Introduction
Breast cancer is the most commonly diagnosed cancer
among women worldwide and remains a leading cause of cancer-related
mortality. Despite advancements in early detection and treatment
strategies, the prognosis of breast cancer varies significantly due
to its heterogeneous nature and the complex interactions between
tumor biology and the host immune response (1). Identifying reliable prognostic factors
is essential for personalized treatment and management, which can
improve survival outcomes and the quality of life of patients with
breast cancer (2).
Inflammation has a crucial role in cancer
development, progression and response to treatment (3). The modified Glasgow prognostic score
(mGPS), a systemic inflammation-based scoring system, has emerged
as a valuable prognostic tool for various cancers (4). The mGPS is derived from two widely
accessible biomarkers: C-reactive protein (CRP) and albumin (Alb)
levels. A score of 0 indicates a low mGPS, representing normal CRP
(≤10 mg/l) and Alb (≥35 g/l) levels. Scores of 1 and 2 correspond
to high mGPS, indicating elevated CRP levels (>10 mg/l) with
normal or decreased Alb levels, respectively. This simple,
non-invasive scoring system has been validated in various cancer
types, including prostate, gynecological, lung and colorectal
cancers, showing a consistent association with poor survival
outcomes (5–10). However, its prognostic utility in
breast cancer remains underexplored.
Recent studies suggest that systemic inflammation
influences the tumor microenvironment and may modulate immune
surveillance and therapeutic response. Elevated CRP levels are
indicative of chronic inflammation, while hypoalbuminemia reflects
malnutrition and systemic inflammation, both of which can impair
the host's ability to mount an effective anti-tumor response
(11–14). Given that breast cancer subtypes,
such as triple-negative breast cancer (TNBC), have distinct
molecular profiles and immune characteristics, understanding the
predictive value of mGPS across these subtypes is crucial for its
clinical applicability.
In breast cancer, several established prognostic
factors include tumor size, lymph node status, histological grade,
hormone receptor status, human epidermal growth factor receptor 2
(HER2) status and 50-gene intrinsic subtype classifier (PAM50)
subtypes (15–17). However, these factors primarily
focus on tumor biology and do not account for the systemic
inflammatory response. By integrating mGPS with these traditional
prognostic markers, it is possible to develop a more comprehensive
risk stratification model that reflects both tumor and host-related
factors. This approach may offer better predictive accuracy for OS
and aid in tailoring therapeutic interventions for different
patient groups.
The current study aims to evaluate the prognostic
value of the preoperative mGPS in patients with breast cancer
undergoing surgery. A retrospective analysis of 300 patients with
breast cancer who underwent surgery and were followed for up to 10
years was conducted. The association between preoperative mGPS and
long-term survival outcomes was assessed using a variety of
statistical methods, including Kaplan-Meier survival analysis,
logistic regression and Cox proportional hazards models. In
addition, a nomogram based on significant factors identified in a
multivariate analysis was constructed to predict 5- and 10-year OS.
By analyzing the impact of mGPS on breast cancer prognosis, the
present study aimed to provide insights into its potential role as
an independent predictor of survival. This study also seeks to
establish whether mGPS, when combined with established clinical and
pathological factors, can improve risk stratification and guide
personalized treatment planning. The findings of this study may
help integrate mGPS into routine clinical practice as a simple,
accessible and effective prognostic tool for patients with breast
cancer.
Patients and methods
Patients
The present study is a retrospective cohort analysis
conducted on patients with breast cancer who underwent surgical
treatment at the Affiliated Cancer Hospital of Xinjiang Medical
University (Urumqi, China) from January 2013 to January 2014. A
total of 300 patients were included based on the following
criteria: i) Histologically confirmed breast cancer; ii) available
preoperative CRP and Alb levels for mGPS calculation; iii) complete
clinicopathological data; and iv) a follow-up period of at least
five years. Patients with concurrent inflammatory diseases or
autoimmune conditions, or those receiving immunosuppressive therapy
were excluded to minimize confounding factors that may influence
systemic inflammation levels. The inclusion of 300 consecutive
patients within this one-year period was based on the hospital's
annual surgical caseload for breast cancer during this timeframe.
Given the large number of breast cancer surgeries conducted at the
hospital annually, this cohort provided an adequate sample size to
conduct meaningful survival analysis while reflecting the
real-world clinical setting. This period allowed for comprehensive
follow-up data collection (up to 10 years) and ensured consistency
in treatment protocols during that time. Therefore, this cohort
size and timeframe were appropriate to investigate the prognostic
value of preoperative mGPS in patients with breast cancer. Among
the enrolled patients, a subset of patients with stage IV breast
cancer was included. Typically, patients with stage IV breast
cancer, due to distant metastasis, are not candidates for curative
surgery. However, certain patients with stage IV in this study
underwent palliative surgery, primarily to alleviate symptoms or
control the primary tumor. These surgeries were conducted in
conjunction with other treatments, such as chemotherapy, targeted
therapy or endocrine therapy. The surgeries were not aimed at
curing the disease but at improving the patients' quality of life
or addressing local complications caused by the primary tumor. All
patients, including those with stage IV disease, underwent
comprehensive clinical evaluation prior to surgery. The decision to
proceed with surgery was made by a multidisciplinary team,
considering the patient's overall health, response to previous
treatments and symptom burden. The inclusion of patients with stage
IV in the present study was intended to explore the OS and
prognostic factors associated with breast cancer, with a focus on
evaluating the role of the mGPS as a prognostic tool. It is
acknowledged that patients with stage IV typically have a shorter
survival period, but their inclusion helps assess the prognostic
predictive value of the mGPS across different stages of breast
cancer.
Data collection
Clinical and pathological data were retrieved from
the electronic medical records of each patient. Collected variables
included age, body mass index (BMI), smoking status, alcohol
consumption, diabetes status, hypertension, family history of
breast cancer, TNM stage (18) and
PAM50 molecular subtype (19).
Treatment modalities, including endocrine therapy, targeted
therapy, chemotherapy and immunotherapy, were also documented. All
patients provided informed consent prior to data collection and the
study was approved by the Ethics Committee of the Affiliated Cancer
Hospital of Xinjiang Medical University (Urumqi, China; approval
no. K-2024056) in accordance with the Declaration of Helsinki.
mGPS calculation
The mGPS was calculated based on preoperative CRP
and serum Alb levels. A score of 0 was assigned if CRP levels were
≤10 mg/l and Alb levels were ≥35 g/l. A score of 1 was assigned if
CRP levels were >10 mg/l with Alb levels ≥35 g/l. A score of 2
was assigned if CRP levels were >10 mg/l and Alb levels <35
g/l. Patients were stratified into three groups based on their
mGPS: mGPS 0 (108 patients), mGPS 1 (120 patients) and mGPS 2 (72
patients), as shown in Table I.
This stratification allowed for comparison of clinical outcomes
across different mGPS categories.
 | Table I.Distribution of patients with breast
cancer based on mGPS. |
Table I.
