Introduction
The immune evasion phenomenon is a crucial factor in
the initiation and progression of cancer and is recognized as one
of its primary characteristics (1).
Immune checkpoints, which comprise co-inhibitory and stimulatory
signals, regulate the immune system as well as shield tumor cells
from immune attack (1–3). Immune checkpoint inhibitors (ICIs)
have demonstrated remarkable and durable antitumor effects by
inhibiting negative immunomodulatory signals (4). This approach has become the standard
therapy for cancer patients (5).
The Food and Drug Administration has approved multiple ICIs,
including anti-programmed cell death 1 (PD-1), anti-PD-1 ligand 1
(PD-L1) and anti-cytotoxic T-lymphocyte associated protein 4
(CTLA-4) antibodies, for a wide range of cancer indications. In
addition, several novel immune checkpoint molecules with
therapeutic potential, such as T-cell immunoglobulin and ITIM
domain, T-cell immunoglobulin and mucin-domain-containing-3, as
well as lymphocyte activation gene-3, have been identified.
Recently developed monoclonal antibodies against these novel
targets have demonstrated encouraging therapeutic efficacy
(4,6).
However, the response rate to ICI treatment varies
greatly depending on the cancer type, typically ranging from 10 to
40%, and the majority of patients eventually progress despite an
initial response (4,7). Besides, immune-related adverse effects
from ICI therapy can be fatal or very severe (8). Early identification of individuals who
do not respond to ICI treatment has recently become a hot topic in
the treatment of malignant tumors in order to avoid ineffective
treatment, reduce the risk of adverse reactions and the economic
burden of patients (9,10). The higher costs of ICIs compared to
traditional therapies, such as chemotherapy or radiotherapy, arise
from expensive drug prices, prolonged treatment regimens, frequent
monitoring, potential severe side effects and limited insurance
coverage in certain cases. While ICIs offer substantial benefits in
certain cancers, their financial impact remains a significant
concern, particularly for patients and healthcare systems with
limited resources (11).
Several predictive biomarkers have been explored for
their association with ICI response, including the nutritional risk
index, intratumoral PD-L1 expression, tumor mutational burden and
T-cell infiltration metrics (12,13).
However, each of these markers has notable drawbacks. The
nutritional risk index is a continuous variable and there is no
universally agreed-upon threshold for defining high or low risk,
which complicates its clinical application. Intratumoral PD-L1
expression, whilst being the only biomarker with regulatory
companion diagnostic approval by the FDA, exhibits significant
intertumoral heterogeneity, and the variability in detection
platforms leads to inconsistent predictive power (14,15).
The tumor mutational burden requires complex and expensive
sequencing technologies, limiting its widespread use in routine
clinical practice. T-cell infiltration metrics are difficult to
standardize across different studies and require invasive biopsy
procedures, which are not always feasible, particularly in patients
who are not candidates for repeated sampling. In addition,
obtaining tumor samples prior to treatment initiation is often
challenging due to technical and ethical considerations. Given
these limitations, there is a clear need for alternative biomarkers
that are more accessible and reliable.
Forrest et al (16) first developed the Glasgow Prognostic
Score (GPS) as a prognostic tool for metastatic non-small cell lung
cancer (NSCLC). A score of 1 is assigned for C-reactive protein
(CRP) of >10 mg/l and/or albumin of <3.5 g/dl, and this
culminates in patients being classified as low (0 points),
intermediate (1 point) or high (2 points) risk (16). The modified (m)GPS has since
improved upon the score's prognostic capabilities. A point is only
awarded for low albumin if the CRP is elevated, thus more heavily
weighting the inflammatory component of the score (17).
The GPS and mGPS have been extensively studied to
predict postoperative outcomes in various cancers (18–22).
Recently, Shimoyama et al (23) compared the prognostic effects of the
prognostic nutritional index, GPS, mGPS, C-reactive
protein-to-albumin ratio, neutrophil-to-lymphocyte ratio,
platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, derived
neutrophil-to-lymphocyte ratio, neutrophil-lymphocyte score,
neutrophil-platelet score, platelet-lymphocyte score,
lymphocyte-monocyte score, prognostic index, systemic
immune-inflammation index, systemic inflammation response index,
lung immune prognostic index and C-reactive protein albumin
lymphocyte index for patients with gastric cancer (GC) treated with
ICIs. The mGPS was found to have the strongest prognostic effect.
However, this was only a single-center study, and the studies by
Diker and Olgun (24), Yamamoto
et al (25) and Freitas
et al (26) confirm that the
mGPS does not predict the efficacy of ICI treatment. Hence, the
present study aimed to systematically evaluate the predictive value
of the GPS and mGPS in ICI-treated patients with cancer. The
findings of the current study can aid in developing effective
treatment strategies that facilitate the administration of precise,
cost-effective treatments with minimal adverse effects.
Materials and methods
Literature search strategies
On May 1st, 2023, the article search was performed
using the PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/) and Cochrane Library
(https://www.cochranelibrary.com/)
databases. Various search terms, including MeSH terms and keywords,
were used to retrieve relevant studies (limited to English
literature), such as ‘immune checkpoint inhibitors [MeSH]’, ‘PD-1
inhibitors’, ‘PD-L1 inhibitors’, ‘CTLA-4 inhibitors’,
‘Pembrolizumab’, ‘Nivolumab’, ‘Atezolizumab’, ‘Ipilimumab’,
‘Avelumab’, ‘Tremelimumab’, ‘Durvalumab’, ‘Cemiplimab’, ‘Glasgow
prognostic score’ and ‘modified Glasgow prognostic score’. A
detailed description of the search strategy is provided in Table SI. In addition, grey literature was
explored using Google Scholar (https://scholar.google.cz/) and the reference lists of
eligible studies were screened manually.
Inclusion and exclusion criteria
The present study followed strict inclusion
criteria, including articles that evaluated the prognostic value of
the GPS or mGPS in patients with cancer who underwent treatment
with ICIs. Only articles that discussed at least one of the
following results were taken into account: Overall survival (OS),
progression-free survival (PFS), objective response rate (ORR) and
disease control rate (DCR). Conference abstracts, comments and case
reports were excluded from the analysis.
To ensure the integrity and originality of the
present findings, it was confirmed that no previous meta-analyses
were included in the current meta-analysis. Instead, only original
research studies were included, which provided primary data on the
prognostic utility of the GPS and mGPS in ICI therapy. The
rationale for this decision was to avoid the duplication of data,
as meta-analyses often aggregate results from the same individual
studies already included in the analysis. Including previous
meta-analyses could lead to the repetition of data and potentially
introduce bias or inflate effect sizes. By focusing solely on
primary studies, the present meta-analysis ensures an independent
and comprehensive evaluation of the available evidence.
Data extraction and quality
assessment
In this study, diverse information was gathered from
the selected articles, including the names of the authors, study
design, duration and location of the study, drugs used for
treatment, cancer type, sample size, patient age and gender,
follow-up time and outcomes. More weight was given to data from
multivariate analyses of hazard ratios (HR) than univariate
analyses. The Newcastle-Ottawa Scale (NOS) was utilized to assess
the quality of observational research and literature with an NOS
score of 6 or above was regarded as high-quality (27). All of the above steps were performed
and cross-checked independently by two authors (HY and MFL).
Statistical methods
Stata 15.0 software (StataCorp LP) was utilized for
statistical analyses. To assess heterogeneity, the I2
was applied. The DerSimonian and Laird method was used for the
random-effects model. Evaluated outcomes comprised hazard ratios
(HRs) for OS and PFS, along with the odds ratio (OR) for the ORR
and DCR. Pooled estimates and their corresponding 95% confidence
intervals (CIs) were computed. HR >1 for OS and PFS indicated
that the event of death is more likely to occur in the high group
compared to the low group, implying a worse survival outcome. By
contrast, an HR <1 would indicate a lower risk of death in the
high group, suggesting a better survival outcome. OS was defined as
the time from initiation of ICI treatment to death from any cause
or the last date of contact. PFS was defined as the time from ICI
treatment initiation to objective disease progression. The ORR was
defined as the proportion of patients achieving a complete response
(CR) or partial response (PR). The DCR was defined as the
proportion of patients achieving a CR, PR or stable disease.
In the present study, the following comparisons were
performed: (m)GPS 2 vs. 0, (m)GPS 1 vs. 0, (m)GPS 1/2 vs. 0 and
(m)GPS 2 vs. 1/0. These comparisons were selected to evaluate the
prognostic value of the GPS across varying levels of inflammatory
and nutritional risk. Specifically, (m)GPS 2 vs. 0 and (m)GPS 1 vs.
0 are standard comparisons that assess the independent effects of
moderate and severe inflammation on clinical outcomes. In addition,
the comparison of (m)GPS 1/2 vs. 0 was performed to explore the
combined impact of moderate and severe inflammation on clinical
outcomes compared to patients with no significant inflammatory
risk. This approach is particularly useful in studies with limited
sample sizes or in instances where combining data provides greater
statistical power. Finally, (m)GPS 2 vs. 1/0 was included to focus
on the distinct impact of severe inflammation compared to patients
with a lower inflammatory risk, providing a more nuanced
understanding of the prognostic utility of the (m)GPS. These
comparisons collectively ensure a comprehensive evaluation of the
stratification capabilities of the (m)GPS, contributing to a deeper
understanding of its prognostic relevance in cancer patients
treated with ICIs.
Both Egger's and Begg's tests were conducted to
estimate publication bias (27). If
bias was found, the ‘trim and fill’ method was applied to assess
its impact on the pooled results (28,29).
In addition, a sensitivity analysis was performed by excluding each
study independently to evaluate the robustness of the findings
(28,29).
