Introduction
Currently, the main treatment modalities for
colorectal cancer (CRC) include surgery, chemotherapy,
radiotherapy, targeted therapy and immunotherapy. Common
chemotherapy drugs include 5-fluorouracil, oxaliplatin and
irinotecan (1). However, cancer
cells can develop resistance to these chemotherapeutic drugs
through mechanisms such as drug inactivation, alterations in drug
influx and efflux and overexpression of ATP-binding cassette
transport proteins (2).
Radiotherapy is primarily used for locally advanced rectal cancer,
with preoperative radiotherapy able to shrink tumors, increase
surgical resection rates and reduce postoperative recurrence
(3). Targeted therapies such as
anti-EGFR (cetuximab) and anti-VEGF (bevacizumab) drugs, have
demonstrated marked efficacy in the treatment of metastatic CRC
(1). Previous studies have also
explored new targets and combinations to enhance the specificity
and effectiveness of these treatments (4,5).
Immunotherapy has made notable progress in CRC, particularly with
immune checkpoint inhibitors (pembrolizumab and nivolumab) showing
efficacy in patients with high microsatellite instability or
mismatch repair deficiency (6).
Despite these treatment options, ~50% of patients with CRC still
experience incurable recurrent CRC. Therefore, there is an urgent
need to develop new targeted therapeutic strategies to meet the
clinical needs of patients with CRC (7).
Natural products have been a notable source of
several novel chemotherapeutic drugs. In previous decades, natural
compounds have served a crucial role in the development of
anticancer drugs, offering several active molecules with novel
structures (8,9). Although natural products and their
analogues, such as paclitaxel and camptothecin (10), have become indispensable in clinical
practice, the treatment of CRC still faces numerous challenges.
Recent studies have suggested that evodiamine may inhibit the
development of CRC by modulating the gut microbiome and suppressing
intestinal inflammation (10–12).
Additionally, bioinformatic methods based on high-throughput DNA
sequencing have identified tumor-specific antigens associated with
single nucleotide variants, offering a new strategy for the
treatment of CRC (13).
Jujuboside B (JUB), a natural saponin compound, is
one of the main active components of Ziziphus jujuba and
possesses a wide range of pharmacological effects and application
values. JUB exhibits multiple biological activities, including
antibacterial, anti-inflammatory and antioxidant properties
(13,14). Previously, JUB has been reported to
induce apoptosis and demonstrate antitumor activity in several
tumor types. In breast cancer, its antitumor effects are associated
with the induction of apoptosis and autophagy (15). Moreover, JUB can inhibit
angiogenesis and tumor growth by blocking the VEGF receptor 2
signaling pathway (16).
Additionally, JUB inhibits intimal hyperplasia and vascular smooth
muscle cell dedifferentiation, proliferation and migration through
activating the adenosine monophosphate-activated protein
kinase/peroxisome proliferator-activated receptor γ signaling
(17). Despite these findings, the
full antitumor potential and underlying mechanisms of JUB remain
unclear.
The mitogen-activated protein kinase (MAPK)
signaling pathway is closely related to several biological
processes, including cell proliferation, differentiation, apoptosis
and angiogenesis. Additionally, MAPK is a fundamental pathway in
mammalian cells (18). Previous
studies have reported that the abnormal activation of certain
proteins within the MAPK pathway is associated with the occurrence
and development of several cancers, such as liver cancer (19) and CRC (20). Hence, targeting proteins associated
with this pathway can serve as an effective strategy for cancer
treatment. However, whether JUB regulates the MAPK pathway in CRC
remains to be elucidated. Therefore, the present study aimed to
investigate the effects of JUB on the proliferation and apoptosis
of human CRC cells, as well as its regulatory impact on the MAPK
pathway.
Materials and methods
Cell culture
The CRC HCT116 cell line was purchased from the
National Collection of Authenticated Cell Cultures. The cells were
cultured in Roswell Park Memorial Institute 1640 medium (HyClone;
Cytiva) containing 10% fetal bovine serum (HyClone; Cytiva) and 100
U/ml penicillin (HyClone; Cytiva) in an incubator at 37°C with 5%
CO2.
Cell viability assay
An MTT assay was utilized to assess the viability of
HCT116 cells treated with varying concentrations of JUB
(Sigma-Aldrich; Merck KGaA). Cells in the logarithmic growth phase
were seeded into a 96-well culture plate at a density of
5×103 cells/well, with a total volume of 100 µl. The
cells were then cultured at 37°C for 48 h in media (Gibco)
containing different concentrations of JUB (0, 5, 10, 20, 40 and 80
µM). Subsequently, the cells were incubated with MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA) in the dark at 37°C for 2 h.
Subsequently, dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was
added to dissolve the formazan crystals. Finally, the optical
density at 570 nm was measured using an ELx800 microplate reader
(BioTek; Agilent Technologies, Inc.).
Cell colony formation assay
A colony formation assay was used to assess cell
proliferation under different experimental treatments. Briefly,
HCT116 cells in the logarithmic growth phase were digested,
centrifuged at 300 × g for 5 min at 4°C, resuspended and counted.
Subsequently, HCT116 cells (1,000 cells/well) treated with 0, 10,
20 and 40 µM JUB were seeded in 6-well plates and cultured at 37°C
for 1 week. Once the colonies became visible to the naked eye, the
plates were taken out and the medium was discarded. After rinsing
with phosphate buffered saline (PBS), the cells were fixed with 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) at room temperature
for 20 min. The paraformaldehyde was then discarded and the cells
were stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA)
at room temperature for 3 min. Upon washing off the crystal violet,
visible cell clusters containing at least 50 cells were captured
and counted using a light microscope (Nikon Corporation) and
manually counted.
Flow cytometry
HCT116 cells were seeded in 6-well plates at a
density of 4×105 cells/well and treated with varying
concentrations of JUB (0, 10, 20 and 40 µM). After 48 h, the cells
were harvested using trypsin and washed twice with PBS before being
collected into Eppendorf tubes. The treated cells were then
resuspended using 195 µl Annexin V-Fluorescein isothiocyanate
binding solution (Beyotime Institute of Biotechnology).
Subsequently, 5 µl Annexin V-Fluorescein isothiocyanate and 10 µl
propidium iodide (Thermo Fisher Scientific, Inc.) staining solution
were added, followed by gentle agitation of the mixture. The cells
were then incubated in the dark at room temperature (20–25°C) for
10–20 min. After adding 400 µl binding buffer, the cells were
analyzed using an LSRFortessa™ flow cytometer (BD Biosciences).
