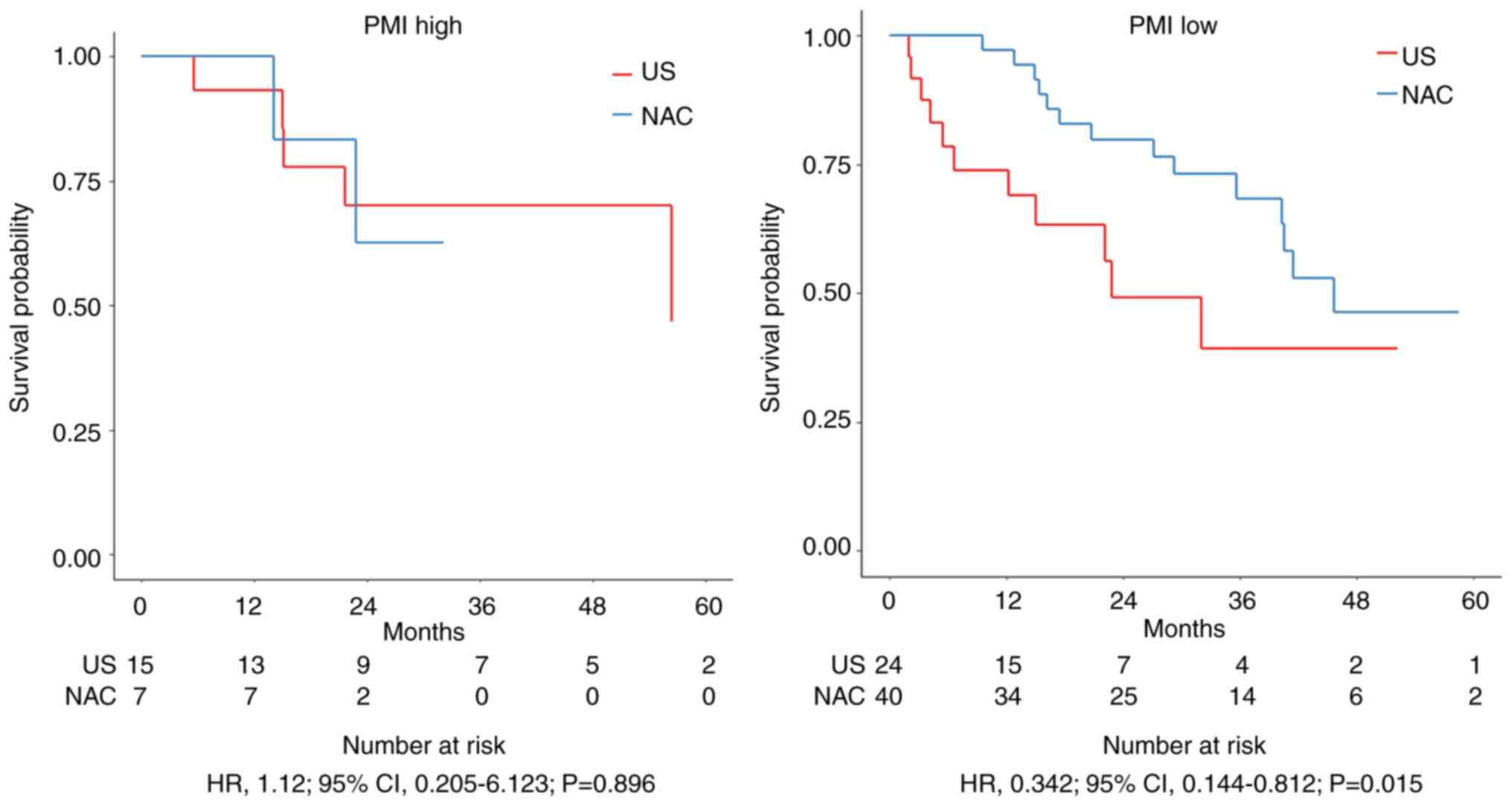
22 patients (25.6%) in the PMI high group and 64 patients (74.4%)
in the PMI low group. No significant difference in 3-year OS was
found in the PMI high group (HR=1.12; 95% confidence interval [CI],
0.205–6.123; P=0.896), but in the PMI low group, it was
significantly prolonged in the NAC group compared to the US group
(HR=0.342; 95% CI, 0.144–0.812; P=0.015) (Fig. 4).
 | Table III.Adverse events in the neoadjuvant
chemotherapy group (n=47). |
Table III.