Results
Characteristics of studies
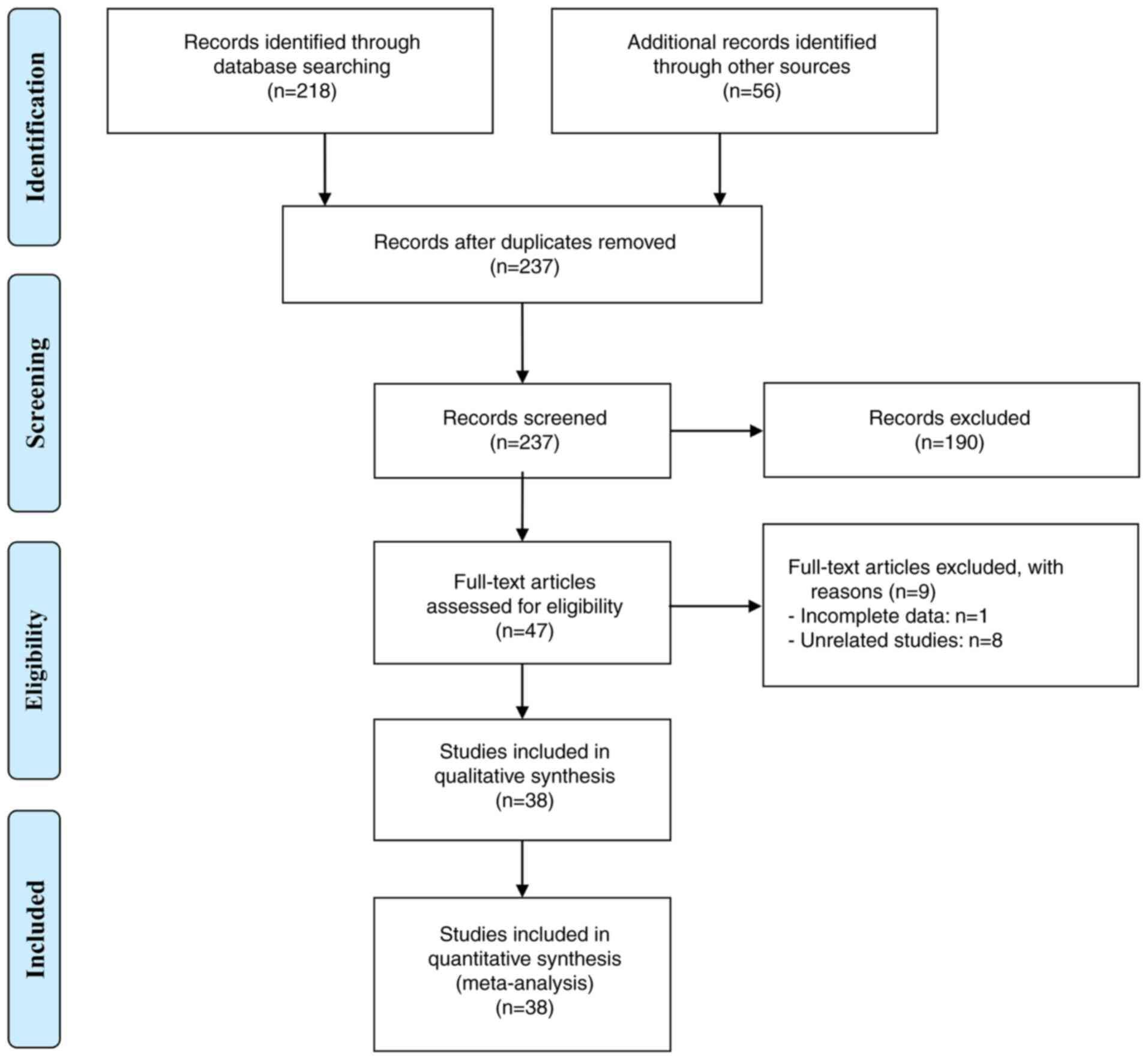
Initially, 274 documents were retrieved. After
eliminating duplicates (37 articles) and evaluating titles and
abstracts (190 articles), 47 articles remained and were assessed in
full-text. Among these, 38 articles were deemed eligible and a
total of 3,772 patients were included in the final analysis
(24–26,30–64).
The study selection process is illustrated in Fig. 1 using a flowchart. The detailed
characteristics of the eligible studies are presented in Table I. The risk of bias in all included
studies was assessed using the NOS, with scores ranging from 6 to
8, indicating a low risk of bias (Tables I and SII). Of the 38 studies, 35 were
retrospective and three were prospective. The included studies
consisted of 13 on NSCLC, five on GC, five on head and neck
squamous cell carcinoma and four on renal cell carcinoma.
Furthermore, 22 studies assessed the predictive value of the GPS,
14 studies estimated the predictive utility of the mGPS and two
studies explored the predictive potential of both metrics.
 | Table I.Main characteristics of the studies
included. |
Table I.
Main characteristics of the studies
included.
| First author,
year | Study design | Study period | Study region | ICI treatment | Cancer type | Sample size | Age, years | Gender
(male/female) | Outcome | Follow-up, months
(median/mean) | NOS score | (Refs.) |
|---|
| Tanaka, 2023 | R |
12/2015-04/2019 | Japan | Nivolumab,
Pembrolizumab | NSCLC | 51 | 79 (75–87)a | 40/11 | GPS (OS, PFS, ORR,
DCR) | 7b | 7 | (56) |
| Takegawa, 2023 | R |
03/2020-09/2021 | Japan | Nivolumab | EC | 37 | 67 (46–84)a | 32/5 | GPS (OS, PFS) | 23.3b | 7 | (63) |
| Madeddu, 2023 | P |
03/2017-08/2021 | Italy | Nivolumab,
Pembrolizumab | NSCLC | 74 | 69.3 ±
11.3c | 54/20 | mGPS (OS, PFS) | 24b | 8 | (42) |
| Kasajima, 2023 | R |
03/2017-01/2022 | Japan | Pembrolizumab,
Atezolizumab | NSCLC | 80 | 71 (44–86)a | 56/24 | GPS (OS, PFS, ORR,
DCR) | 11.1b | 7 | (39) |
| Wasamoto, 2023 | R | 08/2019-
05/2021 | Japan | Atezolizumab | SCLC | 84 | 71 (43–89)a | 70/14 | GPS (OS, PFS, ORR,
DCR) | 12.9b | 8 | (59) |
| Diker, 2022 | R |
03/2017-10/2021 | Cyprus | ICIs | NSCLC | 102 | 67 (35–88)a | 89/13 | mGPS (OS, PFS) | 8.4b | 8 | (24) |
| Zaitsu, 2021 | R |
10/2016-04/2020 | Japan | Nivolumab,
Pembrolizumab, Atezolizumab | LC | 73 |
70.9±9.4c | 52/21 | mGPS (OS, PFS,
DCR) | - | 7 | (62) |
| Takamori, 2021 | R |
01/2016-12/2019 | Japan | Nivolumab,
Pembrolizumab, Atezolizumab | NSCLC | 304 | 66 (31–88)a | 242/62 | GPS (OS, PFS, DCR),
mGPS (OS, PFS, DCR), | 13.8b | 8 | (55) |
| Ogura, 2021 | R |
02/2019-07/2020 | Japan | Pembrolizumab,
Atezolizumab | NSCLC | 34 | 72 (55–81)a | 29/5 | mGPS (OS, PFS) | - | 6 | (52) |
| Kang, 2021 | R |
01/2018-03/2020 | Korea | Pembrolizumab,
Nivolumab, Atezolizumab | NSCLC | 78 |
67.1±9.2c | 64/14 | GPS (OS, PFS) | - | 7 | (37) |
| Imai, 2021 | R |
02/2017-06/2019. | Japan | Pembrolizumab | NSCLC | 142 | 70 (47–86)a | 117/25 | GPS (OS, PFS, ORR,
DCR) | 15.7b | 8 | (36) |
| Freitas, 2021 | R | - | Portugal | Nivolumab,
Pembrolizumab | NSCLC | 77 | 65 (44–87)a | 55/22 | mGPS (OS, PFS) | - | 7 | (26) |
| Araki, 2021 | R |
01/2015-12/2019 | Japan | Nivolumab | NSCLC | 113 | 69 (36–86)a | 87/26 | mGPS (OS) | - | 7 | (31) |
| Ali, 2021 | R |
12/2015-08/2017 | China | Pembrolizumab,
Nivolumab, Camrelizumab, Atezolizumab | NSCLC | 73 | 54 (28–73)a | 51/22 | mGPS (OS, PFS) | 21.2b | 8 | (30) |
| Matsubara,
2020 | R |
01/2018-03/2019 | Japan | Atezolizumab | NSCLC | 24 |
64.5±9.7c | 17/7 | mGPS (OS) | - | 6 | (43) |
| Kasahara, 2019 | R |
01/2016-06/2018 | Japan | Nivolumab,
Pembrolizumab | NSCLC | 47 | 33/14d | 37/17 | GPS (OS, PFS,
DCR) | 14.7b | 7 | (38) |
| Kawakami, 2023 | P | - | Japan | Nivolumab | GC | 439 | 70 (26–90)a | 321/118 | GPS (OS, PFS, ORR,
DCR) | - | 7 | (40) |
| Sakai, 2022 | R |
09/2017-03/2020 | Japan | Nivolumab | GC | 100 | 71 (38–89)a | 78/22 | GPS (OS) | 5.0b | 7 | (53) |
| Tokuyama, 2021 | R |
02/2015-06/2019 | Japan | Nivolumab | GC | 45 | 65 (40–81)a | 31/14 | GPS (OS, ORR) | - | 6 | (57) |
| Namikawa, 2020 | R |
10/2017-12/2019 | Japan | Nivolumab (49–86)a | GC | 29 | 71 | 19/10 | GPS (OS, PFS) | - | 6 | (49) |
| Kurosaki, 2020 | R |
10/2017-03/2019 | Japan | Nivolumab | GC | 80 | 71 (43–87)a | 67/13 | GPS (OS, PFS, ORR,
DCR) | 5.1b | 7 | (41) |
| Matsuo, 2022 | R | 04/2017-032020 | Japan | Nivolumab | HNSCC | 164 | 65 (23–87)a | 127/37 | GPS (OS, ORR,
DCR) | 12.6b | 8 | (45) |
| Minohara, 2021 | R |
04/2017-10/2019 | Japan | Nivolumab | HNC | 126 | 68 (35–90)a | 104/22 | mGPS (OS, PFS) | 7.5b | 7 | (47) |