Transmission electron microscopy
(TEM)
After exposing the human CRC HCT116 cell line to
different concentrations of JUB (0, 10, 20 and 40 µM) for 24 h, the
samples were fixed overnight at 4°C in 2.5% glutaraldehyde (v/v) in
0.1 M PBS buffer, followed by post-fixation with 1% osmium
tetroxide in the same buffer at 4°C for 2 h. The samples were then
dehydrated in graded ethanol series (30, 50, 70, 90, and 100%) at
room temperature for 15 min each, followed by dehydration in
ethanol/epoxy resin gradients (3:1, 1:1, 1:3 and 0:1) at room
temperature for 15 min each before embedding in epoxy resin for 12
h. Ultrathin sections (60–80 nm) were prepared using an
ultramicrotome, stained with 3% uranyl acetate and lead citrate and
observed under a transmission electron microscope to analyze the
ultrastructural changes in the cells.
Western blot
Total proteins were extracted from the cells using
radioimmunoprecipitation assay lysis buffer (cat. no. 89900; Thermo
Fisher Scientific, Inc.) and protein concentration was determined
using a bicinchoninic acid assay. Subsequently, 10 µg protein/lane
and subjected to 10–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis for 120 min, transferred to a polyvinylidene
fluoride membrane (cat. no. LC2005; Thermo Fisher Scientific, Inc.)
using a wet transfer method, and then blocked with 5% skimmed milk
at room temperature for 2 h. Primary antibodies were applied
overnight incubation at 4°C. The primary antibodies (all Abcam)
were as follows: Anti-B-cell lymphoma-2 (Bcl-2, 1:1,000, ab241548),
anti-Bcl-2 associated X-protein (Bax, 1:1,000, ab3191),
anti-cleaved caspase-3 (1:1,000, ab2302), anti-caspase-3 (1:1,000,
ab184787), anti-solute carrier family 7 member 11 (SLC7A11,
1:1,000, ab307601), anti-glutathione peroxidase 4 (GPX4, 1:1,000,
ab252833), anti-acyl-CoA synthetase long chain family member 4
(ACSL4, 1:1,000, ab155282), anti-transferrin receptor 1 (TFR1,
1:1,000, ab214039), anti-MAPK kinase (MEK, 1:1,000, ab265586),
anti-phosphorylated (p)-MEK (1:1,000; cat. no. ab307509),
anti-p-extracellular signal-regulated kinase (ERK, 1:1,000,
ab32537), anti-p-ERK (1:1,000, ab201015) and anti-GAPDH, 1:1,000,
ab8245, Abcam). After washing three times with tris buffered saline
with 0.1% Tween 20 (TBST), the membrane was incubated with
HRP-conjugated goat anti-rabbit IgG (1:1,000, ab181662, Abcam) or
anti-mouse IgG (1:1,000; cat. no. ab181662, Abcam) for 2 h. After
three additional washes with TBST, the protein bands were
visualized using electrochemiluminescence (MilliporeSigma; KGaA)
and recorded with a ChemiDoc EQ System (Bio-Rad Laboratories,
Inc.). The grayscale intensity of the bands was analyzed using
ImageJ software (version 1.53t, National Institutes of Health).
Measurement of reactive oxygen species
(ROS)
Cell suspensions were seeded into 6-well plates at a
density of 2×105 cells per well. After receiving 0 µM
(control group) and 40 µM JUB (JUB group) treatment for 48 h at
37°C, HCT116 cells were pretreated with 1 µM ferrostatin-1 (Fer-1),
a ferroptosis inhibitor (Selleck Chemicals) at 37°C for 2 h.
Subsequently, the cells were treated with 40 µM JUB for another at
37°C 48 h, constituting the JUB + Fer-1 group. Intracellular ROS
levels were then measured using flow cytometry using the
oxidation-activated fluorescent dye DCHF-DA (Beyotime Institute of
Biotechnology). Briefly, cell samples were incubated with PBS
containing 10 µM DCHF-DA at 37°C for 20 min. The cells were then
washed three times with PBS, digested with trypsin and washed twice
with cold PBS. Lastly, the ROS levels were detected using an
LSRFortessa™ flow cytometer (BD Biosciences). Flow cytometry data
were analyzed using FlowJo software (version X.0.7, BD
Biosciences).
Detection of malondialdehyde (MDA),
glutathione (GSH), total iron and ferrous iron (Fe2+)
levels
The levels of MDA, glutathione GSH, total iron and
Fe2+ in HCT116 cells of the control, JUB and JUB + Fer-1
groups were determined using the Lipid Peroxidation Assay Kit, GSH
Colorimetric Assay Kit (cat. no. A006-2), Total Iron Colorimetric
Assay Kit (A040) and Ferrous Iron Colorimetric Assay Kit (A039-2),
respectively (all Nanjing Jiancheng Bioengineering Institute). The
specific detection steps were followed according to the
manufacturer's protocols.
Statistical analysis
SPSS 19.0 (IBM Corp.) was used for data analysis and
data plotting was performed using GraphPad Prism 9.0 (Dotmatics).