Distribution of patients with breast
cancer based on mGPS.
| mGPS | CRP, mg/l | Alb, g/l | Number |
|---|
| Low (0) | ≤10 | ≥35 | 108 |
| High |
|
|
|
| 1 | >10 | ≥35 | 120 |
| 2 | >10 | <35 | 72 |
Statistical analysis
All statistical analyses were conducted using SPSS
software (version 29.0; IBM Corp.) and R software (version 4.0.3; R
Foundation for Statistical Computing). P<0.05 was considered to
indicate statistical significance. Baseline characteristics:
Descriptive statistics were used to summarize the clinical and
pathological characteristics of the study population. Continuous
variables (e.g., age, BMI) were expressed as the mean ± standard
deviation and compared across mGPS groups using one-way ANOVA,
after confirming the normality of the data using the Shapiro-Wilk
test. If the data did not meet the normality assumption, the
Kruskal-Wallis H-test was used as an alternative. For post-hoc
analysis, Tukey's Honestly Significant Difference test was applied
to identify specific group differences. Categorical variables
(e.g., smoking, drinking, TNM stage, PAM50 subtype) were expressed
as frequencies and percentages and compared using the Chi-square
test or Fisher's exact test, as appropriate. Kaplan-Meier survival
analysis: OS was defined as the time from the date of surgery to
the date of death from any cause or the last follow-up. Patients
lost to follow-up were censored at the time of the last available
follow-up. Censoring refers to the inclusion of individuals who did
not experience the event of interest (death) by the end of the
study period or at the time they were lost to follow-up.
Kaplan-Meier survival curves were generated to evaluate the OS of
patients in the mGPS 0, 1 and 2 groups. The log-rank test was
applied to compare survival differences among the groups. The 5-
and 10-year survival rates were recorded for each mGPS category.
Logistic regression analysis: To identify factors associated with
higher mGPS scores, a univariate logistic regression analysis was
performed for each clinical and pathological variable, including
age, BMI, smoking status, alcohol consumption, diabetes,
hypertension, TNM stage and PAM50 subtype. Variables with a
P<0.05 in the univariate analysis were subsequently included in
a multivariate logistic regression model. Adjusted odds ratios
(ORs) and 95% confidence intervals (CIs) were calculated using
logistic regression analysis to determine the independent risk
factors for higher mGPS scores, adjusting for potential confounders
such as age, sex, disease stage, and treatment modalities. Cox
proportional hazards regression analysis: Univariate and
multivariate Cox proportional hazards regression models were used
to assess the association between mGPS and OS. Clinical and
pathological variables and mGPS scores were first analyzed
individually to determine their hazard ratios (HRs) for OS.
Variables with P<0.05 in the univariate analysis were included
in the multivariate analysis. The multivariate model adjusted for
potential confounders to identify independent predictors of OS.
Nomogram and calibration curve: A nomogram was constructed based on
the results of the multivariate Cox regression analysis to predict
the 5- and 10-year OS of patients. The nomogram incorporated the
most significant prognostic factors, including age, smoking status,
TNM stage, PAM50 subtype and mGPS score. The nomogram's predictive
accuracy was evaluated using Harrell's C-index. Calibration curves
were plotted to assess the agreement between predicted survival
probabilities and observed outcomes using bootstrapped resampling
(1,000 repetitions) for internal validation.
Results
Patient characteristics and
differences across mGPS groups
A total of 300 patients with breast cancer were
included in the present study. The mean age of the patients was
51.3 years (range, 34–78 years). All patients were female. Patients
were categorized into three groups based on their preoperative