| Chikuie, 2021 | R |
05/2017-10/2019 | Japan | Nivolumab | HNSCC | 56 | 66 (31–90)a | 40/16 | GPS (OS, PFS) | - | 7 | (34) |
| Ueki, 2020 | R |
03/2017-03/2019 | Japan | Nivolumab | HNSCC | 42 | 68 (43–79)a | 35/7 | GPS (OS) | 9.6b | 7 | (58) |
| Matsuki, 2020 | R |
05/2017-08/2018 | Japan | Nivolumab | HNSCC | 88 | 49/39d | 71/17 | mGPS (OS, PFS,
DCR) | 6.1b | 7 | (44) |
| Yamamoto, 2021 | R | 2015-2019 | Japan | Pembrolizumab | UC | 121 | 74 (50–86)a | 87/34 | GPS (OS), mGPS
(OS) | 7.9e | 8 | (60) |
| Brown, 2021
(UC) | R | 2015-2018 | USA | Atezolizumab,
Pembrolizumab, Nivolumab | UC | 53 | 70 (32–86)a | 45/8 | mGPS (OS, PFS) | 27.1b | 8 | (33) |
| Fujiwara, 2021 | R |
09/2013-08/2019 | Japan | Nivolumab | RCC | 45 | 62 (55–69)b | - | GPS (OS) | 26.4b | 7 | (35) |
| Brown, 2021
(RCC) | R | 2015-2020 | USA | ICIs | RCC | 156 | 64 (23–90)a | 108/48 | mGPS (OS, PFS) | 24.2b | 8 | (32) |
| Yamamoto, 2020 | R | 2016-2018 | Japan | Nivolumab | RCC | 65 | 69 (39–83)a | 51/14 | GPS (OS) | 11.6b | 7 | (25) |
| Noguchi, 2020 | R | - | Japan | Nivolumab | RCC | 64 | 69 (30–86)a | 51/13 | GPS (PFS) | 8.3b | 6 | (51) |
| Minichs-dorfer,
2022 | R |
01/2015-11/2016 | Austria | Pembrolizumab,
Nivolumab | Pan-cancer | 114 | 60 (22–88)a | 74/40 | GPS (OS, PFS) | - | 7 | (46) |
| Tada, 2023 | R |
09/2020-03/2022 | Japan | Atezolizumab | HCC | 421 | 74 (68–79)f | 340/81 | GPS (OS, PFS) | 8.7b | 7 | (54) |
| Yang, 2022 | R |
02/2019-02/2021 | China | Nivolumab,
Pembrolizumab, Toripalimab, Camrelizumab, Sintilimab | ICC | 73 | 57 (31–75)a | 49/24 | GPS (OS) | 11.2e | 8 | (61) |
| Mortensen,
2023 | P | - | Denmark | Nivolumab ±
Ipilimumab | PC | 32 | 68 (47–80)a | 18/14 | mGPS (OS, PFS) | 7.3b | 7 | (48) |
| Tanaka, 2023 | R |
04/2017-12/2020 | Japan | Nivolumab | HNSCC | 42 | 61 (26–81)a | 36/6 | GPS (ORR, DCR) | - | 6 | (64) |
| Niwa, 2020 | R |
05/2017-09/2019 | Japan | Nivolumab | SGC | 24 | 56 (29–82)a | 19/5 | mGPS (OS, PFS) | 6.5b | 6 | (50) |
Baseline GPS levels and OS/PFS
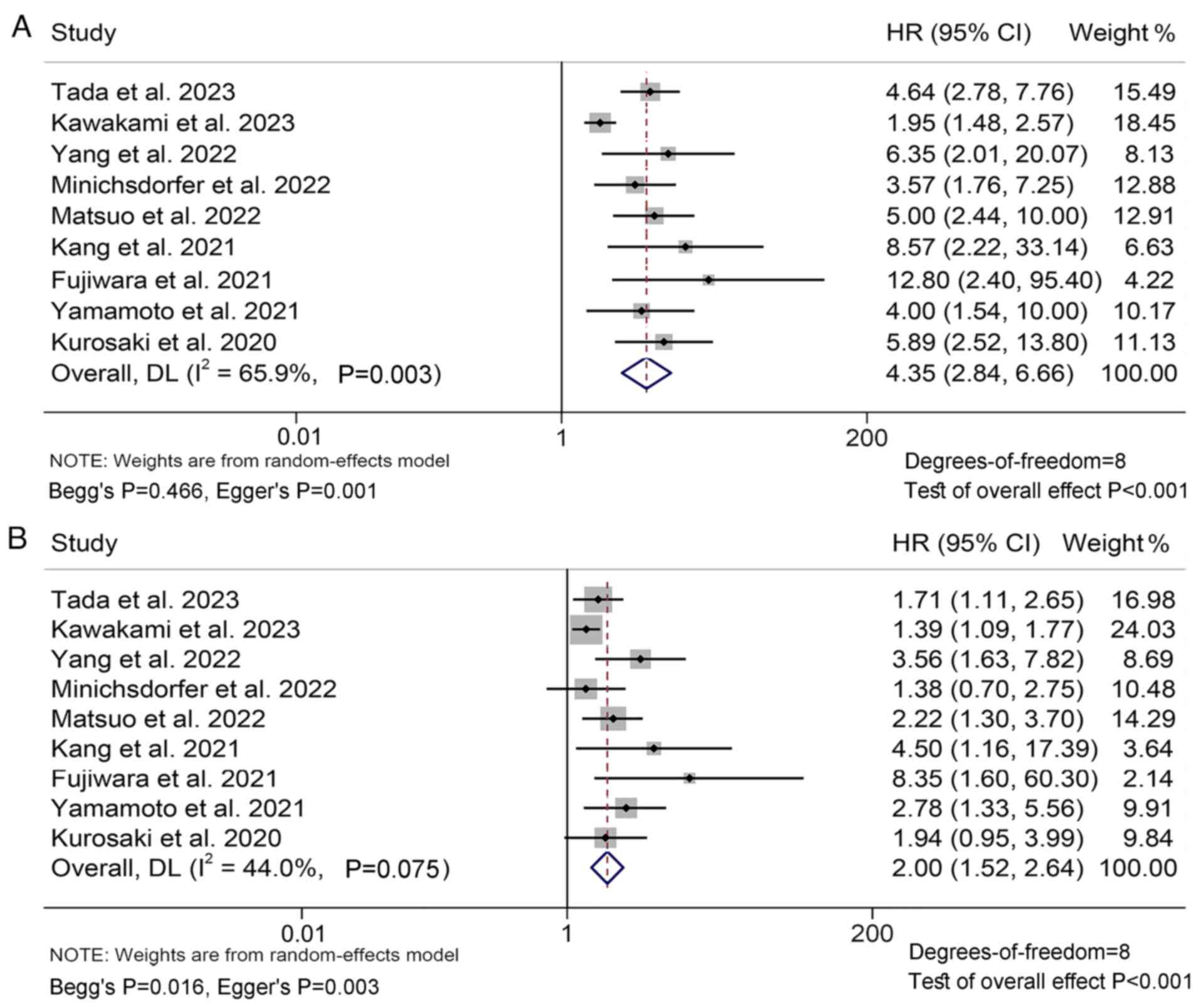
Through the analysis of data from 22 studies with
2,617 patients, the present study aimed to explore the association
between GPS levels and OS in ICI-treated patients with solid
tumors. The results revealed that patients with GPS 2
(I2=65.9%, P=0.003; HR: 4.35, 95% CI: 2.84–6.66,
P<0.001; Fig. 2A) or GPS 1
(I2=44.0%, P=0.075; HR: 2.00, 95% CI: 1.52–2.64,
P<0.001; Fig. 2B) had a shorter
OS compared to patients with GPS 0, and patients with GPS 2 had a
higher risk of death than patients with GPS 1.
Funnel, Begg's, and Egger's tests confirmed the
publication bias of the above results (GPS 2 vs. 0: Begg's P=0.466,
Egger's P=0.001; GPS 1 vs. 0: Begg's P=0.016, Egger's P=0.003;
Fig. S1A and B). To account for
potentially missing studies in the above analysis, the trim and
fill method was applied. However, the results indicated that the
pooled HR did not change significantly even after accounting for
any potential missing studies (Fig.
S1C and D). A sensitivity analysis was conducted to evaluate
the robustness of the present findings by iteratively excluding
each study and examining its impact on the pooled results. It was
revealed that the exclusion of any individual study did not
significantly affect the pooled HR (Fig. S2A and B).
In addition, it was also found that patients with
GPS 2 had a worse prognosis compared to those with GPS 1/0
(I2=0.0%, P=0.724; HR: 2.62, 95% CI: 2.01–3.43,
P<0.001; Fig. S3A). Patients
with GPS 1/2 also had a higher mortality rate compared to patients
with GPS 0 (I2=62.4%, P=0.021; HR: 2.60, 95% CI:
1.57–4.29, P<0.001; Fig. S3B).
Begg's and Egger's tests confirmed the above results without
significant publication bias (GPS 2 vs. 1/0: Begg's P=0.368,
Egger's P=0.175; GPS 2/1 vs. 0: Begg's P=0.452, Egger's P=0.083).
However, the funnel plot revealed publication bias in the GPS 2/1
vs. 0 group (Fig. S3C and D). A
trim and fill method was used, and the results showed that the
combined HR did not change significantly, even after accounting for
any potential missing studies.
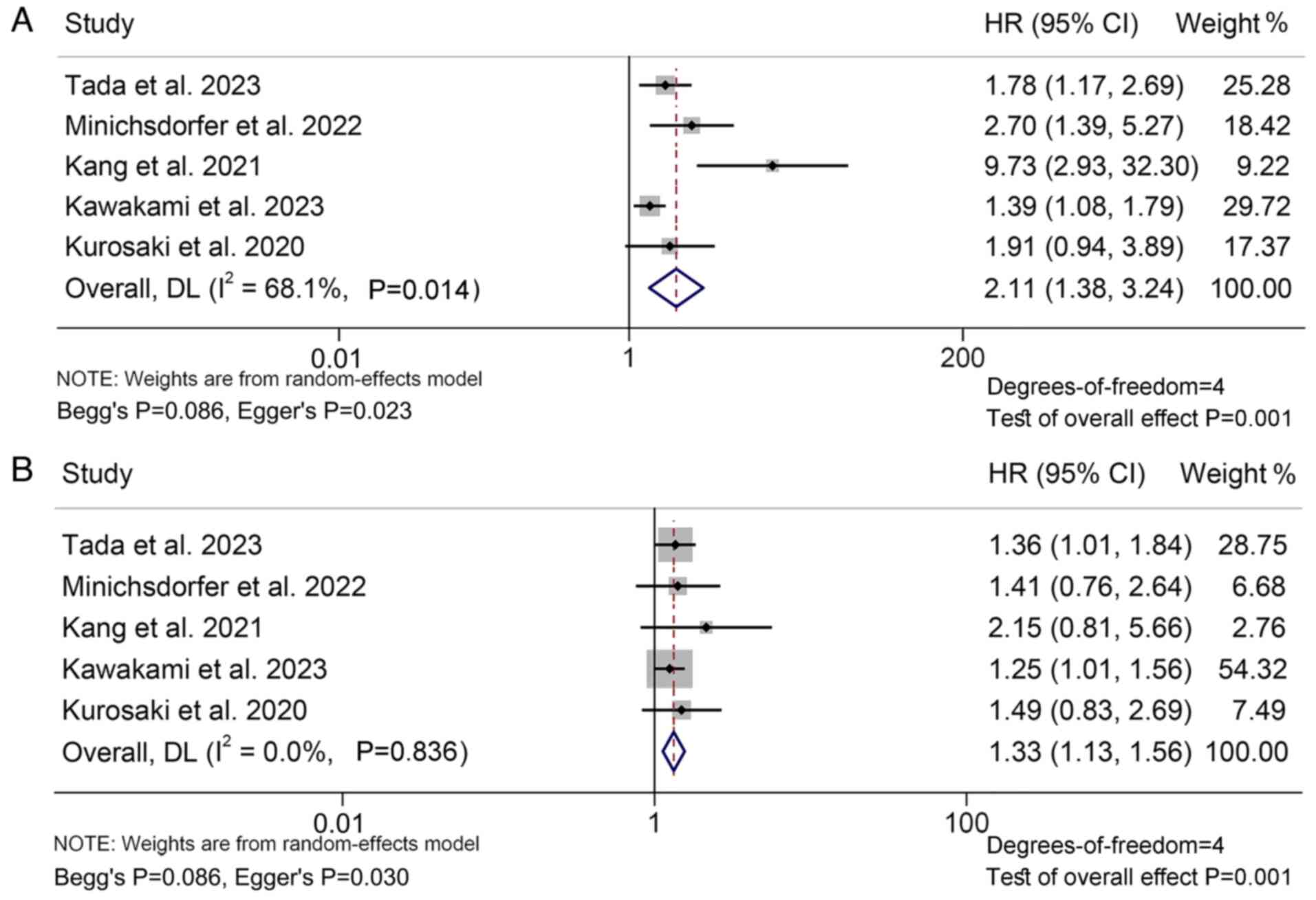
Next, 15 studies with 2,026 patients were analyzed
to investigate the relationship between GPS levels and PFS in
cancer patients treated with ICIs. The findings indicated that
patients with GPS 2 (I2=68.1%, P=0.014; HR: 2.11, 95%
CI: 1.38–3.24, P=0.001; Fig. 3A) or
GPS 1 (I2=0.0%, P=0.836; HR: 1.33, 95% CI: 1.13–1.56,
P=0.001; Fig. 3B) had a
significantly poorer PFS compared to those with GPS 0, and patients
with GPS 2 had a higher risk of progression than patients with GPS
1.