All values are expressed as mean ± standard deviation. A one-way
analysis of variance was performed, followed by a Tukey's HSD post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of JUB on the viability and
proliferation of human CRC cells
To assess the hypothesis that JUB may have
anticancer activity, the effect of JUB on the viability of human
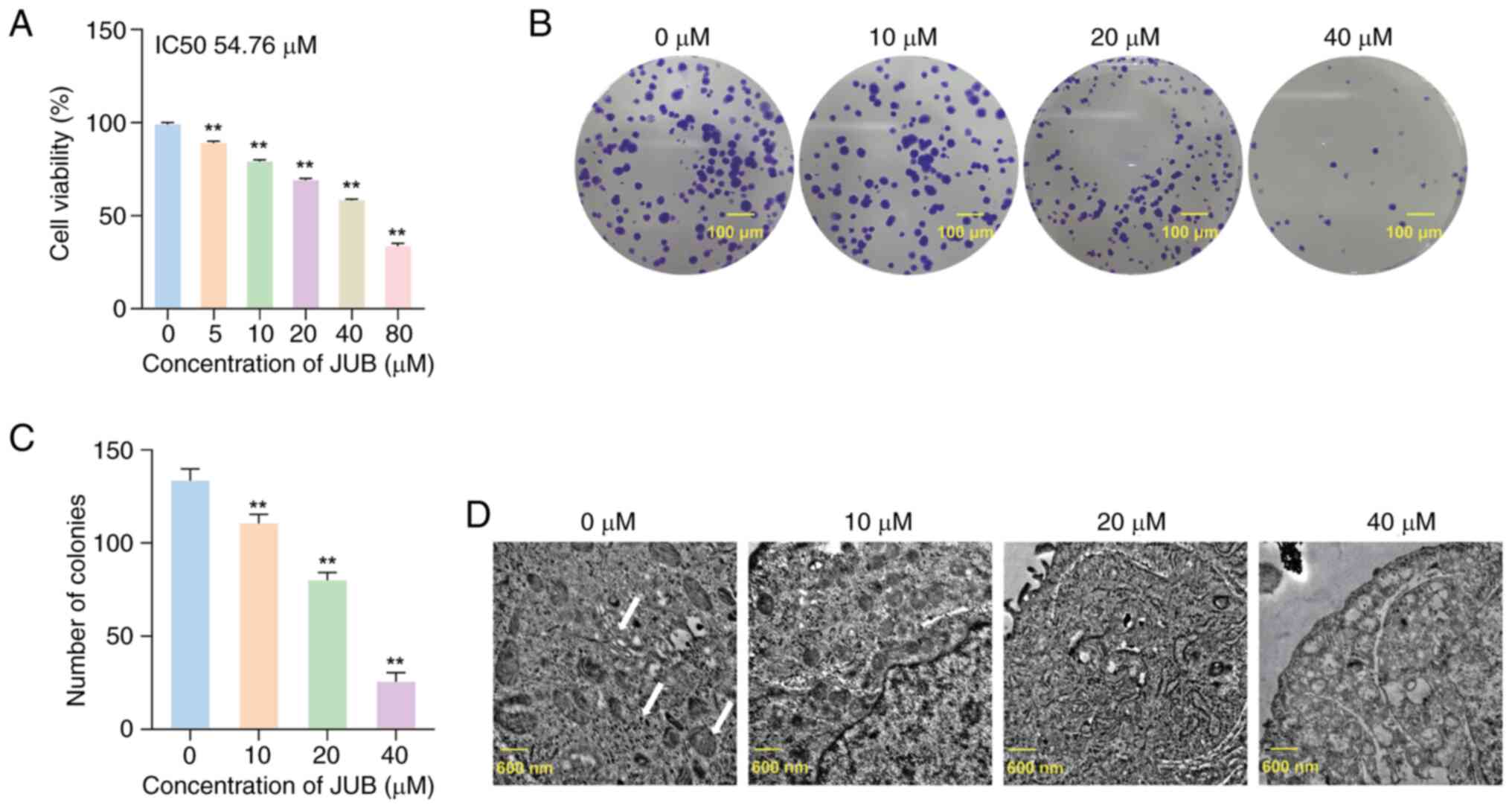
CRC HCT116 cells was assessed. The results of the MTT assay
demonstrated that, compared with the 0 µM group, the survival rate
of HCT116 cells was significantly decreased in the 5, 10, 20, 40
and 80 µM groups (all P<0.01), indicating that JUB had an
inhibitory effect on the viability of HCT116 cells in a
concentration-dependent manner (Fig.
1A). The colony formation assay results demonstrated that the
colony formation of HCT116 cells was significantly reduced in the
10, 20 and 40 µM groups (all P<0.01) in a
concentration-dependent manner compared with the 0 µM group
(Fig. 1B and C). These results
suggested that JUB could inhibit the viability and proliferation of
human CRC cells.
Ultrastructural changes induced by JUB
in promoting apoptosis of HCT-116 cells
High-magnification TEM demonstrated that different
concentrations of JUB induced marked effects on the endoplasmic
reticulum (ER) and mitochondrial structures in HCT-116 cells
(Fig. 1D). In the 0 µM JUB control
group, the ER in HCT-116 cells was neatly arranged with ribosomes
attached and the mitochondria were abundant and structurally
intact, with clearly visible cristae. As the concentration of JUB
increased, the 10 µM treatment group showed that the rough ER
exhibited degranulation, free ribosomes increased and certain
mitochondria became vacuolated. In the 20 µM treatment group, the
vacuolation of mitochondria increased, autophagosomes formed and
the ER expanded. In the 40 µM treatment group, cells exhibited
pronounced autophagic flux, notable mitochondrial swelling, cristae
fragmentation or loss and chromatin condensation with evident
marginalization. These results indicate that JUB induces
concentration-dependent damage to the ER and mitochondrial
structures in HCT-116 cells, with high concentrations of JUB
promoting autophagy and apoptosis-related ultrastructural
changes.
