Introduction
Urothelial carcinoma is the most common histological
type of carcinoma of the urinary tract. The bladder is the most
frequent site of malignancy in the urinary tract. Bladder cancer is
the seventh most commonly diagnosed cancer in men and the tenth
most common for both genders combined (1). Radical cystectomy is considered the
best treatment option for patients with advanced urothelial bladder
cancer and remains the standard of care for muscle-invasive bladder
cancer and high-risk nonmuscle-invasive disease (1–10).
Systematic follow-up after radical treatment allows for proper
diagnosis of possible surgical complications and is also important
in the context of the potential spread of the disease. The most
common complications associated with surgical urinary diversion
after radical cystectomy include uretero-enteric strictures, which
may lead to deterioration of renal function (2). Detection of urothelial cancer
metastases during follow-up after radical treatment is important,
as it is associated with poor prognosis (1,7).
Malignant neoplasms of the appendix are rare and metastatic
involvement of the appendix during the course of any neoplastic
disease is very rare (8,11–13).
The current study presented the case of a patient whose first
metastasis of urothelial carcinoma was found to involve the
appendix.
Case report
A 67-year-old male patient with a history of
hypercholesterolemia, and no other comorbidities, was referred to
the urologist for hematuria in September 2021. The patient
underwent transurethral resection of the bladder tumor and was
diagnosed with pT2 high-grade urothelial bladder cancer. The