Funnel plot, Begg's and Egger's tests demonstrated
the publication bias of the above analysis (GPS 2 vs. 0: Begg's
P=0.086, Egger's P=0.023; GPS 1 vs. 0: Begg's P=0.086, Egger's
P=0.030; Fig. S4A and B). To
address the potential issue of missing studies, the trim and fill
method was employed. However, the pooled HR remained unchanged even
after accounting for any missing studies (Fig. S4C and D). The results of the
sensitivity analysis showed that excluding any of the studies did
not have a significant impact on the pooled HR (Fig. S5A and B).
Furthermore, the present analysis revealed that
patients with a GPS score of 2 had a significantly worse prognosis
than those with a GPS score of 1 and 0 (I2=0.0%,
P=0.509; HR: 2.11, 95% CI: 1.61–2.78, P<0.001; Fig. S6A). Furthermore, patients with a
GPS score of 1 and 2 exhibited a higher progression rate compared
to patients with a GPS score of 0 (I2=0.0%, P=0.414; HR:
1.62, 95% CI: 1.26–2.09, P<0.001; Fig. S6B). Funnel plot, Begg's and Egger's
tests indicated no significant publication bias in the above
results (GPS 2 vs. 1/0: Begg's P=0.086, Egger's P=0.171; GPS 2/1
vs. 0: Begg's P=0.308, Egger's P=0.059; Fig. S6C and D).
Baseline GPS levels and DCR/ORR
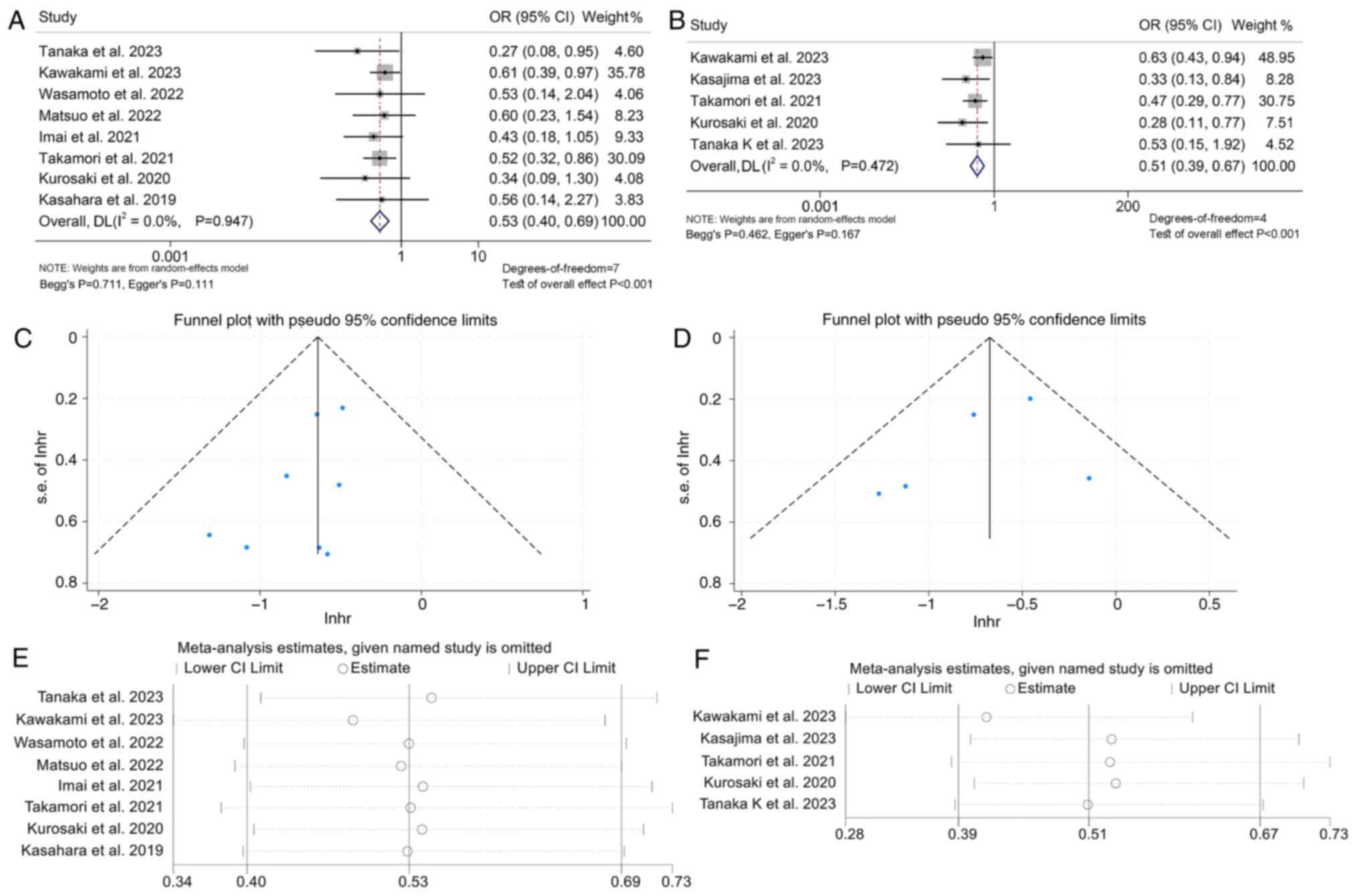
The analysis proceeded to examine the link between
GPS levels and response to ICI treatment in cancer patients through
10 studies with 1,433 patients. The results demonstrated that
patients with GPS 2 had a lower DCR compared to those with GPS 1/0
(OR: 0.53, 95% CI: 0.40–0.69, P<0.001; Fig. 4A). Patients with GPS 1/2 also had a
lower DCR compared to patients with GPS 0 (OR: 0.51, 95% CI:
0.39–0.67, P<0.001; Fig. 4B).
The above analysis did not find a significant publication bias (GPS
2 vs. 1/0: Begg's P=0.711, Egger's P=0.111; GPS 2/1 vs. 0: Begg's
P=0.462, Egger's P=0.167; Fig. 4C and
D). The results of the sensitivity analysis showed that
excluding any of the studies did not have a significant impact on
the pooled HR (Fig. 4E and F). In
addition, high GPS scores were also significantly associated with a
lower ORR in cancer patients (GPS 2 vs. 1/0, I2=52.5%,
P=0.077, OR: 0.53, 95% CI: 0.28–1.00, P=0.049; GPS 2/1 vs. 0,
I2=15.3%, P=0.316, OR: 0.31, 95% CI: 0.16–0.62,
P<0.001; Fig. S7).
Baseline mGPS levels and OS/PFS
A total of 16 studies with 1,474 patients were
analyzed to explore the association between mGPS levels and OS in
patients with solid tumors treated with ICIs. The results showed
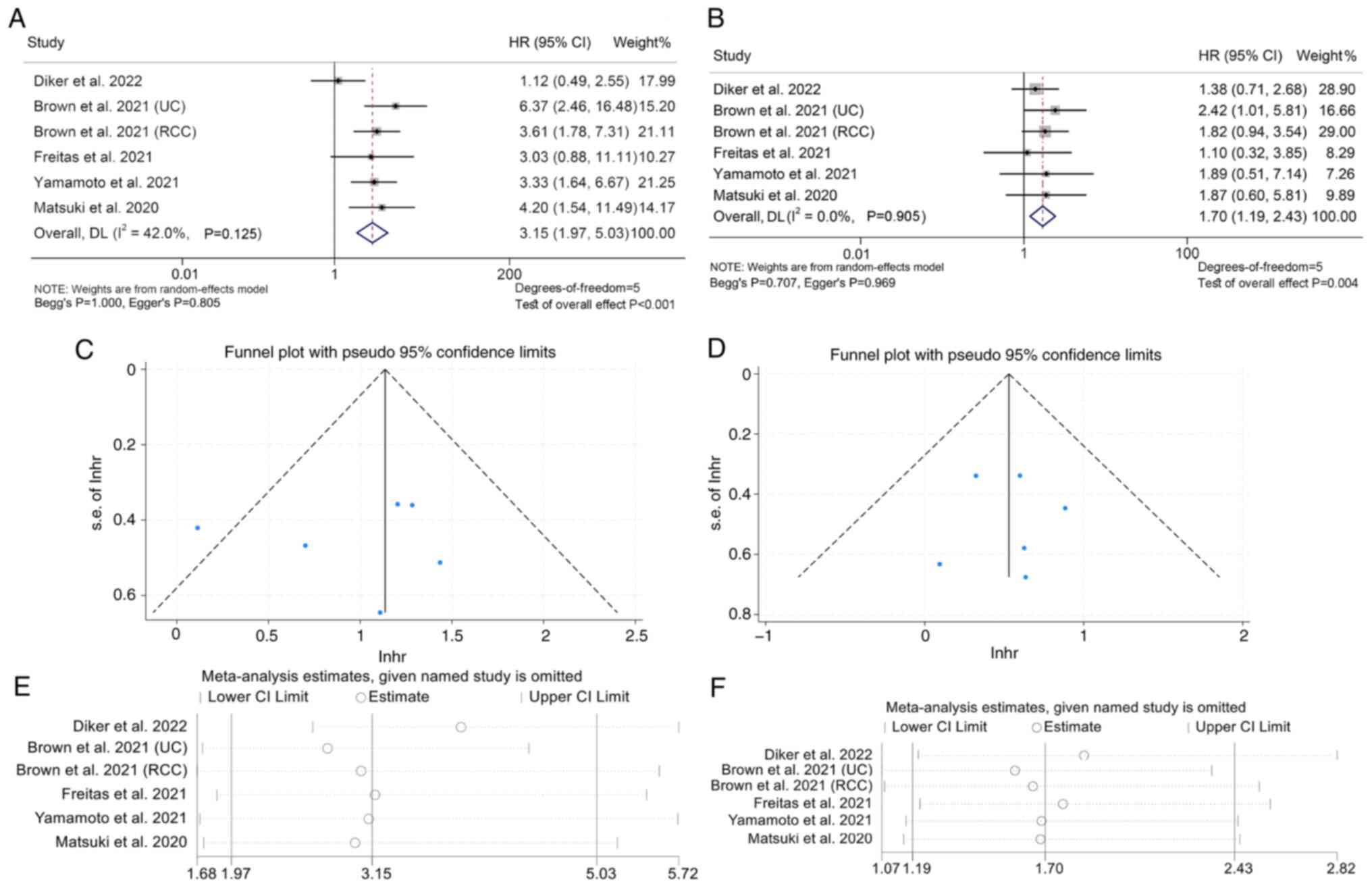
that patients with mGPS 2 (I2=42.0%, P=0.125; HR: 3.15,
95% CI: 1.95–5.03, P<0.001; Fig.