Adverse events in the neoadjuvant
chemotherapy group (n=47).
| Adverse events | No. (%) |
|---|
| All grades (CTCAE
ver 5.0) | 39 (83.0) |
| Grade 2 or
lower |
|
|
Hematologic toxicity | 26 (55.3) |
|
Non-hematologic toxicity | 13 (27.7) |
| Grade 3 or
higher |
|
|
Hematologic toxicity | 20 (42.6) |
|
Leukopenia | 9 (19.1) |
|
Neutropenia | 5 (10.6) |
|
Thrombocytopenia | 3 (6.4) |
|
Anemia | 3 (6.4) |
|
Non-hematologic toxicity | 9 (19.1) |
|
Anorexia | 5 (10.6) |
|
Fatigue | 3 (6.4) |
|
Hyponatremia | 1 (2.1) |
 | Table IV.Surgical results in both groups. |
Table IV.
Surgical results in both groups.
| Variable | Overall (n=86) | NAC (n=47) | US (n=39) |
P-valuea |
|---|
| Median operation
time, min (IQR) | 486 (431, 551) | 481 (436, 530) | 492 (429, 594) | 0.435 |
| Median amount of
blood loss, ml (IQR) | 220 (110, 358) | 165 (80, 320) | 244 (130, 408) | 0.085 |
| Pneumonia (CD ≥II),
n (%) | 19 (22) | 10 (21) | 9 (23) | >0.999 |
| Anastomotic leakage
(CD ≥III), n (%) | 5 (6) | 0 (0) | 5 (13) | 0.017 |
| Recurrent nerve
paralysis (CD ≥II), n (%) | 9 (10) | 5 (11) | 4 (10) | >0.999 |
| Other complications
(CD ≥II), n (%) | 21 (24) | 11 (23) | 10 (26) | >0.999 |
Discussion
Surgery is a particularly invasive treatment for
ESCC. Nevertheless, it has remained the primary form of treatment
for locally advanced ESCC even though perioperative treatment has
intensified and improved the prognosis. Recent advances have
increased the safety of surgical treatment, and more facilities are
actively performing surgery on older patients with ESCC (17). In a study comparing 50 esophageal
cancer patients ≥75 years old with 100 patients <75 years old,
Kanda et al (18) reported
no significant differences in postoperative complications. Morita
et al (19) reported a
morbidity rate of 25% for esophagectomy in patients ≥80 years old
and found the incidences of surgical and medical complications to
be similar to those for patients <70 years old. Moreover, they
reported a decreased morbidity rate even in their patients >80
years old by following strict indications for surgery and
performing a less invasive operation (omitting supraclavicular
lymphadenectomy and performing a two-stage operation for risky
patients). In their study of 5,066 patients aged 75–79 years old
with ESCC, Motoyama et al (20) reported that surgery significantly
prolonged OS compared to chemoradiation therapy or chemotherapy
alone in advanced esophageal cancer of stage II or higher. In
contrast, Miyata et al (21)
reported that among 722 esophageal cancer patients >70 years old
divided into four groups according to age, respiratory and cardiac
complications increased with age. Older patients are particularly
faced with many age-specific problems, such as aspiration pneumonia
from delayed recovery of swallowing function, prolonged
hospitalization due to decreased activities of daily living, and
even progression of dementia.
