Introduction
Hyalinizing clear cell carcinoma (HCCC), also known
as clear cell carcinoma with hyalinization, is a rare, low-grade
malignant neoplasm originating from the minor salivary glands.
Initially described by Batsakis (1)
in 1980, the definition of HCCC was later refined by Simpson et
al (2) and Milchgrub et
al (3). HCCC has been referred
to as clear cell adenocarcinoma, clear cell carcinoma (nonspecific)
or clear cell carcinoma by various authoritative sources, including
the Pathology Atlas Volume of the Military Forces Institute of
Pathology (4), the 3rd edition of
the World Health Organization (WHO) Head and Neck Tumor Pathology
and Genetics Classification (5),
and the 4th edition of the WHO Head and Neck Tumor Classification
(6). In the 5th edition of the WHO
Classification of Head and Neck Tumors in 2022 it was renamed HCCC
(7).
Salivary gland tumors account for 0.5% of all
malignant tumors, with clear cell carcinoma of the salivary gland
representing ~1% of all salivary gland tumors globally. HCCC occurs
only in the minor salivary glands and is characterized by slow
growth, presenting as small inert masses with non-aggressive
biological behavior (8).
Morphologically, HCCC often presents as an irregularly shaped, hard
mass with a rough, grayish-white and grayish-red surface. The tumor
appears grayish-white in histological analysis, with hemorrhage and
necrosis commonly seen in the center. The tumors exhibit poorly
defined boundaries and infiltrate the surrounding tissues, with
diameters typically ranging from 1 to 5 cm. A study in China
involving 10 patients with clear cell carcinoma of the salivary
gland reported tumor diameters measuring 1.5-5.0 cm, with a mean of
3 cm (9). Additionally, Zhang et
al (10) analyzed the
histological morphology of eight cases diagnosed as HCCC of the
salivary gland at the Department of Pathology, The Affiliated
Cancer Hospital of Fudan University between January 2015 and
October 2019. A basal cell-like arrangement was seen in a few
cases, with occasional keratinization in the nests. This previous
study concluded that, histologically, the tumor showed infiltrative
growth, and the tumor cells were arranged in trabecular, cord-like
or solid nest structures.
Because of its rarity, HCCC lacks sufficient
clinical trials to establish standardized treatment protocols.
Moreover, it is not well known to pathologists, leading to frequent
misdiagnoses. Therefore, the present study aimed to provide a
comprehensive understanding of clear cell carcinoma of the salivary
glands from various perspectives, including clinical signs, imaging
features, pathological manifestations, treatment methods and
prognosis, through a case report and literature review.
Case report
Clinical data and medical history
The patient was a 52-year-old woman who presented
with a foreign body sensation at the root of the tongue and
dysphagia for >1 month. The patient denied experiencing pain,
dyspnea, voice changes or any generalized discomfort, and reported
no history of smoking or alcoholism. No enlarged lymph nodes were
detected in the bilateral maxillofacial area and neck. The tongue
mobility was fair, with centered extension, and no apparent signs
of enlargement were observed at the root of the tongue.
A total of 2 weeks before admission, the patient
visited the otorhinolaryngology department of a local hospital,
where a laryngoscopy revealed a new mass at the root of the tongue.
The excised mass was then subjected to a biopsy, and the pathology
results indicated a malignant tumor of small salivary gland origin,
with a high likelihood of mucoepidermoid carcinoma (MEC).
Preoperative imaging
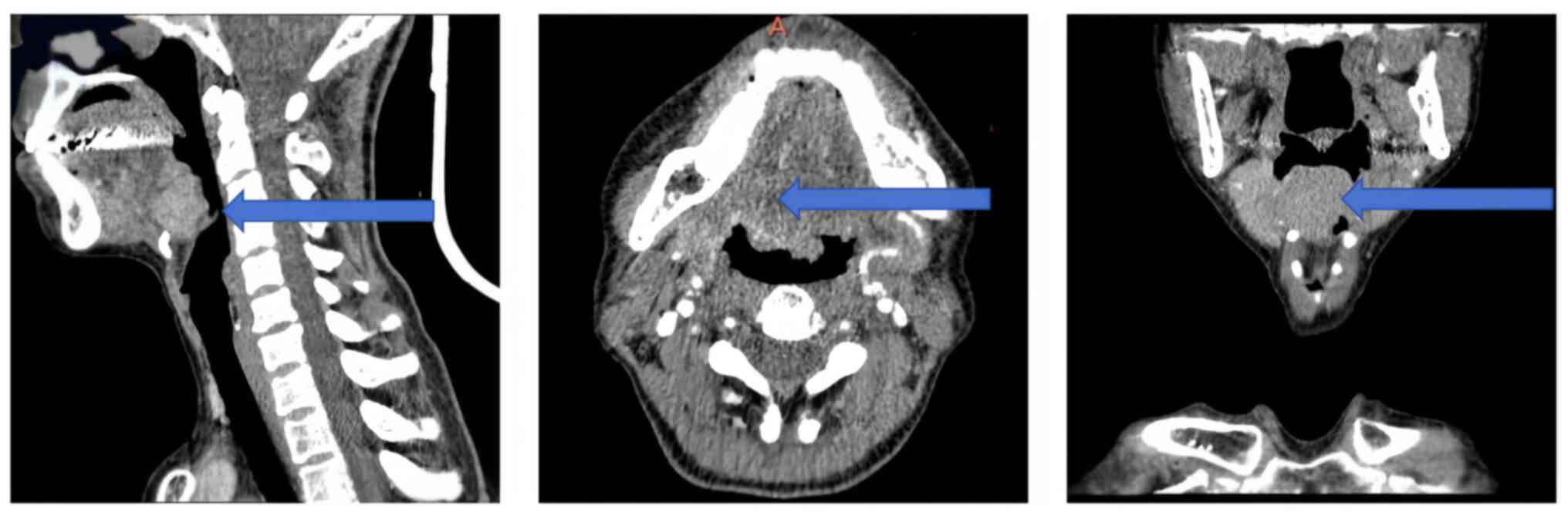
Enhanced head and neck computed tomography (CT)
revealed a homogeneously enhancing mass to the right of the tongue
root, causing narrowing of the right epiglottic vallecular
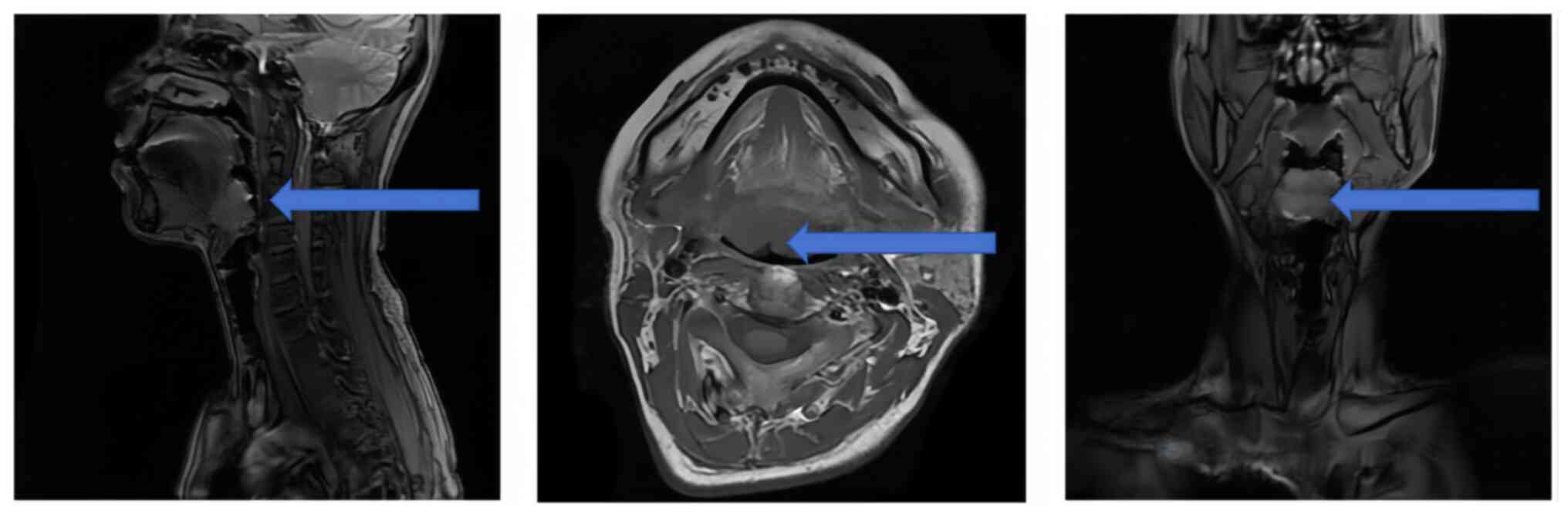
(Fig. 1). Magnetic resonance
imaging (MRI) of the head and neck showed an abnormal signal to the
right of the posterior root of the tongue, suggesting an abnormal
localization (Fig. 2).