5A) or mGPS 1 (I2=0.0%, P=0.905; HR: 1.70, 95% CI:
1.19–2.43, P=0.004; Fig. 5B) had
poorer survival compared to patients with mGPS 0 and patients with
mGPS 2 had a higher risk of death than patients with mGPS 1.
Funnel plot, Begg's and Egger's tests revealed no
publication bias in the above findings (mGPS 2 vs. 0: Begg's
P=1.000, Egger's P=0.805; mGPS 1 vs. 0: Begg's P=0.707, Egger's
P=0.969; Fig. 5C and D).
Furthermore, a sensitivity analysis was employed to test the
robustness of the results by excluding each study iteratively and
assessing its impact on the pooled outcomes. It was found that the
exclusion of any single study did not substantially affect the
pooled HR (Fig. 5E and F).
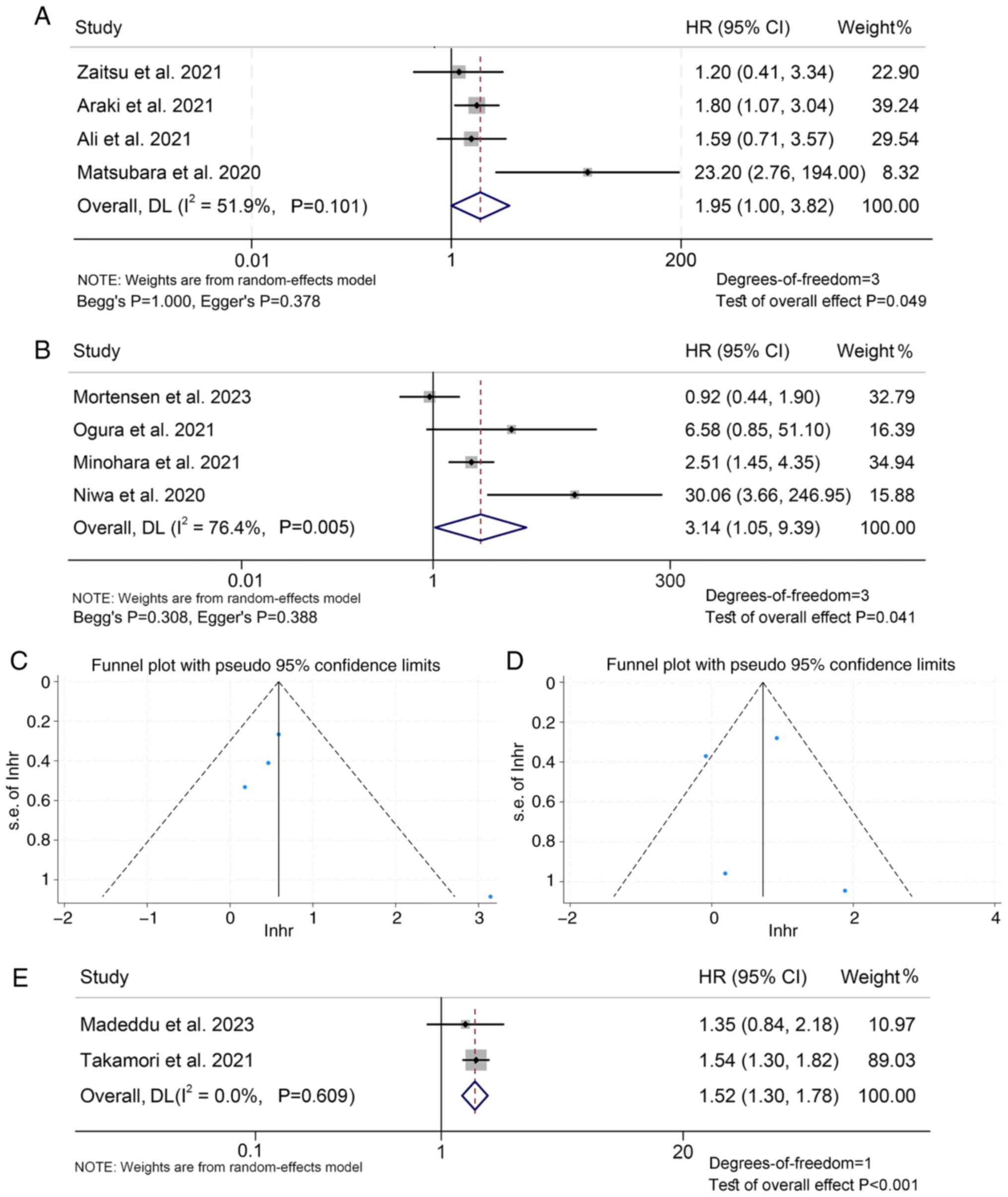
The present analysis further revealed that patients
with mGPS 2 had a poorer prognosis than those with mGPS 1/0, with
an HR of 1.95 (95% CI: 1.00–3.82, P=0.049; Fig. 6A), and patients with mGPS=1/2 also
had a higher mortality rate compared to patients with mGPS=0, with
an HR of 3.14 (95% CI: 1.05–9.39, P=0.041; Fig. 6B). Begg's and Egger's tests
confirmed no significant publication bias was observed (mGPS 2 vs.
1/0: Begg's P=1.000, Egger's P=0.378; mGPS 2/1 vs. 0: Begg's
P=0.308, Egger's P=0.388; Fig. 6C and
D). Finally, it was found that when mGPS is a continuous
variable, a larger mGPS value is associated with shorter patient OS
(HR: 1.52, 95% CI: 1.30–1.78, P<0.001; Fig. 6E).
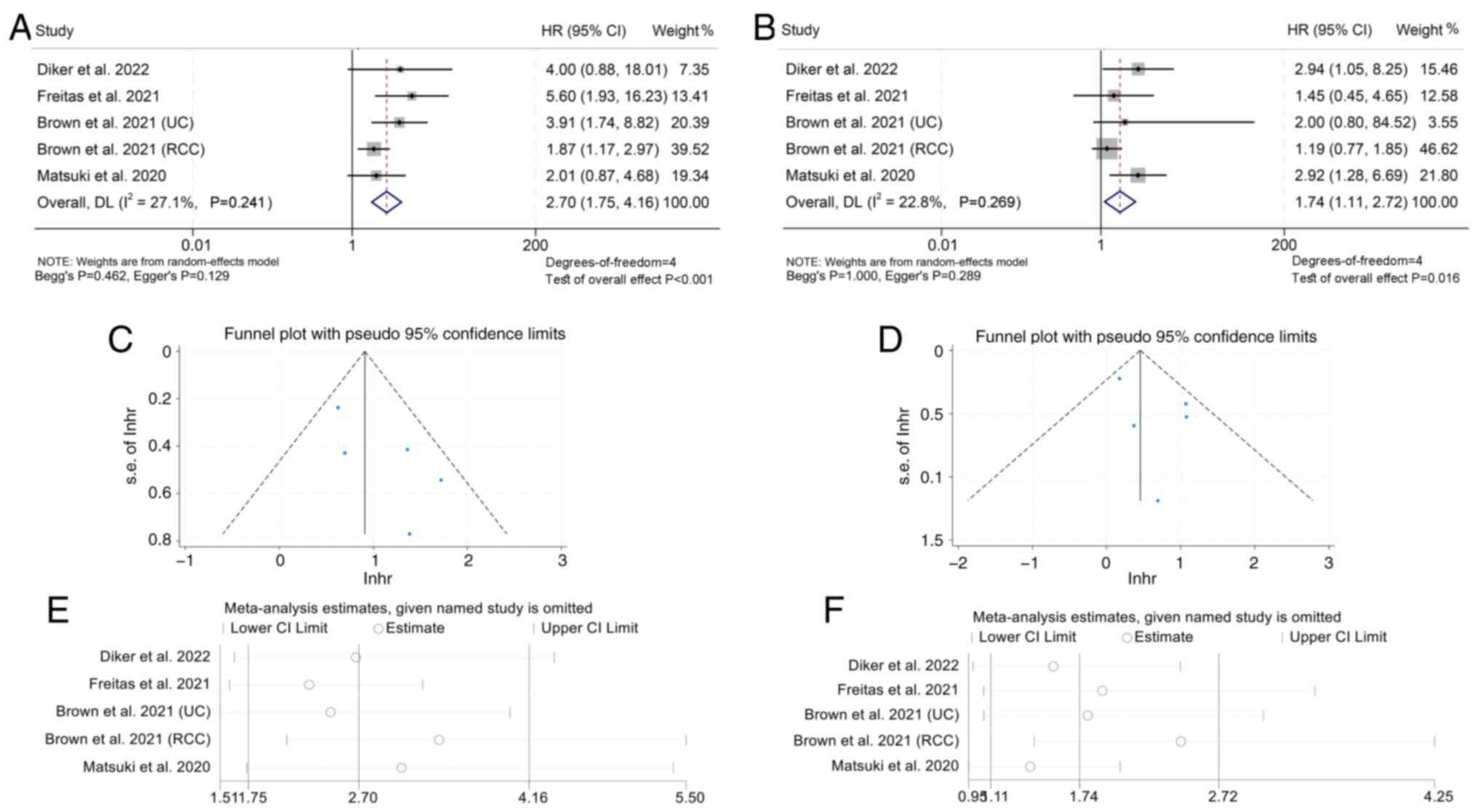
The association between mGPS and PFS in cancer
patients treated with ICIs was then explored using data from 13
studies with 1,216 patients. It was demonstrated that patients with
mGPS 2 (I2=27.1%, P=0.241; HR: 2.70, 95% CI: 1.75–4.16,
P<0.001; Fig. 7A) or mGPS=1
(I2=22.8%, P=0.269; HR: 1.74, 95% CI: 1.11–2.72,
P=0.016; Fig. 7B) had poorer PFS
and patients with mGPS 2 had a higher risk of progression than
patients with mGPS 1.