mGPS: mGPS 0 (n=108), mGPS 1 (n=120) and mGPS 2 (n=72). The
baseline characteristics of the patients are summarized in Table II. Significant differences were
observed among the three mGPS groups in terms of age (P<0.001),
BMI (P=0.011), smoking status (P<0.001), alcohol consumption
(P=0.027), diabetes (P=0.026), TNM stage (P=0.001) and PAM50
molecular subtype (P=0.047). Specifically, higher mGPS scores were
associated with older age, higher BMI, smoking and drinking
history, advanced TNM stage and TNBC subtype. No significant
differences were found for hypertension, family history of breast
cancer or treatment modalities (endocrine therapy, targeted
therapy, chemotherapy and immunotherapy) (P>0.05 for all).
 | Table II.Baseline characteristics of patients
with breast cancer stratified by mGPS scores. |
Table II.
Baseline characteristics of patients
with breast cancer stratified by mGPS scores.
|
|
|
| High mGPS |
|
|---|
| Characteristic | Total (n=300) | Low mGPS 0
(n=108) |
|
|
|---|
| 1 (n=120) | 2 (n=72) | P-value |
|---|
| Age, years |
|
|
|
| <0.001 |
|
<65 | 186 | 84 | 67 | 35 |
|
|
≥65 | 114 | 24 | 53 | 37 |
|
| BMI,
kg/m2 |
|
|
|
| 0.011 |
|
≤18.5 | 83 | 24 | 29 | 30 |
|
|
>18.5, <25 | 142 | 61 | 54 | 27 |
|
|
≥25 | 75 | 23 | 37 | 15 |
|
| Smoking | 167 | 42 | 75 | 50 | <0.001 |
| Drinking | 208 | 65 | 87 | 56 | 0.027 |
| Diabetes | 74 | 15 | 37 | 22 | 0.026 |
| Hypertension | 136 | 43 | 58 | 35 | 0.354 |
| Family history | 45 | 13 | 22 | 10 | 0.395 |
| TNM stage |
|
|
|
| 0.001 |
| I | 159 | 72 | 63 | 24 |
|
| II | 94 | 28 | 36 | 30 |
|
|
III | 35 | 7 | 15 | 13 |
|
| IV | 12 | 1 | 6 | 5 |
|
| PAM50 |
|
|
|
| 0.047 |
| ER+ or
PR+ | 151 | 62 | 56 | 33 |
|
|
HER2+ | 82 | 29 | 38 | 15 |
|
|
TNBC | 67 | 17 | 26 | 24 |
|
| Treatment |
|
|
|
|
|
|
Endocrine therapy | 155 | 62 | 59 | 34 | 0.317 |
|
Targeted therapy | 84 | 30 | 38 | 16 | 0.369 |
|
Chemotherapy | 102 | 36 | 37 | 29 | 0.402 |
|
Immunotherapy | 44 | 14 | 21 | 9 | 0.525 |
High mGPS scores are associated with
poor survival outcomes
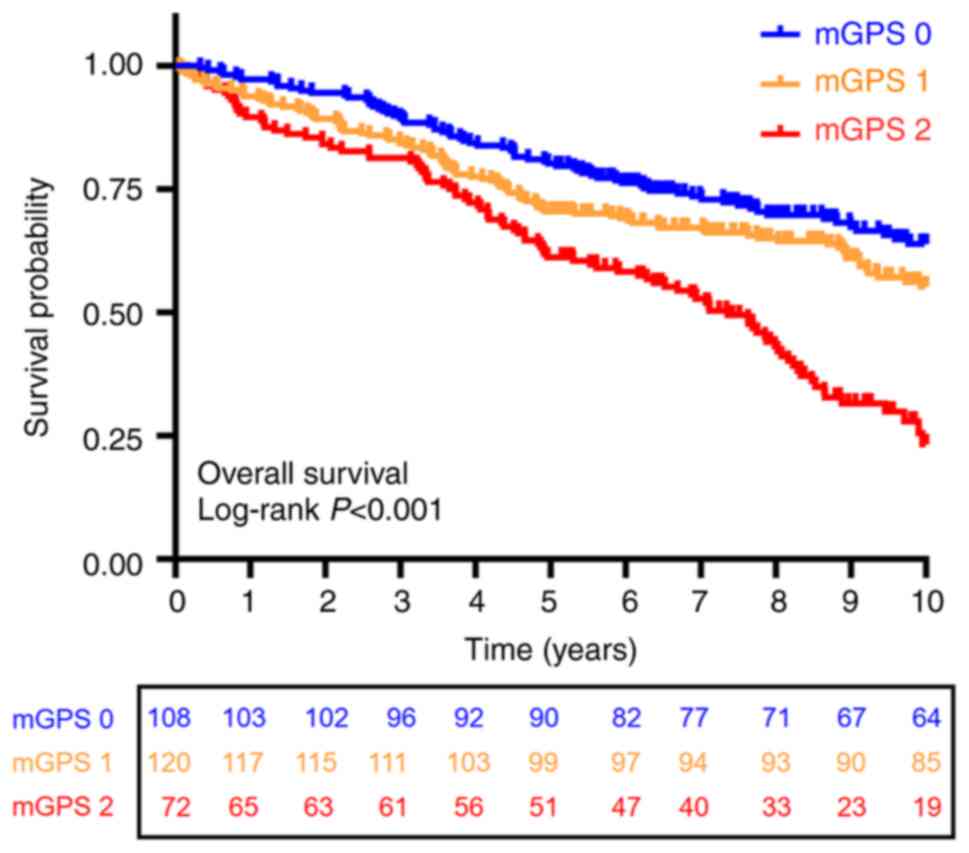
The Kaplan-Meier survival curves for the three mGPS
groups are shown in Fig. 1. The
5-year survival rates were 80, 70 and 55% for the mGPS 0, mGPS 1
and mGPS 2 groups, respectively. The 10-year survival rates were
71, 55 and 22% for these groups. The log-rank test revealed a
significant difference in OS among the three groups (P<0.001).
Further pairwise comparisons showed significant differences between
the following groups: mGPS 0 vs. mGPS 1 (P<0.001), mGPS 1 vs.
mGPS 2 (P=0.025) and mGPS 0 vs. mGPS 2 (P<0.001). Patients with
higher mGPS scores had significantly poorer survival outcomes
compared to those with lower scores. Specifically, the mGPS 2 group
demonstrated the worst survival rates, highlighting the association
between higher mGPS scores and reduced survival.
Risk factors associated with high mGPS
scores
To identify clinical and pathological factors
associated with high mGPS scores, logistic regression analysis was
performed. In the univariate analysis (Table III), factors significantly
associated with increased mGPS scores included age ≥65 years (OR:
2.836, 95% CI: 1.783–4.545, P<0.001), smoking (OR: 3.214, 95%
CI: 1.948–5.267, P<0.001), drinking (OR: 2.180, 95% CI:
1.355–3.486, P=0.002), TNM stage III (OR: 3.145, 95% CI:
1.358–5.765, P<0.001), TNM stage IV (OR: 4.832, 95% CI:
3.227–8.906, P<0.001) and TNBC subtype (OR: 3.123, 95% CI:
1.858–5.251, P<0.001). These variables were included in the
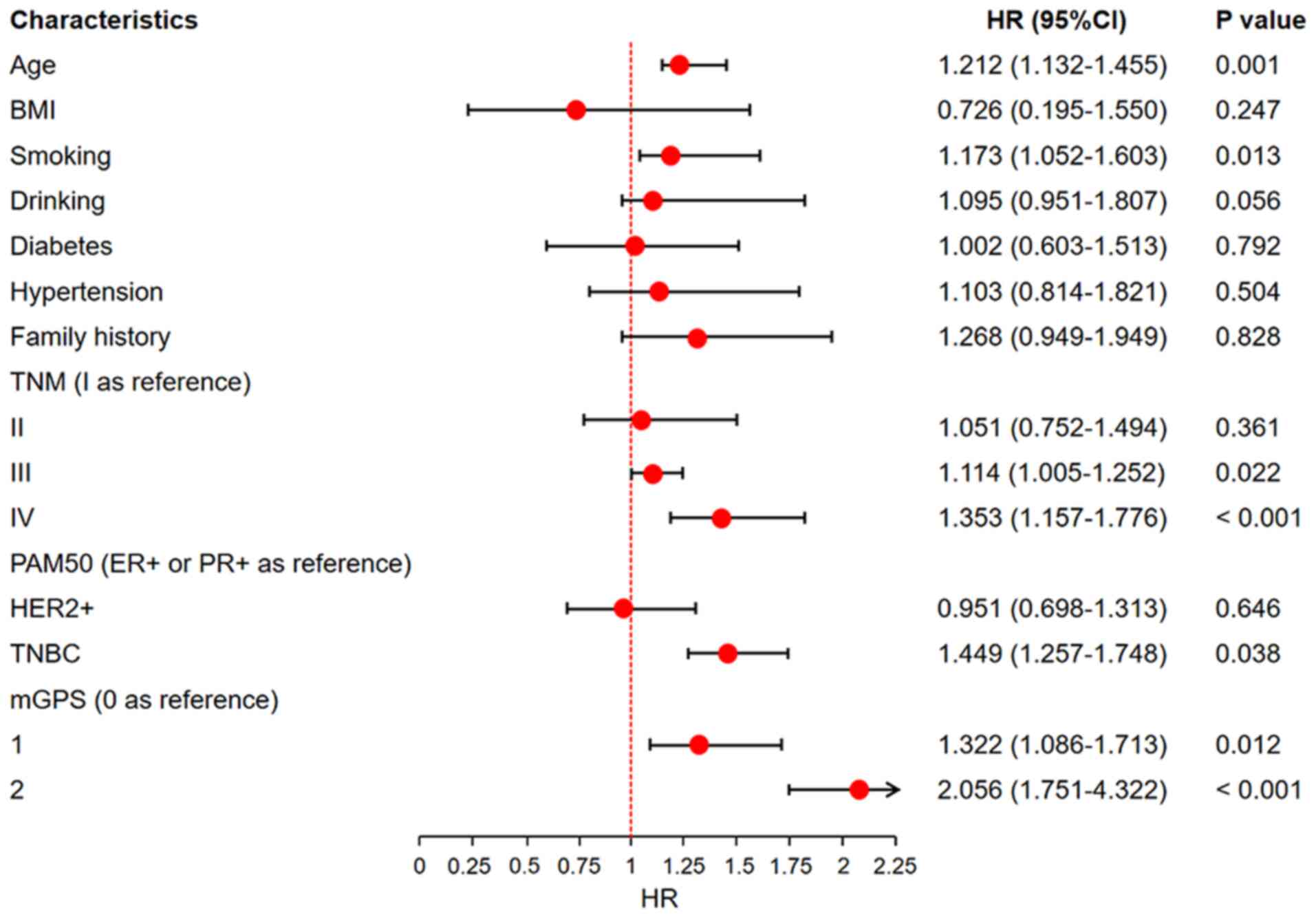
multivariate logistic regression model, which confirmed that age
≥65 years (OR: 1.126, 95% CI: 1.091–1.172, P<0.001), smoking
(OR: 1.395, 95% CI: 1.152–2.102, P=0.008), drinking (OR: 1.477, 95%
CI: 1.268–2.669, P=0.002), TNM stage III (OR: 1.351, 95% CI:
1.185–1.925, P=0.010), TNM stage IV (OR: 2.005, 95% CI:
1.314–7.275, P<0.001) and TNBC subtype (OR: 2.173, 95% CI:
1.683–3.555, P=0.002) were independent risk factors for higher mGPS
scores (Fig. 2).