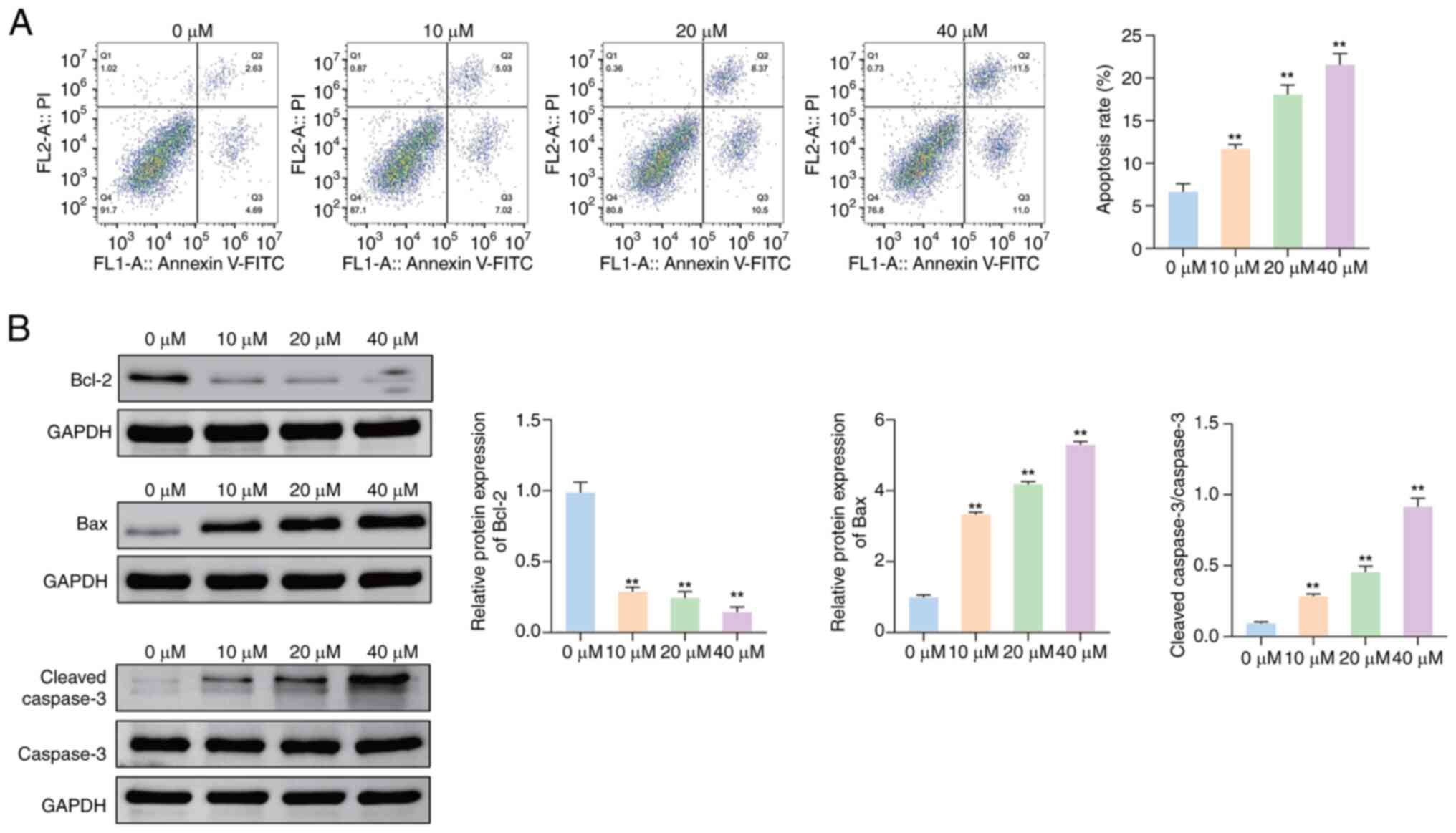
Effects of JUB on the apoptosis of
HCT116 cells
The results of the MTT and colony formation assays
suggested that JUB had an antitumor effect, prompting further
investigation into the underlying mechanisms of JUB. It was
uncertain whether the decreased viability of tumor cells with JUB
treatment was primarily due to apoptosis mechanisms or cell-cycle
dysregulation. Therefore, the rate of apoptosis of HCT116 cells
treated with different concentrations of JUB was evaluated. The
apoptosis rate of HCT116 cells was significantly increased in the
10, 20 and 40 µM groups compared with the 0 µM group (all
P<0.01), with higher concentrations associated with
higher apoptosis rates (Fig.
2A).
To further assess the effect of JUB on cell
apoptosis, the protein expression levels of three apoptotic
biomarkers in cells treated with different concentrations were
evaluated by western blotting. The protein expression level of
Bcl-2 was significantly decreased, whilst the protein expression
levels of Bax and the ratio of cleaved caspase-3/caspase-3 were
significantly increased in the 10, 20 and 40 µM groups compared
with the 0 µM group, in a concentration-dependent manner (Fig. 2B; all P<0.01). These results
indicate that JUB promotes apoptosis in HCT116 cells.
JUB induces apoptosis and ferroptosis
in CRC cells
Subsequently, the role of JUB (40 µM) in the
ferroptosis process in HCT116 cells was assessed. The results
demonstrated that the ROS levels of HCT116 cells were significantly
increased in the JUB group compared with the control group
(Fig. 3A and B; P<0.01) and the
JUB + Fer-1 group showed a significant reduction in ROS levels
compared with the JUB group (P<0.01). Additionally, the
levels of MDA, total iron and Fe2+ were significantly
increased in the JUB group compared with the control group, whilst
the GSH levels were significantly decreased (Fig. 3C-F; all P<0.01). The JUB + Fer-1
group showed a significant decrease in MDA, total iron and
Fe2+ levels, coupled with a significant increase in GSH
levels compared with the JUB group (all P<0.01).