There are several reports on the benefits of NAC to
treat esophageal cancer in older patients. Yamashita et al
(22) compared data on patients
aged ≥75 years with advanced ESCC receiving NAC or not and found a
better prognosis in those patients responding pathologically to
NAC. However, in their patients with a PS of 1 or higher, the
prognostic value of NAC was not clear, and they suggested that this
group could likely undergo surgery alone. Among older patients with
ESCC and a poor PS, Booka et al (23) found NAC to be non-beneficial and
considered an increase in postoperative complications as the reason
for NAC worsening the prognosis of these patients. Matsuda et
al (24) similarly reported no
survival benefit with preoperative DCF, the current standard of
treatment, in patients >76 years old. Furthermore, they reported
that pneumonia and anastomotic leakage as postoperative
complications were negative prognostic factors for shorter OS and
RFS in patients with esophageal cancer who were >75 years old
and had undergone preoperative therapy with DCF (25).
Myelosuppression may be reduced by the divided
administration of TXT and CDDP without greatly changing its
efficacy (10). Neutropenia was the
most common Grade 3 or higher toxicity in 31.3% of the patients in
the biweekly treatment regimen, whereas Kato et al (7) reported that 85% of their patients
developed Grade 3 or higher neutropenia. In the present study, we
limited the NAC regimen to biweekly DCF. Although this regimen was
reported to be a less toxic and potentially effective treatment, it
did not show usefulness as NAC in an older population (9,10).
This result is similar to and supports that reported in the
previous literature (22,23,25).
Although there is no difference in long-term prognosis, it may be
better for older patients with ESCC to undergo US to avoid the side
effects and decreased physical strength resulting from NAC. In our
examination of surgical outcomes, the incidence of failure
resulting in anastomotic leakage was different between the NAC
group and US group. This was presumably due to differences in fine
anastomotic technique and gastric tube construction between
centers.
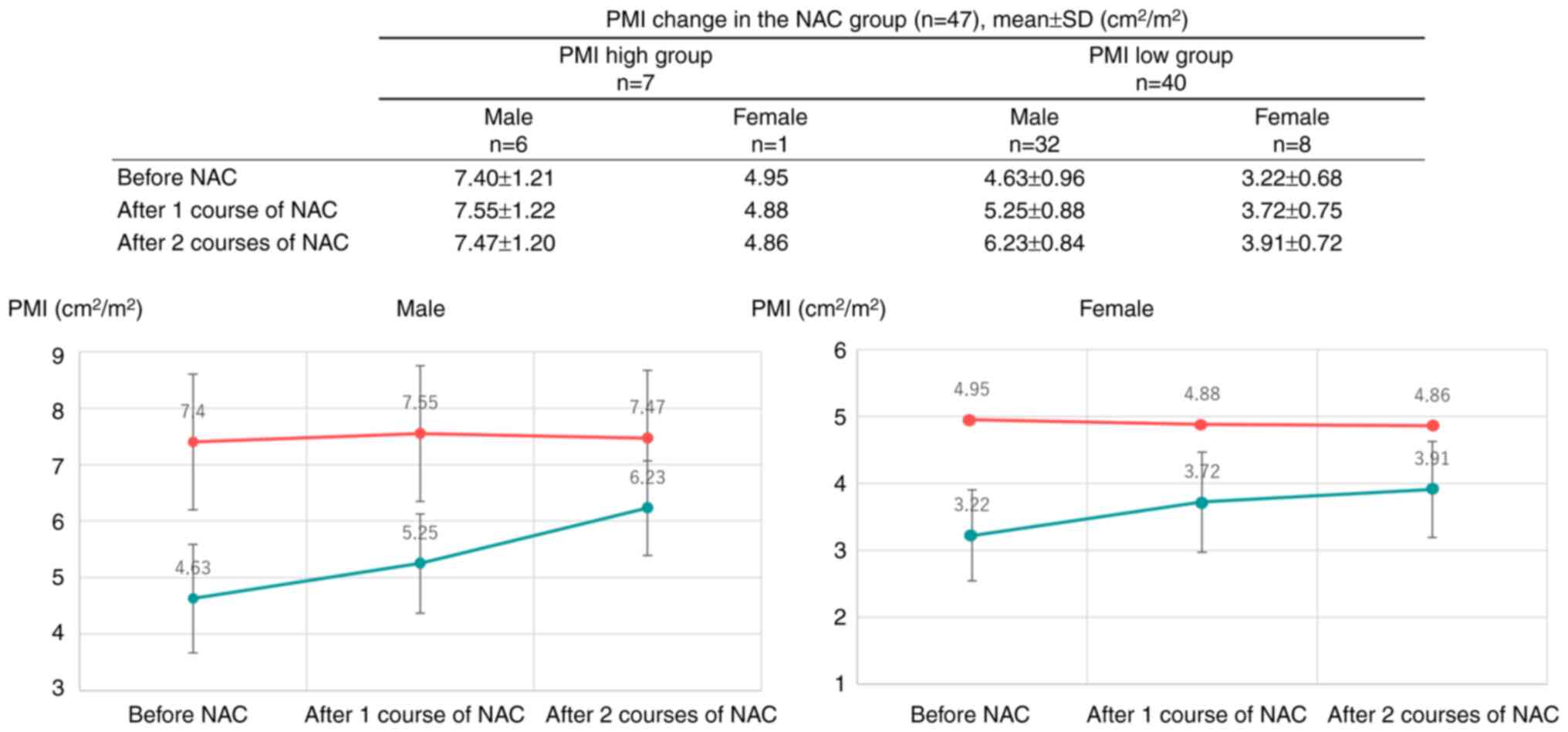
In NAC for ESCC, the PMI has a significant effect on
differences in chemotherapy response rates and adverse event rates
(11,14,26,27).
Our cohort showed significantly prolonged 3-year OS in the PMI low
group of the NAC group compared to that in the US group. The usual
duration of NAC of eight weeks or more is an active period of
nutritional management and intervention with rehabilitation. The
present results suggest that for older patients with ESCC and low
PMI, the duration of NAC may also lead to a period of careful
preoperative preparation, which may result in a favorable outcome
by selecting eligible patients for surgery. In fact, the PMI low