patient was then referred to the Department of Urogenital Cancer at
the Maria Skłodowska-Curie National Research Institute of Oncology
(Warsaw, Poland). Computed tomography (CT) performed in October
2021 due to the diagnosis of muscle-invasive bladder cancer
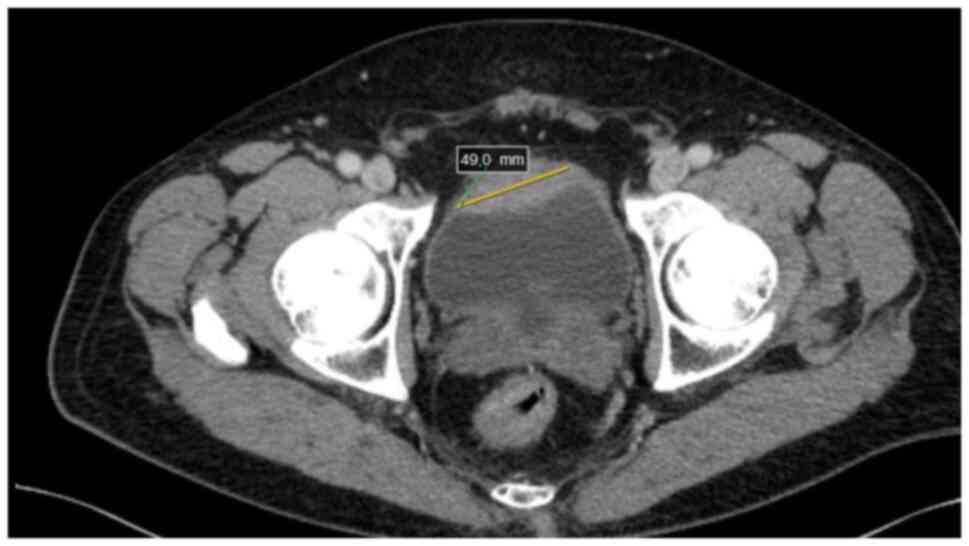
detected a neoplastic infiltration of the anterior wall of the
urinary bladder, measuring ~13×49 mm, extending beyond the bladder
wall, with no lymphadenopathy or metastatic changes in parenchymal
organs or bones (Fig. 1). The
patient received neoadjuvant treatment consisting of four courses
of gemcitabine and cisplatin chemotherapy, followed by a
cystoprostatectomy in March 2022. During the cystoprostatectomy,
the Bricker technique of urinary diversion was used, which involves
spatulating the ends of the ureters and suturing each ureter
separately to the end of an isolated intestinal loop, the so-called
Bricker loop, the other end of which is used as a urostomy
(5). There were no clinical or
intraoperative indications for additional removal of the appendix;
therefore, the appendix was left in place.
The postoperative histopathological examination
showed high-grade pT3a urothelial carcinoma of the urinary bladder.
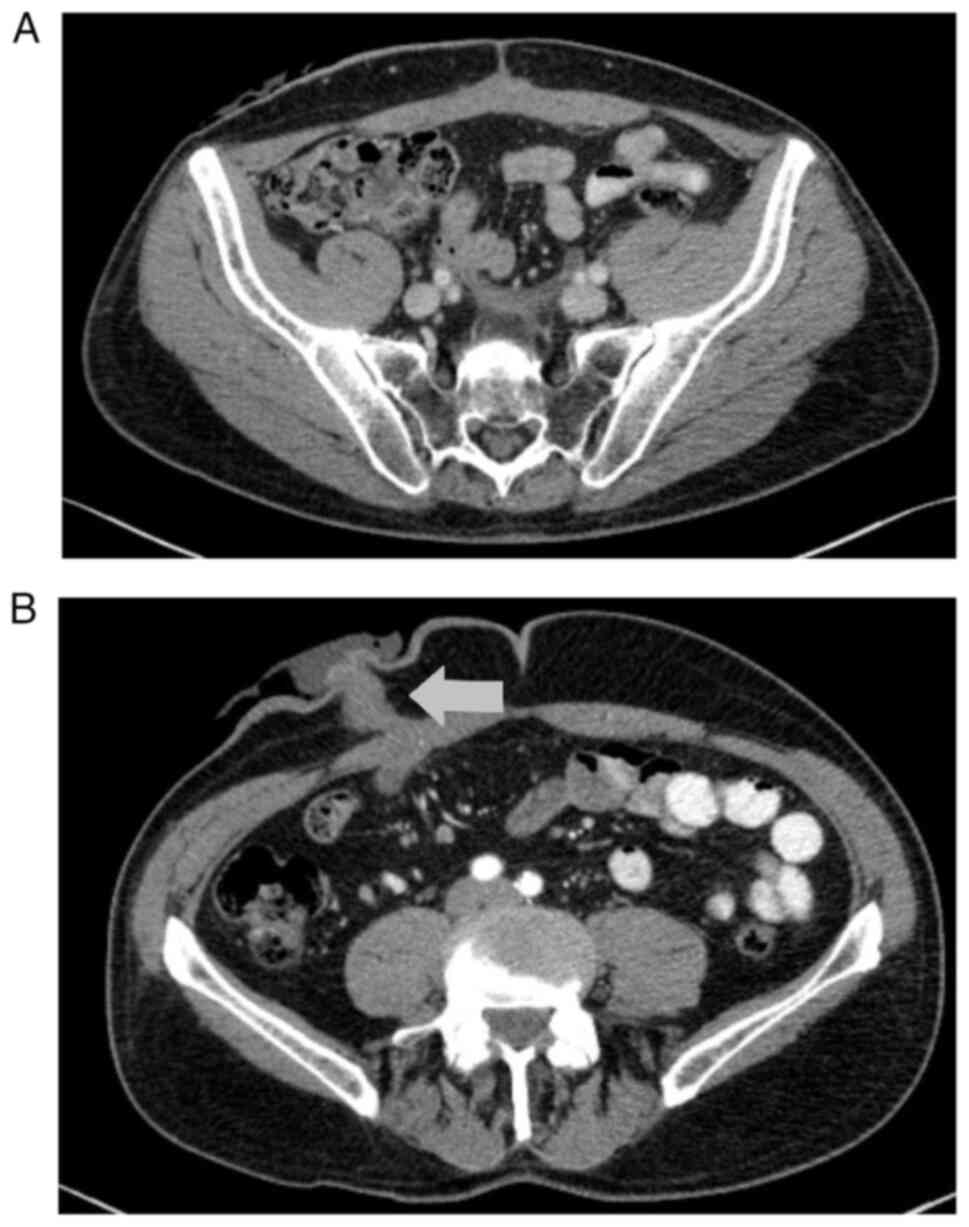
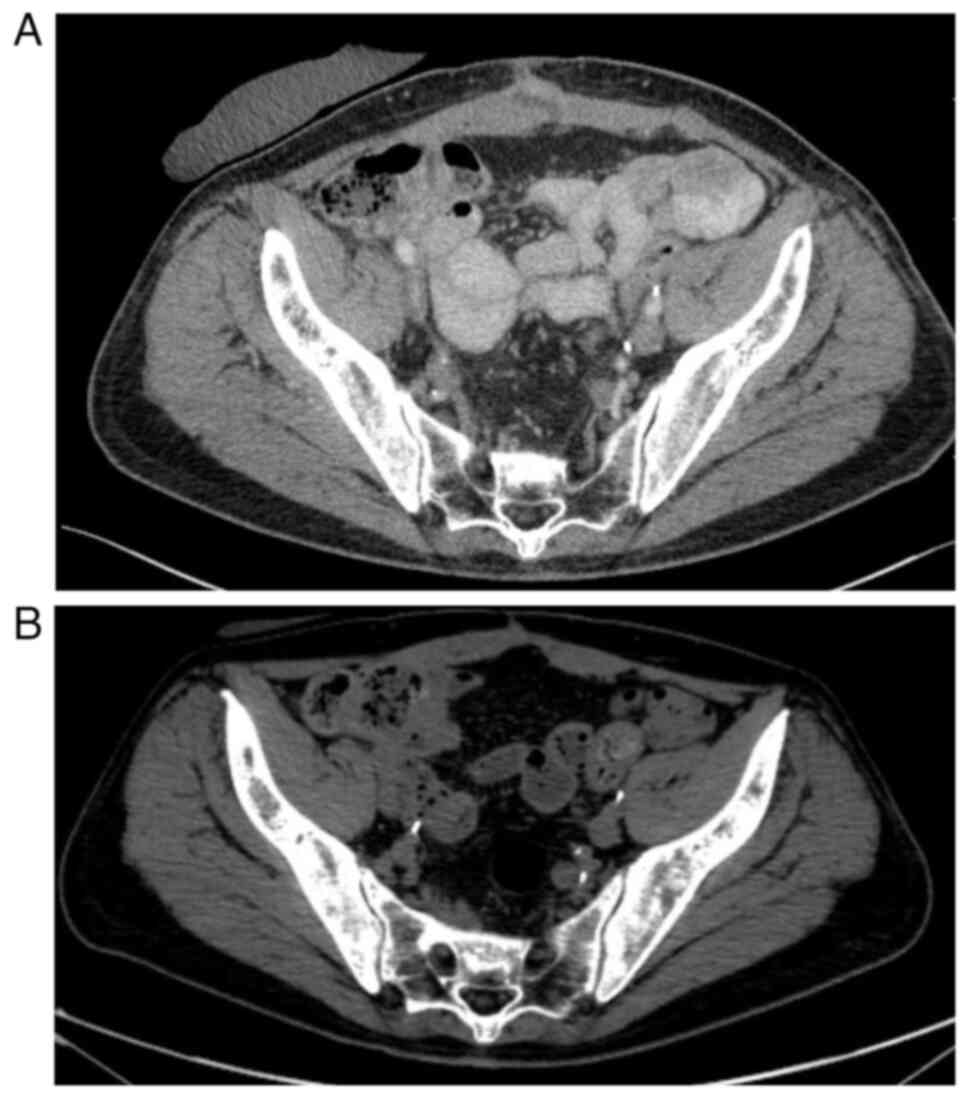
The first postoperative CT scan showed no local recurrence and no
lymphadenopathy or metastases in the parenchymal organs or bones
(Fig. 2). The patient remained
under observation with no signs of progression of the disease.
Regular CT scans were performed every 3 months and other supportive
examinations were performed as part of the oncological follow-up.