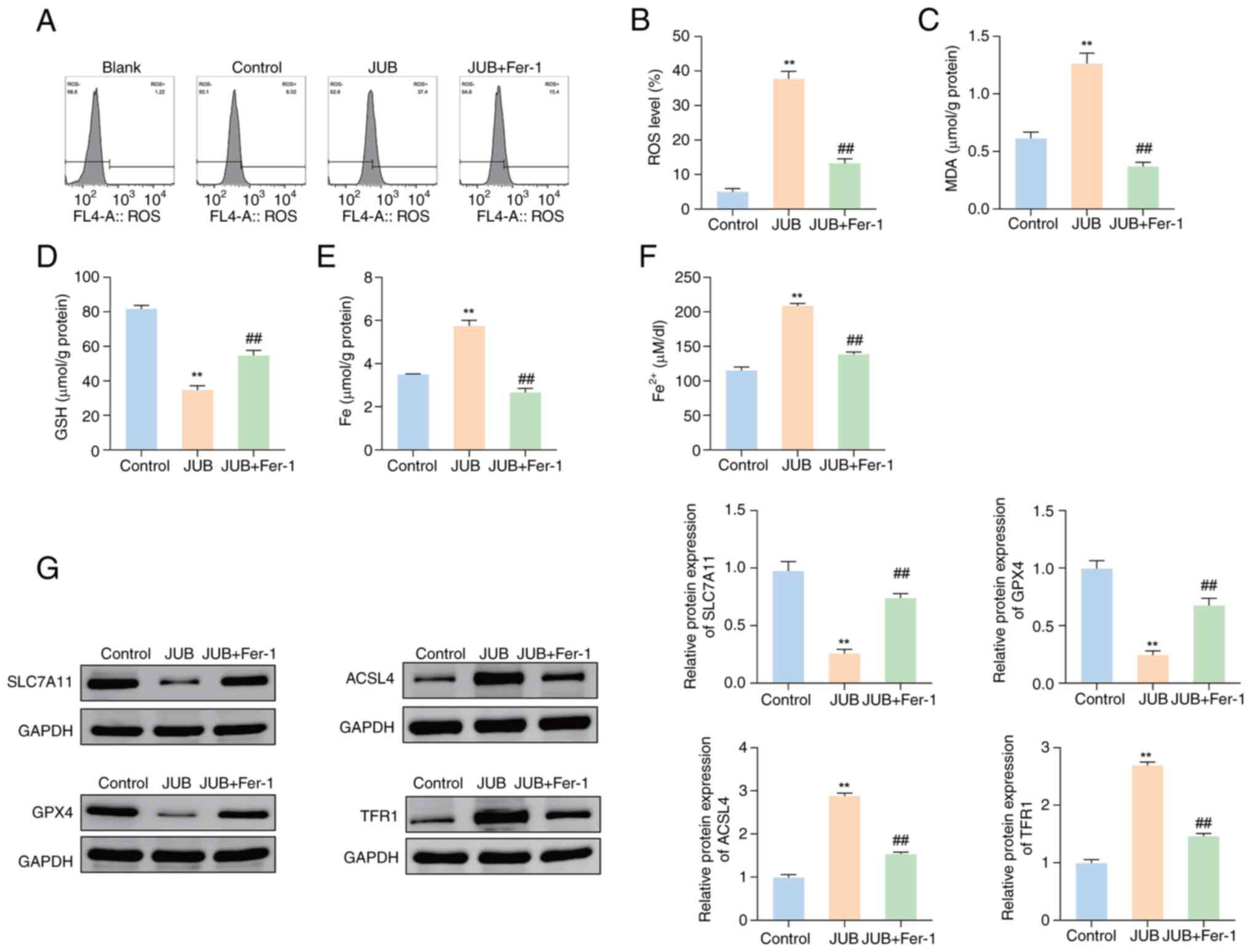 | Figure 3.JUB induces ferroptosis in HCT116
cells. For the JUB + Fer-1 group, HCT116 cells were pre-treated
with 1 µM Fer-1 (ferroptosis inhibitor) for 2 h, followed by
treatment with 40 µM JUB for 48 h. (A) Levels of ROS in HCT116
cells of each group were assessed by flow cytometry and (B)
subsequent quantification. Detection of (C) MDA and (D) GSH levels
was performed. (E) Total iron level in HCT116 cells was determined
using an iron assay kit. (F) Fe2+ level in HCT116 cells
was assessed using a Fe2+ assay kit. (G) Western
blotting was performed to analyze the protein expression levels of
SLC7A11, GPX4, ACSL4 and TFR1. **P<0.01 vs. control;
##P<0.01 vs. JUB. JUB, Jujuboside B; Fer-1,
ferrostatin-1; ROS, reactive oxygen species; MDA, malondialdehyde;
GSH, glutathione; SLC7A11, solute carrier family 7 member 11; GPX4,
glutathione peroxidase 4; ACSL4, acyl-CoA synthetase long chain
family member 4; TFR1, transferrin receptor 1; Fe2+,
ferrous iron. |
To further evaluate the role of JUB in the
ferroptosis process, the expression of four ferroptosis regulatory
factors (SLC7A11, GPX4, ACSL4 and TFR1) in different treatment
groups was assessed. The protein expression levels of SLC7A11 and
GPX4 were significantly downregulated in the JUB group compared
with the control group, whilst the protein expression levels of
ACSL4 and TFR1 proteins were significantly upregulated (Fig. 3G; all P<0.01). In comparison with
the JUB group, the JUB + Fer-1 group demonstrated a significant
increase in the protein expression of SLC7A11 and GPX4, and a
decrease in the expression of ACSL4 and TFR1 (Fig. 3G; all P<0.01). These
results indicate that JUB could induce ferroptosis in HCT116
cells.
Effect of JUB on the expression of
MAPK pathway-related proteins in HCT116 cells
Western blot analysis demonstrated a marked
reduction in the protein expression levels of p-MEK and p-ERK in
HCT116 cells treated with several concentrations of JUB, compared
with the 0 µM group (Fig. 4A).
There were no marked differences in the protein expression levels
of total MEK and ERK between the 0 µM group and other treatment
groups. JUB treatment at all concentrations significantly decreased
the ratios of p-MEK/MEK and p-ERK/ERK compared with the 0 µM
control group, in a concentration-dependent manner (Fig. 4B; all P<0.01). These results
suggest JUB could inhibit the MAPK signaling pathway in HCT116
cells.