group tended to have higher PMI due to multifaceted therapeutic
interventions during the NAC (Fig.
5). However, as low PMI itself is a favorable factor for
adverse events, it is important to perform NAC safely and in
conjunction with the delivery of adequate nutritional therapy and
rehabilitation that maintains muscle mass. It is possible that the
positive impact of lower toxicity by dividing DCF into a biweekly
regimen had an oncological effect in the low PMI group.
This study has several limitations. First, selection
bias was likely present due to the retrospective nature of the
study. Second, although this study focused only on a treatment
regimen of biweekly DCF, dose intensities were not analyzed.
Further, patients unable to undergo esophagectomy with no known
reason for discontinuation, such as disease progression or toxicity
during NAC, were excluded. Third, limited information was collected
about patient characteristics, and preoperative pulmonary function
or other factors were not evaluated. No power calculations were
performed in the PMI study because recruitment was opportunistic.
Fourth, consensus on the indications for postoperative adjuvant
therapy in the US group was lacking. Fifth, there was a relatively
short observation period.
We found that compared to US, a biweekly DCF
treatment regimen did not prolong OS and RFS at all stages in
patients with advanced ESCC who were ≥70 years old. Further
prospective large-scale studies will be required to develop an
optimal treatment strategy that is less toxic to but maintains
efficacy in older patients with advanced ESCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS, YT, RT, TS, RA, TI, MY, NN, DW and NM
contributed to study conception and design. Material preparation,
data collection and analysis were performed by YS, YT, RT, TS, RA,
TI and DW, and MY, NN and NM provided academic advice. YS wrote the
first draft of the manuscript, and all authors commented on
previous versions of the manuscript. YS and NM confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Gifu
University School of Medicine Ethics Committee (ID: 2022-232; Gifu,
Japan). Informed consent was obtained in writing from all
individual participants included in the study.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of this original article and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
NAC
|
neoadjuvant chemotherapy
|
|
DCF
|
docetaxel, cisplatin,
5-fluorouracil
|
|
PS
|
performance status
|
|
TXT
|
docetaxel
|
|
CDDP
|
cisplatin
|
|
5-FU
|
5-fluorouracil
|
|
US
|
upfront surgery
|
|
CT
|
computed tomography
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
PMI
|
psoas muscle index
|
|
IPW
|
inverse probability weighting
|
|
HR
|
hazard ratio
|
References
|
1
|
Watanabe M, Toh Y, Ishihara R, Kono K,
Matsubara H, Miyazaki T, Morita M, Murakami K, Muro K, Numasaki H,
et al: Comprehensive registry of esophageal cancer in Japan, 2015.