 | Figure 2.Risk factors associated with mGPS.
Forest plot displaying the results of logistic regression analysis
identifying clinical and pathological risk factors associated with
a higher mGPS. The x-axis represents the ORs for each variable,
while the y-axis lists the clinical and pathological factors,
including age, smoking status, TNM stage and PAM50 subtype. Error
bars indicate the 95% CIs for each OR. mGPS, modified Glasgow
prognostic score; CI, confidence interval; OR, odds ratio; BMI,
body mass index; ER, estrogen receptor; PR, progesterone receptor;
TNBC, triple-negative breast cancer; PAM50, 50-gene intrinsic
subtype classifier. |
 | Table III.Univariate analysis of risk factors
associated with high mGPS scores. |
Table III.
Univariate analysis of risk factors
associated with high mGPS scores.
| Characteristic | OR | 95% CI univariate
analysis | P-value |
|---|
| Age (≥65 vs. <65
years) | 2.836 | 1.783–4.545 | <0.001 |
| BMI | 0.874 | 0.143–2.522 | 0.068 |
| Smoking | 3.214 | 1.948–5.267 | <0.001 |
| Drinking | 2.180 | 1.355–3.486 | 0.002 |
| Diabetes | 1.357 | 0.268–2.641 | 0.312 |
| Hypertension | 1.851 | 1.129–3.525 | 0.761 |
| Family history | 2.536 | 1.263–3.248 | 0.137 |
| TNM stage (I as
reference) |
|
|
|
| II | 1.512 | 1.051–2.274 | 1.106 |
|
III | 3.145 | 1.358–5.765 | <0.001 |
| IV | 4.832 | 3.227–8.906 | <0.001 |
| PAM50 |
|
|
|
| (ER+ or PR+ as
reference) |
|
|
|
|
HER2+ | 0.928 | 0.403–1.275 | 2.004 |
|
TNBC | 3.123 | 1.858–5.251 | <0.001 |
mGPS is an independent predictor for
OS in breast cancer
To evaluate the impact of clinical characteristics
and the mGPS on OS, univariate and multivariate Cox proportional
hazards regression analyses were conducted. In the univariate
analysis (Table IV), several
factors were associated with a higher risk of mortality, including
age ≥65 years (HR: 3.376, 95% CI: 1.227–5.258, P<0.001), smoking
(HR: 2.045, 95% CI: 1.183–4.904, P=0.001), drinking (HR: 1.762, 95%
CI: 1.254–3.255, P=0.004), family history (HR: 1.827, 95% CI:
1.374–3.359, P=0.025), TNM stage III (HR: 2.659, 95% CI:
1.517–5.043, P=0.031), TNM stage IV (HR: 4.274, 95% CI:
2.654–7.268, P<0.001), TNBC subtype (HR: 3.053, 95% CI:
2.073–6.383, P<0.001) and mGPS scores of 1 (HR: 2.622, 95% CI:
1.674–5.538, P=0.001) and 2 (HR: 4.139, 95% CI: 2.822–9.163,
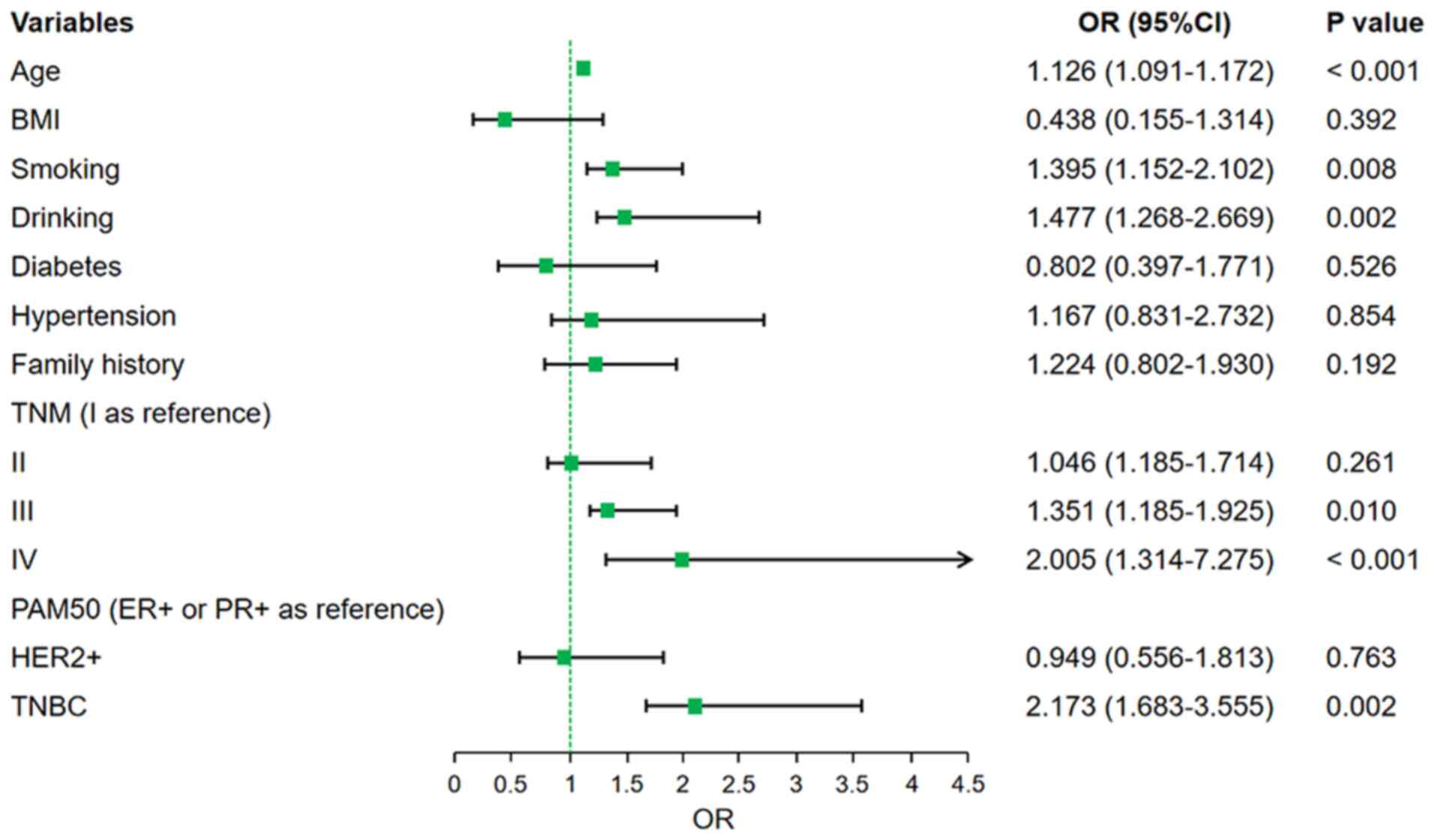
P<0.001). In the multivariate Cox analysis, after adjusting for
confounders such as age, BMI and PAM50 subtype, the mGPS score
remained a significant independent predictor of OS. Patients with
mGPS 1 had an HR of 1.322 (95% CI: 1.086–1.713, P=0.012) and those
with mGPS 2 had an HR of 2.056 (95% CI: 1.751–4.322, P<0.001)
when compared to patients with mGPS 0. Age ≥65 years (HR: 1.212,
95% CI: 1.132–1.455, P=0.001), smoking (HR: 1.173, 95% CI:
1.052–1.603, P=0.013), TNM stage III (HR: 1.114, 95% CI:
1.005–1.252, P=0.022), TNM stage IV (HR: 1.353, 95% CI:
1.157–1.776, P<0.001) and TNBC subtype (HR: 1.449, 95% CI:
1.257–1.748, P=0.038) were also independent predictors of worse OS
(Fig. 3).
 | Table IV.Univariate Cox proportional hazards
regression analysis of overall survival. |
Table IV.
Univariate Cox proportional hazards
regression analysis of overall survival.
| Characteristic | Total (n) | HR (95% CI)
univariate analysis | P-value |
|---|
| Age | 300 | 3.376
(1.227–5.258) | <0.001 |
| BMI | 300 | 0.539
(0.364–2.136) | 0.132 |
| Smoking | 300 | 2.045
(1.183–4.904) | 0.001 |
| Drinking | 300 | 1.762
(1.254–3.255) | 0.004 |
| Diabetes | 300 | 3.318
(0.798–6.527) | 0.003 |
| Hypertension | 300 | 2.434
(1.218–4.663) | 1.672 |
| Family history | 300 | 1.827
(1.374–3.359) | 0.025 |
| TNM stage | 300 |
|
|
| I | 159 | Reference |
|
| II | 94 | 1.268
(1.035–2.186) | 3.846 |
|
III | 35 | 2.659
(1.517–5.043) | 0.031 |
| IV | 12 | 4.274
(2.654–7.268) | <0.001 |
| PAM50 | 300 |
|
|
| ER+ or
PR+ | 151 | Reference |
|
|
HER2+ | 82 | 0.792
(0.512–2.195) | 5.415 |
|
TNBC | 67 | 3.053
(2.073–6.383) | <0.001 |
| mGPS | 300 |
|
|
| 0 | 108 | Reference |
|
| 1 | 120 | 2.622
(1.674–5.538) | 0.001 |
| 2 | 72 | 4.139
(2.822–9.163) | <0.001 |
Nomogram based on mGPS accurately