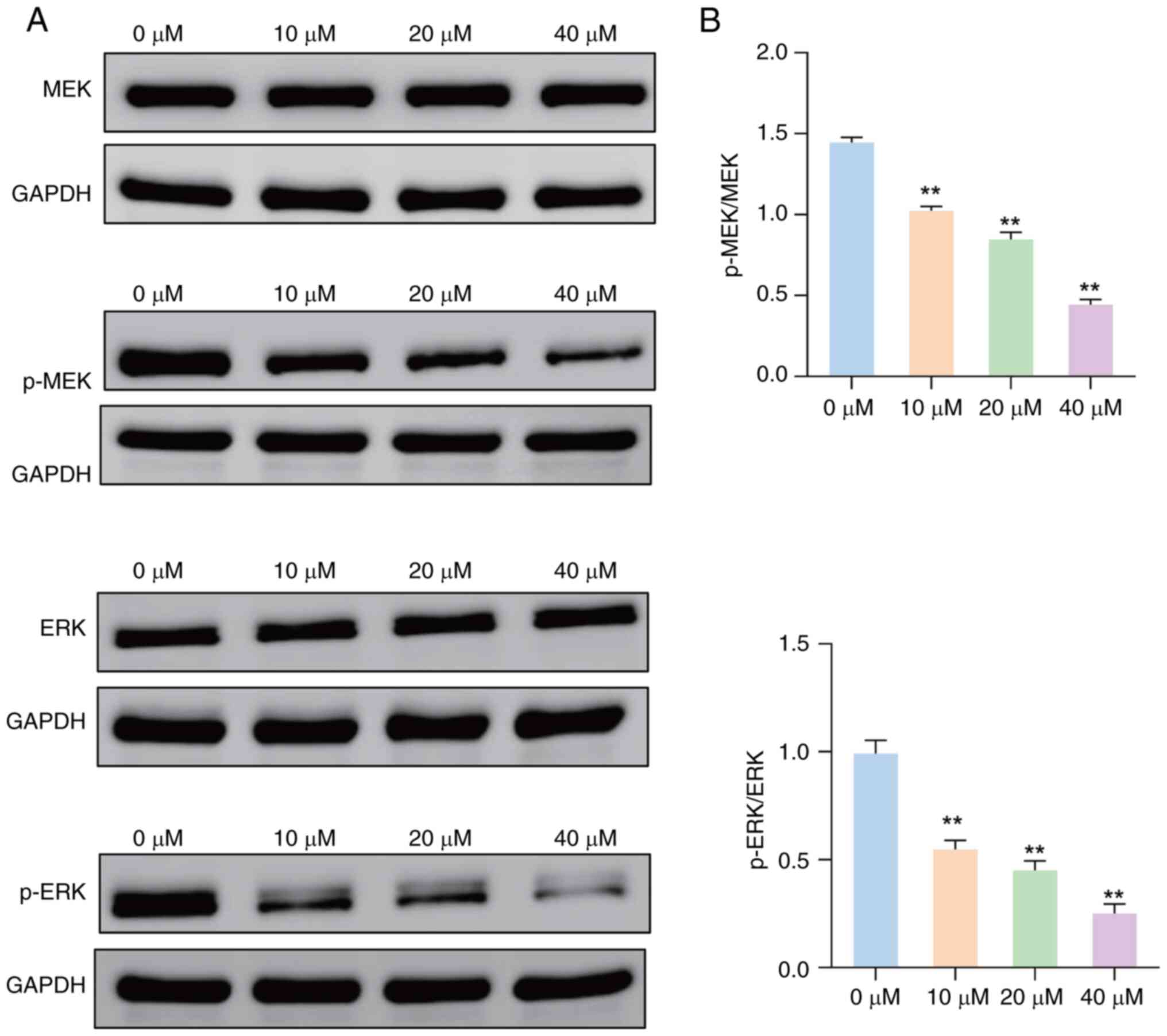 | Figure 4.Effect of JUB on the expression of
MAPK pathway-related proteins in HCT116 cells. (A) Western blotting
was performed to assess the levels of MEK, p-MEK, ERK and p-ERK
proteins in HCT116 cells treated with 0, 10, 20 and 40 µM JUB. (B)
Ratios of p-MEK/MEK and p-ERK/ERK were measured in HCT116 cells
after treatment with 0, 10, 20 and 40 µM JUB. **P<0.01 vs.
control. JUB, Jujuboside B; MAPK, mitogen-activated protein kinase;
MEK, MAPK kinase; p-MEK, phosphorylated-MEK; ERK, extracellular
signal-regulated kinase; p-ERK, phosphorylated-ERK. |
Discussion
As reported in previous studies, Bcl-2 is a common
anti-apoptotic protein in cells, whilst Bax and cleaved caspase-3
are pro-apoptotic proteins. Such proteins are considered the most
important oncoproteins in apoptosis research (21,22).
In the present study, HCT116 cells were treated with different
concentrations of JUB. The results of the present study
demonstrated that cell viability and colony formation were
significantly decreased after JUB treatment, whilst the apoptosis
level was notably increased, both in a concentration-dependent
manner. Western blot analysis indicated that JUB treatment
significantly reduced the expression of Bcl-2, whilst increasing
the expression of Bax and the ratio of cleaved caspase-3/caspase-3.
Additionally, TEM revealed marked ultrastructural changes in the
treated cells, including chromatin marginalization, cell membrane
blebbing, apoptotic body formation, as well as mitochondrial
swelling and cristae disruption or loss. Therefore, the results of
the present study suggest that JUB has anti-CRC effects.
Ferroptosis, a novel form of cell death, possesses a
unique mechanism and can be specifically identified by the
accumulation of Fe2+ and the increase in lipid
peroxidation, particularly polyunsaturated fatty acids (23). Ferroptosis has been reported to be
associated with the occurrence and treatment of several diseases
such as Alzheimer's and Parkinson's, ischemia-reperfusion injury,
liver fibrosis (24). In addition,
a growing body of evidence indicates that ferroptosis is involved
in the occurrence, progression and suppression of tumors.
Ferroptosis suppresses tumors by inducing cell death through lipid
peroxidation and iron metabolism disruption, regulated by factors
like p53. However, in some cases, cancer cells can resist
ferroptosis, allowing them to evade oxidative stress and promote
tumor progression, especially in the tumor microenvironment
(25). In cancer therapy, many
clinical chemotherapeutic agents not only initiate apoptosis, but
also induce ferroptosis and prevent cancer growth (26,27).
In the present study, the inclusion of the Fer-1 treatment group
aimed to further assess the specificity of JUB-induced cell death.
Fer-1 is a well-known ferroptosis inhibitor that effectively
inhibits the ferroptosis process. By pre-treating cells with Fer-1
followed by JUB treatment, significant decreases in the protein
expression levels of ferroptosis-related markers were observed.
These results indicated that the cell death induced by JUB occurs
through the ferroptosis pathway.
In the present study, the effect of JUB on the
expression levels of key ferroptosis markers was explored in HCT116
cells by treatment with Fer-1. Iron metabolism in the ferroptosis
process can cause the accumulation of ROS. The increase in
intracellular ROS can lead to mitochondrial DNA strand breaks and
DNA degradation, thereby resulting in apoptosis (28). The changes in GSH synthesis and MDA
levels are also indicators of ferroptosis-associated lipid
peroxidation (29,30). MDA, one of the products of lipid
peroxidation, is positively associated with ferroptosis, whilst the
association between GSH and ferroptosis is negative (31). The change in iron content is a
notable characteristic of ferroptosis. The accumulation of
Fe2+ can specifically elevate oxidative stress levels,
demonstrating a positive association with ferroptosis (32). The present study demonstrated that,
compared with the control group, JUB treatment significantly
increased the levels of ROS, MDA, total iron and Fe2+ in
HCT116 cells, whilst markedly decreasing the GSH level. However,
after the addition of the ferroptosis inhibitor Fer-1, the levels
of ROS, MDA, total iron and Fe2+ were significantly
reduced compared with the JUB group, whilst the GSH level was
markedly increased. These results demonstrate that JUB could induce
ferroptosis.