Preoperative pathology result
Immunohistochemical staining revealed the following
results: p40 (+), cytokeratin (CK)5/6 (+), p53 (+50%), CK7 (+) and
P16 showing partial positivity. Following consultation with the
local hospital pathology department, the diagnosis was refined to a
salivary gland epithelial tumor with low malignancy.
Surgical procedure
The treatment plan and potential surgical
complications were discussed with the patient and their family, and
an informed consent form was signed. The procedure involved a right
functional neck dissection, localized extended lumpectomy of the
right tongue root, median mandibulotomy and anterolateral thigh
flap transplantation.
A post-cervical lymphatic incision was made on the
right side of the neck, allowing for clearance of the right
functional cervical lymph nodes. The internal jugular vein and
parasympathetic nerves were preserved and the tongue root and mass
were fully exposed through the median mandibular splitting.
Following the No-Tumor Principle (11), a partially enlarged surgical
resection was performed at the edge of the mass, and an
anterolateral thigh flap was used to repair the defective area of
the tongue root. Postoperatively, a tracheostomy was performed, and
the right side of the tongue root mass and the lymphatic tissue
were histopathologically examined.
Postoperative pathological
results
Histopathologically examining the tongue root mass
revealed mucosal irregularities, measuring 6.2×5.4×3.8 cm, with
grayish-white nodules. The tumor, measuring 4.2×2.3×2 cm, exhibited
a relatively firm consistency. Hematoxylin and eosin staining
revealed that the tumor cells were arranged in sheets, nests and
thin cords. The cytoplasm of the tumor cells was transparent, the
stroma around the nests of the cells was reddish-stained, and the
mesenchymal stroma around the tumor showed fibrous changes
(Fig. 3). The fixative was 10%
formalin and the tissues were fixed at room temperature for 24 h.
The thickness of the sections was 4 µm. Staining was performed at
room temperature, with Hematoxylin applied for 5 min and Eosin for
2 min. We used a Nikon Eclipse Ti2 inverted microscope.
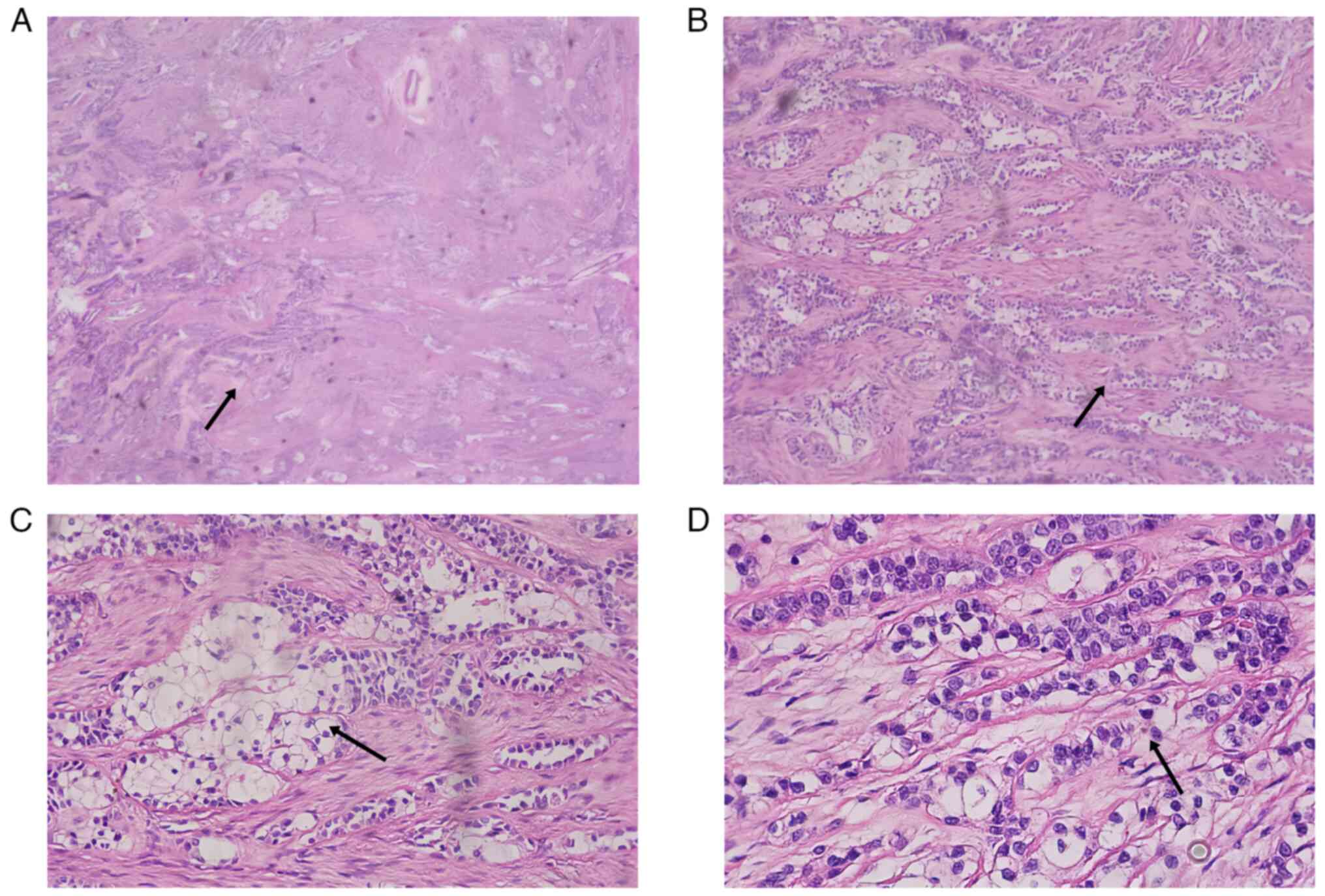
Immunohistochemical staining revealed the following
results: CK5/6 (+), CK7 (+), calponin (−), p63 (+), CD117 (−), p40
(+), Ki-67 (5% +), CD34 (−), S-100 (−) and SOX10 (−; Fig. 4). The tissues used for
immunohistochemistry were paraffin-embedded, and the sections were
cut to a thickness of 4 micrometers. The blocking reagent used was
5% BSA (Thermo Fisher Scientific, Inc.), applied at room
temperature for 1 h. The primary antibody was diluted to 1:200,
obtained from Roche, America, catalogue number CK5/6: 790-4554;
CK7: 790-4462; Calponin: 760-4376; P63: 790-4509; CD117:
08763909001; P40: 790-4950; Ki-67: 790-4286; CD34: 790-2927; SOX10:
790-4968; DOG-1: 760-4590; S-100: 790-2914, and incubated overnight
at 4°C. Secondary antibody dilution: 1:200, catalogue number:
bs-9912R, supplier, conjugate, Bioss, China, temperature: 25°C and
duration of incubation: 1 h. The images were captured using Nikon
Eclipse Ti2 inverted microscope. Although acinic cell carcinoma
(AciCC) and squamous cell carcinoma of the head and neck were
suspected, they were ruled out by a negative DOG1 result (Fig. 4) (12). DOG1, or discovered on
gastrointestinal stromal tumors 1, is an immunohistochemical marker
primarily used to identify AciCC among salivary gland tumors, as
AciCC often exhibits positive DOG1 expression. According to Khurram
and Speight (12), DOG1 is valuable
for differentiating AciCC from tumors with similar histological
features, such as clear cell carcinoma, where DOG1 is typically
negative. In the present case, the negative DOG1 result helped
exclude AciCC, guiding toward a rarer diagnosis of HCCC of the
tongue root. Fluorescence in situ hybridization (FISH)
results revealed positive breakage recombination of the
EWSR1 gene (Fig. S1). Based
on these findings, the morphology and immunophenotype of the mass
were indicative of clear cell carcinoma of the salivary gland. No
metastasis was observed in the cervical lymph nodes (0/5).