predicts survival
Based on the multivariate Cox regression model, a
prognostic nomogram was developed incorporating age, smoking
status, TNM stage, PAM50 subtype and mGPS score to predict 5- and
10-year OS (Fig. 4). The nomogram
demonstrated good predictive accuracy with a concordance index of
0.81 (95% CI: 0.75–0.88). Calibration curves showed strong
agreement between the predicted and observed survival rates,
indicating the model's robustness and applicability in clinical
practice. For internal validation, bootstrapped resampling (1,000
repetitions) was performed, confirming the reliability of the
nomogram.
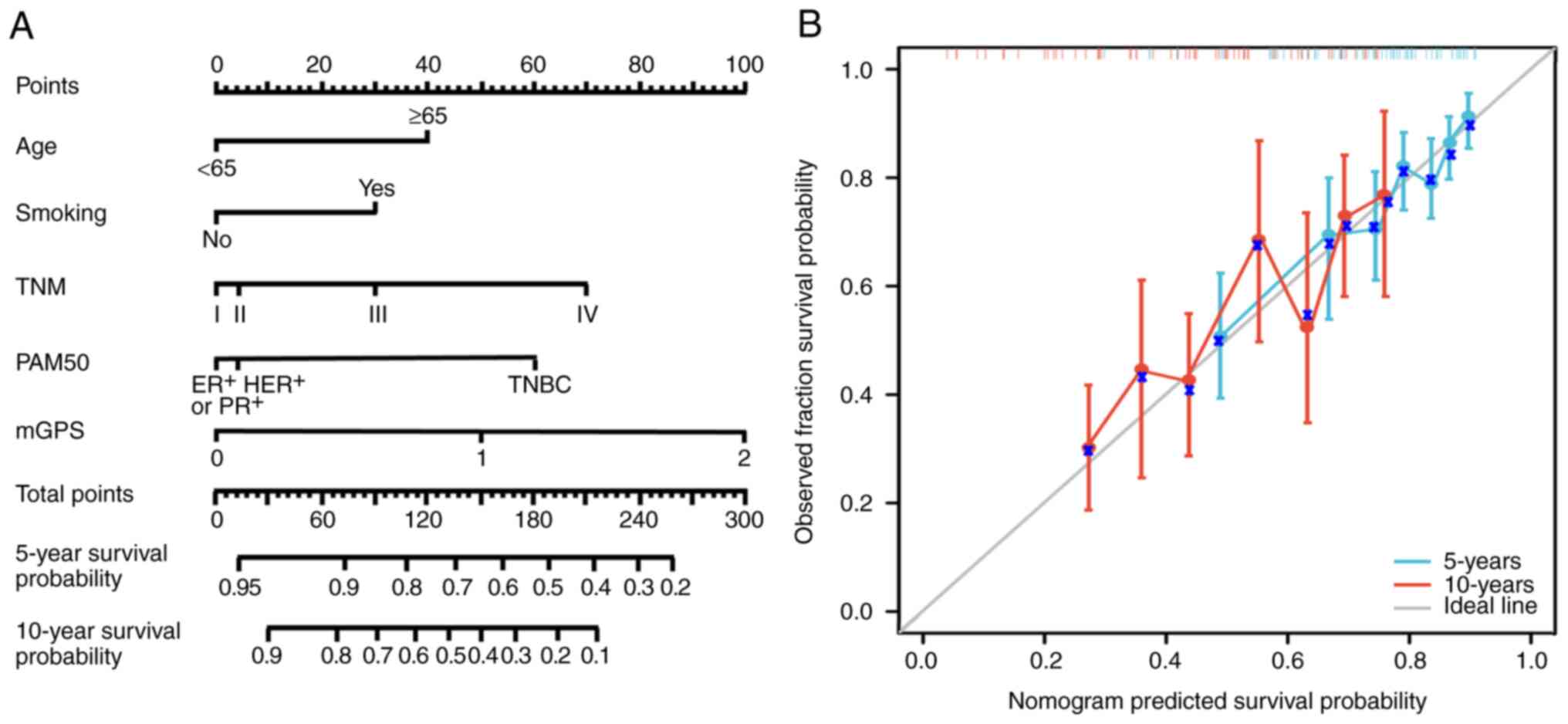 | Figure 4.Nomogram for predicting 5- and
10-year OS. (A) A nomogram developed using multivariate Cox
regression analysis incorporates age, smoking status, TNM stage,
PAM50 subtype and mGPS to predict 5- and 10-year OS. Each variable
is assigned a score, which is summed to estimate survival
probabilities. (B) Calibration curves validate the predictive
accuracy of the nomogram by comparing the predicted OS
probabilities with observed outcomes. The dashed diagonal line
represents perfect agreement and the solid lines indicate the
calibration results for the model. The blue crosses represent
individual patient data-points, showing the comparison between
predicted and observed overall survival probabilities. OS, overall
survival; mGPS, modified Glasgow prognostic score; ER, estrogen
receptor; PR, progesterone receptor; TNBC, triple-negative breast
cancer; HER2, human EGFR 2; TNM, tumor-node-metastasis; PAM50,
50-gene intrinsic subtype classifier. |
Discussion
This study aimed to evaluate the prognostic value of
the preoperative mGPS in patients with breast cancer undergoing
surgery. The present findings firstly demonstrated that higher mGPS
scores are significantly associated with poorer OS of patients with
breast cancer, independent of other established clinical and
pathological factors. This suggests that mGPS, a simple and
cost-effective biomarker of systemic inflammation, may serve as an
effective prognostic tool in clinical practice for patients with
breast cancer.
Inflammation is increasingly recognized as a
critical factor in cancer development and progression. The mGPS,
based on serum CRP and Alb levels, reflects systemic inflammation
and nutritional status. Elevated CRP levels indicate a
pro-inflammatory state, while hypoalbuminemia reflects both
malnutrition and inflammation (20,21).
These factors may collectively impair the host's anti-tumor
response and promote tumor progression. The current findings align
with previous studies demonstrating that high mGPS scores are
associated with poor prognosis in several cancers, including
colorectal, lung and gastric cancers (22–27).
The present study extends the prognostic utility of mGPS to breast
cancer, showing that higher mGPS scores are associated with
significantly lower 5- and 10-year survival rates.
Traditional prognostic markers for breast cancer,
such as tumor size, lymph node status, hormone receptor status,
HER2 status and PAM50 molecular subtype, primarily focus on the
tumor itself (28). However, these
markers do not capture the host's systemic response to the tumor,
which is an important determinant of patient outcomes (29). By integrating the mGPS into the
prognostic assessment, clinicians may obtain a more comprehensive
picture that includes both tumor characteristics and the host's
inflammatory and nutritional status. The present multivariate
analysis confirms that the mGPS is an independent predictor of OS,
even after adjusting for other factors such as TNM stage, age and
PAM50 subtype. This suggests that incorporating the mGPS into
existing risk models may enhance their predictive accuracy and
provide additional information for personalized treatment
planning.
The biological mechanisms underlying the association
between a high mGPS and poor prognosis in breast cancer likely
involve several pathways. Chronic inflammation, as indicated by
elevated CRP levels, is known to promote tumor growth, angiogenesis
and metastasis (30). Inflammatory