SLC7A11 is a specific type of amino acid
transporter, particularly for glutamate and cystine (33). The primary function of SLC7A11 is
inhibition of ferroptosis in cells (34). GPX4 inhibits lipid peroxidation,
thereby protecting cells from oxidative damage and the upregulation
of GPX4 can inhibit ferroptosis (35). Decreased expression of ACSL4 can
reduce the accumulation of lipid peroxidation substrates in cells,
thereby inhibiting ferroptosis (36). TFR1 serves a crucial role in
regulating cellular iron metabolism and maintaining iron balance,
and acts as a specific marker of ferroptosis (37). The results of the present study
demonstrated JUB treatment significantly downregulated the
expression levels of SLC7A11 and GPX4 proteins, whilst markedly
upregulating the levels of ACSL4 and TFR1. However, the combination
of JUB and Fer-1 significantly increased the expression of SLC7A11
and GPX4 but inhibited the expression of ACSL4 and TFR1. The
aforementioned findings suggest that JUB may promote ferroptosis in
HCT116 cells by affecting the expression of SLC7A11, GPX4, ACSL4
and TFR1. JUB treatment appears to downregulate the expression of
SLC7A11 and GPX4, both of which are key regulators of cellular
antioxidant defense, thereby decreasing the ability to counteract
oxidative stress. In contrast, JUB treatment upregulates the
expression of ACSL4 and TFR1, proteins involved in lipid
peroxidation and iron homeostasis, respectively. These changes in
protein expression may enhance lipid peroxidation and increase
intracellular iron levels, contributing to the induction of
ferroptosis.
The MAPK pathway, consisting of ERK1/2, MEK1/2 and
p38 (38), is one of the most
commonly mutated oncogenic pathways in several cancers (39), including colorectal cancer, lung
cancer, and thyroid cancer. The abnormal activation of proteins
such as EGFR, RAS (including KRAS, HRAS, NRAS), and RAF (such as
BRAF) can lead to MAPK pathway dysregulation, resulting in
uncontrolled cellular proliferation and dedifferentiation (40). In colorectal cancer, the MAPK
pathway is generally activated, contributing to the development,
progression, and resistance to therapy (41). In the present study, JUB
significantly reduced the levels of p-MEK and p-ERK proteins, as
well as the ratios of p-MEK/MEK and p-ERK/ERK, in HCT116 cells,
indicating that JUB inhibits MAPK pathway activation. These
findings suggest that the inhibitory effect of JUB on the MAPK
pathway may play a role in reducing CRC cell proliferation, colony
formation, and survival, potentially contributing to its overall
anticancer effects. Alternatively, the anticancer effects of JUB
may be primarily mediated through the induction of ferroptosis or
cell death, rather than solely through MAPK pathway inhibition.
Further studies are required to determine the relative
contributions of ferroptosis induction and MAPK pathway inhibition
to the observed effects of JUB treatment in CRC cells.
Although the antitumor activity of JUB has been
reported in other types of cancer, the present study is the first
to systematically investigate the mechanism of JUB in CRC, to the
best of our knowledge. Not only were the effects of JUB on the
proliferation and apoptosis of HCT116 cells evaluated, but also its
specific molecular mechanisms by regulating the MAPK signaling
pathway and inducing ferroptosis were explored. The present study
is also the first to reveal that the JUB inhibits proliferation,
colony formation, and induces apoptosis and ferroptosis in CRC
cells, to the best of our knowledge.
However, the present study has certain limitations,
such as the lack of animal experiments and the lack of experiments
demonstrating the expression changes of four ferroptosis regulatory
factors (SLC7A11, GPX4, ACSL4 and TFR1) under normal ferroptotic
conditions as a control. Future research should evaluate of the
impact of JUB on normal tissues, and its dose toxicity and safety,
through both in vitro cell experiments on different cell
lines and in vivo animal models. Additionally, incorporating
both MAPK inhibitors and activators would provide more insight into
the mechanisms by which JUB affects the MAPK pathway. Activating
the MAPK pathway during JUB treatment would help confirm whether
the effects of JUB on proliferation, apoptosis, and ferroptosis are
mediated through this pathway. Only detecting activation of the
MAPK pathway does not provide evidence that the mechanism of JUB is
mediated by the MAPK pathway. However, the present study
demonstrate that JUB inhibits the MAPK pathway by reducing the
phosphorylation levels of MEK and ERK in HCT116 cells. The
antitumor effects of JUB on different types of tumors should also
be investigated, validating its anticancer activity both in
vitro and in vivo. Given the varied role of the MAPK
pathway, the impact of JUB on other biological processes dependent
on this pathway should be studied to ensure its safety and
specificity in cancer treatment.
In summary, the results of the present study
demonstrated that JUB can inhibit the proliferation of CRC cells
and promote apoptosis and ferroptosis. The inhibition of the MAPK
pathway suggests that the effects of JUB on apoptosis and
ferroptosis may be related to the regulation of this pathway.
However, further studies are needed to confirm this
association.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KZ and XW conceived and designed the study. KZ and
XW performed the experiments. GL and CC contributed to data
acquisition. GL and CC analyzed the data. GL and CC interpreted the
data. KZ and GL confirm the authenticity of all the raw data. GL
and CC edited the manuscript draft. KZ and XW reviewed and edited
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eng C, Jácome AA, Agarwal R, Hayat MH,
Byndloss MX, Holowatyj AN, Bailey C and Lieu CH: A comprehensive
framework for early-onset colorectal cancer research. Lancet Oncol.
23:e116–e128. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou J, Ji Q and Li Q: Resistance to
anti-EGFR therapies in metastatic colorectal cancer: Underlying
mechanisms and reversal strategies. J Exp Clin Cancer Res.
40:3282021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong Y, Chen X, Wu S, Fang H, Hong L,
Shao L, Wang L and Wu J: Deciphering colorectal cancer
radioresistance and immune microrenvironment: Unraveling the role
of EIF5A through single-cell RNA sequencing and machine learning.