The results of funnel plot, Begg's and Egger's tests
indicated that there was no publication bias in the aforementioned
findings (mGPS 2 vs. 0: Begg's P=0.462, Egger's P=0.129; mGPS 1 vs.
0: Begg's P=1.000, Egger's P=0.289; Fig. 7C and D). Furthermore, a sensitivity
analysis was performed to evaluate the robustness of the results by
iteratively excluding each study and examining its influence on the
pooled outcomes. It was discovered that the exclusion of any
individual study did not significantly alter the pooled HR
(Fig. 7E and F).
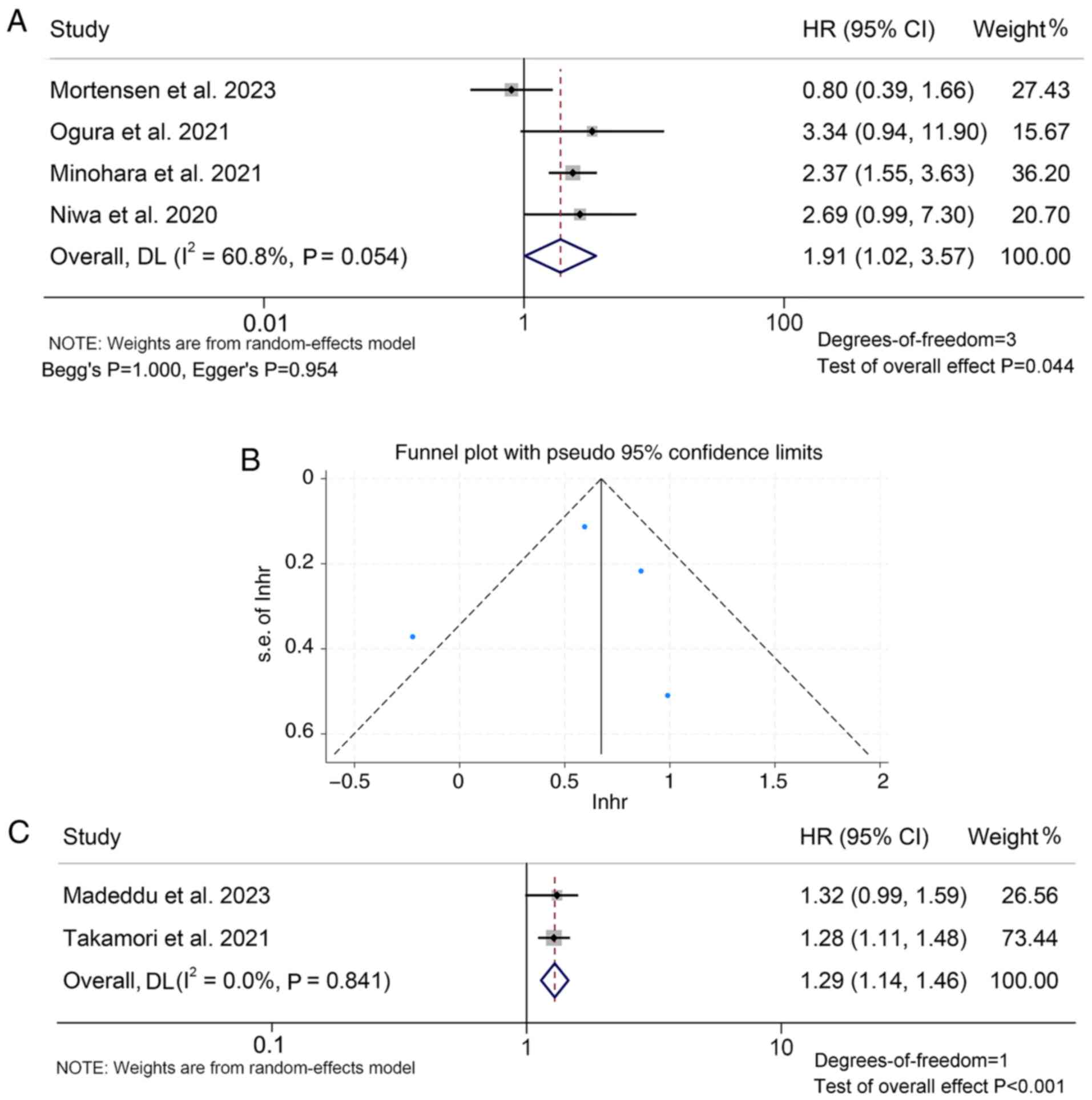
The present analysis further showed that patients
with mGPS 2 had a worse PFS compared to those with mGPS=1/0 (HR:
1.91, 95% CI: 1.02–3.57, P=0.044; Fig.
8A). The funnel plot, Begg's and Egger's tests showed no
significant publication bias (mGPS 2 vs. 1/0: Begg's P=1.000,
Egger's P=0.954, Fig. 8B). It was
also found that when mGPS is a continuous variable, a larger mGPS
value was associated with worse PFS of patients (HR: 1.29, 95% CI:
1.14–1.46, P<0.001; Fig.
8C).
Baseline mGPS levels and DCR
An analysis was performed to investigate the
relationship between mGPS levels and response to ICI therapy in
cancer patients using data from three studies with 465 patients.
The findings demonstrated that cancer patients with mGPS 2
exhibited a lower DCR (I2=0.0%, P=0.644; OR: 0.46, 95%
CI: 0.31–0.70, P<0.001; Fig.
S8A) of ICI therapy than those with mGPS 1/0. Sensitivity
analysis confirmed that the results were stable and reliable
(Fig. S8B).
Discussion
The present investigation aimed to explore the
predictive value of the GPS and mGPS in cancer patients receiving
ICIs. Through this analysis of relevant studies, a strong
relationship between elevated GPS and mGPS and worse OS and PFS, as
well as a lower DCR, was found. As a cost-effective, easy-to-use
tool, the GPS or mGPS status can be used as a predictor to identify
patients who are likely to experience favorable clinical
outcomes.
To provide a comprehensive comparison of the present
findings with previous meta-analyses, including Zhang et al
(65), Wu et al (66) and Wang et al (67), survival outcomes and response
metrics were analyzed across a broader range of studies and cancer
types. Compared to Zhang et al (65), who reported HRs for OS of 3.89 (GPS
2 vs. 0) and 1.48 (GPS 1 vs. 0), the present analysis yielded
higher pooled HRs for OS (4.354 for GPS 2 vs. 0, and 2.004 for GPS
1 vs. 0), indicating a potentially stronger prognostic value of the
GPS. Similarly, for PFS, the HR determined in the present study for
GPS 2 vs. 0 was 2.113, compared to Zhang et al (65)'s 1.48, highlighting a more pronounced
association in the larger dataset of the present study. In terms of
the DCR, a unique aspect of the current study, it was found that
higher GPS and mGPS were significantly associated with lower DCR
and ORR, metrics not previously explored by Zhang et al
(65). This additional analysis
provides new insights into the predictive value of the GPS/mGPS
beyond survival outcomes. Furthermore, unlike prior meta-analyses
that combined pre- and post-treatment GPS/mGPS data or failed to
stratify by baseline characteristics, the current study maintains
methodological rigor by stratifying analyses, reducing
heterogeneity and enhancing clinical interpretability (65–67).
GPS or mGPS values are determined based on CRP and
albumin, which reflect the level of inflammation and nutritional
status of the body. Consistent evidence suggests that
hypoalbuminemia is a significant predictor of poor prognosis in
cancer patients (68–70). Serum albumin has been shown to have
an immunomodulatory role, as it can bind to prostaglandin E2
(PGE2), which is associated with the downregulation of tumor
necrosis factor-α derived from macrophages and contributes to
immunosuppression (71–74). Of note, administering a human
albumin solution has been found to counteract the effects of PGE2,
leading to improved immune function (72). According to earlier research on the
immunological role of serum CRP, elevated CRP levels are linked to
lymphocytopenia, an elevation of proinflammatory cytokines, and
decreased T lymphocyte and macrophage responses (75–77).
The maintenance of tumor growth and tumor invasiveness are both
facilitated by elevated proinflammatory cytokines (78,79).
Inflammation mediators have been demonstrated to impede the
synthesis of albumin, whereas oxidative stress may induce the
denaturation of albumin, thereby fostering a precipitous decline in
serum albumin concentrations among individuals with an inflammatory
milieu (80–82). An additional pivotal aspect in
tumorigenesis pertains to the tumor microenvironment. By
orchestrating the recruitment of T lymphocytes, tumor-associated
macrophages and circulating cytokines, inflammatory mediators can
profoundly modulate various facets of tumor biology, encompassing
cell proliferation and angiogenesis, as well as tumor invasion or
metastasis (2,83,84).
Because of this, GPS and mGPS levels can
significantly predict the efficacy of ICI therapy in cancer
patients. In addition, GPS and mGPS serve as objective, highly
reproducible ways to more accurately classify patients based on a
three-index-scoring indicator. As a result, due to its
well-established impact on the host's inflammation and nutritional
status as well as cancer, the GPS and mGPS could serve as useful
tools in predicting the therapeutic outcomes of ICIs in cancer
patients. Individualized and timely nutritional and immunological
interventions may improve the prognosis of patients with high
baseline GPS and mGPS.
Notably, most studies included in the present
analysis were retrospective, which may have limited the statistical
robustness. In addition, the majority of data were derived from
studies conducted in China and Japan, which may restrict the
generalizability of the findings. The limited number of studies
also precluded subgroup analyses for each cancer type and for
different classes of ICIs. Therefore, further high-quality research
with larger sample sizes, particularly multicenter prospective
studies, is essential to validate and enhance the accuracy of the
present results.
In conclusion, the current meta-analysis indicated
that the GPS and mGPS may be reliable predictors of outcomes in
cancer patients treated with ICIs.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY and ML conceived and designed the study. HY and
ML were responsible for the collection and assembly of data, data
analysis and interpretation. HY was involved in writing the
manuscript and ML revised the manuscript. HY and ML checked and
confirm the authenticity of the raw data (pertaining to the pooled
dataset). All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan Q, Deng D, Pan C, Wei T, Wu Z, Zhang
B, Li S, Yin P and Shang D: Integration of transcriptomics,
proteomics, and metabolomics data to reveal HER2-associated
metabolic heterogeneity in gastric cancer with response to
immunotherapy and neoadjuvant chemotherapy. Front Immunol.
13:9511372022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye F, Dewanjee S, Li Y, Jha NK, Chen ZS,
Kumar A, Vishakha Behl T, Jha SK and Tang H: Advancements in
clinical aspects of targeted therapy and immunotherapy in breast
cancer. Mol Cancer. 22:1052023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morad G, Helmink BA, Sharma P and Wargo
JA: Hallmarks of response, resistance, and toxicity to immune
checkpoint blockade. Cell. 184:5309–5337. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Donnell JS, Teng MWL and Smyth MJ:
Cancer immunoediting and resistance to T cell-based immunotherapy.