cytokines such as interleukin-6 and tumor necrosis factor-α can
create a tumor-promoting environment by enhancing cell
proliferation and inhibiting apoptosis (31–33).
In addition, systemic inflammation may lead to immunosuppression,
reducing the effectiveness of the body's immune surveillance
against tumor cells (34).
Furthermore, hypoalbuminemia, a component of the mGPS, may indicate
malnutrition or an advanced inflammatory state, both of which are
associated with poorer outcomes in cancer patients. Alb has
antioxidant properties and plays a role in maintaining oncotic
pressure and drug binding; its reduction may contribute to poorer
clinical conditions and reduced efficacy of therapies (35–37).
The combination of elevated CRP and low Alb in the mGPS scoring
system may therefore capture a more comprehensive picture of a
patient's inflammatory and nutritional status, which is crucial in
understanding breast cancer prognosis.
Although the present study demonstrates the
independent prognostic value of the preoperative mGPS in assessing
the prognosis of patients with breast cancer, it is important to
acknowledge the limitations of this scoring system. First, mGPS
only considers two factors-systemic inflammation and nutritional
status- and does not account for other key factors that may affect
the prognosis of patients with breast cancer, such as the tumor
microenvironment, immune cell infiltration and genetic mutations or
molecular characteristics. For instance, molecular subtypes of
breast cancer (e.g., TNBC) and the HER2+ status have been shown to
be closely related to survival outcomes (38), but these factors are not included in
the mGPS. Therefore, the mGPS should be considered a supplementary
tool for prognostic evaluation rather than the sole prognostic
criterion. Secondly, as a blood biomarker-based tool, the mGPS does
not reflect the local tumor characteristics or changes in other
clinical factors. Over the follow-up period, patients with breast
cancer may experience various events that impact survival,
including recurrence, metastasis and treatment-related side
effects, none of which are captured by the mGPS. Thus, the mGPS can
only provide a snapshot of the patient's overall health status and
cannot fully replace real-time monitoring of tumor dynamics. In
addition, because the present study is a retrospective cohort
analysis, the quality and completeness of the data collection may
be influenced by the patients' medical records and follow-up data.
Although efforts were made to control potential confounding factors
through strict inclusion criteria and multivariate adjustments, it
is impossible to rule out the possibility that certain important
clinical information was not recorded or considered in the real
clinical environment. The present study was conducted at a single
institution, which may limit the generalizability of the current
findings. Future prospective studies involving multiple centers and
larger patient populations are needed to validate the present
results and explore the potential role of mGPS in guiding treatment
decisions. One of the limitations of the present study is the
inability to monitor the impact of certain factors, such as the
patient's age, the occurrence of other diseases during the 10-year
follow-up period and the recurrence of breast cancer, on survival
outcomes. However, considering that the preoperative mGPS was used
in this study, this limitation may not apply to the preoperative
prognosis, as the factors mentioned here typically occur later
during the follow-up period, after the initial surgery. Therefore,
these factors are more relevant to predicting postoperative
survival outcomes, rather than affecting the preoperative prognosis
assessed by the mGPS. These factors can significantly influence
prognosis and may have confounded the relationship between mGPS
scores and OS. Although the analysis controlled for known clinical
variables, such as TNM stage and PAM50 subtype, the long-term
nature of the follow-up and the lack of detailed data on these
additional factors pose a limitation to the present findings.