In March 2023, the patient was readmitted to the Department of
Urogenital Cancer at the Maria Skłodowska-Curie National Research
Institute of Oncology (Warsaw, Poland) for suspected
uretero-enteric strictures and bilateral hydronephrosis. The
patient qualified for surgical treatment. The last CT scan before
the planned surgery (performed in January 2023) revealed no
recurrence or metastasis of urothelial carcinoma. Laparotomy was
performed, during which an inflammatory infiltration was found
intraoperatively, absorbing the junctions of the ureters with the
Bricker loop and the appendix. The appendix was located in close
proximity to the Bricker loop, but not in direct contact with it;
the surrounding tissues were inflamed. This finding suggested
appendicitis accompanying the ureteral strictures. Macroscopically,
there was no suspicion of cancer recurrence. The ureters were
resected and implanted into the Bricker loop. The inflamed appendix
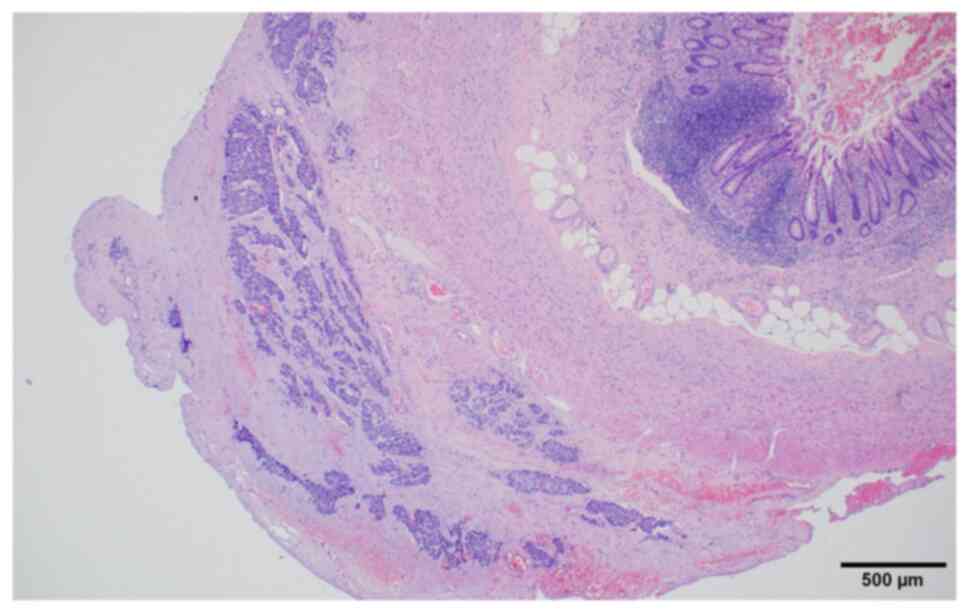
was removed and submitted for histopathological examination, which
revealed neoplastic cells arranged in irregular nests or single
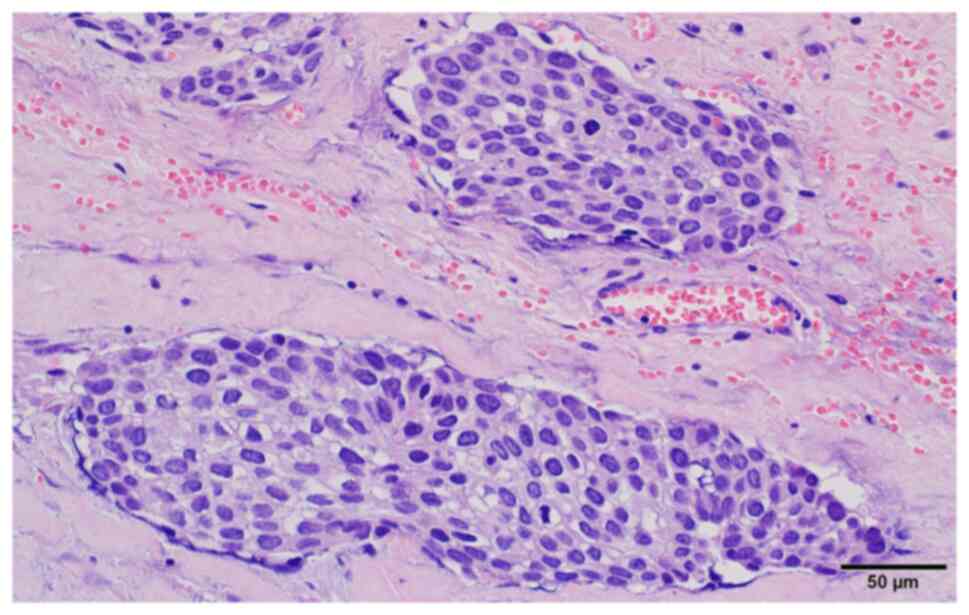
cells within the subserosal connective tissue (Fig. 3). Neoplastic cells showed high-grade