Nat Rev Clin Oncol. 16:151–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dolladille C, Ederhy S, Sassier M, Cautela
J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF,
et al: Immune checkpoint inhibitor rechallenge after immune-related
adverse events in patients with cancer. JAMA Oncol. 6:865–871.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Feng J, Kuang T, Chai D, Qiu Z,
Deng W, Dong K, Zhao K and Wang W: Blood biomarkers predict
outcomes in patients with hepatocellular carcinoma treated with
immune checkpoint Inhibitors: A pooled analysis of 44 retrospective
sudies. Int Immunopharmacol. 118:1100192023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao J, Li D, Xie S, Deng X, Wen X, Li J,
Wu Z, Yang X, Li M, Tang Y, et al: Nomogram for predicting
prognosis of patients with metastatic melanoma after immunotherapy:
A Chinese population-based analysis. Front Immunol. 13:10838402022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vergnenegre A and Chouaid C: Economic
analyses of immune-checkpoint inhibitors to treat lung cancer.
Expert Rev Pharmacoecon Outcomes Res. 21:365–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schalper KA, Carleton M, Zhou M, Chen T,
Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, et al:
Elevated serum interleukin-8 is associated with enhanced intratumor
neutrophils and reduced clinical benefit of immune-checkpoint
inhibitors. Nat Med. 26:688–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Wang K, Kuang T, Deng W, Hu P and
Wang W: Low geriatric nutritional risk index as a poor prognostic
biomarker for immune checkpoint inhibitor treatment in solid
cancer. Front Nutr. 10:12865832023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren J, Wang A, Liu J and Yuan Q:
Identification and validation of a novel redox-related lncRNA
prognostic signature in lung adenocarcinoma. Bioengineered.
12:4331–4348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Havel JJ, Chowell D and Chan TA: The
evolving landscape of biomarkers for checkpoint inhibitor
immunotherapy. Nat Rev Cancer. 19:133–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumour site: A glasgow inflammation outcome study.
Br J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagashima Y, Funahashi K, Kagami S,
Ushigome M, Kaneko T, Miura Y, Yoshida K, Koda T and Kurihara A:
Which preoperative immunonutritional index best predicts
postoperative mortality after palliative surgery for malignant
bowel obstruction in patients with late-stage cancer? A
single-center study in Japan comparing the modified Glasgow
prognostic score (mGPS), the prognostic nutritional index (PNI),
and the controlling nutritional status (CONUT). Surg Today.
53:22–30. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schuettfort VM, Gust K, D'Andrea D, Quhal
F, Mostafaei H, Laukhtina E, Mori K, Rink M, Abufaraj M,
Karakiewicz PI, et al: Impact of the preoperative modified glasgow
prognostic score on disease outcome after radical cystectomy for
urothelial carcinoma of the bladder. Minerva Urol Nephrol.
74:302–312. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsueh SW, Liu KH, Hung CY, Kuo YC, Tsai
CY, Hsu JT, Hung YS, Tsang NM and Chou WC: Significance of the
glasgow prognostic score in predicting the postoperative outcome of
patients with stage III gastric cancer. J Clin Med. 8:14482019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vashist YK, Loos J, Dedow J, Tachezy M,
Uzunoglu G, Kutup A, Yekebas EF and Izbicki JR: Glasgow prognostic
score is a predictor of perioperative and long-term outcome in
patients with only surgically treated esophageal cancer. Ann Surg
Oncol. 18:1130–1138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada S, Fujii T, Yabusaki N, Murotani K,
Iwata N, Kanda M, Tanaka C, Nakayama G, Sugimoto H, Koike M, et al:
Clinical implication of inflammation-based prognostic score in
pancreatic cancer: Glasgow prognostic score is the most reliable
parameter. Medicine (Baltimore). 95:e35822016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimoyama R, Imamura Y, Uryu K, Mase T,
Ohtaki M, Ohtani K, Shiragami M, Fujimura Y, Hayashi M, Shinozaki N
and Minami H: Inflammation-based prognostic markers in patients
with advanced or recurrent gastric cancer treated with nivolumab:
Tokushukai REAl-world Data project 02 (TREAD 02). Mol Clin Oncol.
21:902024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diker O and Olgun P: Association of the
immune-inflammation-nutritional parameters with immune checkpoint
inhibitor outcomes in patients with advanced non-small cell lung
cancer. J Oncol Sci. 8:43–53. 2022. View Article : Google Scholar
|
|
25
|
Yamamoto Y, Matsuyama H, Matsumoto H,
Sakano S, Fuji N, Oba K, Yamamoto M, Kamiryo Y, Hiragino T, Nagao
K, et al: Prognostic value of risk stratification using blood
parameters for nivolumab in Japanese patients with metastatic
renal-cell carcinoma. Jpn J Clin Oncol. 50:214–220. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Freitas C, Jacob M, Tavares N,
Cruz-Martins N, Souto-Moura C, Araújo D, Novais-Bastos H, Santos V,
Fernandes G, Magalhães A, et al: Modified glasgow prognostic score
predicts survival among advanced non-small cell lung carcinoma
patients treated with anti-PD1 agents. Anticancer Drugs.
32:567–574. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu
B and Wang W: Prognostic nutritional index as a prognostic
biomarker for gastrointestinal cancer patients treated with immune
checkpoint inhibitors. Front Immunol. 14:12199292023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Li X, Wang K, Wu M, Liu W and
Wang W: Prognostic impact of body composition in hepatocellular
carcinoma patients with immunotherapy. Ann Med. 56:23950622024.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Xia Z, Li Z, Zhang J, Wang K and
Wang W: Influence of body fat tissue on outcomes in patients
undergoing hepatectomy or liver transplantation. Int J Surg.
111:1167–1181. 2024.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ali WAS, Hui P, Ma Y, Wu Y, Zhang Y, Chen
Y, Hong S, Yang Y, Huang Y, Zhao Y, et al: Determinants of survival
in advanced non-small cell lung cancer patients treated with
anti-PD-1/PD-L1 therapy. Ann Transl Med. 9:16392021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Araki T, Tateishi K, Sonehara K, Hirota S,
Komatsu M, Yamamoto M, Kanda S, Kuraishi H, Hanaoka M and Koizumi
T: Clinical utility of the C-reactive protein:albumin ratio in
non-small cell lung cancer patients treated with nivolumab. Thorac
Cancer. 12:603–612. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brown JT, Liu Y, Shabto JM, Martini D,
Ravindranathan D, Hitron EE, Russler GA, Caulfield S, Yantorni L,
Joshi SS, et al: Modified glasgow prognostic score associated with
survival in metastatic renal cell carcinoma treated with immune
checkpoint inhibitors. J Immunother Cancer. 9:e0028512021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown JT, Liu Y, Shabto JM, Martini DJ,
Ravindranathan D, Hitron EE, Russler GA, Caulfield S, Yantorni LB,
Joshi SS, et al: Baseline modified glasgow prognostic score
associated with survival in metastatic urothelial carcinoma treated
with immune checkpoint inhibitors. Oncologist. 26:397–405. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chikuie N, Hamamoto T, Ueda T, Taruya T,
Kono T, Furuie H, Ishino T and Takeno S: Baseline
neutrophil-to-lymphocyte ratio and glasgow prognostic score are
associated with clinical outcome in patients with recurrent or
metastatic head and neck squamous cell carcinoma treated with
nivolumab. Acta Med Okayama. 75:335–343. 2021.PubMed/NCBI
|
|
35
|
Fujiwara R, Takemura K, Fujiwara M, Yuasa
T, Yasuoka S, Komai Y, Numao N, Yamamoto S and Yonese J: Modified
glasgow prognostic score as a predictor of prognosis in metastatic
renal cell carcinoma treated with nivolumab. Clin Genitourin
Cancer. 19:e78–e83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imai H, Kishikawa T, Minemura H, Yamada Y,
Ibe T, Yamaguchi O, Mouri A, Hamamoto Y, Kanazawa K, Kasai T, et
al: Pretreatment glasgow prognostic score predicts survival among
patients with high PD-L1 expression administered first-line
pembrolizumab monotherapy for non-small cell lung cancer. Cancer
Med. 10:6971–6984. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang HS, Shin AY, Yeo CD, Kim SK, Park CK,
Kim JS, Kim SJ, Lee SH and Kim JW: Significance of glasgow
prognostic scores in NSCLC patients treated with immunotherapy
after platinum-based cytotoxic chemotherapy. In Vivo. 35:3423–3430.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kasahara N, Sunaga N, Tsukagoshi Y, Miura
Y, Sakurai R, Kitahara S, Yokobori T, Kaira K, Mogi A, Maeno T, et
al: Post-treatment glasgow prognostic score predicts efficacy in
advanced non-small-cell lung cancer treated with anti-PD1.
Anticancer Res. 39:1455–1461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kasajima M, Igawa S, Manaka H, Yamada K,
Akazawa Y, Manabe H, Yagami Y, Yamamoto H, Ito H, Kaizuka N, et al:
The glasgow prognostic score predicts outcomes of pembrolizumab or
atezolizumab monotherapy in patients with pretreated non-small cell
lung cancer. Oncology. 101:69–76. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawakami H, Sunakawa Y, Inoue E, Matoba R,
Noda K, Sato T, Suminaka C, Yamaki M, Sakamoto Y, Kawabata R, et
al: Soluble programmed cell death ligand 1 predicts prognosis for
gastric cancer patients treated with nivolumab: Blood-based
biomarker analysis for the DELIVER trial. Eur J Cancer. 184:10–20.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kurosaki T, Kawakami H, Mitani S, Kawabata
R, Takahama T, Nonagase Y, Fumita S, Ozaki T, Chiba Y, Tamura T and
Nakagawa K: Glasgow prognostic score (GPS) and tumor response as
biomarkers of nivolumab monotherapy in third- or later-line setting
for advanced gastric cancer. In Vivo. 34:1921–1929. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Madeddu C, Busquets S, Donisi C, Lai E,
Pretta A, López-Soriano FJ, Argilés JM, Scartozzi M and Macciò A:
Effect of cancer-related cachexia and associated changes in
nutritional status, inflammatory status, and muscle mass on
immunotherapy efficacy and survival in patients with advanced
non-small cell lung cancer. Cancers (Basel). 15:10762023.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsubara T, Takamori S, Haratake N,
Toyozawa R, Miura N, Shimokawa M, Yamaguchi M, Seto T and
Takenoyama M: The impact of immune-inflammation-nutritional
parameters on the prognosis of non-small cell lung cancer patients
treated with atezolizumab. J Thorac Dis. 12:1520–1528. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsuki T, Okamoto I, Fushimi C, Sawabe M,
Kawakita D, Sato H, Tsukahara K, Kondo T, Okada T, Tada Y, et al:
Hematological predictive markers for recurrent or metastatic
squamous cell carcinomas of the head and neck treated with
nivolumab: A multicenter study of 88 patients. Cancer Med.