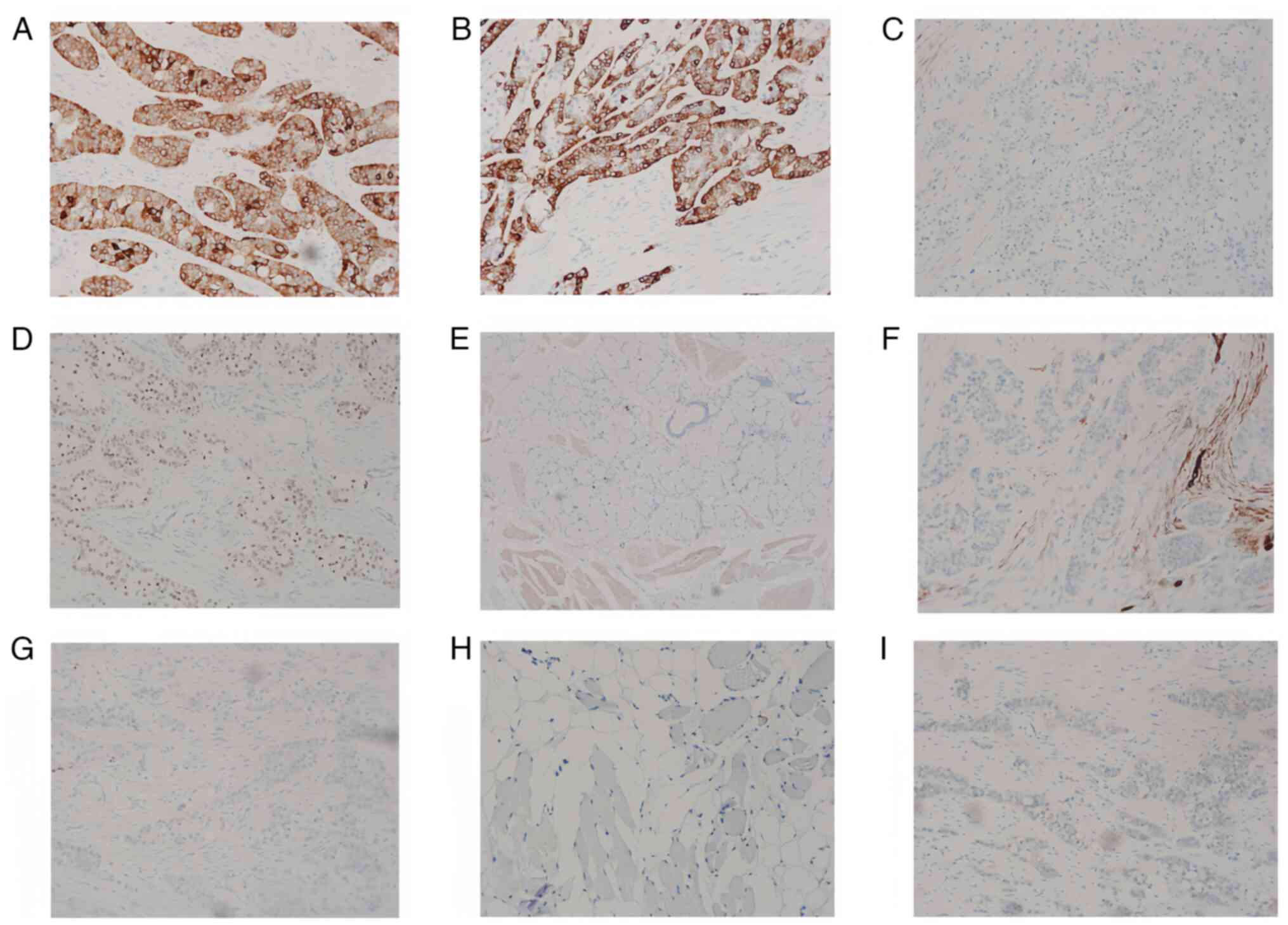 | Figure 4.Immunohistochemical staining results
of the tongue root mass. (A) CK5/6 (+), magnification ×100, (B) CK7
(+), magnification ×100, (C) calponin (−), magnification ×40, (D)
p63 (+), magnification ×40, (E) Ki-67 (5%+), magnification ×40, (F)
CD34 (−), magnification ×40, (G) S-100 (−), magnification ×40, (H)
SOX10 (−), magnification ×40 and (I) DOG-1 (−), magnification ×40.
CK, cytokeratin. |
After consulting with the multidisciplinary tumor
team and considering the extensive nature of the surgery, the
patient received 1 month of adjuvant radiation therapy. The
specific radiation therapy plan, based on the diagnosis of T3N0M0
clear cell carcinoma at the tongue root, outlined the target area
as the preoperative tumor area and bilateral cervical lymph nodes
(areas Ib, II and III). A total dose of 60 Gy was administered over
30 routine irradiation sessions, with each session delivering 2 Gy,
conducted 6 times/week for 5 weeks. Because it was a low-grade
malignant tumor, radiotherapy was not planned for the lymph node
drainage area in zone IV. The target area, including the
oropharyngeal mucosa, was relatively large, and the patient
exhibited a slightly heightened radiation response.