Esophagus. 20:1–28. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oguma J, Ozawa S, Ishiyama K and Daiko H:
Clinical significance of sarcopenic dysphagia for patients with
esophageal cancer undergoing esophagectomy: A review. Ann
Gastroenterol Surg. 6:738–745. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, et al:
Surgery plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: A Japan Clinical
Oncology Group Study-JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitagawa Y, Ishihara R, Ishikawa H, Ito Y,
Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, et al:
Esophageal cancer practice guidelines 2022 edited by the Japan
esophageal society: Part 1. Esophagus. 20:343–372. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitagawa Y, Ishihara R, Ishikawa H, Ito Y,
Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, et al:
Esophageal cancer practice guidelines 2022 edited by the Japan
Esophageal Society: Part 2. Esophagus. 20:373–389. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura K, Kato K, Igaki H, Ito Y,
Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, et
al: Three-arm phase III trial comparing cisplatin plus 5-FU (CF)
versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy
with CF (CF-RT) as preoperative therapy for locally advanced
esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol.
43:752–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato K, Machida R, Ito Y, Daiko H, Ozawa
S, Ogata T, Hara H, Kojima T, Abe T, Bamba T, et al: Doublet
chemotherapy, triplet chemotherapy, or doublet chemotherapy
combined with radiotherapy as neoadjuvant treatment for locally
advanced oesophageal cancer (JCOG1109 NExT): A randomised,
controlled, open-label, phase 3 trial. Lancet. 404:55–66. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka Y, Yoshida K, Sanada Y, Osada S,
Yamaguchi K and Takahashi T: Biweekly docetaxel, cisplatin, and
5-fluorouracil (DCF) chemotherapy for advanced esophageal squamous
cell carcinoma: A phase I dose-escalation study. Cancer Chemother
Pharmacol. 66:1159–1165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Yoshida K, Yamada A, Tanahashi
T, Okumura N, Matsuhashi N, Yamaguchi K and Miyazaki T: Phase II
trial of biweekly docetaxel, cisplatin, and 5-fluorouracil
chemotherapy for advanced esophageal squamous cell carcinoma.
Cancer Chemother Pharmacol. 77:1143–1152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato Y, Tanaka Y, Imai T, Okumura N,
Matsuhashi N, Takahashi T, Shimokawa T and Yoshida K: Serum diamine
oxidase activity derived from response to chemotherapy affects
adverse events and serum amino acid levels. Support Care Cancer.
30:9369–9377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
Wiley-Blackwell; Hoboken, NJ: 2016
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato Y, Tanaka Y, Imai T, Ojio H, Mase J,
Hatanaka Y, Suetsugu T, Fujii H, Okumura N, Matsuhashi N, et al:
Effect of biweekly-DCF neoadjuvant chemotherapy on skeletal muscle
mass of esophageal cancer patients. Gan To Kagaku Ryoho. 49:47–52.