nuclear features, such as nuclear pleomorphism, hyperchromasia, a
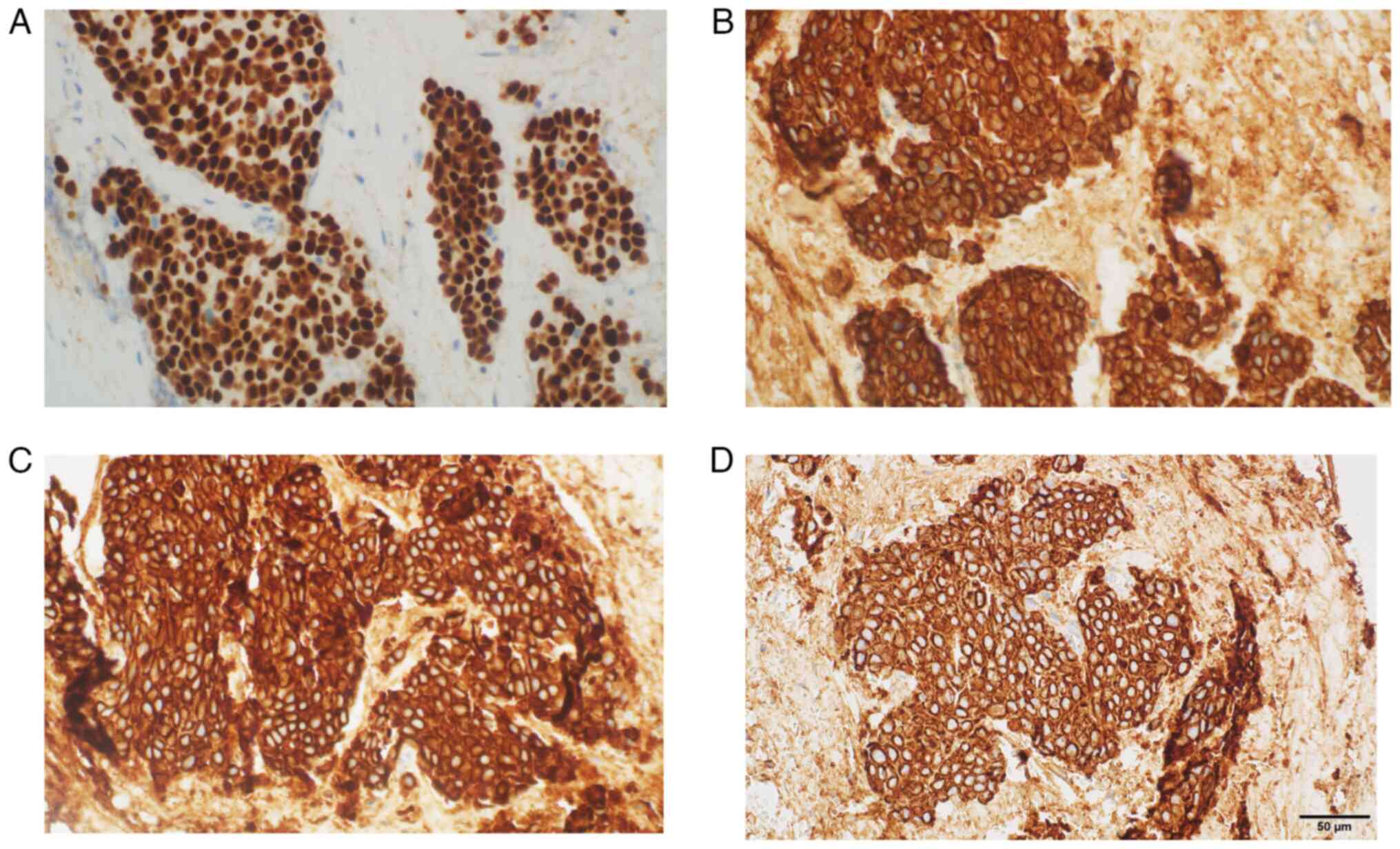
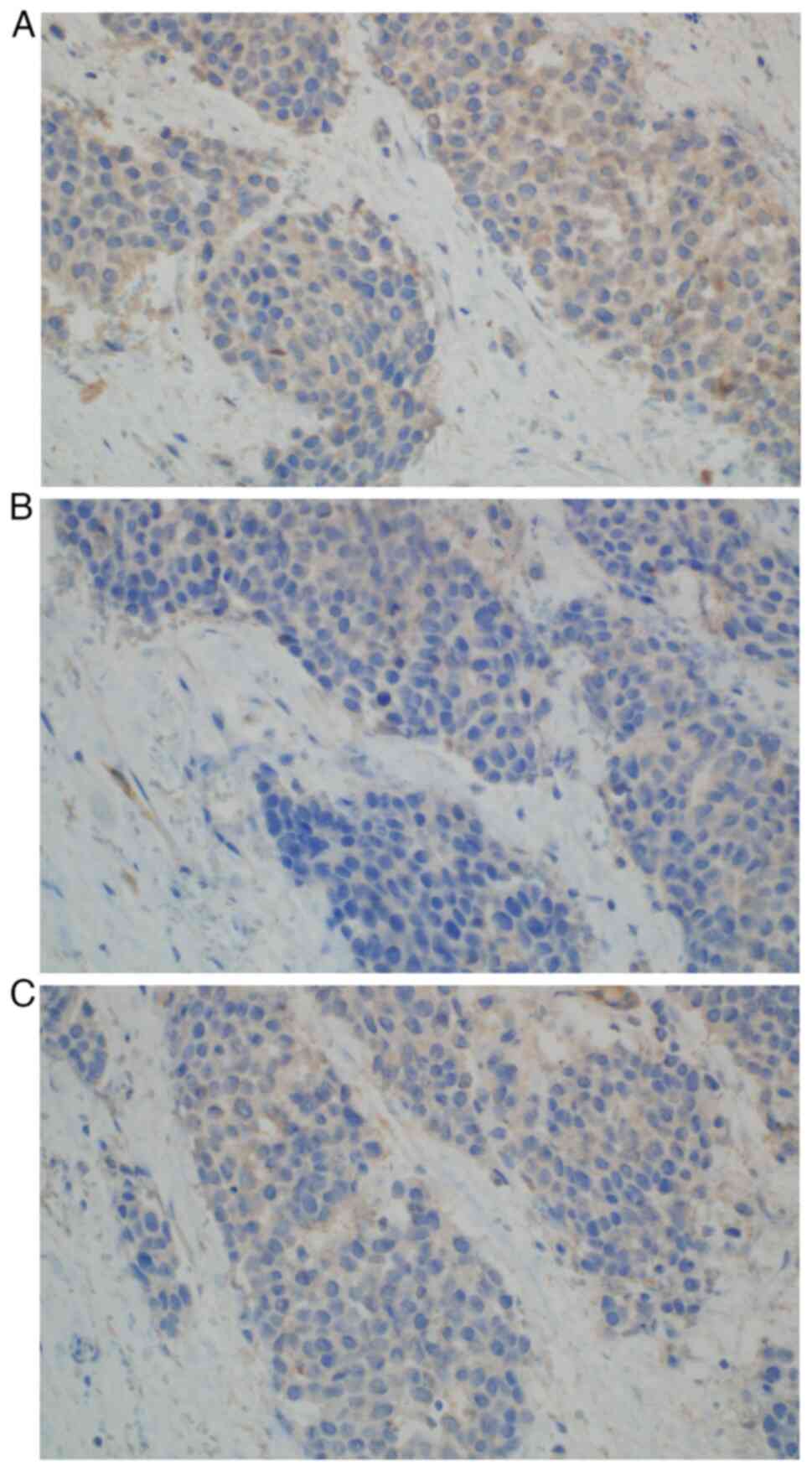
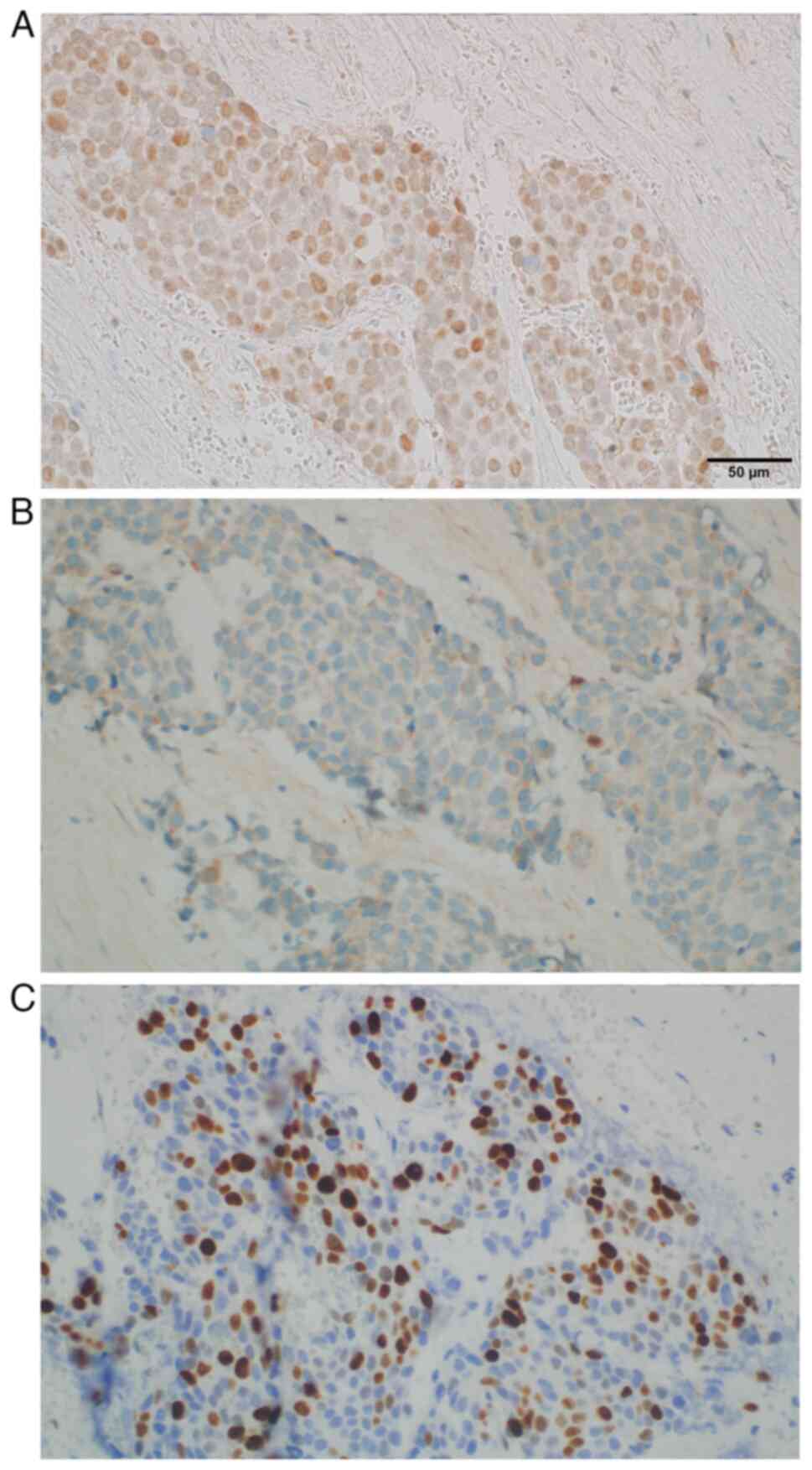
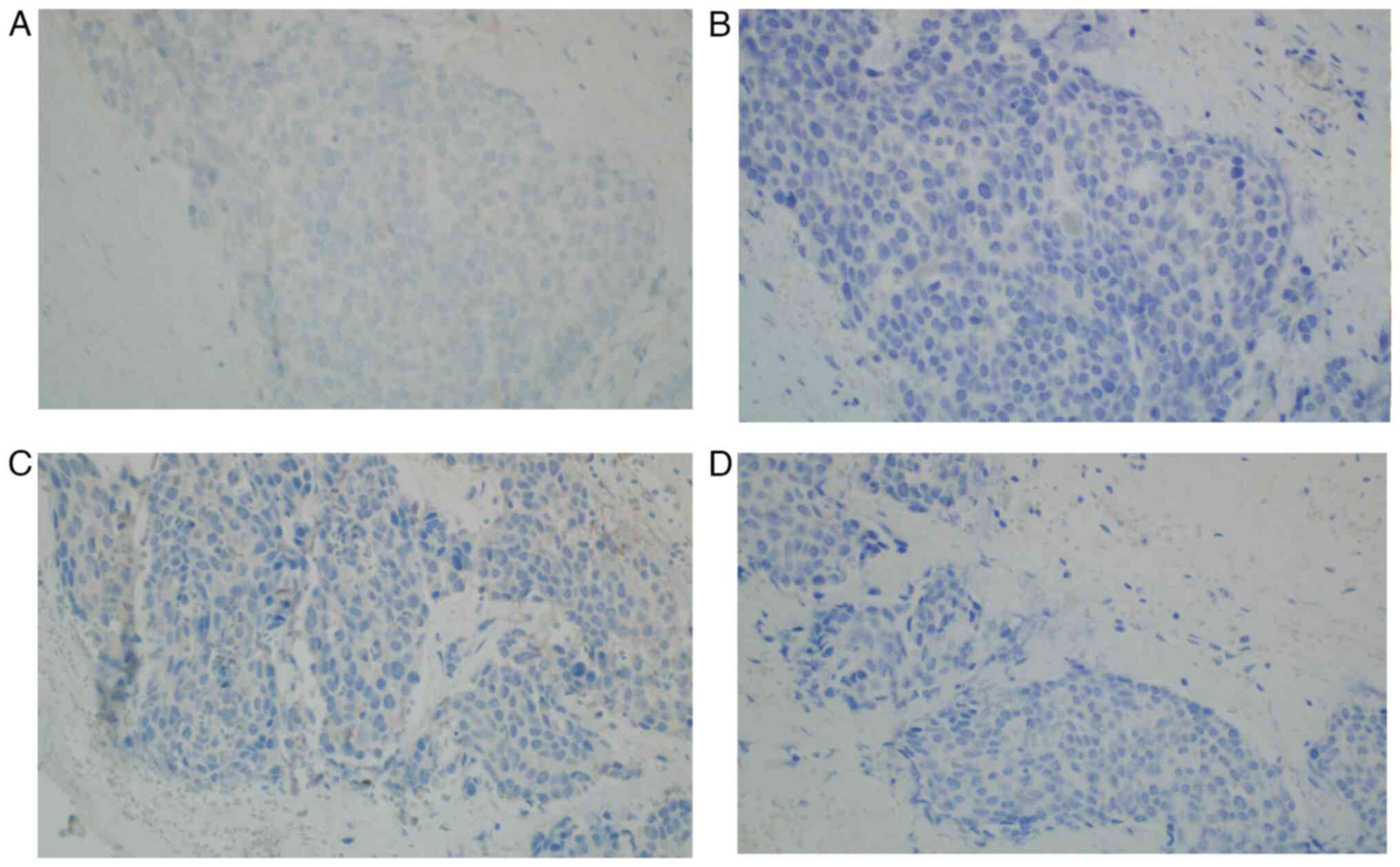
high nuclear-to-cytoplasm ratio and mitotic figures (Fig. 4). The following immunoprofile was
observed: p40d (+), cytokeratin (CK)AE1/AE3 [CKAE1/3 (+)], CK high
molecular weight (+), CK5/6 (+), CK20 (−), CK7 (−), caudal-type
homeobox transcription factor 2 (−), GATA binding protein 3 [GATA-3
(+)], S100 calcium binding protein P (−), Uroplakin III (−),