Future prospective studies should aim to comprehensively monitor
these variables to better understand their effects on the survival
of patients with breast cancer. Finally, there are inherent
limitations to the mGPS itself. For instance, CRP and albumin
levels are influenced by numerous non-cancer-related factors, such
as infection, surgical trauma and other chronic diseases, which may
lead to bias in the mGPS score. While patients with
immunosuppressive therapy or inflammatory diseases were excluded,
further validation of the stability and reliability of the mGPS in
different clinical contexts is still needed. In conclusion, while
the mGPS is a simple and cost-effective prognostic tool, its
clinical application in breast cancer should be combined with other
clinical and pathological features to provide a more comprehensive
and accurate survival prediction. Therefore, it is recommended that
in clinical practice, the mGPS should be used alongside other
molecular biomarkers, immunological indicators and tumor
microenvironment characteristics to further improve the accuracy of
prognostic assessments and the precision of personalized
treatment.
In conclusion, this study was the first to
investigate the impact of the preoperative mGPS on the long-term
prognosis of patients with breast cancer, with follow-up extending
up to 10 years. The current findings show that the mGPS is an
independent predictor of OS, with a higher mGPS associated with
significantly poorer 5- and 10-year survival rates. Combining the
mGPS with other clinical and pathological factors provides an
effective risk stratification tool for guiding personalized
treatment and follow-up strategies. Future studies should validate
these results in larger cohorts and explore mGPS-targeted
interventions to improve patient outcomes.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 82260549).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JJF and BLM contributed to the conception and design
of the study. YC, BXZ and XLW, MA and YYC collected and analyzed
data. YC and BXZ wrote and revised the manuscript. BXZ and YYC
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Cancer Hospital of Xinjiang Medical University
(Urumqi, China; approval no. K-2024056) in accordance with the
Declaration of Helsinki. All of the patients had signed a written
informed consent form, which included consent to participate in the
study, use of their medical data for research purposes and the
publication of anonymized findings.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
mGPS
|
modified Glasgow prognostic score
|
|
CRP
|
C-reactive protein
|
|
Alb
|
albumin
|
|
OS
|
overall survival
|
|
TNM
|
tumor-node-metastasis
|
|
PAM50
|
50-gene intrinsic subtype
classifier
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
TNBC
|
triple-negative breast cancer
|
|
HR
|
hazard ratio
|
|
OR
|
odds ratio
|
|
C-index
|
concordance index
|
|
CI
|
confidence interval
|
|
BMI
|
body mass index
|
References
|
1
|
Nguyen HM, Paulishak W, Oladejo M and Wood
L: Dynamic tumor microenvironment, molecular heterogeneity, and
distinct immunologic portrait of triple-negative breast cancer: An
impact on classification and treatment approaches. Breast Cancer.
30:167–186. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molinelli C, Jacobs F, Agostinetto E,
Nader-Marta G, Ceppi M, Bruzzone M, Blondeaux E, Schettini F, Prat
A, Viale G, et al: Prognostic value of HER2-low status in breast
cancer: A systematic review and meta-analysis. ESMO Open.
8:1015922023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi X, Qiao B, Song T, Huang D, Zhang H,
Liu Y, Jin Q, Yang M and Liu D: Clinical utility of the
pan-immune-inflammation value in breast cancer patients. Front
Oncol. 13:12237862023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Chen S, Chen H and Li W: A
comprehensive analysis of Glasgow Prognostic Score (GPS)/the
modified Glasgow Prognostic Score (mGPS) on immune checkpoint
inhibitor efficacy among patients with advanced cancer. Cancer Med.
12:38–48. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang F, Wu Z, Sun S, Fu Y, Chen Y and Liu
J: POEMS syndrome in the 21st century: A bibliometric analysis.
Heliyon. 9:e206122023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Gao Y, Ying J, Yu H, Guo R, Xiong
J and Jiang H: Bibliometric analysis of global research on breast
reconstruction after mastectomy for breast cancer from 2011 to
2021. J Cosmet Dermatol. 22:2071–2082. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nie D, Zhang L, Wang C, Guo Q and Mao X: A
high Glasgow prognostic score (GPS) or modified Glasgow prognostic
score (mGPS) predicts poor prognosis in gynecologic cancers: A
systematic review and meta-analysis. Arch Gynecol Obstet.
301:1543–1551. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abbass T, Dolan RD, MacLeod N, Horgan PG,
Laird BJ and McMillan DC: Comparison of the prognostic value of
MUST, ECOG-PS, mGPS and CT derived body composition analysis in
patients with advanced lung cancer. Clin Nutr ESPEN. 40:349–356.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Z, Chen Y, Yu G and Ma Y: Research
trends and hotspots in surgical treatment of recurrent
nasopharyngeal carcinoma: A bibliometric analysis from 2000 to
2023. Asian J Surg. 47:2939–2941. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Jia L, Guo R, Xiong J and Jiang
H: A bibliometric and visualized study on global trends of breast
augmentation complications, 2011–2021. Gland Surg. 12:354–365.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boukovala M, Modest DP, Ricard I, Fischer
von Weikersthal L, Decker T, Vehling-Kaiser U, Uhlig J, Schenk M,
Freiberg-Richter J, Peuser B, et al: Evaluation of the
inflammation-based modified Glasgow Prognostic Score (mGPS) as a
prognostic and predictive biomarker in patients with metastatic
colorectal cancer receiving first-line chemotherapy: A post hoc
analysis of the randomized phase III XELAVIRI trial (AIO KRK0110).
ESMO Open. 9:1033742024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goktas Aydin S, Kutlu Y, Muglu H, Aydin A,
Acikgoz O, Hamdard J, Karci E, Bilici A, Olmez OF and Yildiz O:
Predictive significance of inflammatory markers and mGPS in
metastatic castration-resistant prostate cancer treated with
abiraterone or enzalutamide. Cancer Chemother Pharmacol. 93:71–78.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Wu Z, Pan Y, Chen Y, Chu J, Cong Y
and Fang Q: GNL3L exhibits pro-tumor activities via NF-κB pathway
as a poor prognostic factor in acute myeloid leukemia. J Cancer.