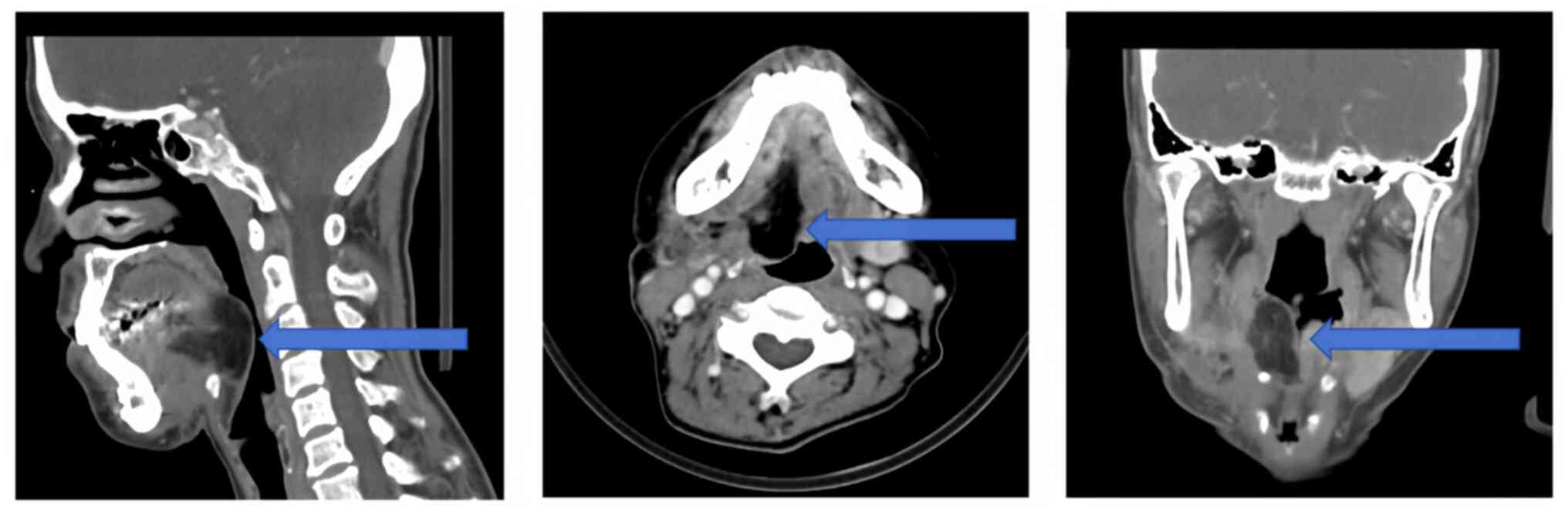
Postoperative imaging examination
A total of 2 months following the operation, an
enhanced CT scan of the tongue was performed. The results showed
the structural disorder of the tongue root and disorganization of
the mandibular operation area. Mixed-density and linear enhancement
from the operative area to the right sternocleidomastoid muscle
tract were also observed. The right submandibular gland was not
visible (Fig. 5), consistent with
the postoperative changes from the localized surgery. There were no
apparent signs of recurrence or metastasis.
Medical history
The patient underwent a biopsy in August 2023, at
the People's Hospital of Changji Hui Autonomous State (Xinjiang
Uyghur Autonomous Region, China. They were admitted to The First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China)
7 days later and were treated by a surgeon 5 days after admission.
The patient was discharged in September 2023. The patient was
followed up at 1 month, three and six months after discharge, with
the most recent follow-up in July 2024. The preoperative
immunohistochemistry and biopsy pathology images are unavailable,
as these diagnostic procedures were performed at another hospital
(People's Hospital of Changji Hui Autonomous State).
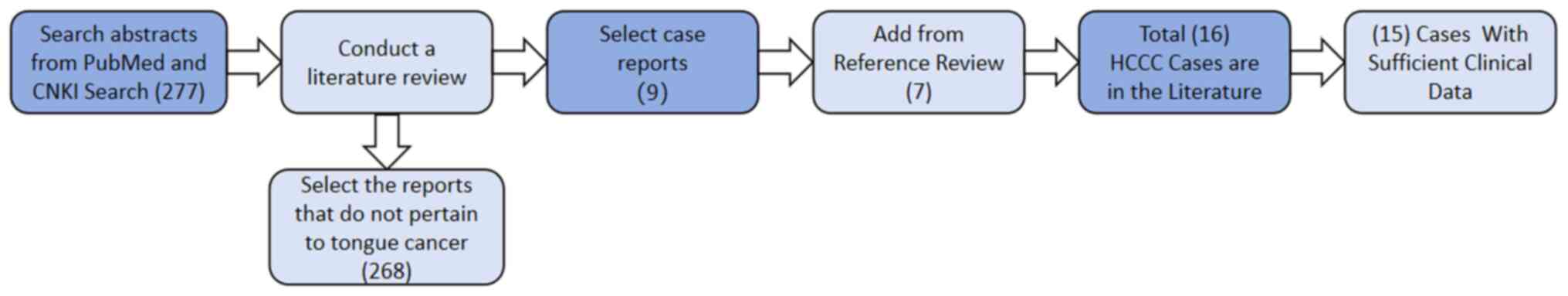
Literature review
A comprehensive literature review was performed by
searching the key words ‘clear cell carcinoma’ and ‘salivary gland’
in the PubMed (pubmed.ncbi.nlm.nih.gov) and CNKI (cnki.net)
databases. After excluding reports on clear cell carcinoma in
non-salivary gland areas and non-tongue primary lesions, 277
reports were retrieved. After thoroughly reviewing these reports
and their relevant references, 15 reports with adequate clinical
data, including complete pre- and postoperative information, were
identified (Fig. 6).
Table I presents the
clinical and statistical features of 16 cases of primary clear cell
carcinoma of the tongue, including the present case (13–25).
Among these patients, nine were female and seven were male, with an
average age of 53 years (range: 33-69 years). Typical symptoms
included dysphagia, a painless mass, tongue root ulceration and a
foreign body sensation in the throat. Dysphagia occurred in eight
cases and a painless mass was noted in three cases, with the
duration of these symptoms ranging from 1 to 6 months. The tumor
size was mentioned in 11 cases, with an average diameter of 3.27 cm
(range: 1.00-5.50 cm). The primary tumor lesion was located in the
tongue in all 16 patients, with 14 in the tongue root and two in
the ventral tongue. Lymph node metastasis was observed in five
patients.
 | Table I.Published cases of primary clear cell
carcinoma of the tongue root. |
Table I.
Published cases of primary clear cell
carcinoma of the tongue root.