insulinoma-associated protein 1 (−), Synaptophysin (−),
Chromogranin (−), prostate-specific acid phosphatase (−) and
antigen Ki-67 [Ki-67 (+)] with ~30% positive nuclei (Fig. 4, Fig.
5, Fig. 6, Fig. 7, Fig.
8, Fig. 9A). A pronounced
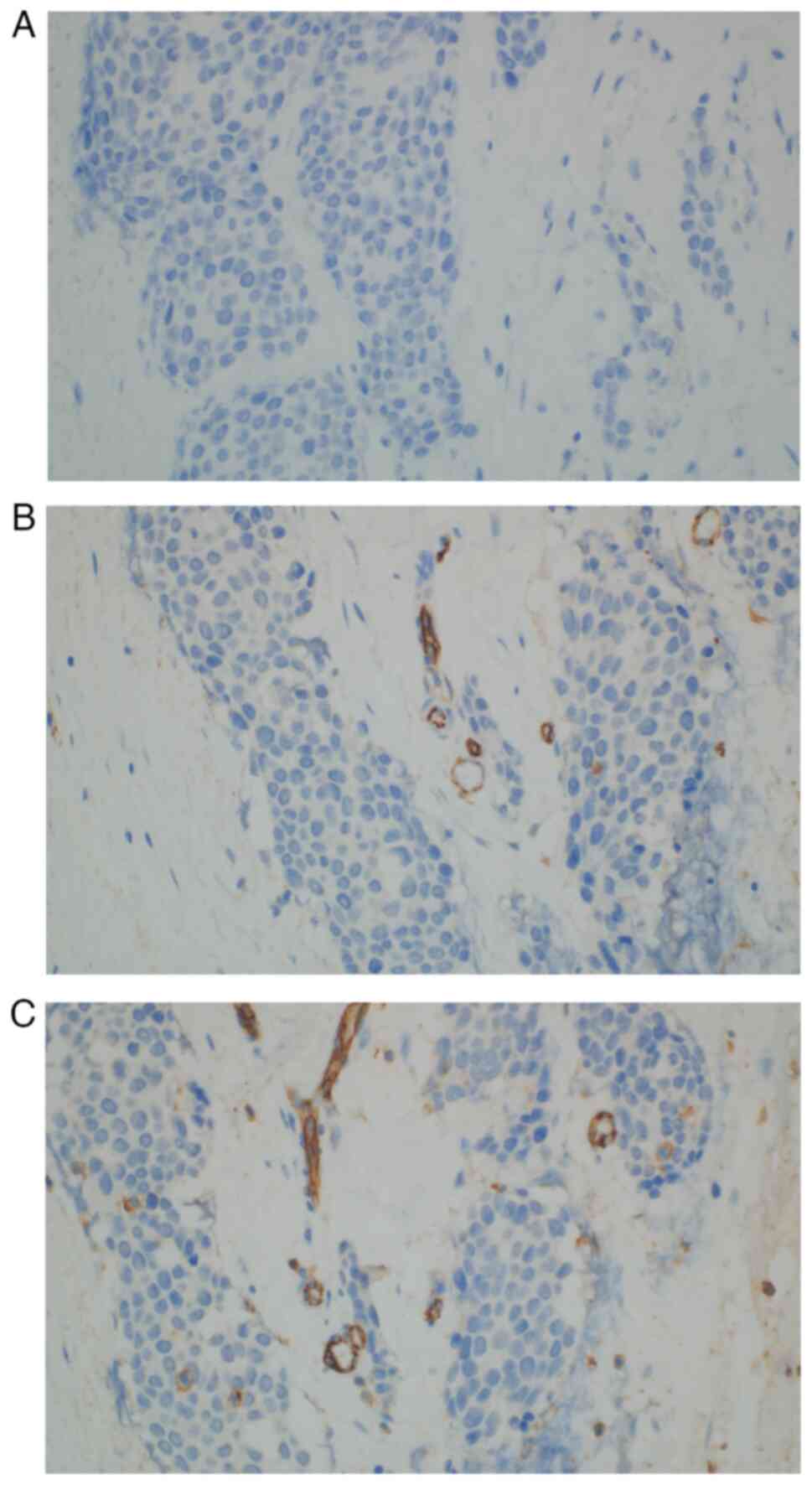
retraction artifact was present; therefore, vascular invasion was
excluded by immunohistochemistry [CD34 (−), CD31 (−); Fig. 9B and C]. All immunohistochemistry
protocols were performed according to the instructions of the
manufacturer and staining platforms. The details of all antibodies
used are provided in Table I.