Front Immunol. 15:14662262024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin
R, He WL, Cai SR, He YL and Ye JN: Anti-EGFR therapy in metastatic
colorectal cancer: Mechanisms and potential regimens of drug
resistance. Gastroenterol Rep (Oxf). 8:179–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Q, Zheng Y, Liu J, Tang X, Wang Y,
Li X, Li H, Zhou X, Tang S, Tang Y, et al: CircIFNGR2 enhances
proliferation and migration of CRC and induces cetuximab resistance
by indirectly targeting KRAS via sponging to MiR-30b. Cell Death
Dis. 14:242023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kashiwagi S, Asano Y, Goto W, Takada K,
Takahashi K, Hatano T, Tanaka S, Takashima T, Tomita S, Motomura H,
et al: Mesenchymal-epithelial transition and tumor vascular
remodeling in eribulin chemotherapy for breast cancer. Anticancer
Res. 38:401–410. 2018.PubMed/NCBI
|
|
9
|
Miyamoto M, Takano M, Kuwahara M, Soyama
H, Kato K, Matuura H, Sakamoto T, Takasaki K, Aoyama T, Yoshikawa T
and Furuya K: Efficacy of combination chemotherapy using irinotecan
and nedaplatin for patients with recurrent and refractory
endometrial carcinomas: Preliminary analysis and literature review.
Cancer Chemother Pharmacol. 81:111–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu LQ, Zhang L, Zhang J, Chang GL, Liu G,
Yu DD, Yu XM, Zhao MS and Ye B: Evodiamine inhibits high-fat
diet-induced colitis-associated cancer in mice through regulating
the gut microbiota. J Integr Med. 19:56–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morazán-Fernández D, Mora J and
Molina-Mora JA: In silico pipeline to identify tumor-specific
antigens for cancer immunotherapy using exome sequencing data.
Phenomics. 3:130–137. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Zhou B, Cong W, Zhang M, Li Z, Li
Y, Liang S, Chen K, Yang D and Wu Z: Amelioration of
AOM/DSS-Induced murine colitis-associated cancer by evodiamine
intervention is primarily associated with gut
microbiota-metabolism-inflammatory signaling axis. Front Pharmacol.
12:7976052021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee IC and Bae JS: Hepatic protective
effects of jujuboside B through the modulation of inflammatory
pathways. Biotechnology and Bioprocess Engineering. 27:336–343.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molagoda IMN, Lee KT, Athapaththu A, Choi
YH, Hwang J, Sim SJ, Kang S and Kim GY: Flavonoid glycosides from
ziziphus jujuba var. inermis (Bunge) rehder seeds inhibit
alpha-melanocyte-stimulating hormone-mediated melanogenesis. Int J
Mol Sci. 22:77012021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo L, Liang Y, Wang S, Li L, Cai L, Heng
Y, Yang J, Jin X, Zhang J, Yuan S, et al: Jujuboside B inhibits the
proliferation of breast cancer cell lines by inducing apoptosis and
autophagy. Front Pharmacol. 12:6688872021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang P, Lai X, Zhu MH, Shi J, Pan H,
Huang Y, Guo RJ, Lu Q, Fang C and Zhao M: Jujuboside B suppresses
angiogenesis and tumor growth via blocking VEGFR2 signaling
pathway. Heliyon. 9:e170722023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji Z, Li J and Wang J: Jujuboside B
inhibits neointimal hyperplasia and prevents vascular smooth muscle
cell dedifferentiation, proliferation, and migration via activation
of AMPK/PPAR-γ signaling. Front Pharmacol. 12:6721502021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moon H and Ro SW: MAPK/ERK signaling
pathway in hepatocellular carcinoma. Cancers (Basel). 13:30262021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pashirzad M, Khorasanian R, Fard MM,
Arjmand MH, Langari H, Khazaei M, Soleimanpour S, Rezayi M, Ferns
GA, Hassanian SM and Avan A: The therapeutic potential of MAPK/ERK
inhibitors in the treatment of colorectal cancer. Curr Cancer Drug
Targets. 21:932–943. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dolka I, Krol M and Sapierzynski R:
Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved
caspase-3 and p53) expression in canine mammary tumors: An
immunohistochemical and prognostic study. Res Vet Sci. 105:124–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wei Z, Pan K, Li J and Chen Q: The
function and mechanism of ferroptosis in cancer. Apoptosis.
25:786–798. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lachaier E, Louandre C, Godin C, Saidak Z,
Baert M, Diouf M, Chauffert B and Galmiche A: Sorafenib induces
ferroptosis in human cancer cell lines originating from different
solid tumors. Anticancer Res. 34:6417–6422. 2014.PubMed/NCBI
|
|
27
|
Yang R, Li Y, Wang X, Yan J, Pan D, Xu Y,
Wang L and Yang M: Doxorubicin loaded ferritin nanoparticles for
ferroptosis enhanced targeted killing of cancer cells. RSC Adv.
9:28548–28553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang D and Kroemer G: Ferroptosis. Curr
Biol. 30:R1292–R1297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Du Y, Zhou Y, Chen Q, Luo Z, Ren Y,
Chen X and Chen G: Iron and copper: Critical executioners of
ferroptosis, cuproptosis and other forms of cell death. Cell Commun
Signal. 21:3272023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koppula P, Zhang Y, Zhuang L and Gan B:
Amino acid transporter SLC7A11/xCT at the crossroads of regulating
redox homeostasis and nutrient dependency of cancer. Cancer Commun
(Lond). 38:122018.PubMed/NCBI
|
|
34
|
Iida Y, Okamoto-Katsuyama M, Maruoka S,
Mizumura K, Shimizu T, Shikano S, Hikichi M, Takahashi M, Tsuya K,
Okamoto S, et al: Effective ferroptotic small-cell lung cancer cell
death from SLC7A11 inhibition by sulforaphane. Oncol Lett.
21:712021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng H, Schorpp K, Jin J, Yozwiak CE,
Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka
JM, et al: Transferrin receptor is a specific ferroptosis marker.
Cell Rep. 30:3411–3423. e34172020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu YC, Lin TJ, Chong KY, Chen GY, Kuo CY,
Lin YY, Chang CW, Hsiao TF, Wang CL, Shih YC and Yu CJ: Targeting
the ERK1/2 and p38 MAPK pathways attenuates Golgi tethering factor
golgin-97 depletion-induced cancer progression in breast cancer.
Cell Commun Signal. 23:222025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Braicu C, Buse M, Busuioc C, Drula R,
Gulei D, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, et al: A
comprehensive review on MAPK: A promising therapeutic target in
cancer. Cancers (Basel). 11:16182019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|