| First author,
year | Age, years/sex | Clinical
symptoms | Tumor size and
location | Metastasis | Treatment | Follow-up | Results | (Refs.) |
|---|
| Present case | 52/F | Foreign body | 4.2×2.3×2.0
cm; | No metastasis | The right-side | No recurrence | CK5/6 (+), CK7
(+), | - |
|
|
| sensation at
the | located on the |
| functional | within 3
months | calponin (−), p63
(+), |
|
|
|
| tongue root | right side
behind |
| neck dissection
+ |
| CD117 (−), p40
(+), |
|
|
|
| with dysphagia | the tongue
root |
| the right side
of |
| Ki-67 (5% +), |
|
|
|
| for 1 month |
|
| the tongue
root |
| CD34 (−), S-100
(−), |
|
|
|
|
|
|
| local extended |
| SOX10 (−) and |
|
|
|
|
|
|
| lumpectomy + |
| DOG-1 (−);
positive |
|
|
|
|
|
|
| median |
| breakage
recom- |
|
|
|
|
|
|
| mandibulotomy
+ |
| bination of
the |
|
|
|
|
|
|
| Anterolateral |
| EWSR1
gene |
|
|
|
|
|
|
| thigh flap |
|
|
|
|
|
|
|
|
| transplantation
+ |
|
|
|
|
|
|
|
|
| tracheostomy + |
|
|
|
|
|
|
|
|
| postoperative |
|
|
|
|
|
|
|
|
| radiotherap |
|
|
|
| Dabas et al,
2023 | 33/F | Dysphagia,
voice | 5.5×4.4×4.1
cm; | Not mentioned | Surgical excision
+ | No recurrence | Not mentioned | (13) |
|
|
| changes and
right | located
bilaterally |
| tracheostomy + | as of 2023 |
|
|
|
|
| ear pain for | at the tongue
root, |
| postoperative |
|
|
|
|
|
| 6 months | extending to
the |
| radiotherapy |
|
|
|
|
|
|
| anterior
tongue, |
|
|
|
|
|
|
|
|
| adjacent to
the |
|
|
|
|
|
|
|
|
| epiglottis and |
|
|
|
|
|
|
|
|
| tonsils |
|
|
|
|
|
| Sento et al,
2020 | 59/M | A painless
mass | 2.8×2.1×1.5
cm; | Not mentioned | Complete tumor | No local | p63 (+), S-100
(−), | (14) |
|
|
| on the
inferior | located on the |
| resection +
partial | recurrence or | αSMA (−), CD10
(−), |
|
|
|
| surface of the | inferior
surface |
| glossectomy
with | metastasis at | GFAP (−), |
|
|
|
| tongue | of the tongue |
| ~1 cm safe margin
+ | the 5-year | vimentin-Ki-67 |
|
|
|
|
|
|
| reconstruction
with | postoperative | <10% and |
|
|
|
|
|
|
| a free forearm
flap | follow-up | EWSR1-ATF1
fusion |
|
| Yoldez et
al, 2022 | 37/M | An ulcerated
and | 3.0×0.5 cm; | Not mentioned | Surgical
excision | Not mentioned | p40 (+), CD10
(−) | (15) |
|
|
| painful indu- | located at the |
|
|
| p40 (+), CD10
(−) |
|
|
|
| ration of the
base | base of the
tongue; |
|
|
|
|
|
|
|
| of the tongue | poorly circum- |
|
|
|
|
|
|
|
|
| scribed with |
|
|
|
|
|
|
|
|
| infiltration of
the |
|
|
|
|
|
|
|
|
| adjacent
tissue |
|
|
|
|
|
| Pillai et
al, 2019 | 42/M | Swallowing
diffi- | 3.0×2.0 cm;a
large, | No neck nodes | Excision biopsy
+ | No local | CK AE1/AE3
(+), | (16) |
|
|
| culty for 5
months | smooth, broad- | were palpable; | coblation + | recurrence at | CK5/6 (+), p63
(+), |
|
|
|
| and feeling of | based mass at
the | no metastasis | postoperative | 1-year | S-100 (−) and |
|
|
|
| choking
sensation | posterior
one-third |
| radiotherapy | follow-up | CD10 (−) |
|
|
|
| in the throat | of the tongue |
|
|
|
|
|
|
|
|
intermittently. | obstructing
the |
|
|
|
|
|
|
|
| No associated | laryngeal
inlet |
|
|
|
|
|
|
|
| voice change
or |
|
|
|
|
|
|
|
|
| dyspnea |
|
|
|
|
|
|
| Lin et al,
2015 | 37/F | Painless
swelling | 1×1 cm nodule
on | Not mentioned | Further
excision | Not mentioned | CKAE1/3 (+), | (17) |
|
|
| on the ventral | the left
ventral |
| with safe
resection |
| p63 (+), SMA
(−), |
|
|
|
| tongue that
had | tongue |
| margin |
| CD10 (−), S-100
(−), |
|
|
|
| been present
for |
|
|
|
| GFAP (−), MSA
(−) |
|
|
|
| months |
|
|
|
| and EWSR1
gene |
|
|
|
|
|
|
|
|
| rearrangement |
|
| Hu and Li,
2005 | 69/M | Restricted
tongue | 5.0 cm maximum | 1/64 | Surgical excision
+ | No local | EMA (+), CK8
(+), | (18) |
|
|
| movement for | diameter at
the |
| postoperative | recurrence or | CK18 (−), |
|
|
|
| 5 months | base of the
tongue |
| radiotherapy | metastasis at | CKHMW (−), |
|
|
|
|
|
|
|
| 4-month | CK10/13 (−), |
|
|
|
|
|
|
|
| follow-up | S-100 (−), SMA
(−), |
|
|
|
|
|
|
|
|
| and calponin
(−) |
|
| Bala- | 35/M | Swallowing | 3.0×2.0 cm; | Cervical | Excisional
biopsy | No local | Immunohisto- | (19) |
| krishnan et
al, |
| difficulty for | smooth and | second abdo- | was performed
with | recurrence or | chemistry was
not |
|
| 2002 |
| 5 months and | elevated
yellowish- | minal lymph | the assistance of
a | metastasis at | performed |
|
|
|
| feeling of
choking | white lesion | nodes and |
microlaryngoscope | 1-year |
|
|
|
|
| sensation in
the | extending from
the | submandibular | and surgical | follow-up. |
|
|
|
|
| throat | midline to the | lymph nodes | microscope; 2
weeks | The patient |
|
|
|
|
| intermittently | lingual groove
of | could be | later, extensive
re- | accepted the |
|
|
|
|
|
| the right
tonsil | palpated on | section of the
lesion | result because |
|
|
|
|
|
|
| the right
side, | through a
trans- | of good |
|
|
|
|
|
|
| each | mandibular | phonetics |
|
|
|
|
|
|
| ~2.0×1.0 cm, | approach was |
|
|
|
|
|
|
|
| with no | performed, with
the |
|
|
|
|
|
|
|
| pressure pain | defect
reconstructed |
|
|
|
|
|
|
|
|
| with a tongue
flap. |
|
|
|
|
|
|
|
|
| A scapulohyoid |
|
|
|
|
|
|
|
|
| supraglottic
cervical |
|
|
|
|
|
|
|
|
| lymph node
dis- |
|
|
|
|
|
|
|
|
| section and
frozen- |
|
|
|
|
|
|
|
|
| section biopsy of
an |
|
|
|
|
|
|
|
|
| isolated lymph
node |
|
|
|
|
|
|
|
|
| were performed |
|
|
|
|
|
|
|
|
| prior to the
resection |
|
|
|
| Chapman et
al, | 68/F | Not mentioned | 3.0 cm; the
left | Not mentioned | Excisional
biopsy | No local | CK5 (+), p63
(+), | (20) |
| 2018 |
|
| base of the
tongue |
| with positive | recurrence or | S-100 (−), SMA
(−), |
|
|
|
|
|
|
| margins. Left
level | metastasis at | negative
MAML2 |
|
|
|
|
|
|
| II–III lymph
node | 18-month | breakage,
positive |
|
|
|
|
|
|
| clearance
showed | follow-up | EWSR1
rearrange- |
|
|
|
|
|
|
| negative
results. |
| ment and intact
ATF1 |
|
| Wang et al,
2018 | 69/M | Not mentioned | The base of
the | 1/64 | Combined left | No local | CK (+), S-100
(+) | (21) |
|
|
|
| left tongue |
| lingual and
cervical | recurrence or | and SMA (−) |
|
|
|
|
|
|
| curettage +
right | metastasis at |
|
|
|
|
|
|
|
| zonal cervical | 42-month |
|
|
|
|
|
|
|
| lymph node | follow-up |
|
|
|
|
|
|
|
| dissection + |
|
|
|
|
|
|
|
|
| pectoralis
major |
|
|
|
|
|
|
|
|
| myocutaneous
flap |
|
|
|
|
|
|
|
|
| repair |
|
|
|
| Wang et al,
2018 | 47/F | Dysphagia | Base of the
left | No metastasis | Extended resection
+ | No recurrence
at | CK (+) and SMA
(−) | (21) |
|
|
|
| tongue |
| adjacent flap
repair | 12-month |
|
|
|
|
|
|
|
| without
cervical | follow-up |
|
|
|
|
|
|
|
| lymph node |
|
|
|
|
|
|
|
|
| dissection |
|
|
|
| Wang et al,
2018 | 67/M | Not mentioned | The base of
the | 0/17 | Extended resection
+ | No recurrence | CK (+), SMA
(−) | (21) |
|
|
|
| right tongue |
| right zonal
cervical | at 6-month | and S-100 (−) |
|
|
|
|
|
|
| lymph node | follow-up |
|
|
|
|
|
|
|
| dissection +
radial |
|
|
|
|
|
|
|
|
| forearm free
flap |
|
|
|
| Al Zadjali et
al, | 38/F | A 2-week
history | 2.9×5.2×3.2
cm; | 2/32 | Tracheostomy + | No recurrence | CK5 (+), CK7
(+), | (22) |
| 2023 |
| of a sore
throat | left root of
the |
| Transcer-vical | at 12-month | p40 (+), p63
(+), |
|
|
|
| superimposed
on | tongue and
left |
|
transmandibular | follow-up | S-100 (−), |
|
|
|
| a 4-year
history | tonsil |
| approach for
wide |
| SOX10 (−) and |
|
|
|
| of hemoptysis. |
|
| excision of
the |
| EWSR1-ATF1
fusion |
|
|
|
| During this
time, |
|
| lesion + neck |
|
|
|
|
|
| they also |
|
| dissection +
radial |
|
|
|
|
|
| experienced |
|
| forearm free flap
+ |
|
|
|
|
|
| progressive |
|
| postoperative |
|
|
|
|
|
| dysphagia and |
|
| adjuvant |
|
|
|
|
|
| odynophagia |
|
| radiotherapy |
|
|
|
| O'Sullivan- | 59/F | Dysphagia | 3 cm; left root
of | No metastasis | Surgical
extended | No recurrence | CK (+), p63
(+), | (23) |
| Mejia et al.