2022.(Japanese). PubMed/NCBI
|
|
15
|
Kaido T, Ogawa K, Fujimoto Y, Ogura Y,
Hata K, Ito T, Tomiyama K, Yagi S, Mori A and Uemoto S: Impact of
sarcopenia on survival in patients undergoing living donor liver
transplantation. Am J Transplant. 13:1549–1556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamaguchi Y, Kaido T, Okumura S, Kobayashi
A, Shirai H, Yao S, Yagi S, Kamo N, Okajima H and Uemoto S:
Proposal for new selection criteria considering pre-transplant
muscularity and visceral adiposity in living donor liver
transplantation. J Cachexia Sarcopenia Muscle. 9:246–254. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamamoto Y, Akutsu Y, Nagashima F,
Hironaka S, Ito Y, Kato K, Hara H, Tsubosa Y, Nakagawa S, Daiko H,
et al: Multicenter questionnaire survey on patterns of care for
elderly patients with esophageal squamous cell carcinoma by the
Japan Esophageal Oncology Group. Jpn J Clin Oncol. 46:111–115.
2016.PubMed/NCBI
|
|
18
|
Kanda M, Koike M, Tanaka C, Kobayashi D,
Hayashi M, Yamada S, Nakayama G, Omae K and Kodera Y: Feasibility
of subtotal esophagectomy with systematic lymphadenectomy in
selected elderly patients with esophageal cancer; a propensity
score matching analysis. BMC Surg. 19:1432019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita M, Egashira A, Yoshida R, Ikeda K,
Ohgaki K, Shibahara K, Oki E, Sadanaga N, Kakeji Y and Maehara Y:
Esophagectomy in patients 80 years of age and older with carcinoma
of the thoracic esophagus. J Gastroenterol. 43:345–351. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motoyama S, Maeda E, Iijima K, Sato Y,
Koizumi S, Wakita A, Nagaki Y, Fujita H, Yoneya T, Imai K, et al:
Does esophagectomy provide a survival advantage to patients aged 80
years or older? Analyzing 5066 patients in the National Database of
Hospital-based Cancer Registries in Japan. Ann Surg. 276:e16–e23.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyata H, Yamasaki M, Makino T, Miyazaki
Y, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M and
Doki Y: Clinical outcome of esophagectomy in elderly patients with
and without neoadjuvant therapy for thoracic esophageal cancer. Ann
Surg Oncol. 22 (Suppl 3):S794–S801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamashita K, Momose K, Tanaka K, Makino T,
Saito T, Yamamoto K, Takahashi T, Kurokawa Y, Nakajima K, Eguchi H
and Doki Y: Indications for neoadjuvant chemotherapy in older
patients undergoing esophagectomy for esophageal cancer. Surg
Today. 54:442–451. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Booka E, Haneda R, Ishii K, Tsushima T,
Yasui H and Tsubosa Y: The negative impact of preoperative
chemotherapy on survival after esophagectomy for vulnerable elderly
patients with esophageal cancer. Ann Surg Oncol. 28:1786–1795.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda S, Kitagawa Y, Takemura R, Okui J,
Okamura A, Kawakubo H, Muto M, Kakeji Y, Takeuchi H, Watanabe M and
Doki Y: Real-world evaluation of the efficacy of neoadjuvant DCF
over CF in esophageal squamous cell carcinoma: Propensity
score-matched analysis from 85 authorized institutes for esophageal
cancer in Japan. Ann Surg. 278:e35–e42. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuda S, Kitagawa Y, Okui J, Okamura A,
Kawakubo H, Takemura R, Muto M, Kakeji Y, Takeuchi H, Watanabe M
and Doki Y: Old age and intense chemotherapy exacerbate negative
prognostic impact of postoperative complication on survival in
patients with esophageal cancer who received neoadjuvant therapy: A
nationwide study from 85 Japanese esophageal centers. Esophagus.
20:445–454. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka Y, Ueno T, Yoshida N, Akutsu Y,
Takeuchi H, Baba H, Matsubara H, Kitagawa Y and Yoshida K: The
effect of an elemental diet on oral mucositis of esophageal cancer
patients treated with DCF chemotherapy: A multi-center prospective
feasibility study (EPOC study). Esophagus. 15:239–248. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka Y, Shimokawa T, Harada K and
Yoshida K: Effectiveness of elemental diets to prevent oral
mucositis associated with cancer therapy: A meta-analysis. Clin
Nutr ESPEN. 49:172–180. 2022. View Article : Google Scholar : PubMed/NCBI
|