Ultimately, the neoplasm's morphology, in correlation with
immunohistochemical results, corresponded to a cancer metastasis to
the appendix, primarily of urothelial origin. The differential
diagnosis included colorectal cancer, neuroendocrine neoplasms and
prostate cancer.
 | Table I.Details of immunohistochemical
staining. |
Table I.
Details of immunohistochemical
staining.
| Protein | Cat. no. | Clone | Dilution | Supplier | Platform |
|---|
| p40d | 6A784 | DAK-p40 | Ready to use | Dako; Agilent
Technologies, Inc. | Omnis |
| CKAE1/AE3 | IR053 | AE1/AE3 colne | Ready to use | Dako; Agilent
Technologies, Inc. | Autostainer |
| CKHMW | IR051 | 34βE12 | Ready to use | Dako; Agilent
Technologies, Inc. | Autostainer |
| CK5/6 | IR780 | D5/16 B4 | Ready to use | Dako; Agilent
Technologies, Inc. | Autostainer |
| CK20 | IR777 |
Ks20.8 | Ready to use | Dako; Agilent
Technologies, Inc. | Autostainer |
| CK7 | IR619 | OV-TL 12/30 | Ready to use | Dako; Agilent
Technologies, Inc. | Autostainer |
| CDX-2 | 6A080 | DAK-CDX2 | Ready to use | Dako; Agilent
Technologies, Inc. | Omnis |
| GATA-3 | 7107749001 | L50-823 | Ready to use | Roche
Diagnostics | BenchMark |
| S100P | 06523935001 | 16/f5 | 1:500 | Roche
Diagnostics | Manual |
| Uroplakin III | 64119232001 | SP73 | Ready to use | Roche
Diagnostics | Ventana
BenchMark |
| INSM1 | G2922 | A-8 | 1:500 | Santa Cruz
Biotechnology, Inc. | Manual |
| Synaptophysin | GA660 | DAK-SYNAP | Ready to use | Dako; Agilent
Technologies, Inc. | Omnis |
| Chromogranin A | MO869 | DAK-A3 | 1:200 | Dako; Agilent
Technologies, Inc. | Autostainer |
| PSAP | MO792 | PASE/4LJ | 1:500 | Dako; Agilent
Technologies, Inc. | Autostainer |
| Ki-67 | GA626 | MIB-1 | Ready to use | Dako; Agilent
Technologies, Inc. | Omnis |
| CD31 | GA610 | JC70A | Ready to use | Dako; Agilent
Technologies, Inc. | Omnis |
| CD34 | GA632 | QBEnd10 | Ready to use | Dako; Agilent
Technologies, Inc. | Omnis |
The first follow-up CT scan performed in April 2023
showed progression of the disease: Foci of sclerotic remodeling
were found in the vertebrae, ribs and pelvis (suggestive of
metastasis; Fig. 10A), though no
local recurrence, lymphadenopathy or hydronephrosis were observed.
The metabolically active nature of the changes observed on the CT
scan was confirmed by a bone scintigram (data not shown), which
diagnosed multifocal active metastasis to the skeletal system. For
this reason, the patient qualified for four cycles of chemotherapy
(consisting of gemcitabine and carboplatin) and received
bisphosphonates as prophylaxis to prevent bone loss and reduce the
risk of skeletal-related events. After four cycles of chemotherapy,
the patient underwent four cycles of maintenance treatment with
avelumab immunotherapy. Follow-up computed tomography examinations
performed in December 2023 revealed further progression of the
disease (Fig. 10B). The patient's
general health gradually deteriorated and the patient did not
return for further therapy.
Discussion
Patients who undergo cystectomy require long-term
monitoring for disease recurrence and evaluation of urinary
diversion function. As far as oncological observation is concerned,
there is no clearly defined protocol for carrying out control
examinations. The Guidelines of the European Association of Urology
point out that current surveillance protocols are based on
recurrence patterns obtained only from retrospective series, most
of which employ different follow-up regimens and imaging techniques
(1).
Deterioration of renal function is the most common
long-term complication of urinary diversion, affecting up to 35% of
patients, mainly due to uretero-enteric strictures (UES) (2,3). The
incidence of UES ranges in the literature from 2.6 to 13%,
depending, among other factors, on the surgical technique.
According to data analyzed by Ericson et al (4), which included 279 open and 689 robotic
cystectomies, UES occurred in 9.3% of patients who underwent open
cystectomy, 11.3% after robotic cystectomy with extracorporeal
urinary diversion and 13.0% after robotic intracorporeal
cystectomy. Known risk factors for UES include an elevated body
mass index, anastomotic technique, preoperative chemotherapy and
early complications of Clavien-Dindo grade ≥III. Preoperative
chemotherapy administration is an independent predictor of
stricture formation (5). The most
commonly used method of treating UES is open surgery due to its
high success rate, though at the cost of significant morbidity.
Endourological procedures are associated with a lower risk of
complications, but a higher risk of recurrence in long-term
follow-up. Robotic-assisted surgery for UES offers success rates
comparable to open surgery while reducing surgical morbidity
(6).
It is generally thought that bladder cancer most
likely spreads to distant organs via the lymphatic system.
Detection of lymph node metastases is an important prognostic
factor for patient survival and the need for adjuvant therapy
(1,8,10).