2009 |
|
| the tongue. |
| resection | during | EMA (+), PAS
(+), |
|
|
|
|
|
|
|
| follow-up | CAM5.2 (weak
+), |
|
|
|
|
|
|
|
|
| S-100 (−), desmin
(−), |
|
|
|
|
|
|
|
|
| TGB (−) and Mu
(−) |
|
| Suzuki et
al, 2006 | 66/F | Dysphagia,
denial | 4×3×2.5 cm; | No metastasis | Tracheostomy + | No recurrence | Not mentioned | (24) |
|
|
| of respiratory | tongue root |
| resection via
the | at 21-month |
|
|
|
|
| distress |
|
| paramedian | follow-up |
|
|
|
|
|
|
|
| mandibulotomy |
|
|
|
|
|
|
|
|
| combined with
a |
|
|
|
|
|
|
|
|
| right-sided
supra- |
|
|
|
|
|
|
|
|
| omohyoid neck |
|
|
|
|
|
|
|
|
| dissection. A
macro- |
|
|
|
|
|
|
|
|
| scopic
surgical |
|
|
|
|
|
|
|
|
| margin was set
at |
|
|
|
|
|
|
|
|
| ~10 mm. Both
the |
|
|
|
|
|
|
|
|
| lingual and |
|
|
|
|
|
|
|
|
| hypoglossal |
|
|
|
|
|
|
|
|
| nerves were |
|
|
|
|
|
|
|
|
| preserved. |
|
|
|
| Zhao et al,
2022 | 67/M | Neck mass
found | The right root
of | Right cervical | Extensive
total | No recurrence | CK5/6 (+), p40
(+), | (25) |
|
|
| for >1 year | the tongue | lymph node | excision of
the | at 26-month | p63 (+), CK7
(+), |
|
|
|
|
|
| metastasis | mass +
cervical | follow-up | EMA (+), |
|
|
|
|
|
| (3/16) | lymph node |
| Ki-67 (5-10%
+), |
|
|
|
|
|
|
| dissection |
| CD117 (−), |
|
|
|
|
|
|
|
|
| CD10 (−), GFAP
(−), |
|
|
|
|
|
|
|
|
| SMA (−), S-100
(−), |
|
|
|
|
|
|
|
|
| calponin (−),
positive |
|
|
|
|
|
|
|
|
| breakage
recom- |
|
|
|
|
|
|
|
|
| bination of
EWSR1 |
|
Treatment was consistent across cases, with all
patients undergoing extended local tumor resection. Additionally,
seven of the 16 patients underwent repair and reconstruction, and
five received postoperative radiotherapy. Postoperative follow-up
information was unavailable for two patients; however, no
recurrence was observed in the other 14 patients during the
follow-up period. The findings suggested that clear cell carcinoma
has a good prognosis when treated with localized mass-enlarged
resection and postoperative adjuvant radiotherapy.
Discussion
HCCC of the salivary glands typically presents as a
slow-growing, painless submucosal mass with no surface ulceration.
Consequently, symptoms are often present for an extended period
before the patient seeks treatment. Most of the aforementioned
cases involve tumors with a size of 3-5 cm in diameter (13–25).
Clear cells are present in a number of other
salivary gland tumors, necessitating differential diagnoses that
rely on a combination of immunohistochemistry, specific staining
and the morphological features of HCCC of the salivary glands. The
histological features observed in the 16 cases reported on in the
present study were as follows: Tumor cells were arranged in sheets,
nests or thin cords with clear boundaries; the cytoplasm was
transparent; the nuclei were round or oval in shape and relatively
uniform in size; the nucleoli were inconspicuous; and mitotic
figures were rare. In addition, nuclear fission was rare; the
stroma around the cell nests was stained red, and the mesenchyme
around the tumor was fibrous. The tumor cells grew infiltratively
into the fibrous mesenchyme. Immunohistochemical results were
positive for epithelial markers, such as CK5/6, CK7 and p63, and
negative for myoepithelial markers, such as S-100 and SOX-10.
The immunohistochemical features of HCCC of the
salivary gland overlap with those of various salivary tumors, such
as MEC and squamous cell carcinoma, all of which are positive for
CK7, p63 and p40, and negativity for S-100 and SOX-10. In the last
decade, advances in molecular techniques have demonstrated
recurrent genetic alterations in some salivary gland tumors,
including the fusion of genes such as ETV6 in secretory
carcinoma, MYB and MYBL1 in adenoid cystic carcinoma,
and MAML2 in MEC (26–28).
Additionally, EWSR1-ATF1 rearrangements have been
found in HCCC, and HRAS exon three mutations are seen in
most cases of epithelial-myoepithelial carcinoma (29,30).
HRAS exon three mutations and a high percentage of
EWSR1 rearrangements are commonly detected in clear cell
subtype myoepithelial carcinoma (31). FISH technology serves a vital role
in pathological research, particularly in detecting recombination
in the EWSR1 gene, a significant genetic alteration commonly
observed across various tumor types (32–34).
FISH allows for the precise detection of EWSR1 gene
recombination, aiding in tumor characterization. The technique has
high sensitivity and specificity for identifying chromosomal
abnormalities, making it an integral part of diagnostic processes
(35–37). In the present case, FISH results
showed positive EWSR1 gene breakage recombination,
confirming the diagnosis of HCCC of the salivary glands and ruling
out MEC.
HCCC can be differentiated from MEC and metastatic
clear cell carcinoma (MCCC) in several ways (31,38).
First, MEC is a malignant tumor with varying proportions of mucous,
intermediate and epidermoid cells. It can occasionally include
columnar cells, clear cells and eosinophils. While the tumor often
demonstrates cystic growth, a clear cell component is generally
rare and atypical. Second, the most common origin site of clear
cell carcinoma is the kidney, and thus, MCCC typically arises from
distant organs, such as the kidneys. Clinically, MCCC presentation
varies depending on the site of metastasis. Imaging studies such as
CT, MRI and pathological evaluations, including immunohistochemical
staining, are crucial for an accurate diagnosis.