9:5015–5024. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Matsuo M, Yasumatsu R, Masuda M, Toh S,
Wakasaki T, Hashimoto K, Jiromaru R, Manako T and Nakagawa T:
Inflammation-based prognostic score as a prognostic biomarker in
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma treated with nivolumab therapy. In Vivo. 36:907–917.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Minichsdorfer C, Gleiss A, Aretin MB,
Schmidinger M and Fuereder T: Serum parameters as prognostic
biomarkers in a real world cancer patient population treated with
anti PD-1/PD-L1 therapy. Ann Med. 54:1339–1349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Minohara K, Matoba T, Kawakita D, Takano
G, Oguri K, Murashima A, Nakai K, Iwaki S, Hojo W, Matsumura A, et
al: Novel prognostic score for recurrent or metastatic head and
neck cancer patients treated with nivolumab. Sci Rep. 11:169922021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mortensen REJ, Holmström MO, Lisle TL,
Hasselby JP, Willemoe GL, Met Ö, Marie Svane I, Johansen J, Nielsen
DL, Chen IM and Andersen MH: Pre-existing TGF-β-specific T-cell
immunity in patients with pancreatic cancer predicts survival after
checkpoint inhibitors combined with radiotherapy. J Immunother
Cancer. 11:e0064322023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Namikawa T, Yokota K, Tanioka N, Fukudome
I, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M
and Hanazaki K: Systemic inflammatory response and nutritional
biomarkers as predictors of nivolumab efficacy for gastric cancer.
Surg Today. 50:1486–1495. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Niwa K, Kawakita D, Nagao T, Takahashi H,
Saotome T, Okazaki M, Yamazaki K, Okamoto I, Hirai H, Saigusa N, et
al: Multicentre, retrospective study of the efficacy and safety of
nivolumab for recurrent and metastatic salivary gland carcinoma.
Sci Rep. 10:169882020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Noguchi G, Nakaigawa N, Umemoto S,
Kobayashi K, Shibata Y, Tsutsumi S, Yasui M, Ohtake S, Suzuki T,
Osaka K, et al: C-reactive protein at 1 month after treatment of
nivolumab as a predictive marker of efficacy in advanced renal cell
carcinoma. Cancer Chemother Pharmacol. 86:75–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ogura Y, Kataoka N, Kunimatsu Y, Tachibana
Y, Sugimoto T, Tani N, Sato I, Hirose K, Kato D and Takeda T:
Predictors of survival among Japanese patients receiving first-line
chemoimmunotherapy for advanced non-small cell lung cancer. Thorac
Cancer. 12:97–105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sakai D, Omori T, Fumita S, Fujita J,
Kawabata R, Matsuyama J, Yasui H, Hirao M, Kawase T, Kishi K, et
al: Real-world effectiveness of third- or later-line treatment in
Japanese patients with HER2-positive, unresectable, recurrent or
metastatic gastric cancer: A retrospective observational study. Int
J Clin Oncol. 27:1154–1163. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tada T, Kumada T, Hiraoka A, Kariyama K,
Tani J, Hirooka M, Takaguchi K, Atsukawa M, Fukunishi S, Itobayashi
E, et al: New prognostic system based on inflammation and liver
function predicts prognosis in patients with advanced unresectable
hepatocellular carcinoma treated with atezolizumab plus
bevacizumab: A validation study. Cancer Med. 12:6980–6993. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takamori S, Takada K, Shimokawa M,
Matsubara T, Fujishita T, Ito K, Toyozawa R, Yamaguchi M, Okamoto
T, Yoneshima Y, et al: Clinical utility of pretreatment glasgow
prognostic score in non-small-cell lung cancer patients treated
with immune checkpoint inhibitors. Lung Cancer. 152:27–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tanaka T, Yoshida T, Masuda K, Takeyasu Y,
Shinno Y, Matsumoto Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N
and Ohe Y: Prognostic role of modified glasgow prognostic score in
elderly non-small cell lung cancer patients treated with anti-PD-1
antibodies. Respir Investig. 61:74–81. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tokuyama N, Takegawa N, Nishikawa M, Sakai
A, Mimura T, Kushida S, Tsumura H, Yamamoto Y, Miki I and Tsuda M:
Pretreatment glasgow prognostic score as a predictor of outcomes in
nivolumab-treated patients with advanced gastric cancer. PLoS One.
16:e02476452021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ueki Y, Takahashi T, Ota H, Shodo R,
Yamazaki K and Horii A: Predicting the treatment outcome of
nivolumab in recurrent or metastatic head and neck squamous cell
carcinoma: Prognostic value of combined performance status and
modified Glasgow prognostic score. Eur Arch Otorhinolaryngol.
277:2341–2347. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wasamoto S, Imai H, Tsuda T, Nagai Y,
Minemura H, Yamada Y, Umeda Y, Kishikawa T, Shiono A, Kozu Y, et
al: Pretreatment glasgow prognostic score predicts survival among
patients administered first-line atezolizumab plus carboplatin and
etoposide for small cell lung cancer. Front Oncol. 12:10807292023.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yamamoto Y, Yatsuda J, Shimokawa M, Fuji
N, Aoki A, Sakano S, Yamamoto M, Suga A, Tei Y, Yoshihiro S, et al:
Prognostic value of pre-treatment risk stratification and
post-treatment neutrophil/lymphocyte ratio change for pembrolizumab
in patients with advanced urothelial carcinoma. Int J Clin Oncol.
26:169–177. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang Z, Zhang D, Zeng H, Fu Y, Hu Z, Pan
Y, Chen J, Wang J, Zhang Y, Zhou Z, et al: Inflammation-based
scores predict responses to PD-1 inhibitor treatment in
intrahepatic cholangiocarcinoma. J Inflamm Res. 15:5721–5731. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zaitsu J, Yamashita Y, Ishikawa A, Saito
A, Kagimoto A, Mimura T, Hirakawa T, Mito M, Fukuhara K, Senoo T,
et al: Systemic inflammatory score predicts response and prognosis
in patients with lung cancer treated with immunotherapy. Anticancer
Res. 41:3673–3682. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Takegawa N, Hirabayashi T, Tanaka S,
Nishikawa M, Tokuyama N, Mimura T, Kushida S, Tsumura H, Yamamoto
Y, Miki I and Tsuda M: The impact of nutritional status in
nivolumab-treated patients with advanced esophageal cancer. PLoS
One. 18:e02853652023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tanaka K, Hirakawa H, Suzuki M, Higa T,
Agena S, Hasegawa N, Kawakami J, Toyama M, Higa T, Kinjyo H, et al:
Biomarkers for predicting anti-programmed cell death-1 antibody
treatment effects in head and neck cancer. Curr Oncol.
30:5409–5424. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang Y, Chen S, Chen H and Li W: A
comprehensive analysis of glasgow prognostic score (GPS)/the
modified glasgow prognostic score (mGPS) on immune checkpoint
inhibitor efficacy among patients with advanced cancer. Cancer Med.
12:38–48. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu J, Wang H, Yang R, Liu D and Li W:
Prognostic significance of the modified Glasgow Prognostic Score in
NSCLC patients undergoing immune checkpoint inhibitor therapy: A
meta-analysis. Front Oncol. 14:14498532024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang H, Yang R, Cheng C, Wang S, Liu D and
Li W: Prognostic value of the glasgow prognostic score in non-small
cell lung cancer patients receiving immunotherapy: A meta-analysis.
Nutr Cancer. 76:187–195. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yao ZH, Tian GY, Yang SX, Wan YY, Kang YM,
Liu QH, Yao F and Lin DJ: Serum albumin as a significant prognostic
factor in patients with malignant pleural mesothelioma. Tumour
Biol. 35:6839–6845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gupta D and Lis CG: Pretreatment serum
albumin as a predictor of cancer survival: A systematic review of
the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Miura K, Hamanaka K, Koizumi T, Kitaguchi
Y, Terada Y, Nakamura D, Kumeda H, Agatsuma H, Hyogotani A,
Kawakami S, et al: Clinical significance of preoperative serum
albumin level for prognosis in surgically resected patients with
non-small cell lung cancer: Comparative study of normal lung,
emphysema, and pulmonary fibrosis. Lung Cancer. 111:88–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang J, Petersen CE, Ha CE and Bhagavan
NV: Structural insights into human serum albumin-mediated
prostaglandin catalysis. Protein Sci. 11:538–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
China L, Maini A, Skene SS, Shabir Z,
Sylvestre Y, Colas RA, Ly L, Becares Salles N, Belloti V, Dalli J,
et al: Albumin counteracts immune-suppressive effects of lipid
mediators in patients with advanced liver disease. Clin
Gastroenterol Hepatol. 16:738–747.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Choe WH and Baik SK: Prostaglandin
E2-mediated immunosuppression and the role of albumin as its
modulator. Hepatology. 61:1080–1082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Arroyo V and Moreau R: Tying up PGE2 with
albumin to relieve immunosuppression in cirrhosis. Nat Med.
20:467–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Du Clos TW and Mold C: C-reactive protein:
An activator of innate immunity and a modulator of adaptive
immunity. Immunol Res. 30:261–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nozoe T, Matsumata T and Sugimachi K:
Preoperative elevation of serum C-reactive protein is related to
impaired immunity in patients with colorectal cancer. Am J Clin
Oncol. 23:263–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Abramovitch R, Marikovsky M, Meir G and
Neeman M: Stimulation of tumour growth by wound-derived growth
factors. Br J Cancer. 79:1392–1398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Canna K, Hilmy M, McMillan DC, Smith GW,
McKee RF, McArdle CS and McNicol AM: The relationship between
tumour proliferative activity, the systemic inflammatory response
and survival in patients undergoing curative resection for
colorectal cancer. Colorectal Dis. 10:663–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Coffelt SB and de Visser KE: Cancer:
Inflammation lights the way to metastasis. Nature. 507:48–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bito R, Hino S, Baba A, Tanaka M, Watabe H
and Kawabata H: Degradation of oxidative stress-induced denatured
albumin in rat liver endothelial cells. Am J Physiol Cell Physiol.
289:C531–C542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu J, Ding P, Wu H, Yang P, Guo H, Tian Y,
Meng L and Zhao Q: Sarcopenia: Molecular regulatory network for
loss of muscle mass and function. Front Nutr. 10:10372002023.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ding P, Guo H, He X, Sun C, Lowe S,
Bentley R, Zhou Q, Yang P, Tian Y, Liu Y, et al: Effect of skeletal
muscle loss during neoadjuvant imatinib therapy on clinical
outcomes in patients with locally advanced GIST. BMC Gastroenterol.
22:3992022. View Article : Google Scholar : PubMed/NCBI
|