15:4072–4080. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Tang S, Biskup E, Zhang Y, Yong
L, Chen L and Cai F: Long-term survival after diverse therapeutic
modalities in malignant phyllodes tumors of the breast. Technol
Cancer Res Treat. 21:153303382211210862022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu XY, Zhang X, Zhang Q, Ruan GT, Liu T,
Xie HL, Ge YZ, Song MM, Deng L and Shi HP: The value of
CRP-albumin-lymphocyte index (CALLY index) as a prognostic
biomarker in patients with non-small cell lung cancer. Support Care
Cancer. 31:5332023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Castelnuovo A, Bonaccio M, Costanzo S,
De Curtis A, Magnacca S, Persichillo M, Panzera T, Bracone F,
Pignatelli P, Carnevale R, et al: The association between
hypoalbuminemia and risk of death due to cancer and vascular
disease in individuals aged 65 years and older: Findings from the
prospective Moli-sani cohort study. EClinicalMedicine.
72:1026272024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapaer T, Chen Y, Pan Y, Wu Z, Tang T,
Zhao Z and Zeng X: Elevated BEAN1 expression correlates with poor
prognosis, immune evasion, and chemotherapy resistance in rectal
adenocarcinoma. Discov Oncol. 15:4462024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou Y, Hu X and Deng X: Distant lymph node
metastases from breast cancer-Is it time to review TNM cancer
staging? JAMA Netw Open. 4:e2120262021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Veerla S, Hohmann L, Nacer DF,
Vallon-Christersson J and Staaf J: Perturbation and stability of
PAM50 subtyping in population-based primary invasive breast cancer.
NPJ Breast Cancer. 9:832023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang
W, Zhai X and Jin B: KIF18A inactivates hepatic stellate cells and
alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell
Mol Life Sci. 81:962024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Yu L and Wang L, Yin X, Liu K, Liu
W, Lin S and Wang L: Integrated analysis of single-cell and bulk
RNA sequencing data reveals immune-related lncRNA-mRNA prognostic
signature in triple-negative breast cancer. Genes Dis. 11:571–574.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reyes-Ruiz A, Calvillo-Rodriguez KM,
Martínez-Torres AC and Rodríguez-Padilla C: The bovine dialysable
leukocyte extract IMMUNEPOTENT CRP induces immunogenic cell death
in breast cancer cells leading to long-term antitumour memory. Br J
Cancer. 124:1398–1410. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shayimu P, Awula M, Wang CY, Jiapaer R,
Pan YP, Wu ZM, Chen Y and Zhao ZL: Serum nutritional predictive
biomarkers and risk assessment for anastomotic leakage after
laparoscopic surgery in rectal cancer patients. World J
Gastrointest Surg. 16:3142–3154. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hua S, Wang W, Yao Z, Gu J, Zhang H, Zhu
J, Xie Z and Jiang H: The fatty acid-related gene signature
stratifies poor prognosis patients and characterizes TIME in
cutaneous melanoma. J Cancer Res Clin Oncol. 150:402024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park YR, Jee W, Park SM, Kim SW, Bae H,
Jung JH, Kim H, Kim S, Chung JS and Jang HJ: Viscum album induces
apoptosis by regulating STAT3 signaling pathway in breast cancer
cells. Int J Mol Sci. 24:119882023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hacker UT, Hasenclever D, Baber R, Linder
N, Busse H, Obermannova R, Zdrazilova-Dubska L, Valik D and Lordick
F: Modified Glasgow prognostic score (mGPS) is correlated with
sarcopenia and dominates the prognostic role of baseline body
composition parameters in advanced gastric and esophagogastric
junction cancer patients undergoing first-line treatment from the
phase III EXPAND trial. Ann Oncol. 33:685–692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Z, Chen Y, Jiang D, Pan Y, Tang T, Ma Y
and Shapaer T: Mitochondrial-related drug resistance lncRNAs as
prognostic biomarkers in laryngeal squamous cell carcinoma. Discov
Oncol. 15:7852024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma L, Gao P, Liu Z, Jiao D, Ling R, Xiao
J, Zhao Y, Wang Y, Yang H, Liu Y, et al: Association of a complete
breast cancer pathologic response with axillary lymph node
metastasis via neoadjuvant chemotherapy: Results from the CSBrS-012
study. Chin Med J (Engl). 137:1369–1371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dimitrakopoulos FI, Goussia A, Koliou GA,
Dadouli K, Batistatou A, Kourea HP, Bobos M, Arapantoni-Dadioti P,
Tzaida O, Koletsa T, et al: Ten-year clinical outcome, toxicity and
compliance of dose-dense sequential adjuvant administration of
cyclophosphamide & epirubicin followed by docetaxel in patients
with early breast cancer: A hellenic cooperative oncology group
observational study (HE 10/10) with concurrent investigation of
significance of tumor infiltrating lymphocytes. Breast.
73:1036682024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Proctor MJ, Talwar D, Balmar SM, O'Reilly
DS, Foulis AK, Horgan PG, Morrison DS and McMillan DC: The
relationship between the presence and site of cancer, an
inflammation-based prognostic score and biochemical parameters.
Initial results of the Glasgow Inflammation Outcome Study. Br J
Cancer. 103:870–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haq ATA, Yang PP, Jin C, Shih JH, Chen LM,
Tseng HY, Chen YA, Weng YS, Wang LH, Snyder MP, et al:
Immunotherapeutic IL-6R and targeting the MCT-1/IL-6/CXCL7/PD-L1
circuit prevent relapse and metastasis of triple-negative breast
cancer. Theranostics. 14:2167–2189. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Farhana A, Alsrhani A, Alghsham RS, Derafa
W, Khan YS and Rasheed Z: Gold nanoparticles downregulate IL-6
expression/production by upregulating microRNA-26a-5p and
deactivating the RelA and NF-κBp50 transcription pathways in
activated breast cancer cells. Int J Mol Sci. 25:14042024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Maitiniyazi G, Li Z, Li T, Liu Y,
Zhang R, Cao X, Gu D and Xia S: TNF-α mediates the association
between dietary inflammatory index and depressive symptoms in
breast cancer. Nutrients. 15:842022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruan GT, Xie HL, Hu CL, Liu CA, Zhang HY,
Zhang Q, Wang ZW, Zhang X, Ge YZ, Lin SQ, et al: Comprehensive
prognostic effects of systemic inflammation and Insulin resistance
in women with breast cancer with different BMI: A prospective
multicenter cohort. Sci Rep. 13:43032023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao W, Li M and Zhang Y:
Fibrinogen/albumin ratio (FAR) in patients with triple negative
breast cancer and its relationship with epidermal growth factor
receptor expression. Onco Targets Ther. 14:5403–5415. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang M, Zhang H, Tian J, Yuan Y, Xu Z and
Chen J: Low serum albumin levels and high neutrophil counts are
predictive of a poorer prognosis in patients with metastatic breast
cancer. Oncol Lett. 24:4322022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang MD, Duan FF, Hua X, Cao L, Xia W and
Chen JY: A novel albumin-related nutrition biomarker predicts
breast cancer prognosis in neoadjuvant chemotherapy: A two-center
cohort study. Nutrients. 15:42922023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|