Immunohistochemistry of MCCC typically shows positivity for PAX8
and CK7, along with increased expression of HIF-1α and VEGF. The
pathological features of MCCC resemble those of primary clear cell
carcinoma, but a thorough medical history, imaging and specific
immunohistochemical markers can help make a proper differentiation.
Based on these differences and the tumor origin, the present study
ruled out a diagnosis of either MEC or MCCC.
Due to the rarity of HCCC in the salivary glands,
there are insufficient clinical trials to determine standardized
treatment protocols. Most malignant salivary gland tumors require
postoperative radiation therapy to reduce the recurrence rate owing
to undesirable features, such as limited margins for resection.
Postoperative radiation therapy is also indicated for some
moderately to highly differentiated tumors with T-stage 3-4 or
lymph node metastases (39). All 16
cases of primary HCCC of the tongue assessed in the present
literature review underwent localized enlarged mass resection; five
cases underwent postoperative radiotherapy, whereas 11 did not.
None of the patients experienced local recurrence or lymph node
metastasis during the follow-up period. Desai et al
(40) specifically analyzed 201 of
254 cases of HCCC of the salivary glands and described the
treatment options. The most common approach was surgical resection
with extensive margins (81.1%). Cervical lymph node dissection was
performed in 10.4% of the cases. Adjuvant treatments were rarely
performed, with radiotherapy or chemotherapy administered in only
17.9% of the cases. Of the 223 cases in which recurrence was
reported, at least one localized recurrence was observed in 15.2%
of cases and more than one recurrence in 3.6%, resulting in a
recurrence rate of 18.8% (40).
Analyses of the salivary gland cases collected by Desai et
al (40), along with the cases
of primary HCCC of the tongue collected in the present study,
showed a low recurrence rate, likely due to the low degree of
malignancy, low biological aggressiveness, and low rate of
lymphatic and distant metastasis of the tumors. It was also
indicated that patients with HCCC of the salivary gland had a
better overall prognosis if they underwent complete localized
extended resection with or without postoperative radiotherapy.
However, despite the low malignancy and recurrence rate of this
type of cancer, lymph node, lung and spinal metastases have been
reported in a number of cases (38,40–43).
Therefore, long-term clinical follow-up after complete tumor
resection is essential.
The present study has one specific limitation;
photographic documentation of the surgical specimen was not
obtained during the procedure. However, detailed written records
and descriptions were meticulously maintained to ensure
comprehensive case documentation.
In conclusion, HCCC is a rare, low-grade malignant
salivary gland tumor characterized by slow clinical progression. It
is often confused with benign or other salivary gland tumors, and
its diagnosis relies on complete histological morphology and
immunohistochemical examination. The FISH test for the fusion of
the MAML2 and EWSR1 genes aids in making a conclusive
diagnosis. The preferred treatment is extended resection of the
localized mass, with radiotherapy based on lymph node metastasis
and pathological examination to minimize local recurrence and
improve the overall patient prognosis.
There are relatively few reports of HCCC occurring
in the maxillofacial region, and the present case provides some new
insights into its diagnosis and treatment. It is necessary to build
a solid foundation to enhance knowledge of this disease, including
its clinical manifestations, imaging features and treatment
options. This will improve differential diagnosis for this rare
disease when patients present with these characteristics. Surgical
treatment of HCCC should be specialized and distinct from standard
procedures. Future research may delve more deeply into the
molecular and genetic mechanisms underlying HCCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BL, LL and XC conceived and designed the study. JW,
YX, YF, XT collected and analyzed data. LL and XC drafted the
initial manuscript and revised it critically for important
intellectual content. LL and BL confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Ethics
Committee of the First Affiliated Hospital of Xinjiang Medical
University (approval no. K202405-30), acknowledging the study's
contribution to medical progress and patient safety. The study
complied with ethical standards, ensuring the patient's autonomy,
privacy and data confidentiality. The imaging and diagnostic data
are used solely for academic and educational purposes. Written
informed consent was obtained from the patient.
Patient consent for publication
The patient provided written informed consent for
publication, authorizing the use of their imaging, pathological and
clinical data for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
FISH
|
fluorescence in situ
hybridization
|
|
HCCC
|
hyalinizing clear cell carcinoma
|
|
WHO
|
World Health Organization
|
References
|
1
|
Batsakis JG: Clear cell tumors of salivary
glands. Ann Otol Rhinol Laryngol. 89:196–197. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simpson RH, Sarsfield PT, Clarke T and
Babajews AV: Clear cell carcinoma of minor salivary glands.
Histopathology. 17:433–438. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milchgrub S, Gnepp DR, Vuitch F, Delgado R
and Albores-Saavedra J: Hyalinizing clear cell carcinoma of
salivary gland. Am J Surg Pathol. 18:74–82. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ellis GL and Auclair PL: Armed Forces
Institute of Pathology (AFIP) Atlas of tumor pathology. Tumors of
the salivary glands (fourth series, fascicle 9). ARP Press;
Maryland: pp. 301–309. 2008
|
|
5
|
Thompson L: World Health Organization
classification of tumors: Pathology and genetics of head and neck
tumors. Ear Nose Throat J. 85:742006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Naggar AK, Chan JKC, Grandis JR, Takata
T and Slootweg PJ: WHO classification of head and neck tumors. 4th
edition. IARC Press; Lyon: pp. 168–169. 2017, PubMed/NCBI
|
|
7
|
WHO Classification of Tumours Editorial
Board, . Head and neck tumours. WHO classification of tumours
series. 5th edition. IARC Press; Lyon: pp. 664–669. 2022
|
|
8
|
Sanjai K, Shivalingaiah D, Sharath R and
Pandey B: Clear cell carcinoma of palatine salivary gland: A
diagnostic challenge. J Oral Maxillofac Pathol. 22:128–131. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gai J and Lu X: Clinicopathologic features
and differential diagnosis of nonspecific clear cell carcinoma of
salivary gland. J Mod Med. 41:520–522. 2013.(In Chinese).
|
|
10
|
Zhang Y, Wang Z, Chen T, Bai Q and Li X:
Clinicopathological characterization of 8 cases of vitelliform
clear cell carcinoma of salivary gland. Chin J Cancer. 33:168–173.
2023.(In Chinese).
|
|
11
|
Kamat M, Rai BD, Puranik RS and Datar UV:
A comprehensive review of surgical margin in oral squamous cell
carcinoma highlighting the significance of tumor-free surgical
margins. J Cancer Res Ther. 15:449–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khurram SA and Speight PM:
Characterisation of DOG-1 expression in salivary gland tumours and
comparison with myoepithelial markers. Head Neck Pathol.
13:140–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dabas SK, Menon NN, Ranjan R, Tiwari S,
Gurung B, Shukla H, Dua A, Sharma A, Sinha A, Singla R, et al:
Hyalinizing clear cell carcinoma of base of the tongue-case report
and review of literature. Ann Indian Acad Otorhinolaryngol Head
Neck Surg. 7:11–17. 2023. View Article : Google Scholar
|
|
14
|
Sento S, Kudo Y, Hibiya K, Ishimaru N,
Sasabe E, Kitamura N and Yamamoto T: Hyalinizing clear cell
carcinoma of the anterior lingual salivary gland: A case report and
review of the literature. J Oral Maxillofac Surg Med Pathol.