Previous reports emphasize the role of lymphangiogenesis and
lymphatic vessel density, suggesting that both processes may be
potential prognostic markers and mechanisms of metastatic spread in
patients with urothelial bladder cancer (10). Local and distant recurrences after
radical treatment of bladder cancer are associated with a poor
prognosis (1,10). The reported rate of local pelvic
recurrence is 5–15%, usually occurring within 6 to 18 months after
surgery. Local recurrence of transitional cell carcinoma after
radical cystectomy may occur in the surgical bed or pelvic lymph
nodes. Up to 50% of patients experience distant recurrences,
usually diagnosed within two years after surgery, in the
surrounding lymph nodes above the aortic bifurcation, lungs, liver
and bones (1,9). Therefore, tumors in the appendix
should be considered distant metastases, alongside bone metastases
detected on CT scans.
Malignant neoplasms of the appendix are rare. Among
primary tumors, the most common are neuroendocrine neoplasms,
followed by adenocarcinomas, particularly mucinous carcinomas
(11). Metastatic involvement of
the appendix in any neoplastic disease is rare. A search of the
Medline database found no reports of the appendix as the primary
site of metastasis for urothelial carcinoma. A small number of
cases of metastases from various primary tumors have occasionally
been reported as single case reports (12). In the literature, most of these
cases manifested as acute appendicitis. The proposed mechanism for
the development of acute appendicitis caused by tumor metastasis is
the attachment of cancer cells to the serosa, followed by
infiltration into the muscularis propria, mucosa, obstructive
luminal narrowing and secondary inflammation. The described
mechanism distinguishes this condition from primary appendix
cancer, which usually originates in the mucosa and infiltrates
outward (12). In a review of 7,970
appendectomies examined by Connor et al (13), metastasis of other cancers to the
appendix was found in only 11 cases, most commonly (55%) in
patients with primary colorectal disease. To the best of our
knowledge, bladder cancer metastasis to the appendix has not been
previously reported.
In conclusion, cancer metastases to the appendix are
rare. The current study presented a unique case of urothelial
carcinoma metastasis to the appendix, which was detected in an
inflamed appendix removed during surgical treatment for UES.
Inflammatory infiltration around the appendix, adjacent to the
Bricker loop, drew in the uretero-intestinal anastomoses, causing
bilateral hydronephrosis in a patient one year after radical
cystectomy. Distant metastases of urothelial carcinoma are
associated with poor prognosis and require systemic treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PN wrote the manuscript, obtained data and made
substantial contributions to the conception and design of the
manuscript. PN, BA and TK performed the operation (TK was the first
surgeon who performed the operation), provided tissues that could
be used as material for the study and were involved in the
acquisition of data. OKS carried out microscopic examinations of
the tissue, acquired data and made substantial contributions to the
interpretation of data. OKS carried out the histopathology
examination. PK and TD made significant contributions to the
conception and content of the manuscript, in particular PK
contributed to the Case report section and TD to the Discussion
section. TK and TD confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent for
participation in the study. The informed consent procedures were in
compliance with the Helsinki Declaration.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
EAU Guidelines, . Edn. presented at the
EAU Annual Congress Paris. 2024.ISBN 978-94-92671-23-3.
|
|
2
|
Eisenberg MS, Thompson RH, Frank I, Kim
SP, Cotter KJ, Tollefson MK, Kaushik D, Thapa P, Tarrell R and
Boorjian SA: Long-term renal function outcomes after radical
cystectomy. J Urol. 191:619–625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin XD, Roethlisberger S, Burkhard FC,
Birkhaeuser F, Thoeny HC and Studer UE: Long-term renal function
after urinary diversion by ileal conduit or orthotopic ileal
bladder substitution. Eur Urol. 61:491–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ericson KJ, Thomas LJ, Zhang JH, Knorr JM,
Khanna A, Crane A, Zampini AM, Murthy PB, Berglund RK, Pascal-Haber
G and Lee BHL: Uretero-enteric anastomotic stricture following
radical cystectomy: A comparison of open, robotic extracorporeal,
and robotic intracorporeal approaches. Urology. 144:130–135. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krafft U, Mahmoud O, Hess J, Radtke JP,
Panic A, Püllen L, Darr C, Kesch C, Szarvas T, Rehme C, et al:
Comparative analysis of bricker versus wallace ureteroenteric
anastomosis and identification of predictors for postoperative
ureteroenteric stricture. Langenbecks Arch Surg. 407:1233–1240.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albisinni S, Aoun F, Mjaess G, Abou Zahr
R, Diamand R, Porpiglia F, Esperto F, Autorino R, Fiori C, Tubaro
A, et al: Contemporary management of benign uretero-enteric
strictures after cystectomy: A systematic review. Minerva Urol
Nephrol. 73:724–730. 2021.PubMed/NCBI
|
|
7
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mari A, Campi R, Tellini R, Gandaglia G,
Albisinni S, Abufaraj M, Hatzichristodoulou G, Montorsi F, van
Velthoven R, Carini M, et al: Patterns and predictors of recurrence
after open radical cystectomy for bladder cancer: A comprehensive
review of the literature. World J Urol. 36:157–170. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghoneim MA, Abdel-Latif M, el-Mekresh M,
Abol-Enein H, Mosbah A, Ashamallah A and el-Baz MA: Radical
cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5
years later. J Urol. 180:121–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu ZS, Ding W, Cai J, Bashir G, Li YQ and
Wu S: Communication of cancer cells and lymphatic vessels in
cancer: Focus on bladder cancer. Onco Targets Ther. 12:8161–8177.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lyss AP: Appendiceal malignancies. Semin
Oncol. 15:129–137. 1988.PubMed/NCBI
|
|
12
|
Yadav S, Mittal N, Kumar R, Janu A and
Prabhash K: Metastasis to appendix presenting as acute
appendicitis-A rare case report and review of literature. J
Gastrointest Cancer. 52:1114–1118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Connor SJ, Hanna GB and Frizelle FA:
Appendiceal tumors: Retrospective clinicopathologic analysis of
appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum.
41:75–80. 1998. View Article : Google Scholar : PubMed/NCBI
|