32:267–274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoldez H, Rahma Y and Maha D: An
unmistakable tumour of the tongue. J Oral Health Craniofac Sci.
7:20–21. 2022. View Article : Google Scholar
|
|
16
|
Pillai N, Balasundaram P and Isaac N:
Hyalinizing clear cell carcinoma: Base of tongue. Indian J
Otolaryngol Head Neck Surg. 71 (Suppl 1):S239–S242. 2019.
View Article : Google Scholar
|
|
17
|
Lin JC, Liao JB, Fu HT, Chang TS and Wang
JS: Salivary gland hyalinizing clear cell carcinoma. J Pathol
Transl Med. 49:351–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y and Li J: Clinicopathologic analysis
of 10 cases of clear cell carcinoma of salivary gland. Chin J
Stomatol. 40:54–57. 2005.(In Chinese).
|
|
19
|
Balakrishnan R, Nayak DR, Pillai S and Rao
L: Hyalinizing clear cell carcinoma of the base of the tongue. J
Laryngol Otol. 116:851–853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chapman E, Skalova A, Ptakova N, Martinek
P, Goytain A, Tucker T, Xiong W, Leader M, Kudlow BA, Haimes JD, et
al: Molecular profiling of hyalinizing clear cell carcinomas
revealed a subset of tumors harboring a novel EWSR1-CREM fusion:
Report of 3 cases. Am J Surg Pathol. 42:1182–1189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Shen Y and Sun J: Diagnosis and
treatment of clear cell carcinoma of salivary gland. Chin J Oral
Maxillofac Surg. 266–269. 2008.(In Chinese).
|
|
22
|
Al Zadjali F, Alsaffar H, Odell M,
Wasserman JK, Tohme A and Johnson-Obaseki S: Base of the tongue
hyalinizing clear cell carcinoma: Case report and literature
review. SAGE Open Med Case Rep. 11:2050313X2312096702023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Sullivan-Mejia ED, Massey HD, Faquin WC
and Powers CN: Hyalinizing clear cell carcinoma: Report of eight
cases and a review of literature. Head Neck Pathol. 3:179–185.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki H, Katoh A, Udaka T, Shiomori T,
Fujimura T, Fujimura K and Kitamura T: Hyalinizing clear cell
carcinoma arising from the base of the tongue. Acta Otolaryngol.
126:653–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao S, Zhu Y, Pan MH, Hua HJ, Yang QY, Li
X and Li H: Clinicopathologic features of head and neck salivary
gland-type clear cell carcinoma. J Chin J Pathol. 51:494–499.
2022.
|
|
26
|
Toper MH and Sarioglu S: Molecular
pathology of salivary gland neoplasms: Diagnostic, prognostic, and
predictive perspective. Adv Anat Pathol. 28:81–93. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaur K, Mehta S, Vanik S, Trivedi P,
Banerjee N, Dhar H, Datta S and Karanjai S: The evolving role of
molecular pathology in the diagnosis of salivary gland tumours with
potential pitfalls. Eur Arch Otorhinolaryngol. 279:3769–3783. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pei J, Flieder DB, Patchefsky A, Talarchek
JN, Cooper HS, Testa JR and Wei S: Detecting MYB and MYBL1 fusion
genes in tracheobronchial adenoid cystic carcinoma by targeted
RNA-sequencing. Mod Pathol. 32:1416–1420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirose K, Usami Y, Kohara M, Sato S,
Iwamoto Y, Murakami S, Uchihashi T, Oya K, Fukuda Y, Hori Y, et al:
Clear cell carcinoma of palatal minor salivary gland harboring a
novel EWSR1-ATF1 fusion gene: report of a case and review of the
literature. Head Neck Pathol. 15:676–681. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nojima S, Kohara M, Harada H, Kajikawa H,
Hirose K, Nakatsuka SI, Nakagawa Y, Oya K, Fukuda Y, Matsunaga K,
et al: Clear cell carcinoma in the oral cavity with three novel
types of EWSR1-ATF1 translocation: A case report. Head Neck Pathol.
16:560–566. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Skalova A, Leivo I, Hellquist H, Simpson
RHW, Vander Poorten V, Willems SM, Mosaieby E, Slouka D and Ferlito
A: Clear cell neoplasms of salivary glands: A diagnostic challenge.
Adv Anat Pathol. 29:217–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dragoescu E, Jackson-Cook C, Domson G,
Massey D and Foster WC: Small cell osteosarcoma with Ewing sarcoma
breakpoint region 1 gene rearrangement detected by interphase
fluorescence in situ hybridization. Ann Diagn Pathol. 17:377–382.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ariasi C, Romanò C, Ghini I, Licata G,
Rubelli L, Artelli GL, Calzavara-Pinton P and Arisi M: Cutaneous
syncytial myoepithelioma with positive CD34 immunohistochemical
staining: An unusual tumor and a challenging diagnosis.
Dermatopathology (Basel). 10:259–265. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krystel-Whittemore M, Taylor MS, Rivera M,
Lennerz JK, Le LP, Dias-Santagata D, Iafrate AJ, Deshpande V,
Chebib I, Nielsen GP, et al: Novel and established EWSR1 gene
fusions and associations identified by next-generation sequencing
and fluorescence in-situ hybridization. Hum Pathol. 93:65–73. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukuda H, Kato I, Furuya M, Tanaka R,
Takagi T, Kondo T and Nagashima Y: A novel partner of TFE3 in the
Xp11 translocation renal cell carcinoma: Clinicopathological
analyses and detection of EWSR1-TFE3 fusion. Virchows Arch.
474:389–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vergara-Lluri ME, Stohr BA, Puligandla B,
Brenholz P and Horvai AE: A novel sarcoma with dual
differentiation: Clinicopathologic and molecular characterization
of a combined synovial sarcoma and extraskeletal myxoid
chondrosarcoma. Am J Surg Pathol. 36:1093–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sidiropoulos M, Busam K, Guitart J, Laskin
WB, Wagner AM and Gerami P: Superficial paramucosal clear cell
sarcoma of the soft parts resembling melanoma in a 13-year-old boy.
J Cutan Pathol. 40:265–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Atkins MB and Tannir NM: Current and
emerging therapies for first-line treatment of metastatic clear
cell renal cell carcinoma. Cancer Treat Rev. 70:127–137. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pfister DG, Spencer S, Adelstein D, Adkins
D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ,
et al: Head and neck cancers, version 2.2020, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
18:873–898. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Desai A, Faquin WC, Iafrate AJ, Rivera MN,
Jaquinet A and Troulis MJ: Clear cell carcinoma: A comprehensive
literature review of 254 cases. Int J Oral Maxillofac Surg.
51:705–712. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang B, Brandwein M, Gordon R, Robinson R,
Urken M and Zarbo RJ: Primary salivary clear cell tumors-a
diagnostic approach: A clinicopathologic and immunohistochemical
study of 20 patients with clear cell carcinoma, clear cell
myoepithelial carcinoma, and epithelial-myoepithelial carcinoma.
Arch Pathol Lab Med. 126:676–685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin R, Craddock KJ, Irish JC,
Perez-Ordonez B and Weinreb I: Recurrent hyalinizing clear cell
carcinoma of the base of tongue with high-grade transformation and
EWSR1 gene rearrangement by FISH. Head Neck Pathol. 6:389–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Newman WC, Williams L, Duvvuri U, Clump DA
II and Amankulor N: Hyalinizing clear cell carcinoma with
biopsy-proven spinal metastasis: Case report and review of
literature. World Neurosurg. 90:699.e7–699.e10. 2016. View Article : Google Scholar : PubMed/NCBI
|